Abstract
Background
Anemia remains a public health problem in Rwanda, affecting 38% of young children and 17% of reproductive-aged women (Demographic and Health Survey [DHS] 2010). The importance of iron deficiency (ID) as a cause of anemia in Rwanda is not known.
Objective
We aimed to estimate the prevalence of ID and iron deficiency anemia (IDA) among young children and women in 2 provinces of Rwanda.
Methods
We conducted a cluster randomized survey, selecting 408 rural households each in the Northern and Southern Provinces of Rwanda in 2010. Anemia was defined as hemoglobin <110 g/L in children and <120 g/L in nonpregnant women after correction for altitude. We defined ID as (1) serum transferrin receptor (TfR) >8.3 mg/L or (2) serum ferritin (SF) <12 µg/L in children and <15 µg/L in nonpregnant women after correction for inflammation.
Results
The prevalence of anemia was 30.9% (95% confidence interval [CI], 26.4-35.8) in children (n = 577) and 11.2% (95% CI, 8.4-14.7) in women (n = 595). The prevalence of ID in children was 3.1% (95% CI, 1.8-5.1) as defined by high TfR and 5.9% (95% CI, 4.0-8.4) as defined by low SF. Similarly, 3.0% (95% CI, 1.8-4.8) of women had high TfR and 4.8% (95% CI, 3.2-7.2) had low SF. The prevalence of IDA (low SF with concurrent anemia) ranged from 1.4% (95% CI, 0.5-3.6) among women in the North to 5.6% (95% CI, 3.1-10.0) among children in the South.
Conclusions
ID is likely not an important contributor to anemia in the Northern and Southern Provinces of Rwanda. This finding warrants further investigation into other causes of anemia.
Keywords: anemia, iron deficiency, prevalence, Rwanda
Introduction
Iron deficiency (ID) is considered the leading cause of anemia globally.1,2 The World Health Organization (WHO) estimates that 800 million women and young children worldwide were anemic in 2011 and that 42% of anemia in children and 50% of anemia in women could be eliminated with improved iron intake.3,4 Iron deficiency results from blood loss due to parasitic infestations and other gastrointestinal conditions or when iron demands are not met by insufficient bioavailable dietary iron.5 Life stages with high physiological demands associated with ID include pregnancy, periods of rapid growth and development in infancy and childhood, and onset of menarche.5 Thus, infants, young children, and reproductive-aged women are among the most atrisk population groups. There are a number of health risks associated with ID, including poor pregnancy outcomes, reduced cognitive function, decreased physical work productivity, and anemia.5,6 The estimated global burden of iron deficiency anemia (IDA) resulted in 45 million disability-adjusted life years in 2010.7
Despite recognition that ID poses a significant public health burden worldwide, there is very little survey data regionally or nationally using specific methods to measure iron status and estimate ID. Rather, anemia prevalence based on international hemoglobin cutoff values is frequently used for population assessment of iron status because it is inexpensive and user-friendly in field conditions. However, there is increasing awareness that the etiology of anemia varies significantly by region and country.8 Low hemoglobin levels can only be used speculatively for estimation of the actual attributable portion of anemia due to ID, particularly in developing countries. Other important causes of anemia include vitamin A and other nutrient deficiencies; infections such as malaria, hookworm, and schistosomiasis; and hemoglobinopathies.2,5
In Rwanda, anemia is also a public health concern, affecting 37% of children <5 years, 23% of pregnant women, and 19% of reproductive-age women who are nonpregnant and nonlactating.9 Demographic Health Surveys over the last 10 years show that the prevalence of anemia has declined substantially since 2005 when 52% of children and 26% of women were anemic.10 However, the prevalence of anemia has plateaued in recent years,9 suggesting additional efforts are needed to understand the causes and to effectively control anemia in Rwanda, where chronic hunger is significant as reflected in a 38% prevalence of stunting in children <5 years.9 The Rwandan diet is based on cereals, legumes, and tubers that are poor sources of readily absorbed iron, and there appears to be very little iron supplementation.11 Malaria prevalence is very low among children (1.4%) and women (0.7%),9 and the prevalence of intestinal parasites (eg, hookworm) or urinary parasites (eg, schistosoma) associated with chronic blood loss is also low.12 Therefore, inadequate dietary iron intake could be an important cause of anemia in Rwanda. As part of the preliminary research carried out in preparation for an efficacy trial of iron-biofortified beans, we conducted a cross-sectional, representative survey to characterize the iron status and determine the prevalence of ID and IDA among children <5 years and women of childbearing age in 2 predominantly rural provinces of Rwanda.
