Abstract
Introduction
Macroscopic anatomy has traditionally been taught using cadaveric material, lectures and a variety of additional resources including online modules and anatomical models. Traditional plastic models are effective educational tools yet they have significant drawbacks such as a lack of anatomical detail, a lack of texturisation and cost. Three-dimensional printed models stand to solve these problems and widen access to high-quality anatomical teaching. This paper outlines the use of three-dimensional multiplanar imaging (computed tomography) in the development of an accurate model of the hepatobiliary system.
Materials and methods
Computed tomography scans were used to construct a virtual three-dimensional model of the hepatobiliary system. This was printed locally as a full-size colour model. We give a complete account of the process and software used.
Discussion
This study is among the first of a series in which we will document the newly formed Oxford Library of Anatomy. This series will provide the methodology for the production of three-dimensional models from computed tomography and magnetic resonance imaging scans, and the library will provide a complete collection of the most complex anatomical areas. We hope that these models will form an important adjunct in teaching anatomy to medical students and surgical trainees.
Keywords: Anatomy, Education, Printing, Three-dimensional, Liver, Biliary tract, Pancreas
Introduction
The use of cadavers for teaching anatomy to undergraduate and postgraduate students has classically been the undisputed gold standard.1,2 Despite changing attitudes to the clinical applicability of using such methods for undergraduate teaching, issues with cost and access to bodies, and recent technological advances,3,4 it is still widely accepted that cadaveric methods should be maintained and recent advances used as adjuncts to learning.5 Indeed, students learn best when multiple pedagogical resources are used to complement one another.6 Examples of such adjuncts are numerous and can largely be split into non-digital and digital. Plastinated specimens, such as von Hagens,7 and plastic models represent the bulk of the former, while three-dimensional (3D) imaging techniques, virtual reality and augmented reality represent examples of the latter.8–10
Plastic models are prevalent in medical education and have consistently been shown to be effective for the teaching of anatomy.11–13 They can help to mitigate the work of maintaining large libraries of physical cadaveric models, but costs are often high, anatomical variation non-existent, and anatomical accuracy only as good as the stylised caricature produced by the artist.14
Consistent with studies on plastic models, a growing body of evidence points to the usefulness of 3D printed models in the teaching of anatomy. Studies show that 3D printed models are just as good as, and in some places better than, classical cadaveric or book-based teaching resources.15–17 In addition, 3D printing presents solutions to the aforementioned issues with traditional plastic models. Where ‘in-house’ production is possible, costs can be lower and numerous models of the same system can depict differing anatomical variation.14 Systems can also be printed in isolation to demonstrate complex anatomy rarely shown on traditional plastic models. Further, for complex anatomy, it is feasible to print each student an individual copy for future study.18
Recent advances in 3D printing have meant that hardware costs have fallen significantly and the selection of colours and materials has increased.19 At the same time, open-source software for designing models and, in some cases, even detailed open-source models files for printing have become available.20 Clearly, the uptake of this technology by anatomy departments will increase in the coming years.
In this project, we used the hepatobiliary system as the focal point and aimed to provide a comprehensive road map detailing how to use computed tomography (CT) imaging data to produce a highly accurate 3D printed model for teaching medical students.
