Abstract
Introduction
Necrotising fasciitis with sepsis is a life threatening disease. The aim of this study was to analyse the association between international normalised ratio (INR) and mortality in sepsis patients with necrotising fasciitis.
Methods
A retrospective review was undertaken of 106 patients suffering from necrotising fasciitis with sepsis between November 2007 and December 2016. Data on comorbidities, clinical manifestations, laboratory findings, causative microbiological organisms, APACHE II (Acute Physiology and Chronic Health Evaluation II) score and outcomes were extracted. Logistic regression was carried out to examine the factors affecting mortality.
Results
Forty patients (37.7%) died. There was no significant difference in the white blood count (WBC) for the survivor and non-survivor groups. Non-survivors had a lower mean oxygenation index (OI) (288.7mmHg vs 329.4mmHg, p=0.032) and platelet count (PC) (139.5 vs 214.8 x 109/l, p=0.028), and a higher mean INR (1.9 vs 1.3, p=0.000), activated partial thromboplastin time (APTT) (54.6 vs 44.2 seconds, p=0.005) and serum creatinine (2.3mg/dl vs 1.4mg/dl, p=0.007). Mortality in patients with INR >1.5 was significantly higher than in those with INR <1.5 when all risk factors (WBC, PC, OI, INR, APTT, creatinine) were considered (odds ratio: 4.414, 95% confidence interval: 1.263–15.428, p=0.020). Even after adjusting for age, sex, bacteraemia, diabetes and hepatic disorders, the data still exhibited elevated mortality for patients with INR >1.5 (odds ratio: 5.600, 95% confidence interval: 1.415–22.166, p=0.014).
Conclusions
INR is a significant independent predictor of mortality in sepsis patients diagnosed with necrotising fasciitis.
Keywords: Necrotising fasciitis, Sepsis, International normalised ratio, Risk factors
Introduction
Necrotising fasciitis (NF) is a severe soft tissue infection that causes extensive necrosis of subcutaneous tissue and fascia but mostly sparing the muscle and skin tissue. Despite recent improvement in the clinical care of patients with NF, mortality remains high. Multiple factors such as age, female sex, diabetes mellitus, bacteraemia and liver disease have been documented as contributing to NF related mortality.1–3 Interval from admission to surgical debridement is the only potentially modifiable risk factor that has been identified in numerous studies. Early recognition of patients at increased risk of NF allows earlier and more aggressive care. In particular, early surgical intervention is crucial in preventing mortality.4 Multiple laboratory and radiographic tests have been employed in the evaluation of NF but none are optimal and all may lead to delays in therapy. Currently, the gold standard for diagnosis is operative exploration of the suspected area.
International normalised ratio (INR) is a laboratory parameter that measures the clotting of blood. It is used to determine the effects of oral anticoagulants on the clotting system. INR is therefore routinely used to evaluate the coagulation status of a patient for adjusting therapy. Furthermore, elevated INR is associated with severe morbidity and mortality in the presence of liver disease. However, few previous studies have examined the association between NF and INR. The aim of our retrospective study was to investigate the association between INR and NF at Wenzhou Medical University in China using data spanning a period of nine years.
Methods
Patients diagnosed with NF and sepsis in the upper or lower limbs between November 2007 and December 2016 were included in the study. Diagnosis of NF was based on intraoperative findings (Fig 1). Sepsis was defined using the criteria from the international sepsis conference in 2016, also known as the ‘sepsis-3’ definition.5 Patients were excluded from the study if they were aged <18 years, were taking any form of anticoagulation before admission, had any underlying haematological disorders or refused treatment. In addition, patients given low molecular weight heparin, warfarin or unfractionated heparin during their admission before taking blood samples were excluded from the analysis.
Figure 1.
