Abstract
Purpose:
Discoidin domain receptor 1 (DDR1) belongs to a novel class of receptor tyrosine kinases. Previous evidence indicates that DDR1 overexpression promotes the aggressive growth of bladder cancer (BC) cells. This study aimed to investigate the molecular mechanisms by which DDR1 influences BC.
Methods:
DDR1 was transfected into human BC RT4 cells. DDR1, COL4A1, and MMP-2 expression in 30 BC tissues and paired adjacent tissues were examined by real-time polymerase chain reaction (RT-PCR) and immunohistochemistry. Transwell assays were conducted to determine cell migration and invasion. RT-PCR and western blot (WB) were also used to measure the DDR1, COL4A1, MMP-2, and EMT-related gene (ZEB1 and SLUG) expression in RT4 cells after DDR1 overexpression.
Results:
COL4A1 and MMP-2 interacted with DDR1 in the PPI network. RT-PCR and immunohistochemistry results showed that both mRNA and protein levels of DDR1 and COL4A1 were significantly increased in BC tissue, while the expression of MMP-2 was increased only at the mRNA level (P < 0.05). Overexpression of DDR1 in RT4 cells significantly promoted their migratory and invasive capabilities in vitro (P < 0.05). Moreover, overexpression of DDR1 in RT4 cells increased the mRNA and protein expression of ZEB1, SLUG, COL4A1, and MMP-2 (P < 0.01). DDR1-mediated migration and invasion of RT4 cells were reversed after COL4A1-siRNA treatment.
Conclusion:
DDR1 may be a potential therapeutic target in BC patients.
Keywords: bladder cancer, DDR1, migration, invasion, EMT, COL4A1/DDR1/MMP-2
Introduction
Bladder cancer (BC) is the fourth most common malignancy worldwide and the second most common cause of cancer-related deaths among urinary tumors.1 As estimated by the GLOBOCAN 2018 cancer report, a total of 549,393 newly diagnosed cases and 199,922 deaths were due to BC.2 Typically, cigarette smoking, obesity, urinary tract infection, and exposure to certain chemicals are thought to be risk factors of BC.3-5 Despite clinical advances in surgical surgery, radiation therapy, immunotherapy, chemotherapy, or combined therapies, the 5-year survival rate of BC remains bleak.6,7 Tumor invasion and metastasis are still the major causes of treatment failure and patient death. Therefore, it is urgent to investigate the potential molecular mechanisms involved in metastatic BC progression.
Recently, accumulating evidence have indicated that epithelial-mesenchymal transition (EMT) is involved in the carcinogenesis and metastasis of multiple cancers.8,9 During EMT, polarized epithelial cells lose tight intercellular connections and gradually differentiate into cells with mesenchymal attributes.10 Previous studies demonstrated that EMT-inducing transcription factors (TFs), including TWIST1, SLUG, SNAIL1, ZEB1, and ZEB2 were used as biomarkers of EMT11,12 and may facilitate effective treatments for metastatic cancer.13-17 The ECM was thought to act as a cell scaffolding to promote migration, and collagen is a major part of this cellular microenvironment.18
Current studies indicated that DDRs support cell-collagen interactions, and are thus thought of as microenvironment sensors, playing a vital role in cell migration and invasion.19-21 Discoidin domain receptors (DDRs), a subfamily of tyrosine kinases, are comprised of the proteins DDR1 and DDR2.22 Previous studies suggested that abnormal expression of DDR1 was found in several different types of cancer, such as renal cancer.23,24 lung cancer,25 and breast cancer.26 A growing amount of research demonstrated that overexpression of DDR1 promoted migration and invasion in certain malignancies.25,27,28 However, the underlying mechanisms that promote BC migration and invasion are still not well documented.
A previous study demonstrated that a high expression of DDR1 in BC tissue was correlated with poor patient outcomes, and DDR1 overexpression promoted cell invasion and tumor xenograft growth. ZEB1 and SLUG were also implicated in the increase in cell invasion.29 In the current study, we aimed to comprehensively investigate the mechanisms by which DDR1 promotes BC migration and invasion. The proteins that interacted with DDR1 were analyzed using the STRING database. The expression of DDR1 and the proteins that interact with it was determined. DDR1-overexpressing BC cells were established to investigate the effects of DDR1 overexpression on cell invasion and migration and the expression of related proteins in vitro. The results implicate that DDR1 contributes to invasion and migration via EMT-associated proteins and the COL4A1/DDR1/MMP-2 signaling axis.
Methods
Tissue Samples and Cell Lines
A total of 30 BC tumors and paired adjacent normal tissues were isolated from patients with stage IV BC who underwent surgical resection at the Ruijin Hospital. Written informed consent was acquired from all patients before conducting the research, and the present study was approved by the Institutional Review Board of Ruijin Hospital. The human embryonic kidney 293 T (HEK293 T) cell line and a BC cell line (RT4) were acquired from American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in RPMI1640 medium (Gibco, Garlsbad, CA, USA) with 15% fetal bovine serum (Invitrogen, USA), and maintained in a humidified atmosphere at 37°C with 5% CO2.