Materials and methods
This 3D printed anatomically correct model was formed following a collaboration between the University of Oxford and 3D LifePrints, a medical 3D printing and technology organisation. The team consisted of the director of anatomy, a specialist surgeon, an anatomical artist, a radiologist and medical students. The model consists of the following components:
liver
gall bladder and bile duct
stomach
duodenum
kidneys
pancreas
descending abdominal aorta and branches
inferior vena cava
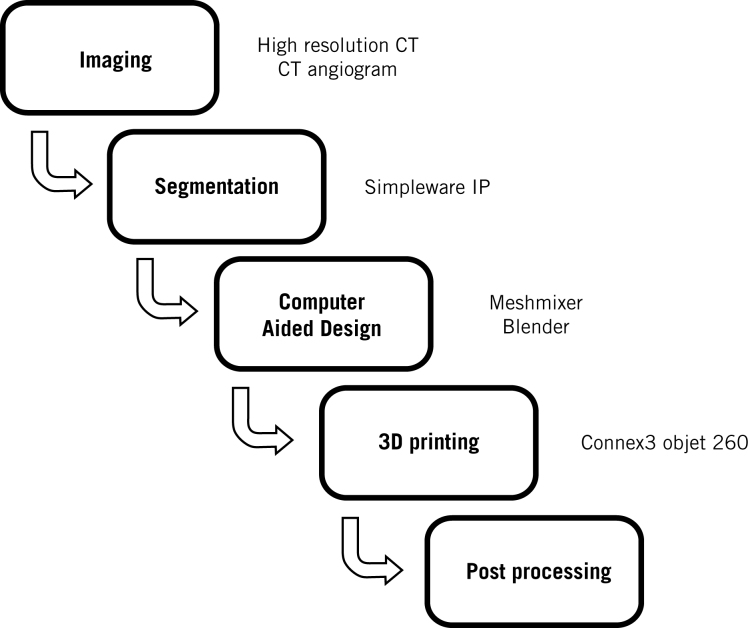
The project workflow is shown in Figure 1 and is described below.
Figure 1.
The production workflow
Results
Imaging
To provide true anatomical representation, our model was recreated from high-resolution CT abdomen scan images in Digital Imaging and Communication in Medicine (DICOM) format. To preserve anatomical detail the slice thickness of our scans was 0.625mm. During imaging, we performed a CT angiogram to allow appreciation of the complex blood supply to the hepatobiliary system.
Segmentation of anatomical structures
Anonymised DICOM data were uploaded to Simpleware ScanIP (www.simpleware.com/software/scanip) in which the user semiautomatically classifies anatomical structures using Hounsfield units to determine tissue density. This allows for differing anatomical structures to be segmented into separate 3D objects, which ultimately allows the whole model to be printed with differing anatomical structure in different colours and/or materials. Tissue classification happens in a slice-by-slice manner and so, owing to the absence of imaging data for the space between slices, forms a scaffold that defines the outline of the anatomical structure and defines the object’s geometry. Formation of the 3D objects requires the interpolation of a scaffold into a 3D mesh which takes the shape of the selected tissue type.
Owing to the low-contrast gradients between some abdominal anatomical structures on CT imaging, semiautomatic segmentation was not possible for some anatomical structures. Manual segmentation may have taken a number of weeks,21 and so the following organs were not extracted from CT images: gall bladder, biliary tract, oesophagus, stomach, duodenum, falciform ligament and venous system.
Figure 2 shows the 3D mesh representing the anatomical structures that were segmented on Simpleware ScanIP. Where appropriate these 3D meshes were digitally optimised using Meshmixer® (version 3.5; www.meshmixer.com). Meshmixer allows for meshes exported from Simpleware ScanIP to be repaired, modified and smoothed. This largely consisted of removing surface irregularities and sharp edges which presented as artefacts from the creation of the mesh. This was particularly important in the case of the pancreas.
Figure 2.