Methods
Study Design and Inclusion Criteria
We conducted a cross-sectional, representative survey in the Northern and Southern Provinces of Rwanda. Thirty-four villages were selected from each province. There were 2748 villages in the Northern Province and 3517 villages in the Southern Province. Selection of villages was random, based on probability proportional to sample size—that is, the probability of a village being randomly selected was equivalent to the village population divided by the total population of the province. Within each village, 12 households that met the inclusion criteria were randomly selected. The inclusion criteria were having at least 1 reproductive-aged woman (15-44 years), or 1 child (6-59 months old), who was a usual resident of the household. Random selection of households was by systematic sampling. A route through the village, in which every household was passed, was predetermined. Then at the starting point of that route, a random household was selected from the 1st through kth household, and every kth household after that, where:
k =(n households in village)÷ 12 and the value of k was rounded down to the nearest integer.
All analyses of blood data were weighted according to the probability of the individual being selected, and thus all results presented are statistically representative of the Northern or Southern Provinces, or the 2 provinces combined.
A sample of 408 households per province was targeted to yield a minimum of 198 nonpregnant and nonlactating women and 248 children 6 to 59 months of age after accounting for expected household composition, a design effect of 1.5 and a refusal rate of 10%. Sample size was calculated to estimate the prevalence of anemia in nonpregnant and nonlactating women and children 6 to 59 months of age with precision of ± 7.5%. The inclusion criterion into the survey was having at least 1 woman 15 to 44 years of age or 1 child 6 to 59 months age residing in the household. We excluded pregnant women from analysis of ID because currently there are no internationally agreed upon diagnostic criteria for ID during pregnancy.13,14
Ethics Considerations
A statement of the research purpose was communicated in the local language to eligible women and eligible children’s guardians. Written or verbal consent was obtained from all participating women, and informed consent was obtained from the mothers or guardians of all participating children. The research was approvedbythe National Ethics Committee of Rwanda (280/RNEC/2010), the research commission of the National University of Rwanda Faculty of Medicine and the HealthBridge Research Ethics Board (HBREB 2010_1).
Data Collection Procedures
Households were visited in November and December 2010. Upon consent, participants were registered, and information on age, medication use, dietary supplement use, morbidity status, pregnancy, lactation, family size, and socioeconomic status was collected. A venous blood sample (≥3 mL) was drawn by venipuncture and stored in a collection tube inside a cool box while in the field. The blood was allowed to settle and serum pipetted out and aliquoted into 0.2 mL tubes at the end of the day.
Hemoglobin (Hb) was measured on site using HemoCue Hb201+ (HemoCue AB, Ängelholm, Sweden) within 3 hours of blood collection. The HemoCue machines were calibrated and checked for accuracy daily before and after analysis by using 3-level Coulter controls provided by the manufacturer (Beckman Coulter, Krefeld, Germany).
Frozen serum samples were air shipped in a Styrofoam box with dry ice to Germany where the sandwich enzyme-linked immunosorbent assay technique was used to determine serum ferritin (SF), transferrin receptor (TfR), C-reactive protein (CRP), and a1-acid glycoprotein (AGP).15
Biomarkers and Definitions Used to Assess Iron Status
Iron status was evaluated with Hb, SF, TfR, and total body iron (TBI) stores. The Hb values were adjusted for altitude, using the methods described by the Centers for Disease Control and Prevention.16 The SF was adjusted according to an individual’s inflammation status, based on CRP and AGP measures.17 The inflammatory state of each individual was classified as “healthy” if neither CRP nor AGP were raised (CRP ≤5 mg/L; AGP ≤1 g/L); “incubating” if only CRP was raised (SF multiplied by correction factor [CF] of 0.77); “early convalescence” if both CRP and AGP were raised (CF = 0.53); and “late convalescence” if only AGP was raised (CF = 0.75). We calculated TBI using Cook’s formula: body iron (mg/kg) = – (log10(TfR/SF) – 2.8229) / 0.1207.18 Anemia was defined as an Hb concentration adjusted for altitude less than 110 g/L for children and less than 120 g/L for women, according to internationally recognized definitions.13 Iron deficiency was defined using the following criteria, using inflammation-adjusted indicators when appropriate: SF concentration <12 µg/L for children or <15 µg/L for women; TfR concentration >8.3 µg/mL; and TBI <0 mg iron/kg body weight. Iron deficiency anemia was defined as anemia with concurrent ID using the definitions for anemia and low SF concentrations as defined above. We further considered applying the WHO definition for ID in children (SF <30 µg/L) which is recommended for use when chronic infections are widespread.13 However, the literature on infectious disease prevalence in Rwanda9,12 and our findings on morbidity and inflammation did not justify its use.