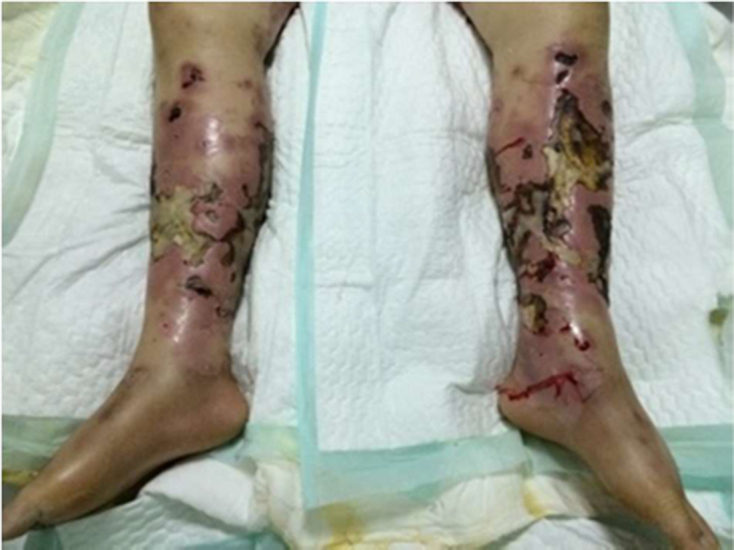
Lower limbs of a patient infected by Staphylococcus leading to fascial necrosis and sepsis
Age, sex, presentation, duration of symptoms, underlying chronic diseases, causative microbiological organisms and laboratory parameters (white blood count [WBC], platelet count [PC], oxygenation index [OI], INR, activated partial thromboplastin time [APTT], serum creatinine) were reviewed. The severity of illness on admission was assessed using the APACHE II (Acute Physiology and Chronic Health Evaluation II) scoring system.
Statistical analysis
The data were analysed using SPSS® version 15.0 (SPSS, Chicago, IL, US). Baseline demographic characteristics, laboratory findings, clinical presentation and bacteriology were compared between survivors and non-survivors. The significance of differences between groups in continuous variables was tested using either Student’s t-test or the Mann–Whitney U test. Categorical variables were analysed with either the chi-squared test or Fisher’s exact test. Multivariate logistic regression was used to evaluate the association between the diagnostic criteria of sepsis and death.
Results
The baseline characteristics of the 106 patients included in the study are summarised in Table 1. The mean patient age was 58.3 years (standard deviation [SD]: 13.8 years, range: 19–83 years) and 70% of the patients were male. Underlying chronic diseases included diabetes mellitus (n=48, 45%), liver cirrhosis (n=49, 46%), malignancy (n=6, 6%) and immunocompromised status (n=5, 5%). The infected sites were the lower limbs (n=95, 90%) or the upper limbs (n=11, 10%). On admission, the mean APACHE II score for the whole study cohort was 18.7 (SD: 7.4).
Table 1.