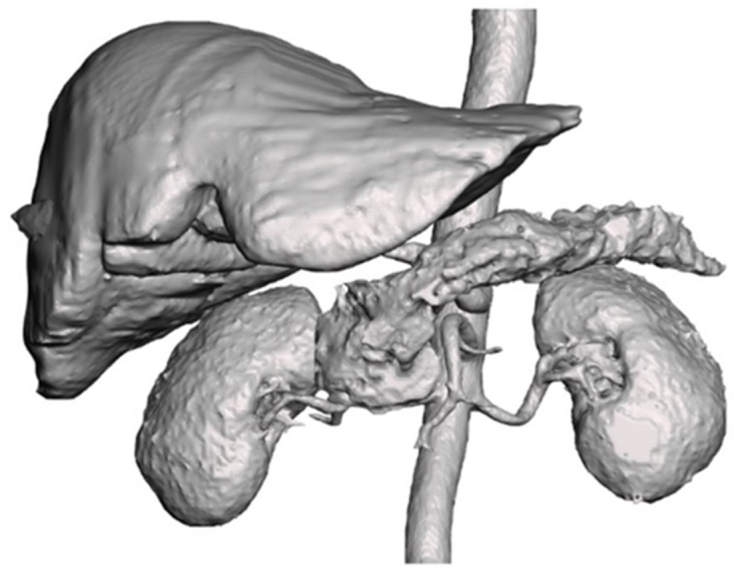
The three-dimensional (3D) mesh representing the anatomical structures that were segmented into 3D objects from the computed tomography scan
Computer-aided design
The modified 3D meshes that were exported from Meshmixer were redesigned in Blender (version 2.82; www.blender.org) by an expert anatomy artist under the guidance of the director of anatomy. Blender was used to ‘create’ the anatomy that was not extracted from CT images in Simpleware ScanIP (gall bladder, biliary tract, oesophagus, stomach, duodenum, falciform ligament, the venous system, and the lobes of the liver). In addition, the liver was sectioned in such a way as to allow appreciation of the segmental anatomy of the liver. Blender also allowed for the selection of colours to match the anatomical structures, and for the depiction of the eight Couinaud segments (I–VIII) that represent the functional liver anatomy.
The redesigned 3D meshes were then exported out of Blender and back into Meshmixer. Minor positional changes were made to ensure all component pieces were aligned correctly, thus forming the digital prototype (Figures 3 and 4) of many objects that would be printed together to form a 3D model. This alignment is critical as it avoids the overlapping of objects which causes printing errors. Meshmixer was also used to create a stand for the model that was based on the anatomy and was used to insert connectors to support the model (see Fig 5).
Figure 3.
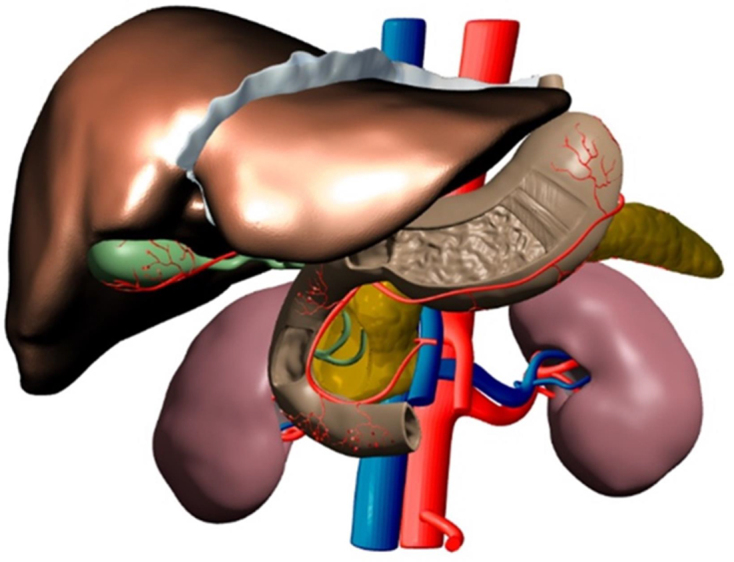
The main view of the digital prototype displaying anatomy from a computed tomography scan
Figure 4.