Statistical Analyses
Household and sociodemographic characteristics of all individuals participating in the survey at enrollment were reported as means and standard deviations or percentages. Biochemical data were checked for normality and transformed as necessary on the logarithmic scale. Weighted arithmetic or geometric means, percentages, and associated 95% confidence intervals (CI) were estimated. We used STATA v.13 software (StataCorp) set of survey data commands to account for the multistage cluster sampling design and sampling weights in the point estimates and variance estimation methods.19 All results on iron status and deficiency are representative at the provincial level and of the 2 provinces combined.
Results
In total, 362 households from the Northern Province and 346 households from the Southern Province participated in the study, resulting in 679 women and 650 children completing the survey and 595 nonpregnant women and 577 children providing valid biochemical results. Characteristics of households, women, and children are presented in Table 1. Approximately one-third of households reported having access to piped water, 5% with electricity, and 10% with cement floors. Women were, on average, 31 years old and children were 34 months old. Forty percent of children in the Northern Province and 47% in the Southern Province were currently breastfeeding at the time of the survey. Very few women and children (2.1% and 1.3%, respectively) reported having taken iron supplements in the past 6 months, and use of insecticide-treated nets was reported to be very high.
Table 1.
Household and Participant (Children and Women) Characteristics at Enrollment.
| Northern Province | Southern Province | Total | |
|---|---|---|---|
| Altitude in meters, min and max | 1393-2422 | 1450-2403 | - |
| Household characteristics | - | - | - |
| n | 362 | 346 | 708 |
| Agriculture is primary source of income, % | 68.3 | 65.6 | 67.0 |
| Primary water source from pipe, % | 36.6 | 31.4 | 34.2 |
| Electricity in home, % | 5.8 | 3.2 | 4.5 |
| Cement floor in household, % | 8.0 | 10.7 | 9.3 |
| Children | |||
| n | 307 | 343 | 674 |
| Age in months, mean (SD) | 35.0 (15.4) | 33.5 (14.7) | 34.3 (15.1) |
| Sex (boys), % | 48.2 | 45.8 | 47.5 |
| Breastfeeding, % | 39.6 | 47.2 | 43.4 |
| Iron supplement use in past 6 months, % | 0.6 | 2.0 | 1.3 |
| Antimalarial drugs in past 6 months, % | 8.0 | 16.3 | 12.1 |
| Deworming drugs in past 6 months, % | 65.8 | 36.4 | 51.3 |
| Use of insecticide-treated nets, % | 92.2 | 91.4 | 91.8 |
| Symptoms of illness yesterday, % | - | - | - |
| Fever | 10.9 | 14.3 | 12.6 |
| Diarrhea | 5.5 | 10.4 | 7.9 |
| Women | |||
| N | 357 | 322 | 679 |
| Age in years, mean (SD) | 30.9 (6.3) | 30.9 (6.9) | 30.9 (6.6) |
| Pregnant, % | 10.6 | 10.9 | 11.2 |
| Lactating, % | 56.0 | 64.8 | 60.6 |
| Iron supplement use in past 6 months, % | 2.2 | 0.7 | 2.1 |
| Antimalarial drugs in past 6 months, % | 10.1 | 9.0 | 9.6 |
| Deworming drugs in past 6 months, % | 16.0 | 6.8 | 11.6 |
| Use of insecticide-treated nets, % | 91.6 | 90.7 | 91.2 |
| Symptoms of illness yesterday, % | |||
| Fever | 5.9 | 9.0 | 7.4 |
| Diarrhea | 2.2 | 4.3 | 3.2 |
Approximately 30% of children and 15% of women had some form of inflammation (Table 2). Estimated mean concentrations of iron status indicators and prevalence of anemia, ID, and IDA are presented in (Table 3). After adjustment for altitude, mean Hb concentrations were 4.3 g/L greater among children (P < .001) and 3.4 g/L greater among women (P < .05) living in the Northern Province than children and women from the Southern Province. Similarly, mean SF concentrations adjusted for inflammation were significantly greater in the Northern Province than in the Southern Province: 43.4 µg/L versus 35.2 µg/L (P < .05) among children and 66.8 µg/L versus 49.3 µg/L (P < .001) among women. There were no significant differences between provinces in mean TfR concentrations among either children or women.