Lentiviral Vector Construction in RT4 Cell Line
Lentiviral vectors expressing GFP were purchased from Genechem (Shanghai, China). The full-length human DDR1 coding sequences were obtained by PCR amplification and then accurately cloned to the lentiviral vector (Genechem, Shanghai, China). Using Lipofectamine 2000 (Invitrogen), the recombinant lentiviral vector was transfected into HEK293 T cells (Lentivirus-DDR1) for 48 h, followed by infection with RT4 cells. The stably transfected RT4 cells were screened using culture medium containing 6 μg/mL puromycin (Sigma, USA). RT4 cells transfected with the empty vector (GenePharma, Shanghai, China) were designed as a negative control (RT4-NC). The GFP density imparted by the lentiviruses was utilized to detect transfection efficiency. In addition, COL4A1-specific siRNA and the control siRNA were synthesized by Sangon Biotech (Shanghai) Co., Ltd. and transfected into cells using Lipofectamine 2000 (Invitrogen) to inhibit COL4A1 in cells.
Protein–Protein Interaction (PPI) Network
Functional interactions for DDR1 were analyzed using PPIs in the Search Tool for the Retrieval of Interacting Genes (STRING) database (https://version-10-5.string-db.org/cgi/network.pl?taskId=jDdJHRC1vsJe).30 The protein interaction network with a combined score of 0.4 was constructed, and no more than 10 interactions were set.
Immunohistochemical Staining
Immunohistochemical analysis was conducted on 30 BC tumor and adjacent non-tumorous tissues. Paraformaldehyde-fixed paraffin sections with a thickness of 6 µm were deparaffinized with xylene, and incubated for 15 min with 0.3% hydrogen peroxide to eliminate endogenous peroxidase activity. The sections were then treated with a blocking solution containing sheep serum for 50 min to block non-specific protein binding. After washing, the slides were stained with primary antibodies against DDR1 (Catalog #sc-21790, 1:200, Abcam), COL4A1 (eBioscience, Catalog# ab86042, 1:50, Abcam), and MMP-2 (Catalog # ab97779, 1:300, Abcam) overnight at 4°C. Cells were then incubated with rabbit anti-Mouse IgG H&L (1:5000, ab6728; 1:5,000; Abcam) or donkey anti-rabbit IgG H&L (1:5000, ab6802, Abcam, USA) conjugated with horseradish peroxidase at 37°C for 30 min. Finally, the sections were stained with 3′-diaminobenzidine and subsequently observed and photographed under an inverted microscope (IX71; Olympus Corporation). The integrated optical density (IOD) of the immunohistochemically stained samples from representative fields in each pathological section were calculated using Image-Pro Plus 6 software. The results were assessed semi-quantitatively.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from RT4 cells and 18 pairs of frozen tissue specimens using TRIZolì Reagent (Gibco BRL, Gaithersburg, MD, USA), and then transcribed into cDNA using PrimeScript™RT reagent Kit (Takara, Japan). About 1.0 µg of total RNA was subjected to first-strand cDNA synthesis by PrimerScript™RT Master Mix (Takara Biotechnology Co., Ltd., Dalian, *China), followed by PCR amplification via the SYBR® Premix Ex Taq™ Kit (TaKaRa, Japan). PCR was performed with an initial denaturation for 30 seconds at 95°C, followed by 40 cycles at 95°C for 5 seconds and 60°C for 34 seconds. GAPDH was used as an internal control. The primers used for PCR are listed in Table 1. PCR quantification was performed using ViiA™7 (Applied Biosystems, Foster City, CA) and the mRNA expression levels were calculated based on the 2-ΔΔCt method.
Table 1.
Sequences of the Primers Used in this Study.
| Primer | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| DDR1 | GATCTCGACTCCGCTTCAAGGA | CAAAGGGTGTCCCTTACGCACA |
| COL4A1 | TGTTGACGGCTTACCTGGAGAC | GGTAGACCAACTCCAGGCTCTC |
| MMP-2 | AGCGAGTGGATGCCGCCTTTAA | CATTCCAGGCATCTGCGATGAG |
| ZEB1 | GGCATACACCTACTCAACTACGG | TGGGCGGTGTAGAATCAGAGTC |
| SLUG | ATCTGCGGCAAGGCGTTTTCCA | ATCTGCGGCAAGGCGTTTTCCA |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
Western Blot (WB) Analysis
Cells were harvested at the logarithmic growth phase after overexpression treatment. The obtained cells were lysed in RIPA buffer supplemented with PMSF (RIPA: PMSF ratio of 99:1, Solarbio, Beijing, China), and total protein was quantified with the bicinchoninic acid (BCA) method (Biorad, China). The extracted proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then transferred onto nitrocellulose membranes, blocked with non-fat milk (BD, USA) for 1 h at room temperature, and then incubated with primary antibodies against DDR1 (Catalog #sc-21790, Abcam), COL4A1 (eBioscience, Catalog# ab86042, Abcam), MMP-2 (Catalog # ab97779, Abcam), SLUG (Catalog #ab97779, Abcam), ZEB1 (Catalog #ab203829, Abcam), and β-actin polyclonal antibody (Catalog # ab179467, Abcam). β-actin was used as a control. The membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibodies rabbit anti-Mouse IgG H&L (1:5000, ab6728; 1:5000; Abcam) or donkey anti-rabbit IgG H&L (1:5000, ab6802, Abcam, USA). Using enhanced chemiluminescence (ECL) chromogenic substrate (Thermo Fisher), the antibody-reactive protein bands were visualized by the SuperSignal™ West Dura system (Pierce Biotechnology, Inc., Rockford, IL, USA), and UVITech imager (UVITech, Inc., Cambridge, UK) was used to photograph the membrane.
