Abstract
Background:
A large proportion of patients eventually experience disease progression despite treatment with immune checkpoint inhibitors (ICIs), but subsequent treatment options are limited for this population. Retreatment with the same or different types of ICIs is a possible strategy, but the clinical efficacy and safety data are limited. This systematic review aims to evaluate the efficacy and safety of ICIs retreatment in patients with solid tumors after disease progression to previous ICIs.
Methods:
We searched MEDLINE, EMBASE, the Cochrane Library, and major meeting libraries for prospective studies. The primary outcomes included the objective response rate (ORR), disease control rate (DCR), median overall survival (mOS), and the incidence of grade ⩾3 immune-related adverse events (irAEs).
Results:
We identified 22 prospective studies including 1865 patients. For disease progression after CTLA-4 inhibitors, three studies evaluated anti-CTLA-4 retreatment. The ORR was 12–23%, the DCR was 48.4–67.7%, and the mOS was 12 months. The incidence of grade ⩾3 irAEs was 5.9–25%. Four studies evaluated anti-programmed cell death protein 1 (PD-1) retreatment. The ORR was 22–36%, the DCR was 40–64%, and the mOS was 13.4–20.6 months. The incidence of grade ⩾3 irAEs was <10%. For disease progression after PD-(L)1 inhibitors, 13 studies evaluated anti-PD-(L)1 retreatment. The ORR was 5–53%, the DCR was 38–83%, and the mOS was 13.9 months. The incidence of grade ⩾3 irAEs was 0–15% for patients retreated with single anti-PD-(L)1 agent, but was higher (0–64%) for those retreated with ICIs combined with other agents. Two studies evaluated anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) retreatment. The ORR was 0–22.4%, the DCR was 50–72%, and the mOS was 4–21 months. The incidence of grade ⩾3 irAEs was 26–61%.
Conclusion:
Retreatment with ICIs is feasible for cancer patients considering its encouraging efficacy and tolerable safety. Further prospective trials are needed to explore more promising strategies and identify suitable populations for retreatment.
Keywords: immune checkpoint inhibitor, CTLA-4, PD-1, rechallenge, retreatment
Background
The use of immune checkpoint inhibitors (ICIs) has revolutionized the treatment paradigm for advanced cancers. Currently, the US Food and Drug Administration (FDA) has passed more than 50 approvals for the use of ICIs on the basis of extensive evidence from clinical trials.1 For most diseases, a durable response with significant survival benefit has been achieved in 10–25% of the patients, but a large proportion of patients still do not respond to ICIs.2–6 Moreover, patients who initially responded to ICIs may show disease progression over time even with continued treatment; the incidence of disease progression varies from 10% to 70% depending on the types of disease.2 Thus, the risk of disease progression after ICIs is high, and subsequent treatment options should be considered.
Unfortunately, only a few treatment options are available for patients who show disease progression after the use of ICIs. In real-world clinical practice, systemic treatment including targeted therapy and chemotherapy are empirically applied, but the efficacy is limited.7–10 Thus, considering the dynamic nature of the immune response and long-term benefit of ICIs, retreatment with the same or another ICI seems a suitable treatment option. Although several studies have evaluated the efficacy and safety of ICI retreatment, different regimens were administered to heterogeneous populations.11–13 Since the clinical application of ICI retreatment requires further analysis, we conducted the current systematic review to evaluate the efficacy and safety of retreatment with ICIs for patients with solid tumors who had disease progression after the first treatment with ICIs.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.14 The protocol was registered in PROSPERO on 28 April 2020 (CRD42020166902).
Literature search
We performed a literature search of electronic databases including MEDLINE, EMBASE, and the Cochrane Library for relevant records published between 1 January 2005 and 26 September 2020. Annual meeting proceedings from the American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) were retrieved, and clinical trial registers including ClinicalTrials.gov were also reviewed for ongoing trials. The keywords used for the literature search included ipilimumab, tremelimumab, pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, cemiplimab, retreatment, rechallenge, and reintroduction (Supplemental Table 1). The references of included records were manually searched by one author (KLY) to identify additional relevant studies. The language was restricted to English.
Study selection
Two authors (JRL and KLY) independently screened records obtained during the literature search. We only included prospective studies that investigated retreatment with ICIs after disease progression following ICI treatment. Patients were restricted to those with advanced solid tumors. For one trial with multiple publications, the most recent publication was included. Any discrepancies were solved by discussion. A third author (LZ) participated if a consensus could not be reached.
Data extraction and analysis
The study characteristics and the outcomes of the included studies were independently extracted by two authors (JRL and KLY) using a standardized data collection form. The primary outcomes of the current systematic review included the objective response rate (ORR), disease control rate (DCR), median overall survival (mOS), and the incidence of grade ⩾3 immune-related adverse events (irAEs). The ORR was defined as the percentage of patients who achieved a complete response (CR) or partial response (PR). The DCR was defined as the percentage of patients who achieved CR, PR, or stable disease (SD).
Two authors (JRL and KLY) independently assessed the methodological quality of the included studies by using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I). A third independent reviewer (LZ) participated to resolve discrepancies between the two reviewers.