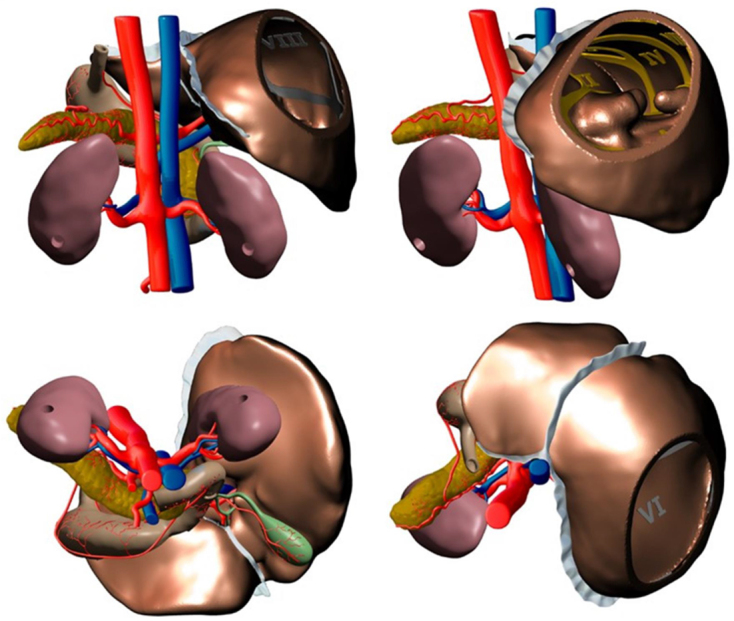
Alternative views of the digital prototype
Figure 5.
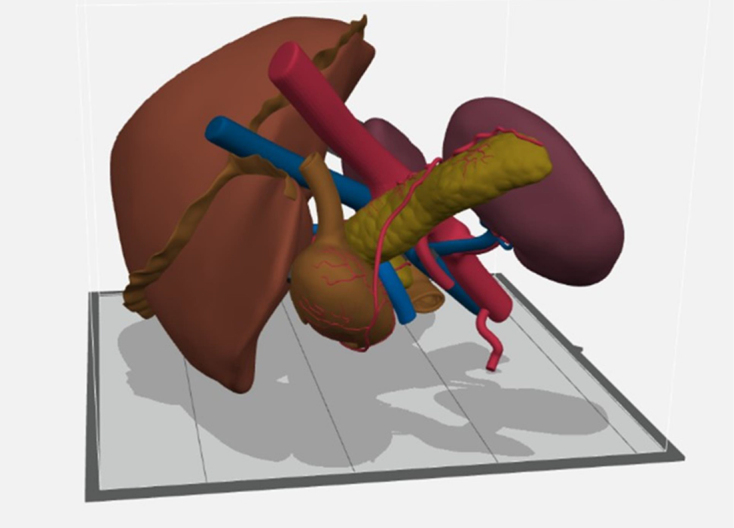
The model as viewed in GrabCAD immediately prior to printing
Once the digital 3D model was completed the file was converted to the Standard Tessellation Language (STL) file format and uploaded to the 3D printer software GrabCAD (https://grabcad.com) for production.
Material selection
The colour range of the model was determined by the material selection, which was determined by the 3D printing hardware available to 3D LifePrints. The printer was a Connex3 Objet 260 (Stratasys, Eden Prairie, MN), which allows for three material to be simultaneously printed. The materials chosen were VeroMagenta™, VeroYellow™ and VeroCyan™, which can be mixed to give a colour palette.
Printing process
GrabCAD calculates the most economical print orientation considering the need to support the overhanging 3D part of the model with a removable support polymer (SUP 706B support). After this calculation the printing process took 58 hours and the model was printed preassembled. The model used 1,835ml of plastic material, comprising 154ml of VeroCyan, 1,177ml of VeroMagenta and 505ml of VeroYellow. The removal support required 4,874ml of SUP 706B. The cost of the raw plastic materials alone was £1,343. After printing was complete, post-processing involved the use of a chemical solution to remove the support polymer. For added stability and better surface finish, the model was then spray coated with five coats of acrylic matt gloss paint, after which it was mounted in a case.
Results
We have described the production process of a 3D printed patient specific model of the hepatobiliary system for anatomical education purposes. Similar models are being developed across another 20 anatomical regions, including the brachial plexus, retroperitoneum, and base of skull. Ultimately the Oxford Library of Anatomy will represent a comprehensive library of some of the most complex anatomical structures.