Transwell Migration Assay and Invasion Assays
The Transwell migration assay (Corning Life Sciences, Tewksbury, MA, USA) was performed as follows. Briefly, a total of 5 × 105 cells suspended in 100 µl serum-free RPMI-1640 medium were transferred into the upper chamber (8 µm pore size, Millipore, USA). About 600 µl RPMI-1640 medium supplemented with 10% fetal bovine serum was placed in the lower chamber of the transwell. After 24 h incubation at 37°C in 5% CO2, the cells in the upper chambers were gently removed, and the adherent tumor cells in the lower chambers were fixed with paraformaldehyde and stained with 0.1% crystal violet. Cells in the invasion assay were seeded in the upper chamber covered with Matrigel (BD, Catalog #356234, San Diego, CA, USA). The experimental procedures were the same as that with the migration experiment. Using a light microscope, the number of migratory and invasive cells were counted in random fields of view.
Statistical Analysis
All statistical calculations were performed using SPSS 22.0 software (IBM Corporation, Armonk, NY, USA). Data were presented as means ± standard deviation (SD) from 3 independent experiments. Comparisons between 2 groups were analyzed using Student’s t-test, and comparisons involving more than 2 groups were analyzed with ANOVA. A P-value less than 0.05 was considered as statistically significant.
Results
Expression of DDR1 Is Upregulated in BC Tissues
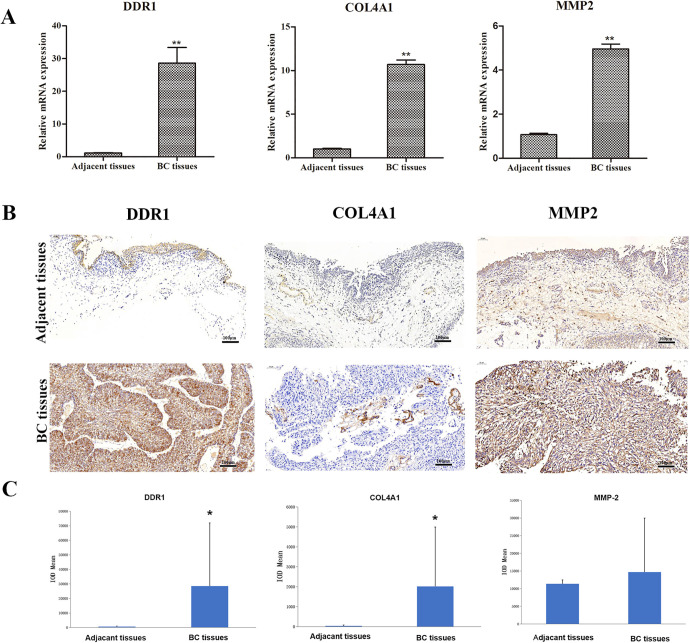
To investigate whether DDR1 expression was correlated with malignant BC progression, paired human BC tissues and adjacent normal tissues were subject to gene expression analysis via PCR. The results showed that the mRNA expression of DDR1 was upregulated in BC tissues compared with adjacent normal tissues (Figure 1A, P < 0.01). The expression of DDR1 protein in BC tissues was further determined using immunohistochemistry. As shown in Figure 1B, DDR1 was localized to the cytoplasm and intracellular spaces. Further analysis indicated that the DDR1 protein expression level in BC tissues showed an increased level than those in adjacent normal tissues (Figure 1B and C, P < 0.05). The proteins interacting with DDR1 were then predicted using the STRING database. As shown in Figure 2, DDR1 interacted with MMP-2 and various types of collagens (COL2A1, COL3A1, COL4A1, COL5A1). We selected MMP-2 and COL4A1 in the current study. The results of RT-PCR and immunohistochemistry showed that the expression of COL4A1 in BC tissue was significantly increased at both the mRNA (P < 0.01) and protein levels (P < 0.05). The expression of MMP-2 was also increased at the mRNA level (P < 0.01), but there was no significant difference in MMP-2 protein expression in BC tissues (P > 0.05).
Figure 1.
The expression of DDR1, COL4A1, and MMP-2 in tissue samples. (A) The mRNA levels of DDR1, COL4A1, and MMP-2 in BC tissue and paired adjacent tissues determined by RT-PCR. (B) Representative images of immunohistochemistry staining of DDR1, COL4A1, and MMP-2 in BC and adjacent normal tissues. (C) Immunohistochemical staining quantification showed the expression levels of DDR1, COL4A1, and MMP-2 in BC tissues and adjacent tissues. *P < 0.05; **P < 0.01.
Figure 2.