Results
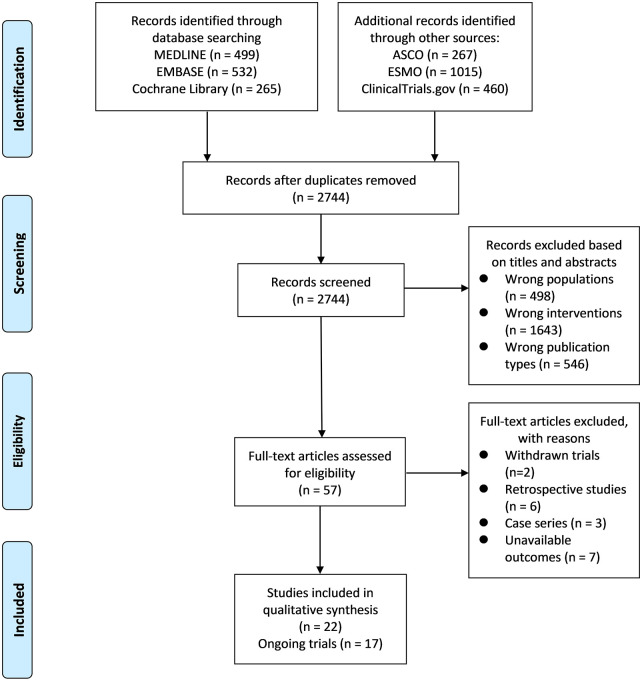
A comprehensive literature search generated 3038 records. After removing duplicates and screening titles and abstracts, 57 records were identified for full-text review. Finally, 22 prospective studies with 1865 patients were included in the qualitative analysis (Figure 1). The initial retrieval of clinical trial registers generated 460 records. After excluding irrelevant records (n = 434) and withdrawn trials (n = 2), seven relevant trials with published articles and 17 relevant ongoing trials were identified (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of literature search.
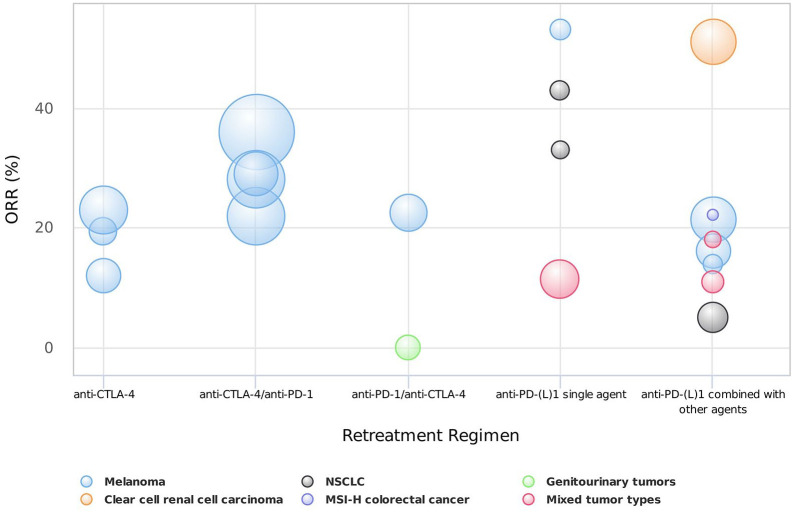
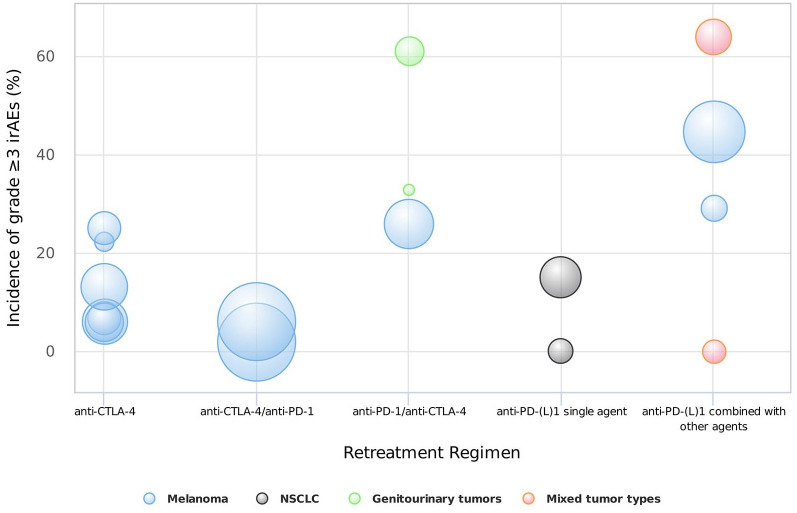
The main characteristics of the 22 included studies are listed in Table 1. Among the 22 included prospective studies, two were randomized controlled clinical trials comparing the efficacy and safety of ICI retreatment with systemic chemotherapy, and 20 were non-randomized studies investigating the clinical outcomes of retreatment with ICIs. According to the treatment regimen, eligible studies were divided into the following four categories: anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) treatment after previous CTLA-4 inhibitors (anti-CTLA-4 retreatment), anti-programmed cell death protein 1 (PD-1) treatment after previous CTLA-4 inhibitors (anti-CTLA-4/anti-PD-1 retreatment), anti-PD-1 or anti-programmed cell death ligand 1 (PD-L1) treatment after previous PD-1 or PD-L1 inhibitors [anti-PD-(L)1 retreatment], and anti-CTLA-4 treatment after previous PD-1 inhibitors (anti-PD-1/anti-CTLA-4 retreatment). The ORR outcomes of each included study according to different treatment strategies were summarized in Figure 2, and the incidence of grade ⩾3 irAEs was summarized in Figure 3.