Baseline characteristics of necrotising fasciitis patients with sepsis
|
Survivors (n=66) |
Non-survivors (n=40) |
p-value | |
| Mean age (years) | 58.4 (SD: 13.4) | 58.0 (SD: 14.7) | 0.883 |
| Male sex | 45 (68%) | 29 (73%) | 0.639 |
| Infection in lower limbs | 56 (85%) | 39 (98%) | 0.082 |
| Median duration of symptoms before admission (days) | 2 (IQR: 1–7) | 2 (IQR: 2–4) | 0.876 |
| Mean APACHE II score on admission | 15.3 (SD: 5.3) | 24.1 (SD: 7.0) | 0.000 |
| Coexisting conditions | |||
| Diabetes mellitus | 29 (44%) | 19 (48%) | 0.721 |
| Hepatic disorders | 25 (38%) | 24 (60%) | 0.027 |
| Immunocompromised status | 2 (3%) | 3 (8%) | 0.562 |
| Malignancy | 5 (8%) | 1 (3%) | 0.508 |
| Signs and symptoms on admission | |||
| Body temperature >38.3°C or <36°C | 38 (58%) | 25 (63%) | 0.617 |
| Blood pressure <90/60mmHg | 28 (42%) | 22 (55%) | 0.209 |
| Laboratory findings on admission | |||
| Mean WBC (x 109/l) | 13.2 (SD: 7.1) | 11.7 (SD: 9.9) | 0.402 |
| Mean PC (x 109/l) | 214.8 (SD: 176.1) | 139.5 (SD: 154.8) | 0.028 |
| Mean OI (mmHg) | 329.4 (SD: 95.8) | 288.7 (SD: 89.4) | 0.032 |
| Mean INR | 1.3 (SD: 0.3) | 1.9 (SD: 0.9) | 0.000 |
| Mean APTT (seconds) | 44.2 (SD: 11.4) | 54.6 (SD: 20.5) | 0.005 |
| Mean creatinine (mg/dl) | 1.4 (SD: 1.7) | 2.3 (SD: 1.6) | 0.007 |
| Outcomes | |||
| Amputation | 4 (6%) | 2 (5%) | 1.000 |
| Mean length of hospital stay (days) | 41.4 (SD: 19.3) | 11.4 (SD: 13.9) | 0.000 |
APACHE = Acute Physiology and Chronic Health Evaluation; APTT = activated partial thromboplastin time; INR = international normalised ratio; IQR = interquartile range; OI = oxygenation index; PC = platelet count; SD = standard deviation; WBC = white blood count
The prevalence of hepatic disorders was higher in non-survivors than in survivors (60% vs 38%, p=0.027). WBC was not significantly different between surviving and non-surviving patients. Non-survivors presented with a lower mean PC (139.5 [SD: 154.8] vs 214.8 [SD: 176.1] x 109/l, p=0.028) and OI (288.7mmHg [SD: 89.4mmHg] vs 329.4mmHg [SD: 95.8mmHg], p=0.032), and a higher mean INR (1.9 [SD: 0.9] vs 1.3 [SD: 0.3], p=0.000), APTT (54.6 [SD: 20.5] vs 44.2 [SD: 11.4] seconds, p=0.005) and creatinine (2.3mg/dl [SD: 1.6mg/dl] vs 1.4mg/dl [SD: 1.7mg/dl], p=0.007). Survivors had a lower mean APACHE II score (15.3 [SD: 5.3] vs 24.1 [SD: 7.0], p=0.000). The mean length of hospital stay was longer in the survivor group than in the non-survivor group (41.4 [SD: 19.3] vs 11.4 [SD: 13.9] days, p=0.000). Forty patients died, giving an overall mortality rate of 37.7%.
Microbiological findings
Monobacterial Gram negative organisms were the most frequently identified (30.2%), followed by monobacterial Gram positive organisms (10.4%) and fungus (6.6%) (Table 2). Among Gram negative bacteria, Vibrio (17.9%) and Proteus (13.2%) were the most common organisms. Staphylococcus (14.2%) was the organism most often identified among Gram positive bacteria. In non-survivors, the presence of positive blood cultures was significantly higher than in survivors (42.5% vs 19.7%, p=0.012). The results of the blood cultures were: Vibrio vulnificus (n=10, 9.4%), Staphylococcus (n=8, 7.5%), Klebsiella (n=4, 3.8%), Candida (n=3, 2.8%), Acinetobacter baumannii (n=2, 1.9%), Proteus (n=2, 1.9%), and Streptococcus pyogenes, Aeromonas hydrophila, Escherichia coli, Pseudomonas aeruginosa, Corynebacterium, Klebsiella aerogenes and Shewanella algae. (all n=1, 0.9%).
Table 2.