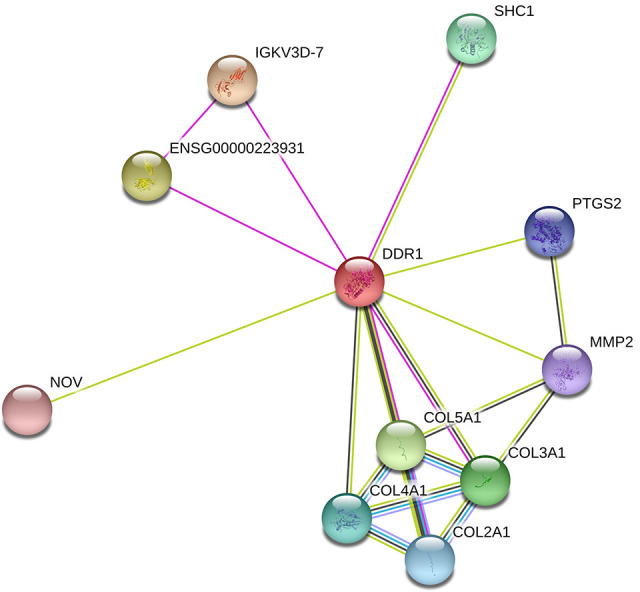
The protein-protein interactions for DDR1 as predicted by the STRING database. Nodes represent proteins encoded by genes and lines represent the interactions between 2 nodes.
Overexpression of DDR1 Induced mRNA and Protein Expression in RT4 Cells
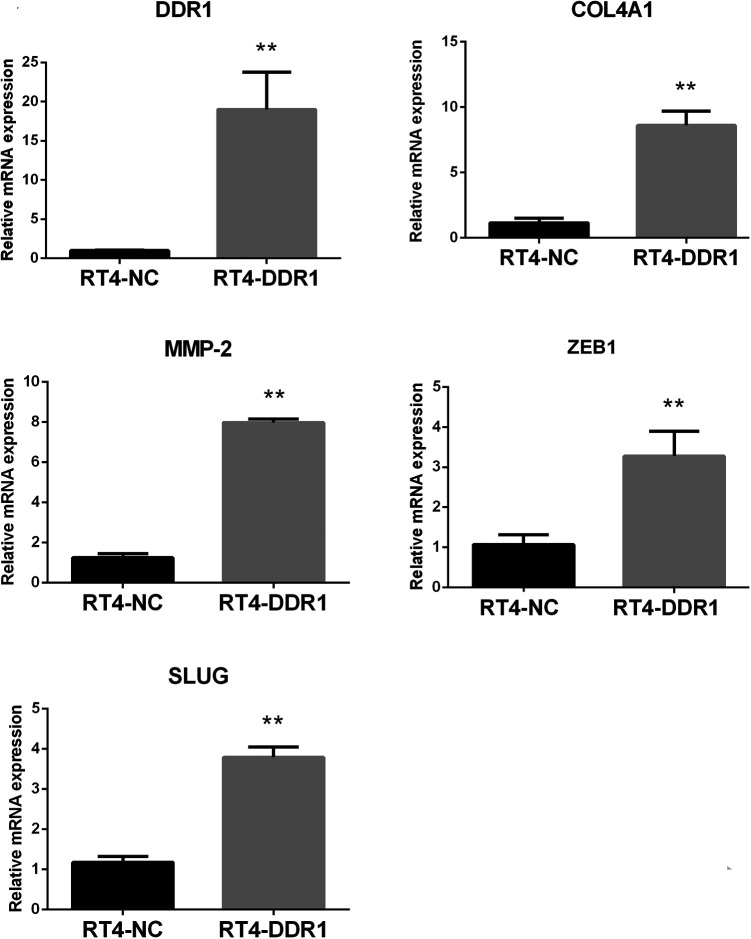
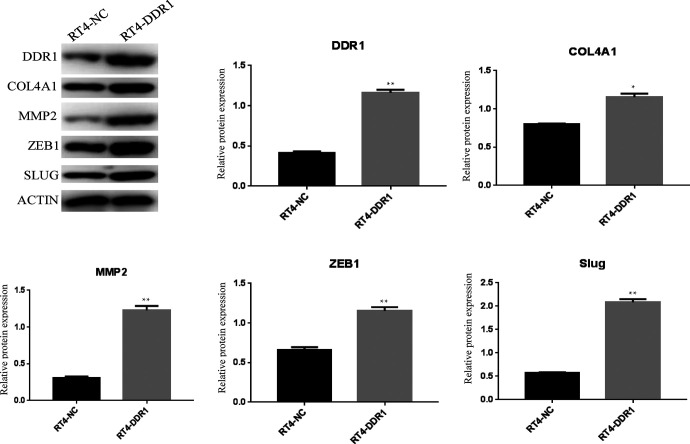
To explore the potential function of DDR1, the expression level of DDR1 following lentiviral transduction was detected in RT4 cells, DDR1-overexpressing, and control cells using PCR and WB (RT-DDR1 vs. RT-NC). As shown in Figure 3, the relative mRNA expression levels of DDR1 in RT4-DDR1 cells with the same level of β-actin was significantly increased compared with the levels in the RT4-NC cells (P < 0.01). To prove that this upregulation of DDR1 mRNA translated into increased levels of DDR1 protein, WB was performed. DDR1 protein expression in RT4-DDR1 was found to be higher than in RT4-NC (Figure 4).
Figure 3.
The mRNA expression of DDR1, COL4A1, MMP-2, ZEB1, and SLUG after transfection. **P < 0.01, compared with RT4-NC.
Figure 4.
Representative images and statistical graphs of DDR1, COL4A1, MMP-2, ZEB1, and SLUG protein expression determined by western blot. *P < 0.05, compared with RT4-NC; **P < 0.01, compared with RT4-NC.