Table 2.
Inflammation Statusa in Rwandan Women and Children.
| Healthy Reference(No APPb Raised),% | Incubation(Raised CRPc Only),% | Early Convalescence(Raised CRP and AGPc,d),% | Late Convalescence(Raised AGP Onlyd),% | ||
|---|---|---|---|---|---|
| N | |||||
| Children | 577 | 70.9 | 1.9 | 11.8 | 15.4 |
| Women | 595 | 84.5 | 3.2 | 4.2 | 8.1 |
Inflammation status as defined in Thurnham et al.17
APP ¼ acute-phase protein; APP refers to C-reactive protein and α1-acid glycoprotein.
CRP ¼ C-reactive protein; cutoff value CRP >5.0 mg/L.
AGP ¼ α1-acid glycoprotein; cutoff value AGP >1.0 g/L.
Table 3.
Prevalence of Anemia and Iron Deficiency Through the Application of Several Definitions.
| Northern Province | Southern Province | Total | |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Children | |||
| n | 290 | 287 | 577 |
| Hemoglobin (Hb; g/L) | 123.5 (121.7-125.4) | 118.2 (116.3-120.1) | 121.0 (119.7-122.4) |
| Hb (g/L), adjusted for altitudea | 116.6 (114.7-118.5)f | 112.3 (110.6-114.0) | 114.6 (113.3-115.9) |
| Serum ferritin (SF; µg/L) | 48.2 (43.3-53.7)d | 40.4 (35.7-45.8) | 44.4 (40.9-48.1) |
| SF (µg/L), adjusted for inflammationb | 43.4 (38.9-48.4)e | 35.2 (31.5-39.3) | 39.3 (36.4-42.4) |
| Transferrin receptor (TfR; µg/mL) | 4.0 (3.7-4.4) | 3.9 (3.6-4.1) | 3.9 (3.7-4.2) |
| Total body iron (TBI; mg/kg body weight) | 7.5 (7.0-7.9) | 7.1 (6.6-7.6) | 7.2 (6.9-7.6) |
| TBI (mg/kg body weight), adjusted for inflammation | 7.0 (6.4-7.5) | 6.5 (6.0-7.0) | 6.8 (6.4-7.2) |
| Women | |||
| n | 313 | 282 | 595 |
| Hb (g/L) | 142.8 (141.1-144.5) | 138.3 (136.2-140.5) | 140.8 (139.4-142.2) |
| Hb (g/L), adjusted for altitudea | 135.9 (134.0-137.9)d | 132.5 (130.4-134.5) | 134.4 (132.9-135.8) |
| SF (µg/L) | 70.5 (62.9-79.1)f | 52.5 (47.0-58.6) | 61.8 (57.0-67.0) |
| SF (µg/L), adjusted for inflammationb | 66.8 (59.4-75.2)f | 49.3 (44.0-55.1) | 58.3 (53.7-63.3) |
| TfR (µg/mL) | 3.9 (3.7-4.2) | 3.6 (3.3-3.9) | 3.8 (3.6-4.0) |
| TBI (µg/kg body weight) | 8.90 (8.40-9.41)d | 8.71 (8.20-9.22) | 8.6 (8.2-8.9) |
| TBI (µg/kg body weight), adjusted for inflammation | 8.17 (7.67-8.67)d | 7.94 (7.43-8.45) | 8.4 (8.0-8.7) |
Adjustment for altitude using methods proposed by CDC.16
Corrected for inflammation status.17
Cutoff used is 8.3 based on equivalency to Ramco assay.15
P value <.05 for differences between Northern and Southern Provinces.