Micro-organisms involved in necrotising fasciitis
|
Overall (n=106) |
Survivors (n=66) |
Non-survivors (n=40) |
p-value | |
| Positive wound culture | 63 (59.4%) | 40 (60.6%) | 23 (57.5%) | 0.752 |
| Positive blood culture (bacteraemia) | 30 (28.3%) | 13 (19.7%) | 17 (42.5%) | 0.012 |
| Polybacterial infection | 28 (26.4%) | 20 (30.3%) | 8 (20.0%) | 0.244 |
| Monobacterial Gram positive | 11 (10.4%) | 6 (9.1%) | 5 (12.5%) | 0.819 |
| Monobacterial Gram negative | 32 (30.2%) | 18 (27.3%) | 14 (35.0%) | 0.401 |
| Fungus | 7 (6.6%) | 2 (3.0%) | 5 (12.5%) | 0.134 |
| Gram positive | ||||
| Staphylococcus | 15 (14.2%) | 12 (18.2%) | 3 (7.5%) | 0.126 |
| Streptococcus | 8 (7.5%) | 6 (9.1%) | 2 (5.0%) | 0.694 |
| Enterococcus | 9 (8.5%) | 6 (9.1%) | 3 (7.5%) | 1.000 |
| Gram negative | ||||
| Vibrio | 19 (17.9%) | 9 (13.6%) | 10 (25.0%) | 0.139 |
| Proteus | 14 (13.2%) | 11 (16.7%) | 3 (7.5%) | 0.177 |
| Enterobacter | 10 (9.4%) | 8 (12.1%) | 2 (5.0%) | 0.383 |
| Klebsiella | 10 (9.4%) | 7 (10.6%) | 3 (7.5%) | 0.851 |
| Pseudomonas | 8 (7.5%) | 5 (7.6%) | 3 (7.5%) | 1.000 |
| Acinetobacter | 7 (6.6%) | 5 (7.6%) | 2 (5.0%) | 0.909 |
Multivariate analysis for the major predictors of mortality
The patients were divided into high and low risk groups for each parameter according to their sequential organ failure assessment (SOFA)6 score and diagnostic criteria for sepsis.7 High risk was defined as SOFA score ≥2 (PC ≤100 x 109/l, OI ≤300mmHg, creatinine ≥2mg/dl), INR >1.5, WBC <4 x 109/l or >12 x 109/l and APTT >60 seconds. The mortality in the higher risk INR group was significantly higher than in the lower risk INR group (odds ratio: 4.414, 95% confidence interval: 1.263–15.428, p=0.020) when all risk factors (WBC, PC, OI, INR, APTT, creatinine) were considered. WBC, PC, OI, APTT and creatinine were not significantly associated with mortality. Even when adjusting for age, sex, age, bacteraemia, diabetes mellitus and hepatic disorders, the data still exhibited elevated mortality for the higher risk INR group (odds ratio: 5.600, 95% confidence interval: 1.415–22.166, p=0.014) (Table 3).
Table 3.
Multivariate analysis for the major predictors of mortality
| Model 1* | Model 2† | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| WBC | 0.785 (0.309–1.994) | 0.611 | 1.032 (0.369–2.880) | 0.953 |
| PC | 0.855 (0.252–2.906) | 0.802 | 1.394 (0.312–6.233) | 0.664 |
| OI | 1.419 (0.566–3.563) | 0.456 | 1.205 (0.456–3.182) | 0.707 |
| INR | 4.414 (1.263–15.428) | 0.020 | 5.600 (1.415–22.166) | 0.014 |
| APTT | 2.028 (0.574–7.168) | 0.273 | 2.284 (0.550–9.486) | 0.256 |
| Creatinine | 1.688 (0.629–4.535) | 0.299 | 1.922 (0.687–5.380) | 0.214 |
APTT = activated partial thromboplastin time; CI = confidence interval; INR = international normalised ratio; OI = oxygenation index; OR = odds ratio; PC = platelet count; WBC = white blood count
*Adjusted for WBC, PC, OI, INR, APTT and creatinine
†Further adjusted for age, sex, bacteraemia, diabetes mellitus and hepatic disorders
Discussion
This study analysed the association between INR and mortality in sepsis patients with limb NF. Sepsis is defined as life threatening organ dysfunction caused by a dysregulated host response to infection.5 A literature search identified ten studies published between 2005 and 2016 looking at mortality in NF patients (Table 4).8–17 WBC, PC, INR, APTT and creatinine appeared to be the main risk factors for NF although there is no consensus among these studies for specific predictors of mortality. WBC, PC and creatinine do not have uniform criteria for evaluation of disease and prognosis.