Discussion
Flexibility of design benefits the surgical trainee
A key benefit of 3D printing is the flexibility it allows in the design and production of models. Premade plastic models typically come in a ‘one size fits all’ form, whereas 3D printing allows educators to personalise the model according to the level of education of the students. A crucial distinction here would be undergraduates and postgraduates, the latter often being surgical trainees who require a more intimate knowledge of anatomy and a knowledge of key surgical landmarks. For example, undergraduates need knowledge about the structure of the biliary tree and the effect of blockages, while surgical trainees must have an intimate understanding of anatomical relationships of the structures forming the cystohepatic triangle. This model will allow trainees a safe and accessible environment to develop confidence in their knowledge, and an accurate visualisation of the anatomy. Looking forward, such customisations of the model to match the level of knowledge required could be achieved by morphing the STL meshes.
For those needing anatomical knowledge to perform clinical procedures, there is no substitute for knowing the anatomical changes that precede the need for the procedure. Extracting new data from the CT imaging of patients with pathology would allow the use of 3D printed models of pathological anatomy (eg dilated bile ducts) in study and endoscopic training.22 Likewise, there is no substitute for exposure to anatomical variation for trainee surgeons. The ability to print real-life anatomical variations will benefit surgical trainees as it will speed up their training by helping prepare them for the jump from theoretical anatomical learning to the practical application of their knowledge in theatres.
Production of this model
The development of this model took considerable time and skill from all those involved. Discounting these development costs, the costs involved in running and maintaining the 3D printer, the costs of running the business the printer was owned by, the cost of the plastic materials for this model was £1,343. This falls under the US$2,000 mark that a 2018 review (US$2,000 was equivalent to c. £1,550 at the time of writing) found to be the upper limit of cost for models of the liver printed for surgical planning.23 It is a cost that is also comparable to the sum of plastic models of the same anatomy. For example, excluding postage and value added tax, a plastic model of the liver and gall bladder from a well-known manufacturer costs £329, a model of the pancreas with spleen and duodenum costs £260, a model of the stomach costs £406, and a model of a single kidney costs £275 (prices at 12 March 2020). It is worth noting that our 3D printed model is a true to size model, and so any scale model would be both cheaper and faster to print; a half-size model would use one-eighth the volume of plastic, so the cost of the plastic materials would be under £200. In addition, as with most new technology, prices of production are likely to fall as uptake becomes more widespread.19
While the cost of 3D printing to the standard required for teaching anatomy (as opposed to providing spatial perspective to surgeons planning operations) as described in the study is not an instant gamechanger; the technique does reduce ethical and safety concerns. The modern 3D printer can be housed in an office and printing comes with few, if any, ethical or safety concerns. By comparison, sourcing cadavers has become ever more difficult.24,25 Further, the storage of cadavers is complex, and wear and tear is to be expected, so a team of skilled anatomical prosectors must be employed to maintain the supply for teaching.
Issues with production
The semiautomatic segmentation method described above is helpful, yet for those anatomical tissues between which there is low contrast, it has limitations. As such a number of structures were drawn in following coordination between our senior anatomist, a software engineer and sometimes a medical artist. We believe this goes some way to explaining why many of the 3D models that have been published relate to areas with a stark distinction between tissues: blood vessels can use angiography, cardiac structures are easily seen on magnetic resonance imaging and the bone is well demarcated from soft tissue.21
It would be remiss to champion the uptake of 3D printing without some consideration of the environment. Worldwide and across many disciplines, 3D printing is likely to reduce CO2 emissions and lead to a more sustainable approach to manufacturing.26 This conclusion will be more nuanced if we are to use 3D printing as an additional teaching tool, especially if we consider printing personalised models for students. Either way, educators should be aware of the environmental and social costs of 3D printing.27
Limitations with this model
As seen in Figures 3–5, this model, designed for the teaching of undergraduates, does not have the same level of detail as is found in the human body. This same issue is found in other plastic models when compared with plastinated specimens of preserved cadavers.2,28 While a model with clinical applicability in the form of education useful for surgical trainees, as discussed above, is eminently printable, we acknowledge there are additional complexities. The depiction of the intrahepatic arteries, portal vein and hepatic vein branches would require alterations to the design, however the use of transparent plastic for the liver would make this endeavour worthwhile.