P value <.01 for differences between Northern and Southern Provinces.
P value <.001 for differences between Northern and Southern Provinces.
The overall prevalence of anemia was 30.9% (95% CI, 26.4-35.8) among children and 11.2% (95% CI, 8.4-14.7) among women (Table 4). The prevalence of anemia tended to be higher in the Southern Province than the Northern Province, although differences did not reach statistical significance. The prevalence of ID among children ranged from 3.1% (95% CI, 1.8-5.1) as assessed by elevated TfR concentrations to 5.9% (95% CI, 4.0-8.4) as assessed by low SF concentrations. Similarly, the prevalence of ID among women ranged from 3.0% (95% CI, 1.8, 4.8) using the TfR definition to 4.8% (95% CI, 3.2, 7.2) using the SF definition. The Southern Province had a higher prevalence of low SF concentrations among children (8.9% vs 3.1%; P < .05) and women (6.8% vs 3.3%; P > .05). There were no differences between provinces in the prevalence of elevated TfR. In all groups, the prevalence of ID with concurrent anemia was very low, ranging from 1.4% (95% CI, 0.5-3.6) among women in the Northern Province to 5.6% (95% CI, 3.1-10.0) among children in the Southern Province. The prevalence of ID without concurrent anemia was also very low, ranging from 1.2% (95% CI, 0.4-3.8) among children in the Northern Province to 3.8% (95% CI, 1.9-7.3) among women in the Southern Province.
Table 4.
Prevalence of Iron Deficiency Anemia and Iron Deficiency Without Anemia in Women and Children in the Northern and Southern Provinces of Rwanda.
| Northern Province | Southern Province | Total | |
|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | |
| Children | |||
| n | 290 | 287 | 577 |
| Anemia (Hb <110 g/L)a | 26.5 (20.7-33.3) | 35.8 (29.1-43.1) | 30.9 (26.4-35.8) |
| Iron deficiency, by definition | |||
| Low SF (<12 µg/L)b | 3.1 (1.6-5.9)d | 8.9 (5.7-13.7) | 5.9 (4.0-8.4) |
| High TfR (>8.3 µg/mL)c | 2.9 (1.2-6.8) | 3.3 (1.8-5.8) | 3.1 (1.8-5.1) |
| Low TBI (<0 µg/kg body weight) | 2.2 (0.9-5.1) | 5.3 (3.0-9.4) | 3.7 (2.3-5.9) |
| Iron deficiency (SF <12 µg/L) with anemiaa,b | 1.9 (0.8-4.5) | 6.2 (3.5-10.9) | 3.9 (2.4-6.4) |
| Iron deficiency (SF <12 µg/L) without anemiaa,b | 1.2 (0.4-4.8) | 2.7 (1.3-5.4) | 1.9 (1.0-3.5) |
| Women | |||
| N | 313 | 282 | 595 |
| Anemia (Hb <120 g/L)a | 7.9 (5.1-11.9)d | 15.3 (10.4-21.8) | 11.2 (8.4-14.7) |
| Iron deficiency, by definition | |||
| Low SF (<15 µg/L)b | 3.3 (1.5-7.1) | 6.8 (4.3-10.5) | 4.8 (3.2-7.2) |
| High TfR (>8.3 µg/mL)c | 3.0 (1.6-5.6) | 3.0 (1.5-6.0) | 3.0 (1.8-4.8) |
| Low TBI (<0 µg/kg body weight) | 1.7 (0.5-5.4) | 1.7 (0.6-4.5) | 1.7 (0.8-4.7) |
| Iron deficiency (SF <15 µg/L) with anemiaa,b | 1.4 (0.5-3.7) | 3.0 (1.6-5.7) | 2.1 (1.2-3.6) |
| Iron deficiency (SF <15 µg/L) without anemiaa,b | 1.9 (0.8-4.3) | 3.8 (1.9-7.3) | 2.7 (1.6-4.6) |
Abbreviations: Hb, hemoglobin; TfR, transferrin receptor; TBI, total body iron.