Table 4.
Summary of studies investigating mortality in patients with necrotising fasciitis
| Paper | Country | Study period | Mortality | Site of infection | WBC | PC | OI | INR | APTT | Creatinine |
| Lee, 20168 | Taiwan | 2004–2012 | 66.7% (total n=18) |
Limbs | – | <150 x 109/l | – | – | – | – |
| Khamnuan, 20159 | Thailand | 2009–2012 | 19.3% (total n=1,504) |
Limbs, Fournier gangrene, head and neck, trunk, multiple sites | – | – | – | – | – | ≥1.6mg/dl |
| Krieg, 201410 | Germany | 1996–2011 | 32.8% (total n=64) |
Limbs, perineum, trunk | – | – | – | – | – | ≥4mg/dl |
| Lee, 201411 | Taiwan | 1996–2011 | 18% (total n=100) |
Limbs, multiple sites | <10 x 109/l | <100 x 109/l | – | – | – | ≥1.3mg/dl |
| Lin, 201312 | Taiwan | 2005–2011 | 16% (total n=75) |
Limbs, trunk | <10 x 109/l | <100 x 109/l | – | >1.5 | – | – |
| Yu, 201313 | South Korea | 2001–2012 | 33.7% (total n=83) |
Limbs, buttocks, head and neck, trunk | – | <50 x 109/l | – | – | – | – |
| Huang, 201114 | Taiwan | 2003–2009 | 12.1% (total n=472) |
Limbs, perineum, head and neck, trunk | Band polymorphonuclear neutrophils >10% | – | – | – | ≥60 seconds | ≥2mg/dl |
| Yeung, 201115 | Hong Kong | Not known | 28% (total n=29) |
Limbs | – | <150 x 109/l | – | – | – | ≥1.5 x normal |
| Tsai, 201016 | Taiwan | 2002–2008 | 25.7% (total n=70) |
Limbs | Increased band forms of leucocytes | <80 x 109/l | – | – | – | – |
| Anaya, 200517 | US | 1996–2001 | 16.9% (total n=166) |
Limbs, perineum, buttocks, head and neck, trunk | >30 x 109/l | – | – | – | – | ≥2mg/dl |
APTT = activated partial thromboplastin time; INR = international normalised ratio; OI = oxygenation index; PC = platelet count; WBC = white blood count
OI was not analysed in these studies but hyperbaric oxygen therapy is an effective tool in the treatment of NF.18 On univariate analysis of our cohort of NF patients presenting to the emergency department, OI was found to significantly influence mortality. We therefore feel that OI should be considered as a potential major risk factor together with other risk factors that have been well documented previously (WBC, PC, INR, APTT and creatinine). The correlation between these risk factors was also examined, and INR was shown to be significantly and independently associated with risk of mortality. To our knowledge, this is the first study to examine the correlation between WBC, PC, OI, INR, APTT and creatinine.