Table 1.
Main characteristics and outcomes of included studies.
| Author | Study | Design | Sample size | Cancer type | Main inclusion criteria | Prior treatment | Retreatment regimen | Efficacy of retreatment | Incidence of grade 3/4 irAEs | Methodological quality | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-CTLA4-retreatment | |||||||||||
| Chiarion-Sileni et al.11 | NR | Expanded access program | 51 | Advanced melanoma | PD after initial disease control of prior ICI;a No grade ⩾3 irAEs | Ipilimumab | Ipilimumab | ORR | 12% | 6% | Poor |
| DCR | 55% | ||||||||||
| mOS | 12 months | ||||||||||
| Robert et al.12 | CA180-002 (NCT00094653) | Second course of a phase III randomized trial | 31 | Advanced melanoma | PD after initial disease control of prior ICI; No grade 3 non-skin irAEs or any grade 4 irAEs | Ipilimumab | Ipilimumab plus gp100 peptide vaccine or placebo | ORR | 19.4%b | 6.9% for ipilimumab plus gp100; 22.2% for ipilimumab plus placebo | Moderate |
| DCR | 67.7%b | ||||||||||
| Lebbé et al.13 | CA184-025 (NCT00162123) | Companion study for six phase II trials | 122 | Advanced melanoma | PD after initial disease control of prior ICI; No grade ⩾3 irAEs | Ipilimumab | Ipilimumab | ORR | 23% | 5.9–25%c | Poor |
| DCR | 48.4% | ||||||||||
| Anti-CTLA-4/anti-PD-1 treatment | |||||||||||
| Hamid et al.15 | KEYNOTE-001 (NCT01295827) | Phase IIb open-label trial | 342 | Advanced melanoma | PD after two or more ipilimumab doses; PD after BRAF or/and MEK inhibitors if BRAFV600 mutant-positive; Resolution of all irAEs to grade 0 or 1; No previous anti-PD-(L)1 treatment | Ipilimumab | Pembrolizumab 2 mg/kg or 10 mg/kg | ORR | 36% | NR | Moderate |
| DCR | 64% | ||||||||||
| Hamid et al.16 | KEYNOTE-002 (NCT01704287) | Phase II randomized trial | 361 | Advanced melanoma | PD after two or more ipilimumab doses; PD after BRAF or MEK inhibitors if BRAFV600 mutant-positive | Ipilimumab | Pembrolizumab 2 mg/kg or 10 mg/kg versus chemotherapy (ctx) | ORR | 22% (2 mg/kg), 28% (10 mg/kg), 4% (ctx)13.4 months (2 mg/kg), 14.7 months (10 mg/kg), 11.0 months (ctx) | 2% (2 mg/kg), 6% (10 mg/kg); 13.5% (2 mg/kg) 16.8% (10 mg/kg) versus 26.3% (ctx)d | Moderate |
| mOS | |||||||||||
| Larkin et al.17 | CheckMate 037 (NCT01721746) | Phase III open-label randomized trial | 272 | Advanced melanoma | PD after ipilimumab; PD after BRAF inhibitors if BRAFV600 mutant positive; No previous anti- PD-(L)1 treatment; No grade 4 toxicity during prior ICI treatment | Ipilimumab | Nivolumab versus chemotherapy | ORR | 27% versus 10% | 14% versus 34%d | Moderate |
| DCR | 47% versus 38% | ||||||||||
| mOS | 16 versus 14 months | ||||||||||
| Weber et al.18 | NCT01176461 | Phase I/II trial | 92 | Advanced melanoma | PD without prior response to ipilimumab | Ipilimumab | Nivolumab | ORR | 29% | NR | Moderate |
| DCR | 40% | ||||||||||
| mOS | 20.6 months | ||||||||||
| Anti-PD-(L)1 retreatment | |||||||||||
| Long et al.19 | KEYNOTE-006 (NCT01866319) | Second course of a phase III randomized trial | 15 | Advanced melanoma | PD after completing 2-year pembrolizumab with initial disease control; Ipilimumab-naive | Pembrolizumab | Pembrolizumab | ORR | 53% | NR | Moderate |
| DCR | 73% | ||||||||||
| Herbst et al.20 | KEYNOTE-010 (NCT01905657) | Second course of a phase II/III open-label randomized trial | 14 | Advanced non-small cell lung cancer (NSCLC) | PD after completing 35 cycles of pembrolizumab; TPS ⩾1% | Pembrolizumab | Pembrolizumab | ORR | 43% | NR | Moderate |
| DCR | 79% | ||||||||||
| Brahmer et al.21 | KEYNOTE-024 (NCT02142738) | Second course of a phase III randomized trial | 12 | Advanced NSCLC | PD after completing 2-year pembrolizumab or confirmed CR; PD-L1 TPS ⩾50%; No sensitizing EGFR/ALK alteration | Pembrolizumab | Pembrolizumab | ORR | 33% | 0 | Moderate |
| DCR | 83% | ||||||||||
| Diaz et al.22 | KEYNOTE-164 (NCT02460198) | Second course of a phase II trial | 9 | Microsatellite instability-high (MSI-H) colorectal cancer | PD after completing 2-year pembrolizumab or confirmed CR | Pembrolizumab | Pembrolizumab | ORR | 22.2% | NR | Moderate |