Anemia is defined as an abnormal hemoglobin concentration of <120 g/L for nonpregnant women and <110 g/L for children and pregnant women, adjusted for altitude.16
SF ¼ serum ferritin, corrected for inflammation status.17
Cutoff used is 8.3 mg/mL based on equivalency to Ramco assay reference.15
P value <.05 for difference between Northern and Southern Provinces.
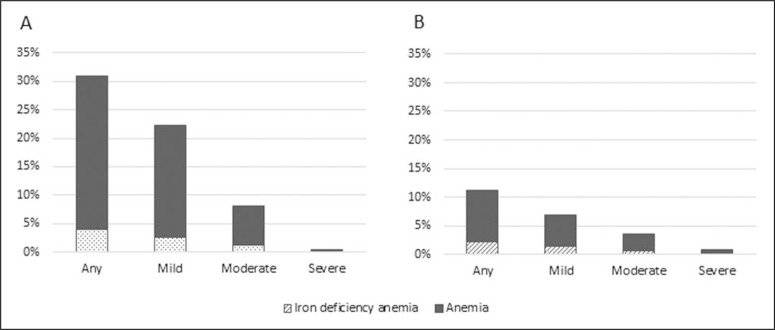
Most forms of anemia were mild or moderate (Figure 1). Severe anemia was rare in young children and women. Iron deficiency anemia constituted a small proportion of anemia among children and women, about 13% and 19% of total anemia, respectively, with slight variation across mild and moderate categories of anemia severity.
Figure 1.
Prevalence of anemia and iron deficiency anemia, by severity of anemia,a among children <5 years (Panel A) and reproductive-age women (Panel B) in the Northern and Southern Provinces of Rwanda. aSevere anemia is defined as Hb <70 g/L and moderate anemia is defined as Hb 70 to 99 g/L for both children <5 years and women; mild anemia is defined as Hb 100 to 109 g/L for children <5 years and Hb 100 to 119 g/L for nonpregnant women. Prevalence of anemia is adjusted for altitude using CDC formulas16 and prevalence of iron deficiency anemia is further corrected for inflammation status.17 Hb indicates hemoglobin; CDC, Centers for Disease Control and Prevention.
Discussion
Rwanda. Guidelines on the interpretation of SF and TfR concentrations in population surveys To our knowledge, this is the first representative suggest that if the proportion of SF values below survey characterizing the iron status and prevathe threshold is <20% and the proportion with lence of ID among women and children in TfR values above threshold is <10%, then ID is not of public health concern.14 In our study, we found that ID was prevalent among 3% to 6% of children and 3% to 5% of women, depending on the definition of ID used. In comparison, we estimated the prevalence of anemia at 31% among children and 11% among women, respectively. The prevalence of IDA was very low ranging at 4% and 2% of children and women, respectively.
Regionally, we found the Southern Province had a greater burden of ID and anemia. The prevalence of anemia among children in the Southern Province was nearly 10% greater than in the North (P = .053). Similarly, 7% more women in the Southern Province were anemic (P < .05) than in the North. We found a 3-fold higher prevalence of ID as defined by low SF concentrations among children in the Southern Province than in the Northern Province (P < .05); however, regional differences among women were not statistically significant. Similarly, we did not find regional differences in other iron status indicators.
The children in the Northern Province were equally likely to have ID with or without anemia, while children from the Southern Province were more likely to have ID with concurrent anemia. This suggests that the etiologies of anemia are different between provinces. Extant reports indicate that the prevalence of malaria is very low, but it is higher in the Southern Province (4.4% among children <5 years and 0.9% among women) than the Northern Province (0.0% among children and 0.1 % among women),9 and helminth infection is higher in the Northern Province where school children have been reported as carriers of Ascaris lumbricoides twice as frequently (38%) as in the Southern Province (19.5%).20
Our results do not markedly differ from subsequent studies carried out by our research team in Rwanda (unpublished data). In a convenience sample of 1022 young women (age range 12-26 years) attending secondary-level boarding schools in Kigali, ID and IDA were present in 12.3% and 4.8% of participants, respectively. In a second convenience sample of 1000 nonpregnant, nonlactating women attending the National University of Rwanda (age range 18-39 years), ID and IDA affected 19.5% and 6.7% of women, respectively. Although the prevalence of ID is higher in comparison to what we found in our survey of the Northern and Southern Provinces, the proportion of women who were iron deficient was still low. Furthermore, the data from female students are not representative of the target group.