Based on our review of the existing literature, there are several possible explanations for our findings. Owing to the post-thrombolytic coagulation seen in acute ischaemic stroke,19 the INR is elevated, which renders the patient prone to excessive bleeding. It has been estimated that the risk of bleeding almost doubles for each one-point increase in INR above 3.0,20 with the risk of intracranial haemorrhage similarly doubling for every unit rise in INR.21
While antimicrobial therapy and supportive treatment are essential early interventions when dealing with NF, surgery is thought to be key to improving the survival rate. When the wound is infected, it can release mass toxins and enzymes. This leads to tissue damage and necrotic tissue is an ideal breeding ground for bacteria. Consequently, the infection becomes more serious, leading to a vicious cycle. Debridement should be performed immediately because the time taken from admission to surgical intervention is related to survival rate in patients with NF.4 The insufficiency of coagulation of the blood increases the possibility of severe bleeding during the operation and patients have a higher chance of losing their lives.
An elevated INR might also indicate the presence of fatigue, in which inflammation has been reported to play a role.22 NF is a life threatening soft tissue infection that is accompanied by a rapid increase in inflammation and necrosis of the skin, which can progress to sepsis. Sepsis is commonly associated with a net procoagulant state and secondary consumptive conditions of thrombocytopenia. Large amounts of cytokines are released into the circulation, including tumour necrosis factor, interleukin 1 and interleukin 6.23 These cytokines activate the coagulation system and affect anticoagulant mechanisms. As a result, microvascular clots are deposited, which may lead to tissue ischaemia and organ dysfunction.
If too many coagulation factors and platelets are consumed, bleeding is difficult to stop. This phenomenon is known as disseminated intravascular coagulation, which manifests itself as widespread microvascular thrombosis and haemorrhage. An elevated INR may therefore be associated with a greater severity of sepsis, which may explain the increased risk of mortality.
The SOFA score is a useful tool to assess the severity of sepsis. It based on six variables, representing coagulation, liver and kidney function, and the respiratory, cardiovascular and neurological systems. In addition, the laboratory risk indicator for necrotising fasciitis (LRINEC) is a robust scoring system capable of detecting NF even at an early stage.24 Unfortunately, neither the SOFA score nor the LRINEC score incorporate INR.
Study limitations
One of the major limitations of our study is the retrospective study design. Medical records are not designed for the purposes of research and do not include all variables of interest to researchers. Moreover, this study did not investigate and cannot prove the pathogenic mechanism for our observed association between elevated INR and mortality in patients with NF. Finally, our patients were from a single medical centre in Wenzhou, China. The value of INR in predicting clinical outcomes may be different in other populations of NF patients. Further prospective studies with larger patient cohorts from multiple centres are necessary to more accurately evaluate the prognostic role of elevated INR as a predictor of mortality in patients with NF.
Conclusions
The mortality rate for NF with sepsis is high. INR appears to be a significant and independent predictor of mortality in sepsis patients with NF. This is not only because of the association between INR and sepsis but also because of the role INR plays in abnormal coagulation, which increases the possibility of severe bleeding intraoperatively, resulting in higher mortality. INR appears to be a laboratory risk indicator of NF that aids in pointing towards the initiation of early surgery and predicting a higher risk of death. It is unfortunate that the SOFA and LRINEC scoring systems do not take account of INR.
Funding information
This article was supported by the Science and Technology Project of Wenzhou (Grant No. Y20160328), the science and technology innovation team project of Foshan (2015IT100062), the study on biological three-dimensional printing tissue repair technology and clinical application—the aspect of full-thickness vascularized skin biological three-dimensional printing tissue repair technology and clinical application (2017C01054).