DDR1 Overexpression Promoted Cell Migration and Invasion of RT4 Cells In Vitro
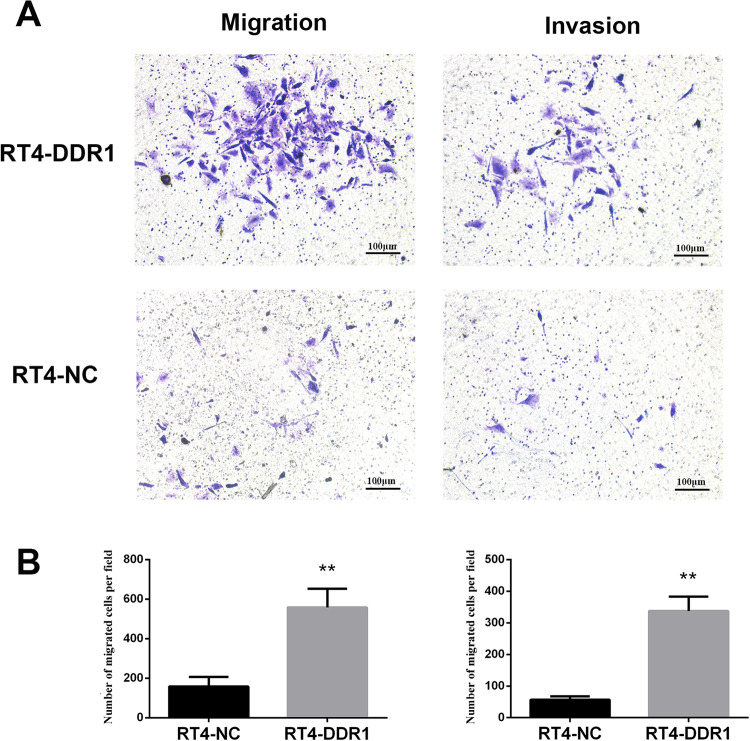
To evaluate the role of DDR1 in regulating cell migration and invasion, a transwell assay was performed. It was found that DDR1 overexpression in RT4 cells was related to a higher number of invading cells (Figure 5A and B, RT4-DDR1 vs. RT4-NC, P < 0.01). The migration capacity was also markedly higher in RT4-DDR1 cells compared with RT4-NC cells (Figure 5A and B, P < 0.01).
Figure 5.
Effect of the overexpression of DDR1 on BC cell migration and invasion. (A) Representative images of migration and invasion for RT4-DDR1 and RT4-NC cells. DDR1 overexpression resulted in a significantly increased number of migratory or invasive RT4 cells. **P < 0.01.
DDR1 Regulates the Expression of EMT-Related Genes COL4A1 and MMP-2 in RT4 Cells
ZEB1 and SLUG are TFs and key regulators of the EMT process, therefore, their expression were tested in BC cell lines. The relative expression levels of ZEB1 and SLUG mRNA were dramatically increased (Figure 3, P < 0.01). In accordance with PCR, Western blot revealed that the expression level of ZEB1 and SLUG protein in the cells of the lentivirus-infected DDR1 overexpression group was higher than the RT4-NC group (Figure 4, P < 0.01). To further explore the possible mechanisms by which DDR1 induces cell migration and invasion in RT4 cells, PCR and WB were performed to verify the expressions of the interacting genes COL4A1 and MMP-2 during DDR1 overexpression. COL4A1 and MMP-2 are closely associated with DDR1 in the PPI network. PCR results showed that there was a significant increase in COL4A1 and MMP-2 mRNA and protein expression in RT4-DDR1 cells compared to that in RT4-NC cells (Figures 3–4, P < 0.01, only P < 0.05 for COL4A1 protein).
COL4A1 Was Implicated in DDR1-Mediated Increase in Cell Migration
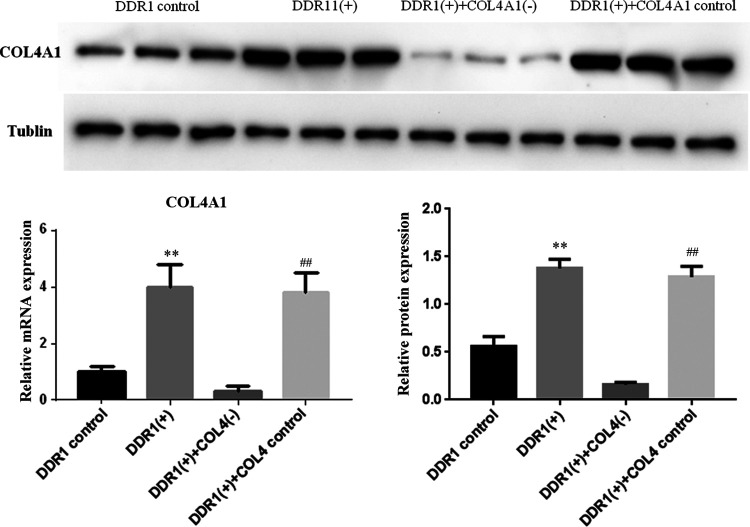
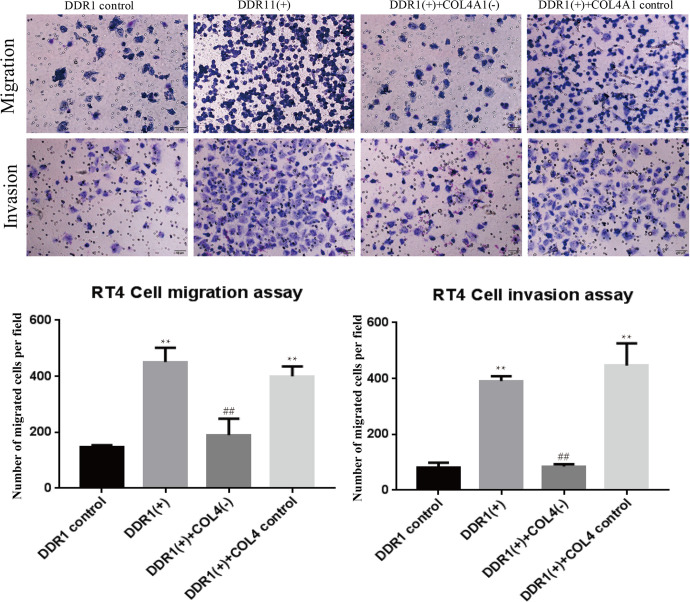
To further confirm whether COL4A1 was implicated in DDR1-mediated increase in cell migration, COL4A1-siRNA was used to inhibit the expression of COL4A1. As shown in Figure 6, overexpression of DDR1 significantly upregulated the expression of COL4A1 as expected. Conversely, such increase in COL4A1 expression was significantly decreased by COL4A1-siRNA treatment. Furthermore, the migration and invasion of RT4 cells were promoted after overexpressing DDR1, and these were reversed by COL4A1-siRNA treatment (Figure 7).