| Sheth et al.23 | NCT01693562 | Second course of a phase I/II trial | 70 | Advanced solid tumors | PD after completing 1-year durvalumab with clinical benefit | Durvalumab | Durvalumab | ORR | 11.4% | NR | Moderate |
| DCR | 47.1% | ||||||||||
| Garassino et al.24 | ATLANTIC (NCT02087423) | Second course of a phase II trial | 40 | Advanced non-small-cell lung cancer (NSCLC) | PD after completing 1-year durvalumab with initial disease control | Durvalumab | Durvalumab | NR | NR | 15% | Moderate |
| Lee et al.25 | NCT02501096 | Phase II open-label trial | 104 | Metastatic clear cell renal cell carcinoma | PD on/after prior ICI | Anti-PD-(L)1 treatment | Lenvatinib plus pembrolizumab | ORR | 51% | NR | Poor |
| Fernandez et al.26 | LEAP-004 (NCT03776136) | Phase II open-label trial | 103 | Advanced melanoma | PD within 12 weeks after the last dose of prior ICI | Anti-PD-(L)1 treatment alone or combined with other therapies | Lenvatinib plus pembrolizumab | ORR | 21.4% | 44.7%d | Moderate |
| mOS | 13.9 months | ||||||||||
| Sandhu et al.27 | NCT03178851 | Phase Ib trial | 50 | Advanced melanoma | BRAFV600 wild type PD on/after prior ICI |
Anti-PD-1 treatment | Cobimetinib plus atezolizumab | ORR | 16% | NR | Poor |
| DCR | 38% | ||||||||||
| Robert et al.28 | NR | Phase I trial | 14 | Advanced melanoma | Male patients with resistance to anti-PD-1 treatment | Anti-PD-1 treatment | Triptorelin plus nivolumab | ORR | 14% | 29%d | Poor |
| DCR | 50% | ||||||||||
| Ahn et al.29 | NCT02964013 | Phase I trial | 38 | Advanced NSCLC | PD on prior ICI | Anti-PD-(L)1 treatment | Vibostolimab plus pembrolizumab | ORR | 5% | NR | Moderate |
| Marquez-Rodas et al.30 | NCT02828098 | Phase I trial | 28 | Advanced solid tumors | Primary resistance to anti-PD-1 treatment | Anti-PD-1 treatment | BO-112 plus previous anti-PD-1 treatment | ORR | 11% | 64%d | Poor |
| DCR | 72% | ||||||||||
| Pedrazzoli et al.31 | NCT04122625 | Phase Ib trial | 11 | Advanced solid tumors | PD on/after prior ICI | Anti-PD-(L)1 treatment | Debio 1143 plus nivolumab | ORR | 18% | 0 | Poor |
| DCR | 54% | ||||||||||
| Anti-PD-1/anti-CTLA-4 retreatment | |||||||||||
| Niglio32 | NCT02496208 | Phase I trial | 24 | Metastatic urothelial carcinoma and other genitourinary tumors | PD after previous ICI treatment with initial disease control | Carboplatin plus nivolumab alone (CarboNivo) or with ipilimumab (CarboNivoIpi) | Ipilimumab | ORR | 0 | 61% (CarboNivo), 33% (CarboNivoIpi) | Moderate |
| DCR | 72% (CarboNivo), 50% (CarboNivoIpi)13.9 months (CarboNivo), 4 months (CarboNivoIpi) | ||||||||||
| mOS | |||||||||||
| Haymaker et al.33 | ILLUMINATE-204 (NCT02644967) | Phase I/II trial | 62 | Advanced melanoma | PD on/after prior ICI | Anti-PD-1 treatment | Tilsotolimod (IMO-2125) plus ipilimumab | ORR | 22.4% | 26% | Moderate |
| DCR | 71.4% | ||||||||||
| mOS | 21 months | ||||||||||
Initial disease control was defined as CR, PR or DCR ⩾3 months if not specially pointed out.
Pooled results for all patients receiving ipilimumab retreatment.
The incidence of grade ⩾3 irAEs was 25% for patients previously receiving ipilimumab 0.3 mg/kg, 5.9% for patients previously receiving ipilimumab 3 mg/kg, and 13.2% for patients previously receiving ipilimumab 10 mg/kg.
Incidence of grade 3 or more treatment-related adverse events (TRAEs).
ORR, objective response rate; DCR, disease control rate; mOS, median overall survival; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; CR, complete response; PD, progressive disease; TPS, tumor proportion score; NR, not reported.
Figure 2.
The objective response rates of different retreatment strategies. Note that the bubble size indicates the sample size of each study.
MSI-H, microsatellite instability-high; NSCLC, non-small cell lung cancer.
Figure 3.
The incidence of grade ⩾3 irAEs of different retreatment regimens. Note that the bubble size indicates the sample size of each study.
irAEs, immune-related adverse events; NSCLC, non-small cell lung cancer.