Despite the comparatively lower level of detail, this hepatobiliary model has good anatomical fidelity. However, the same cannot be said about its ability to replicate the tissue itself. Despite using state of the art printing methods, the final output from the STL file is printed in multi-colour rigid plastic. Therefore, this model would not be suitable for surgical training requiring dissection, cutting and suturing, and does not give students an appreciation of the texture, density and pliability of human tissue. Constructing models upon which practical training can be done has been well received in endoscopic training,29 and should be a target for future developments to this model. Bioprinting is an emerging field that seeks to 3D print whole functioning organs.30 While this would clearly be beyond the function necessary to teach anatomy at any level, the middle ground of a material found between this dream and our current reality would seem perfect.
Future perspective
This study is among the first of a series in which we will document the newly formed Oxford Library of Anatomy. When completed this will be a repository of a comprehensive selection of some of the most complex anatomical structures. As barriers to entry regarding in-house 3D printing continue to fall, it is hoped that medical students and schools around the world with access to 3D printing technology will be able to download and print the required models from our library. We hope to develop a new generation of 3D models that incorporate haptic technology such that a student will be presented with information (audially or visually) about different parts of the model as they lay their hands on lay their hands on it.
Conclusion
Here we give a methodology for printing a model of the hepatobiliary system to be used as an adjunct to cadaveric teaching. This printed model offers a tangible alternative to traditional cadaveric teaching while offering benefits over and above traditional plastic models. We will proceed with a study comparing the learning outcomes of medical students and core surgical trainees studying the hepatobiliary system anatomy for their respective exams using conventional methods with and without the addition of a 3D printed model.
References
- 1.Korf HW, Wicht H, Snipes RL et al. The dissection course: necessary and indispensable for teaching anatomy to medical students. Ann Anat 2008; : 16–22. [DOI] [PubMed] [Google Scholar]
- 2.Balta JY, Cronin M, Cryan JF, O’Mahony SM. The utility of cadaver-based approaches for the teaching of human anatomy: a survey of British and Irish anatomy teachers. Anat Sci Educ 2017; : 137–143. [DOI] [PubMed] [Google Scholar]
- 3.Howe A, Campion P, Searle J, Smith H. New perspectives: approaches to medical education at four new UK medical schools. BMJ 2004; : 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghazanfar H, Rashid S, Hussain A et al. Cadaveric dissection a thing of the past? The insight of consultants, fellows, and residents. Cureus 2018; : e2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memon I. Cadaver dissection is obsolete in medical training! A misinterpreted notion. Med Princ Pract 2018; : 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estai M, Bunt S. Best teaching practices in anatomy education: a critical review. Ann Anat 2016; : 151–157. [DOI] [PubMed] [Google Scholar]
- 7.von Hagens G. Impregnation of soft biological specimens with thermosetting resins and elastomers. Anat Rec 1979; : 247–255. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson SB, Hendricks BK, Cohen-Gadol A. Immersive three-dimensional modeling and virtual reality for enhanced visualization of operative neurosurgical anatomy. World Neurosurg 2019; : 313–320. [DOI] [PubMed] [Google Scholar]
- 9.Tam MD. Building virtual models by postprocessing radiology images: a guide for anatomy faculty. Anat Sci Educ 2010; : 261–266. [DOI] [PubMed] [Google Scholar]
- 10.Moro C, Stromberga Z, Raikos A, Stirling A. The effectiveness of virtual and augmented reality in health sciences and medical anatomy. Anat Sci Educ 2017; : 549–559. [DOI] [PubMed] [Google Scholar]