Figure 6.
The mRNA and protein expression of COL4A1 after DDR1 overexpression and COL4A1 inhibition. **P < 0.01, compared with DDR1 control; ## P < 0.01, compared with DDR1(+)+COL4A1(-) cells.
Figure 7.
Cell migration and invasion after DDR1 overexpression and COL4A1 inhibition. **P < 0.01, compared with DDR1 control; ## P < 0.01, compared with DDR1(+)+COL4A1(-) cells.
Discussion
In this study, we investigated the potential role of DDR1 in BC. Accordingly, RT4 cells overexpressing DDR1 were established. The data showed that overexpression of DDR1 not only enhanced cell motility and invasiveness in vitro, but also facilitated the expression of EMT-inducing TFs in RT4 cells, as shown by the increased expression of ZEB1 and SLUG. Furthermore, the overexpression DDR1 also induced increased levels of COL4A1 and MMP-2 in RT4 cells. In addition, the DDR1-mediated increase in migration and invasion of RT4 cells were reversed after COL4A1-siRNA treatment. Thus, we propose that COL4A1/DDR1/MMP-2 is likely to be involved in cell migration and invasion.
Dysregulated DDRs play an important role in the cellular processes of tumor development and progression, such as differentiation, migration, and malignant transformation.31-33 DDR2 is also the member of DDR subfamily, and is overexpressed in bladder, lung, prostate, and stomach cancers.34 Meanwhile, DDR2 knockdown impaired cellular viability, migration, and invasion.35 The results of our study support current evidence that DDR1 facilitates cell migration and invasion in BC cells.
Although the oncogenic function of DDR1 in BC has been clearly demonstrated, the molecular mechanism behind the DDR1 regulation in BC development remains largely unknown. Subsequently, mechanical analysis demonstrated that DDR1 controlled EMT transcription factor expression in BC cells.
Accumulating evidence indicate that TFs (such as Snail, Slug, ZEB1, SIP1, and Twist), as crucial modulators of EMT, are intracellular elements that drive cellular proliferation, longevity, and invasion.36,37 Sayan et al. indicated that ZEB1 protein expression is associated with increased migration in vitro, and functional enrichment of ZEB2 was muscle-invasive in human urothelial cancer and other primary human tumors.38 High expression of SLUG was found to be correlated with poor clinical outcomes during tumor metastasis, such as in oral tongue squamous cell carcinoma,39 hepatocellular carcinoma,40 and human gliomas.41 A negative correlation was observed between DDR1 and ZEB1 in female-derived cancer cell lines.42 A previous study suggested that DDR1 may partly enhance the invasive ability of BC by regulating ZEB1 and SLUG,43 and there are a number of studies reporting direct evidence for migration and invasion between DDR1, ZEB1, and SLUG.
MMP-2 and COL4A1 are closely related with DDR1 in the PPI, and further study can show the potential functions of interacting genes. MMPs are involved in tumor growth, invasion, metastasis and neovascularization.44 MMP-2 mRNA levels were increased after DDR1 overexpression in renal clear cell carcinoma.23 An aberrantly high expression of MMP-2 may play important roles in the regulation of cell migration and invasive capabilities of lung adenocarcinoma cancer stem-like cells.45 The collagen IV family contains 6 homologous α chains, α1–6, encoded by the COL4A1–6 genes, respectively.46 A previous study indicated that MMP-2 and MMP-9 are the major collagen IV collagenases involved in tumor invasion and metastasis.47 DDR1 is mostly activated by type I and IV collagens.21,32 Furthermore, type IV collagen can efficiently bind to DDR1 and activate intracellular protein kinase B to promote cell migration and adhesion of myeloid leukemia cells.48 Meanwhile, a COLIV-DDR1-MMP-9-COLIV feed-forward loop was suggested to promote the migration and adhesion of myeloid leukemia cells in the bone marrow.48 These evidence imply that the COL4A1 and MMP-2 are important for BC initiation and development. Thus, we speculated that the regulatory effect of DDR1 in BC may depend on the COL4A1/DDR1/MMP-2 signaling axis. However, the current experiments are still preliminary, and further investigations are required to determine the specific molecular mechanism of DDR1 in BC invasion and metastasis in vivo. In addition, studies have demonstrated that collagen or collagen matrices activate the expression of DDR1.49,50 For instance, Lai et al. suggested that collagen contributed to cell proliferation and migration in head and neck cancer through DDR1.51 As a type IV collagen alpha protein, it should be investigated whether COL4A1 could activate DDR1 in BC. Besides, overexpression of DDR1 in RT4 cells upregulated the expression of MMP-2 at both mRNA and protein levels, while the protein expression of MMP-2 showed no significant difference between BC tissue and paired adjacent tissues. Further in vivo experiments are needed to confirm the correlation of DDR1 and MMP-2.