Our estimates of anemia in children are very similar to what is reported in the 2010 Demographic and Health Survey (DHS), which was conducted the same year, indicating validity of our methods. We report a prevalence of 26.5% in the Northern Province and 35.8% in the Southern Province, whereas the DHS reports 30.6% and 37.5%, respectively. Our confidence intervals overlap the DHS estimates, and the regional trend is the same. Similarly, our estimates of anemia prevalence in women in the Northern and Southern Provinces are 4 and 2 percentage points lower than what is reported in the 2010 DHS; however, our estimates exclude pregnant women, which may explain the discrepancy.
This is not the first survey to report very low prevalence of ID in areas where anemia is a public health problem.21 - 23Surveys conducted in rural areas of Bangladesh,21Cambodia,23and the Democratic Republic of the Congo22report that ID was very low or nonexistent. Further investigations in Bangladesh found that elevated iron concentrations in groundwater contributed substantial amounts of iron to the diet, which explained why ID was not found in this population21. Hemoglobinopathies,21,23 higher parity among women,21and zinc and folic acid deficiencies23were some of the determinants of anemia in the surveys that explored additional pathways.
Because the etiology of anemia is multifactorial, it is possible that the moderately high rates of anemia in our study may be due to other micronutrient deficiencies, chronic infections, and hemoglobinopathies. A multiple micronutrients status and hemoglobinopathies survey among reproductive-aged women and preschool children could more conclusively address the question on the etiology of anemia among Rwandan children and women as sickle cell anemia, thalassemia, or other hemoglobinopathies may also be important causes of anemia in this population.24,25 In addition, a thorough investigation of the concentration and bioavailability of iron from groundwater could further elucidate why the prevalence of ID and IDA is very low in this population. However, we conducted a water and soil iron concentration study (unpublished) in an area of Rwanda with very low ID prevalence and found no correlation between soil iron concentration and iron status.
Our findings are consistent with national trends showing that the quality of life has been improving in Rwanda. Over the past 2 decades, Rwanda has made steady progress in economic and social development and in health access and health equity.26,27 Under 5 mortality rates have declined from 150 in 1990 to 76 in 2010 and further to 42 deaths per 1000 live births in 2015.28 Fever among children <5 years has also declined since 2005, although reports of children experiencing diarrhea within 2 weeks of the survey remained relatively stagnant from 14.5% in 2005 to 12.3% in 2014.9 A decline in the prevalence of anemia has also been shown, although recent DHS suggest the trend has reached a plateau.9 In addition to the low malaria prevalence among children and women in Rwanda, the prevalence of HIV in Rwandan women is very low, affecting 2.5% of women in the North and 3.2% of women in the South.9
We cannot rule out that our ID prevalence estimates may be higher than measured. Errors in measurement of SF, TfR, and Hb are possible. However, a second blood sample was drawn from 96 women and 76 children, and SF and TfR were analyzed by a different, independent lab and results were not different from the first round of data reported here, thus it is unlikely that they would both have large errors in the same direction.
Conclusions
The primary objective of this research was to serve as a background nutrition study for the planned roll out of biofortified beans and an effectiveness study by HarvestPlus. Rwanda is relatively understudied, in terms of the causes and underlying factors of anemia, with little biochemical data. This study helps fill the gap by providing the first report of ID among women and children in the predominantly rural Northern and Southern Provinces of Rwanda. We find little evidence that ID is a public health problem among this population. However, anemia is a public health problem in these provinces, and further research is needed to determine its causes.