- 11.Lombardi SA, Hicks RE, Thompson KV, Marbach-Ad G. Are all hands-on activities equally effective? Effect of using plastic models, organ dissections, and virtual dissections on student learning and perceptions. Adv Physiol Educ 2014; : 80–86. [DOI] [PubMed] [Google Scholar]
- 12.Preece D, Williams SB, Lam R, Weller R. ‘Let's get physical’: advantages of a physical model over 3D computer models and textbooks in learning imaging anatomy. Anat Sci Educ 2013; : 216–224. [DOI] [PubMed] [Google Scholar]
- 13.Yammine K, Violato C. The effectiveness of physical models in teaching anatomy: a meta-analysis of comparative studies. Adv Health Sci Educ Theory Pract 2016; : 883–895. [DOI] [PubMed] [Google Scholar]
- 14.John R. Fredieu JK, Mark Herron et al. Anatomical models: a digital revolution. Med Sci Educ 2015; : 183–194. [Google Scholar]
- 15.Chen S, Pan Z, Wu Y et al. The role of three-dimensional printed models of skull in anatomy education: a randomized controlled trail. Sci Rep 2017; : 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner JA, Jethwa B, Jackson J et al. A three-dimensional print model of the pterygopalatine fossa significantly enhances the learning experience. Anat Sci Educ 2020; : 568–580. [DOI] [PubMed] [Google Scholar]
- 17.Lim KH, Loo ZY, Goldie SJ et al. Use of 3D printed models in medical education: a randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat Sci Educ 2016; : 213–221. [DOI] [PubMed] [Google Scholar]
- 18.Backhouse S, Taylor D, Armitage JA. Is this mine to keep? Three-dimensional printing enables active, personalized learning in anatomy. Anat Sci Educ 2019; : 518–528. [DOI] [PubMed] [Google Scholar]
- 19.Smith ML, Jones JFX. Dual-extrusion 3D printing of anatomical models for education. Anat Sci Educ 2018; : 65–72. [DOI] [PubMed] [Google Scholar]
- 20.Ganry L, Hersant B, Bosc R et al. Study of medical education in 3D surgical modeling by surgeons with free open-source software: example of mandibular reconstruction with fibula free flap and creation of its surgical guides. J Stomatol Oral Maxillofac Surg 2018; : 262–267. [DOI] [PubMed] [Google Scholar]
- 21.Garcia J, Yang Z, Mongrain R et al. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn 2018; : 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhir V, Itoi T, Fockens P et al. Novel ex vivo model for hands-on teaching of and training in EUS-guided biliary drainage: creation of “Mumbai EUS” stereolithography/3D printing bile duct prototype (with videos). Gastrointest Endosc 2015; : 440–446. [DOI] [PubMed] [Google Scholar]
- 23.Perica E, Sun Z. A systematic review of three-dimensional printing in liver disease. J Digit Imaging; 2018; : 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habicht JL, Kiessling C, Winkelmann A. Bodies for anatomy education in medical schools: an overview of the sources of cadavers worldwide. Acad Med 2018; : 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangata H, Ntaba P, Akol P, Louw G. The reliance on unclaimed cadavers for anatomical teaching by medical schools in Africa. Anat Sci Educ 2010; : 174–183. [DOI] [PubMed] [Google Scholar]
- 26.Gebler M, Schoot Uiterkamp AJM, Visser C. A global sustainability perspective on 3D printing technologies. Energy Policy 2014; : 158–167; corrigendum 2015; 85: 511. [Google Scholar]
- 27.Behm JE, Waite BR, Hsieh ST, Helmus MR. Benefits and limitations of three-dimensional printing technology for ecological research. BMC Ecol 2018; : 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogali SR, Yeong WY, Tan HKJ et al. Evaluation by medical students of the educational value of multi-material and multi-colored three-dimensional printed models of the upper limb for anatomical education. Anat Sci Educ 2018; : 54–64. [DOI] [PubMed] [Google Scholar]
- 29.Waran V, Narayanan V, Karuppiah R et al. Neurosurgical endoscopic training via a realistic 3-dimensional model with pathology. Simul Healthc 2015; : 43–48. [DOI] [PubMed] [Google Scholar]
- 30.Tappa K, Jammalamadaka U. Novel biomaterials used in medical 3D printing techniques. J Funct Biomater. 2018; : 17. [DOI] [PMC free article] [PubMed] [Google Scholar]