The methodological quality of most included studies was moderate (Table 1, Supplemental Table 2). The major risk of bias was owing to confounding factors, deviations from intended interventions, and missing data.
Anti-CTLA-4 retreatment
Three studies with 204 patients evaluated retreatment with CTLA-4 inhibitors after disease progression.11–13 All the patients had advanced melanoma and progressive disease after achieving initial disease control for ⩾3 months following prior ipilimumab treatment. The ORR was 12–23%, and the DCR was 48.4–67.7%.
In the Italian expanded access programme (EAP), the ORR was 12% and the DCR was 55% in 51 patients who received ipilimumab retreatment; one patient with SD, which was the best response after prior ipilimumab, achieved CR after retreatment.11 The mOS from retreatment was 12 months, and the 1-year OS rate was 50%. In the phase III CA180-002 study, a total of 31 patients received ipilimumab combined with gp100 vaccine or placebo for retreatment after disease progression.12 Retreatment achieved an ORR of 13% in the vaccine group and 37.5% in the placebo group. For all patients who underwent retreatment, the ORR was 19.4% and the DCR was 67.7%. Compared to the response observed in the previous treatment, four patients achieved a better response during retreatment, as the response changed from PR to CR in one patient and from SD to PR in three patients. Lebbé et al. reported the results of ipilimumab retreatment for patients who had received ipilimumab in six phase II studies with a follow-up of more than 5 years.13 Among 122 patients who underwent retreatment, seven achieved CR and 21 achieved PR, showing an ORR of 23% and a DCR of 48.4%.
According to the inclusion criteria of these trials, patients who discontinued ipilimumab owing to toxicity during previous therapy were not permitted to receive retreatment in these three studies. The incidence of grade ⩾3 irAEs was 5.9–25% during retreatment, and the most common grade 3 or 4 irAEs were diarrhea, colitis, and rash.11–13 No new types of toxicities were observed during retreatment, and all irAEs were generally reversible.
Anti-CTLA-4/anti-PD-1 retreatment
Four studies including 1067 patients with melanoma used PD-1 inhibitors after prior anti-CTLA-4 therapy.15–18 The ORR was 22–36%, the DCR was 40–64%, and the mOS was 13.4–20.6 months.
The KEYNOTE-001 trial included 342 patients with ipilimumab-refractory disease for pembrolizumab retreatment.15 The ORR was 36% after a 5-year follow-up. This trial also analyzed a gene expression profile (GEP) consisting of 18 genes associated with T-cell function. The GEP was similar among patients who responded to pembrolizumab regardless of the previous ipilimumab exposure, which was different from that of patients who did not respond to pembrolizumab. Two randomized trials compared ICIs with chemotherapy in patients with progressive disease after ipilimumab treatment. In the KEYNOTE-002 trial, patients were randomly allocated to receive 2 mg/kg or 10 mg/kg pembrolizumab every 3 weeks or investigator-choice chemotherapy (carboplatin, carboplatin plus paclitaxel, dacarbazine, paclitaxel alone, or oral temozolomide).16 Compared with the ORR during chemotherapy, the ORR was significantly higher in patients receiving 2 mg/kg pembrolizumab (22% versus 4%, p < 0.0001) or 10 mg/kg pembrolizumab (28% versus 4%, p < 0.0001), but the difference in OS was not significant among the different treatment regimens. The CheckMate037 trial compared the efficacy of nivolumab with investigator-choice chemotherapy (dacarbazine, or carboplatin plus paclitaxel) for retreatment.17 Patients who received nivolumab had a higher ORR than those who received chemotherapy regimens (27% versus 10%). Weber et al. investigated the population who did not respond to prior ipilimumab.18 A total of 92 patients obtained an ORR of 29% on nivolumab retreatment. The mOS was 20.6 months with a 1-year OS rate of 68.4%. Pre-treatment peripheral blood was analyzed in this trial for potential biomarkers, and higher levels of myeloid-derived suppressor cells (MDSCs) were associated with a lower response rate and shorter survival of patients receiving anti-CTLA-4/anti-PD-1 retreatment.
In the KEYNOTE-002 trial, grade ⩾3 irAEs occurred in 2% of patients receiving 2 mg/kg pembrolizumab and in 6% of patients receiving 10 mg/kg pembrolizumab.16 In addition, the incidence of grade ⩾3 treatment-related adverse events (TRAEs) was also lower in patients receiving 2 mg/kg pembrolizumab (13.5%) or 10 mg/kg pembrolizumab (16.8%) than in patients receiving chemotherapy (26.3%). Consistently, in the CheckMate037 trial, grade ⩾3 TRAEs were fewer in the nivolumab group than in the chemotherapy group (14% versus 34%).17 The phase I/II trial performed by Weber et al. included 21 patients with grade 3 or 4 irAEs during prior ipilimumab treatment.18 Four patients (19%) had episodes of grade 3 irAEs on retreatment, including two patients with rash, one with pneumonitis, and one with arthralgia. All of them were managed successfully with corticosteroids, and the treatment was discontinued only in the patient with late-onset grade 3 arthralgia in week 96.