Acknowledgments
We thank Dr Aflodis Kagaba and Dr Joseph Nkurunziza from Health Development Initiative Rwanda (www.hdirwanda.org) who facilitated project logistics and transportation throughout the survey. We also thank Jackie Kungu who coordinated Health-Bridge’s work in Rwanda, including training, arranging for blood analysis, and supervising the first week of data collection.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia In: Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004:163-209. [Google Scholar]
- 2.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. . A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization The Global Prevalence of Anaemia in 2011. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 4.Stevens GA, Finucane MM, De-Regil LM, et al. ; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Heal. 2013;1(1):e16-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370(9586):511-520. [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Victora CG, Walker SP, et al. ; Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427-451. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJ, Vos T, Lozano R, et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197-2223. [DOI] [PubMed] [Google Scholar]
- 8.Suchdev PS, Namaste SM, Aaron GJ, Raiten DJ, Brown KH, Flores-Ayala R; BRINDA Working Group. Overview of the biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Adv Nutr. 2016;7(2):349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute of Statistics of Rwanda (NISR), Ministry of Health (MOH) [Rwanda], ICF International. Rwanda Demographic and Health Survey 2014–15. Rockville, MD: NISR, MOH, and ICF International; 2015. [Google Scholar]
- 10.Institut National de la Statistique du Rwanda (INSR) and ORC Macro Rwanda Demographic and Health Survey 2005. Calverton, MD: INSR and ORC Macro; 2006. [Google Scholar]
- 11.Ministry of Health R of R National Nutrition Policy. Rwanda: Kigali; 2005. [Google Scholar]
- 12.Mupfasoni D, Karibushi B, Koukounari A, et al. . Polyparasite helminth infections and their association to anaemia and undernutrition in Northern Rwanda. PLoS Negl Trop Dis. 2009;3(9): e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 14.World Health Organization/Centers for Disease Control and Prevention Assessing the Iron Status of Populations: Including Literature Reviews: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. 2nd ed Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 15.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004; 134(11):3127-3132. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep; 1989;38(22):400-404. [PubMed] [Google Scholar]
- 17.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92(3):546-555. [DOI] [PubMed] [Google Scholar]
- 18.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9): 3359-3364. [DOI] [PubMed] [Google Scholar]
- 19.StataCorp Survey Data Reference Manual: Release 13. College Station, TX: Stata Press; 2013. [Google Scholar]
- 20.Dushimimana A, Condo J, Munyanshongore C. La Situation Nutritionnelle et infections parasitaires chez les Enfants de l’ecole primaire au Rwanda. Final Report. Kigali City, Rwanda: Institut National de la Statistique; 2008. [Google Scholar]
- 21.Merrill RD, Shamim AA, Ali H, et al. . High prevalence of anemia with lack of iron deficiency among women in rural Bangladesh: a role for thalassemia and iron in groundwater. Asia Pac J Clin Nutr. 2012;21(3):416-424. [PubMed] [Google Scholar]
- 22.Harvey-Leeson S, Karakochuk CD, et al. Hawes M, Anemia and micronutrient status of women of childbearing age and children 6–59 months in the Democratic Republic of the Congo Nutrients. 2016;8(2):98-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieringa F, Sophonneary P, Whitney S, et al. . Low prevalence of iron and vitamin A deficiency among Cambodian women of reproductive age. Nutrients. 2016;8(4):197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munyanganizi R, Cotton F, Vertongen F, Gulbis B. Red blood cell disorders in Rwandese neonates: screening for sickle cell disease and glucose-6-phosphate dehydrogenase deficiency. J Med Screen. 2006;13(3):129-131. [DOI] [PubMed] [Google Scholar]
- 25.Gahutu JB, Musemakweri A, Harms G, Mocken-haupt FP. Prevalence of classic erythrocyte polymorphisms among 749 children in Southern Highland Rwanda. Trans R Soc Trop Med Hyg. 2012;106(1):63-65. [DOI] [PubMed] [Google Scholar]
- 26.US Agency for International Development Rwanda: Nutrition Profile. Washington DC: USAID; 2014. https://www.usaid.gov/what-we-do/global-health/nutrition/countries/rwanda-nutrition-profile. Accessed 20 July 2016. [Google Scholar]
- 27.Binagwaho A, Farmer PE, Nsanzimana S, et al. . Rwanda 20 years on: investing in life. Lancet. 2014;384(9940):371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UN Inter-agency Group for Child Mortality Estimation Levels and Trends in Child Mortality: Report 2015. New York, NY: UNICEF; 2015. [Google Scholar]