Conclusion
Taken together, this study demonstrated that overexpression of DDR1 may enhance the progression, invasion, and metastasis in BC cells. Furthermore, DDR1 may promote cell invasion partly by regulating levels EMT-related molecule and COL4A1/DDR1/MMP-2 expression in RT4 cells. Our study suggests that DDR1 acts as a potential target for BC treatment.
Footnotes
Authors’ Contributions: Conception and design of the research: YZ, XX; acquisition of data: XX, NZ, XW, WR; analysis and interpretation of data: XX, HH, WR; statistical analysis: XX, HH; drafting the manuscript: XX, HH; revision of manuscript for important intellectual content: YZ, DX. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: This study was approved by the Ruijin Hospital Ethics Committee Shanghai JiaoTong University School of Medicine (approval no. 2016-155). All patients provided written informed consent prior to enrollment in the study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yu Zhu, MD  https://orcid.org/0000-0002-7709-4071
https://orcid.org/0000-0002-7709-4071
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. Ca Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. [DOI] [PubMed] [Google Scholar]
- 3. Masaoka H, Matsuo K, Ito H, et al. Cigarette smoking and bladder cancer risk: an evaluation based on a systematic review of epidemiologic evidence in the Japanese population. Jpn J Clin Oncol. 2016;46(3):273–283. [DOI] [PubMed] [Google Scholar]
- 4. Turati F, Bosetti C, Polesel J, et al. Family history of cancer and the risk of bladder cancer: a case–control study from Italy. Cancer Epidemiol. 2017;48:29–35. [DOI] [PubMed] [Google Scholar]
- 5. Sun J-W, Zhao L-G, Yang Y, Ma X, Wang Y-Y, Xiang Y-B. Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PloS One. 2015;10(3):e0119313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Y, Wang F, Huang H, Zhang Y, Xie H, Men T. lncRNA SLCO4A1-AS1 promotes growth and invasion of bladder cancer through sponging miR-335-5p to upregulate OCT4. Onco Targets Ther. 2019;12:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang Y, Wei T, Li W, Zhang R, Chen M. Circular RNA hsa_circ_0002024 suppresses cell proliferation, migration, and invasion in bladder cancer by sponging miR-197-3p. Am J Transl Res. 2019;11(3):1644. [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Chen L, Li Y, Guan X-Y. Overexpression of cathepsin Z contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. PloS One. 2011;6(9):e24967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang J, Zhu Z, Wu H, et al. PODXL, negatively regulated by KLF4, promotes the EMT and metastasis and serves as a novel prognostic indicator of gastric cancer. Gastric Cancer. 2019;22(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitschke J, Burk UC, Reinheckel T. The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer Metastasis Rev. 2019;38(3):431–444. [DOI] [PubMed] [Google Scholar]
- 11. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. [DOI] [PubMed] [Google Scholar]
- 12. Fu J, Zhang L, He T, et al. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int J Biol Sci. 2012;8(4):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wushou A, Hou J, Zhao Y-J, et al. Twist-1 up-regulation in carcinoma correlates to poor survival. Int J Mol Sci. 2014;15(12):21621–21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Nes JG, de Kruijf EM, Putter H, et al. Co-expression of SNAIL and TWIST determines prognosis in estrogen receptor–positive early breast cancer patients. Breast Cancer Res Treat. 2012;133(1):49–59. [DOI] [PubMed] [Google Scholar]
- 15. Manshouri R, Coyaud E, Kundu ST, et al. ZEB1/NuRD complex suppresses TBC1D2b to stimulate E-cadherin internalization and promote metastasis in lung cancer. Nat Commun. 2019;10(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krebs AM, Mitschke J, Losada ML, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. [DOI] [PubMed] [Google Scholar]
- 17. Imani S, Hosseinifard H, Cheng J, Wei C, Fu J. Prognostic value of EMT-inducing transcription factors (EMT-TFs) in metastatic breast cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:28587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120(Pt 12):1955–1958. [DOI] [PubMed] [Google Scholar]
- 19. Itoh Y. Discoidin domain receptors: microenvironment sensors that promote cellular migration and invasion. Cell Adh Migr. 2018;12(4):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu H-L, Valiathan RR, Arkwright R, et al. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013;288(11):7430–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wasinski B, Sohail A, Bonfil RD, et al. Discoidin domain receptors, DDR1b and DDR2, promote tumour growth within collagen but DDR1b suppresses experimental lung metastasis in HT1080 xenografts. Sci Rep. 2020;10(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu S, Xu H, Wang W, et al. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song J, Chen X, Bai J, et al. Discoidin domain receptor 1 (DDR1), a promising biomarker, induces epithelial to mesenchymal transition in renal cancer cells. Tumor Biol. 2016;37(8):11509–11521. [DOI] [PubMed] [Google Scholar]