Anti-PD-(L)1 retreatment
Anti-PD-(L)1 single agent retreatment
Thirteen studies with 508 patients evaluated the outcomes of anti-PD-(L)1 retreatment. In six trials, patients received anti-PD-(L)1 retreatment if they had achieved initial disease control but then showed disease progression after completing their previous treatment course.19–24 The ORR was 11.4–53%, and the DCR was 47.1–83%.
In the KEYNOTE-006 trial, three patients achieved CR and five showed PR among 15 patients with melanoma during their second course pembrolizumab with an ORR of 53% and a DCR of 73%.19 The KEYNOTE-010 trial evaluated the efficacy of pembrolizumab retreatment in 14 patients with advanced non-small cell lung cancer (NSCLC).20 The ORR was 43% and the DCR was 79%. The efficacy of pembrolizumab retreatment in patients with NSCLC was also reported in the 5-year follow-up of yhe KEYNOTE-024 trial, resulting in an ORR of 33% and DCR of 83% in 12 patients.21 Notably, the PD-L1 tumor proportion score (TPS) of the included patients was ⩾1% in the KEYNOTE-010 trial and ⩾50% in the KEYNOTE-024 trial.20,21 In the KEYNOTE-164 trial, nine patients with microsatellite instability-high (MSI-H) colorectal cancer received a second course of pembrolizumab, and two patients (22.2%) achieved PR.22 In a phase I/II trial that evaluated the efficacy of durvalumab for patients with advanced solid tumors, durvalumab was resumed for 70 patients after a previous 1-year treatment, resulting in an ORR of 11.4% and a DCR of 47.1%.23 The ORR varied across different tumor types, which was 14.3% in 21 patients with NSCLC, 0% in 12 patients with MSI-H tumors, 37.5% in eight patients with bladder cancer, and 8.7% in patients with other tumor types.
Two studies evaluated the safety profile of anti-PD-(L)1 single agent retreatment in patients with advanced NSCLC.21,24 No grade ⩾3 irAEs occurred during pembrolizumab retreatment in the KEYNOTE-024 trial.21 Among 40 patients with advanced NSCLC who underwent durvalumab retreatment in the ATLANTIC trial, six patients (15%) had grade ⩾3 irAEs and two died of treatment-related complications (i.e. respiratory failure and cardiac arrest in one patient each).24
Anti-PD-(L)1 retreatment combined with other agents
The preliminary outcomes of retreatment with PD-1 or PD-L1 inhibitors combined with other agents were evaluated in seven studies.25–31 The ORR was 5–51%, and the DCR was 38–72%.
A phase II study evaluated the efficacy of pembrolizumab combined with lenvatinib [a vascular endothelial growth factor (VEGF) receptor inhibitor] for patients with metastatic clear cell renal cell carcinoma (mccRCC) whose disease progressed after prior treatment with PD-1 inhibitors.25 The efficacy was evaluated at week 24 in 104 patients, resulting in an ORR of 51%. Three studies investigated anti-PD-(L)1 retreatment combined with other agents in patients with advanced melanoma.26–28 The combination of pembrolizumab and lenvatinib was applied in the phase II LEAP-004 trial; 21.4% of patients achieved CR or PR with a mOS of 13.9 months.26 Atezolizumab combined with cobimetinib [a mitogen-activated protein kinase kinase (MEK) inhibitor] was evaluated in a phase Ib study with 50 patients; the ORR was 16% and the DCR was 38%.27 The tumor expression status of PD-L1 was available for seven patients with a confirmed PR. Six of them were positive for PD-L1, while one was negative for PD-L1. In addition, in a phase I study, 14 male patients with melanoma with resistance to PD-1 inhibitors were retreated with nivolumab combined with triptorelin, a gonadotropin-releasing hormone agonist.28 Two patients achieved PR with an ORR of 14%. For patients with NSCLC, a phase I trial evaluated the efficacy of pembrolizumab retreatment combined with vibostolimab [an anti-T-cell immunoreceptor with Ig and ITIM domains (TIGIT) antibody] in 38 patients, resulting in an ORR of 5%.29 Marquez-Rodas investigated the efficacy of anti-PD-1 retreatment with intratumoral double-stranded RNA (dsRNA) for patients with advanced solid tumors showing primary resistance to anti-PD-1 inhibitors.30 The ORR was 11% and the DCR was 72% in 18 patients who were eligible for response assessment. Another phase Ib trial applied nivolumab combined with Debio 1143 [an antagonist of inhibitor of apoptosis proteins (IAPs)] in 11 patients with advanced solid tumors.31 PR was achieved in one patient with colorectal cancer and one patient with gastric cancer (ORR: 18%), and the DCR was 54%.