References
- 1.Cheng NC, Tai HC, Chang SC et al. Necrotizing fasciitis in patients with diabetes mellitus: clinical characteristics and risk factors for mortality. BMC Infect Dis 2015; : 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taviloglu K, Cabioglu N, Cagatay A et al. Idiopathic necrotizing fasciitis: risk factors and strategies for management. Am Surg 2005; : 315–320. [PubMed] [Google Scholar]
- 3.Yii YC, Hsieh VC, Lin CL et al. Alcohol use disorder increases the risk of necrotizing fasciitis: a nationwide retrospective cohort study. Medicine 2017; : e7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao WN, Tsai CF, Chang HR et al. Impact of timing of surgery on outcome of Vibrio vulnificus-related necrotizing fasciitis. Am J Surg 2013; : 32–39. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes A, Evans LE, Alhazzani W et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; : 304–377. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence Sepsis: Recognition, Diagnosis and Early Management (NG51). London: NICE; 2016. [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; : 580–637. [DOI] [PubMed] [Google Scholar]
- 8.Lee CY, Li YY, Huang TW et al. Synchronous multifocal necrotizing fasciitis prognostic factors: a retrospective case series study in a single center. Infection 2016; : 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khamnuan P, Chongruksut W, Jearwattanakanok K et al. Necrotizing fasciitis: risk factors of mortality. Risk Manag Healthc Policy 2015; : 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieg A, Dizdar L, Verde PE, Knoefel WT. Predictors of mortality for necrotizing soft-tissue infections: a retrospective analysis of 64 cases. Langenbecks Arch Surg 2014; : 333–341. [DOI] [PubMed] [Google Scholar]
- 11.Lee YC, Hor LI, Chiu HY et al. Prognostic factor of mortality and its clinical implications in patients with necrotizing fasciitis caused by Vibrio vulnificus. Eur J Clin Microbiol Infect Dis 2014; : 1011–1018. [DOI] [PubMed] [Google Scholar]
- 12.Lin JN, Chang LL, Lai CH et al. Group A streptococcal necrotizing fasciitis in the emergency department. J Emerg Med 2013; : 781–788. [DOI] [PubMed] [Google Scholar]
- 13.Yu SN, Kim TH, Lee EJ et al. Necrotizing fasciitis in three university hospitals in Korea: a change in causative microorganisms and risk factors of mortality during the last decade. Infect Chemother 2013; : 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang KF, Hung MH, Lin YS et al. Independent predictors of mortality for necrotizing fasciitis: a retrospective analysis in a single institution. J Trauma 2011; : 467–473. [DOI] [PubMed] [Google Scholar]
- 15.Yeung YK, Ho ST, Yen CH et al. Factors affecting mortality in Hong Kong patients with upper limb necrotising fasciitis. Hong Kong Med J 2011; : 96–104. [PubMed] [Google Scholar]
- 16.Tsai YH, Hsu RW, Huang KC, Huang TJ. Laboratory indicators for early detection and surgical treatment of vibrio necrotizing fasciitis. Clin Orthop Relat Res 2010; : 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anaya DA, McMahon K, Nathens AB et al. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg 2005; : 151–157. [DOI] [PubMed] [Google Scholar]
- 18.Shaw JJ, Psoinos C, Emhoff TA et al. Not just full of hot air: hyperbaric oxygen therapy increases survival in cases of necrotizing soft tissue infections. Surg Infect 2014; : 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee VH, Conners JJ, Cutting S et al. Elevated international normalized ratio as a manifestation of post-thrombolytic coagulopathy in acute ischemic stroke. J Stroke Cerebrovasc Dis 2014; : 2139–2144. [DOI] [PubMed] [Google Scholar]
- 20.Dentali F, Ageno W. Management of coumarin-associated coagulopathy in the non-bleeding patient: a systematic review. Haematologica 2004; : 857–862. [PubMed] [Google Scholar]
- 21.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; : 897–902. [DOI] [PubMed] [Google Scholar]
- 22.Karshikoff B, Sundelin T, Lasselin J. Role of inflammation in human fatigue: relevance of multidimensional assessments and potential neuronal mechanisms. Front Immunol 2017; : 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med 2010; : S26–S34. [DOI] [PubMed] [Google Scholar]
- 24.Bechar J, Sepehripour S, Hardwicke J, Filobbos G. Laboratory risk indicator for necrotising fasciitis (LRINEC) score for the assessment of early necrotising fasciitis: a systematic review of the literature. Ann R Coll Surg Engl 2017; : 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]