- 24. Krazinski BE, Kiewisz J, Sliwinska-Jewsiewicka A, et al. Altered expression of DDR1 in clear cell renal cell carcinoma correlates with miR-199a/b-5p and patients’ outcome. Cancer Genomics Proteomics. 2019;16(3):179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valencia K, Ormazábal C, Zandueta C, et al. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res. 2012;18(4):969–980. [DOI] [PubMed] [Google Scholar]
- 26. Toy KA, Valiathan RR, Núñez F, et al. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie R, Wang X, Qi G, et al. DDR1 enhances invasion and metastasis of gastric cancer via epithelial-mesenchymal transition. Tumor Biol. 2016;37(9):12049–12059. [DOI] [PubMed] [Google Scholar]
- 28. Jing H, Song J, Zheng J. Discoidin domain receptor 1: new star in cancer-targeted therapy and its complex role in breast carcinoma. Oncol Lett. 2018;15(3):3403–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie X, Rui W, He W, et al. Discoidin domain receptor 1 activity drives an aggressive phenotype in bladder cancer. Am J Transl Res. 2017;9(5):2500–2507. [PMC free article] [PubMed] [Google Scholar]
- 30. Damian S, Andrea F, Stefan W, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2014;43:D1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeh Y-C, Lin H-H, Tang M-J. Dichotomy of the function of DDR1 in cells and disease progression. Biochim Biophys Acta Mol Cell Res. 2019;1866(11):118473. [DOI] [PubMed] [Google Scholar]
- 32. Rammal H, Saby C, Magnien K, et al. Discoidin domain receptors: potential actors and targets in cancer. Front Pharmacol. 2016;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 2014;310:39–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim D, Ko P, You E, Rhee S. The intracellular juxtamembrane domain of discoidin domain receptor 2 (DDR2) is essential for receptor activation and DDR2-mediated cancer progression. Int J Cancer. 2014;135(11):2547–2557. [DOI] [PubMed] [Google Scholar]
- 35. Tsai M-C, Li W-M, Huang C-N, et al. DDR2 overexpression in urothelial carcinoma indicates an unfavorable prognosis: a large cohort study. Oncotarget. 2016;7(48):78918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12(4):361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. [DOI] [PubMed] [Google Scholar]
- 38. Sayan AE, Griffiths TR, Pal R, et al. SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci U S A. 2009;106(35):14884–14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng M, Jiang Y-P, Chen W, et al. Snail and Slug collaborate on EMT and tumor metastasis through miR-101-mediated EZH2 axis in oral tongue squamous cell carcinoma. Oncotarget. 2015;6(9):6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun Y, Song GD, Sun N, Chen JQ, Yang SS. Slug overexpression induces stemness and promotes hepatocellular carcinoma cell invasion and metastasis. Oncol Lett. 2014;7(6):1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang HW, Menon LG, Black PM, Carroll RS, Johnson MD. SNAI2/Slug promotes growth and invasion in human gliomas. BMC Cancer. 2010;10:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim SY, Lee S, Lee E, et al. Sex–biased differences in the correlation between epithelial–to–mesenchymal transition–associated genes in cancer cell lines. Oncol Lett. 2019;18(6):6852–6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie X, Rui W, He W, Shao Y, Zhu Y. Discoidin domain receptor 1 activity drives an aggressive phenotype in bladder cancer. Am J Transl Res. 2017;9(5):2500–2507. [PMC free article] [PubMed] [Google Scholar]
- 44. Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825(1):29–36. [DOI] [PubMed] [Google Scholar]
- 45. Xin Y-H, Bai-shi-jiao Bian X-J, Yang WC, et al. POU5F1 enhances the invasiveness of cancer stem-like cells in lung adenocarcinoma by upregulation of MMP-2 expression. PloS One. 2013;8(12):e83373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parkin JD, San Antonio JD, Pedchenko V, Hudson B, Jensen ST, Savige J. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutat. 2011;32(2):127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Veidal SS, Karsdal MA, Nawrocki A, et al. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Favreau AJ, Vary CP, Brooks PC, Sathyanarayana P. Cryptic collagen IV promotes cell migration and adhesion in myeloid leukemia. Cancer Med. 2014;3(2):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Juskaite V, Corcoran DS, Leitinger B. Collagen induces activation of DDR1 through lateral dimer association and phosphorylation between dimers. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saby C, Collin G, Sinane M, et al. DDR1 and MT1-MMP expression levels are determinant for triggering BIK-Mediated Apoptosis by 3D Type I collagen matrix in invasive basal-like breast carcinoma cells. Front Pharmacol. 2019;10:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lai SL, Tan ML, Hollows RJ. Collagen induces a more proliferative, migratory and chemoresistant phenotype in head and neck cancer via DDR1. Cancers (Basel). 2019;11(11):1766. [DOI] [PMC free article] [PubMed] [Google Scholar]