The incidence of grade ⩾3 TRAEs was 0–64% when PD-(L)1 inhibitors were combined with other agents for retreatment. No grade ⩾3 TRAEs occurred in patients retreated with nivolumab plus Debio 1143 at a median treatment duration of 11.6 weeks.31 For patients with melanoma receiving atezolizumab combined with cobimetinib, five episodes of grade 3 TRAEs occurred in four patients with an incidence of 29%, and all of them were resolved by treatment interruption.27 In the LEAP-004 trial, the incidence of grade ⩾3 TRAEs was 44.7%, and 7.8% of patients discontinued retreatment because of TRAEs.26 In the phase I trial by Marquez-Rodas, the incidence of grade 3 or 4 TRAEs was 64%.30
Anti-PD-1/anti-CTLA-4 retreatment
The strategy of anti-PD-1/anti-CTLA-4 retreatment was investigated in two studies.32,33 The phase I/II ILLUMINATE-204 study applied ipilimumab combined with IMO-2125 [a Toll-like receptor (TLR) 9 agonist] in 62 patients with melanoma.33 The ORR was 22.4%, the DCR was 71.4%, and the mOS was 21 months. Grade ⩾3 irAEs occured in 26% of the patients. A phase I study evaluated the efficacy of ipilimumab in 24 patients with genitourinary tumors that progressed on carboplatin combined with nivolumab alone (CarboNivo) or carboplatin combined with nivolumab plus ipilimumab (CarboNivoIpi) after initial disease control.32 After a median follow-up of 29.2 months, there was no objective response. For 18 patients receiving CarboNivo as initial treatment, 13 (72.2%) had SD during retreatment, and grade ⩾3 irAEs occurred in 61% of them. For six patients receiving CarboNivoIpi as initial treatment, three (50%) had SD and the incidence of grade ⩾3 irAEs was 33%.
Ongoing trials
We identified 17 ongoing trials that evaluated the efficacy and safety of ICI retreatment after disease progression (Table 2). There were two randomized trials: a phase II trial (NCT03697304) and the phase III ILLUMINATE-301 trial (NCT03445533); others were non-randomized phase II trials. Three trials included patients with primary resistance to ICIs, while the response to previous ICIs was not specified in the 14 other trials. As a retreatment regimen, ICIs were administered alone (n = 3) or combined with a different type of ICI (n = 7), chemotherapy (n = 2), targeted therapy (n = 4), or radiotherapy (n = 1).
Table 2.
Ongoing trials investigating retreatment with immune checkpoint inhibitors after disease progression in solid tumors.
| Trial | Phase | Cancer type | Prior treatment | Retreatment regimen | Inclusion criteria | Sample size | Primary endpoint |
|---|---|---|---|---|---|---|---|
| UNISON (NCT03177239) | II | Non-clear cell renal cell carcinoma (nccRCC) | Nivolumab | Nivolumab plus ipilimumab | Progressive disease | 36 | ORR |
| SENSITIZE (NCT03278665) | Ⅰb/II | Melanoma | Anti-PD-1 treatment | Pembrolizumab plus domatinostat (a histone deacetylase inhibitor) | Primary resistance | 40 | Incidence of adverse events |
| PRIME002 (ACTRN12618000053224) | II | Melanoma | Anti-PD-1 treatment | Azacitidine plus carboplatin; followed by avelumab | Primary resistance | NR | ORR, SD, CBR |
| OPTIM (2017-003349-14) | II | Squamous carcinoma of the head and neck (SCCHN) | Nivolumab | Nivolumab plus ipilimumab versus chemotherapy | Progressive disease | 157 | ORR |
| ILLUMINATE 301 (NCT03445533) | III | Melanoma | Anti-PD-1 treatment | Ipilimumab plus IMO-2125 [a Toll-like receptor (TLR) 9 agonist] versus ipilimumab | Progressive disease during or after anti-PD-1 therapy | 454 | OS, ORR |
| Replay (NCT03526887) | II | Non-small cell lung cancer (NSCLC) | Anti-PD-(L)1 treatment | Pembrolizumab | Progressive disease | 110 | ORR |
| NCT03126331 | II | Renal cell carcinoma (RCC) | Nivolumab or nivolumab plus ipilimumab | Nivolumab or nivolumab plus ipilimumab | Progressive disease | 40 | Proportion of patients able to receive intermittent therapy, ORR |
| NCT03085719 | II | SCCHN | Anti-PD-1 treatment | Radiation plus pembrolizumab | Progressive disease | 26 | ORR |
| NCT04322643 | II | Urothelial carcinoma | Pembrolizumab or atezolizumab or durvalumab or nivolumab avelumab | Same agent as previous treatment | Progressive disease | 20 | Number of participants that sustain a response post ICI suspension |
| NCT03697304 | II | Advanced solid tumors | Anti-PD-(L)1 treatment | BI 754091 (an anti-PD-1 agent) plus BI 754111 [an anti-lymphocyte activation gene-3 (LAG-3) agent] versus BI 754091 plus BI 836880 [an anti-vascular endothelial growth factor (VEGF) agent] | Primary or secondary resistance | 260 | ORR |
| HUDSON (NCT03334617) | II | NSCLC | Anti-PD-(L)1 therapy | Durvalumab plus AZD9150 or AZD6738 or vistusertib or olapanib or oleclumab or trastuzumab deruxtecan or cediraniba | Progressive disease | 320 | ORR |
| NCT03737123 | II | Urothelial carcinoma | Anti-PD-(L)1 treatment | Atezolizumab plus carboplatin and gemcitabine or docetaxel | Progressive disease | 33 | PFS |
| NCT04088500 | II | RCC | Nivolumab plus ipilimumab; followed by nivolumab | Nivolumab plus ipilimumab | Progressive disease | 100 | DCR |
| NCT03003676 | II | Melanoma | Anti-PD-1 treatment | ONCOS-102 (an oncolytic adenovirus) followed by pembrolizumab | Progressive disease | 21 | Incidence of adverse events |
| NCT03177239 | II | NccRCC | Nivolumab | Nivolumab plus ipilimumab | Progressive disease | 85 | ORR |
| NCT03262779 | II | NSCLC | Anti-PD-(L)1 treatment | Nivolumab plus ipilimumab | Progressive disease | 50 | ORR |
| NCT02743819 | II | Melanoma | Anti-PD-(L)1 treatment | Pembrolizumab plus ipilimumab | Progressive disease or stable disease | 70 | ORR |
Vistusertib is a mammalian target of rapamycin (mTOR) inhibitor; olapanib is a poly (ADP-ribose) polymerase (PARP) enzyme inhibitor; oleclumab is an anti-CD73 monoclonal antibody; trastuzumab deruxtecan is an antibody-drug conjugate; cediranib is a VEGF signaling inhibitor.
CBR, clinical benefit rate; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; SD, stable disease.
Discussion
This systematic review outlines the up-to-date evidence on the efficacy and safety of retreatment with ICIs for patients with solid tumors. Despite the relatively high heterogeneity of included studies, retreatment with ICIs exhibited encouraging efficacy and manageable safety profiles for patients with solid tumors whose disease progressed after prior treatment with ICIs.
Retreatment after anti-CTLA-4 treatment
Although ipilimumab is no longer recommended as the first-line treatment option for advanced melanoma, a large number of patients still receive ipilimumab because of clinical trial design or limited drug availability in real-world practice.34–37 Therefore, treatment for progression after prior ipilimumab treatment is still a clinical issue that requires investigation.
This systematic review evaluated two possible strategies: anti-CTLA-4 retreatment and anti-CTLA-4/anti-PD-1 retreatment. An overall higher ORR was achieved in anti-CTLA-4/anti-PD-1 retreatment than in anti-CTLA-4 retreatment (22–36% versus 12–23%, Figure 2). This result is expectable as CTLA-4 and PD-1 convey inhibitory effects at different stages of the immune response. CTLA-4 reduces immune response at the early stage of T-cell activation, while the major role of PD-1 is to limit the activity of effector T cells within the tumor microenvironment.38,39 Thus, CTLA-4 inhibitors and PD-1 inhibitors can be considered as different types of drugs, and the resistance to one ICI does not interfere the activity of the other. Furthermore, the efficacy of PD-1 inhibitors is inherently higher than CTLA-4 inhibitors regardless of previous treatment lines,40–42 and this higher efficacy may also exist in the retreatment setting.
In addition, retreatment with PD-1 inhibitors and chemotherapy was also compared in two studies. Retreatment with PD-1 inhibitors exhibited superior ORR and fewer grade ⩾3 TRAEs than chemotherapy. Thus, the FDA accelerated the approval of nivolumab and pembrolizumab for ipilimumab-refractory melanoma.43,44 Based on these encouraging results, we consider that PD-1 inhibitors is a better choice than CTLA-4 inhibitor for patients with melanoma who have disease progression after previous anti-CTLA-4 treatment.
Retreatment after anti-PD-(L)1 treatment
Patients could receive anti-PD-(L)1 retreatment or anti-PD-1/anti-CTLA-4 retreatment after disease progression to PD-(L)1 inhibitors. The ORR was similar in anti-PD-(L)1 retreatment administered alone (11.4–53%) or combined with other agents (5–51%). Notably, the combination strategy could achieve an objective response in patients with primary resistance to prior ICIs.30 As both preclinical and clinical evidence have demonstrated enhanced efficacy with combination strategies for cancer patients,45–47 ICIs combined with other agents might be effective for retreatment. Currently, 14 ongoing trials are evaluating the efficacy of different combinations including ICIs combined with chemotherapy, targeted therapy, radiotherapy, or a different ICI (Table 2). These strategies are used in broad clinical settings, including different cancer types and resistance status. We can expect that more clinically useful treatment regimens for retreatment will be identified in the future.
Two studies evaluated the efficacy of anti-PD-1/anti-CTLA-4 retreatment. In patients with metastatic genitourinary tumors, no objective response was achieved at a median follow-up of 21.2 months, while an ORR of 22.4% was observed in patients with melanoma receiving ipilimumab plus IMO-2125. Ipilimumab as single agent is only applied in melanoma patients, while it is combined with PD-(L)1 inhibitors in most clinical scenarios.1 It is reasonable to expect the poor response to ipilimumab retreatment in non-melanoma tumors. For melanoma patients, the ORR of ipilimumab ranged from 10% to 15% in treatment-naive patients,48,49 and the higher ORR during retreatment may be attributed to the addition of IMO-2125 (a TLR 9 agonist) which upregulates the production of endogenous interferons and enhances the activity of ICIs.33,50 Therefore, we consider PD-(L)1 inhibitors are better regimens for retreatment than CTLA-4 inhibitors, especially in non-melanoma tumor types, while the use of ipilimumab in combination with other agents is also promising and requires further investigation.
Predictive factors for ICIs retreatment
The efficacy of ICI retreatment depends on many case-specific factors, which have not been much investigated to date. The response to prior ICIs might be a predictive factor. A previous retrospective study showed that a progression-free survival of 90 days or more in prior ipilimumab treatment predicted better responses for subsequent pembrolizumab treatment.51 In this review, eight studies investigated the efficacy of ICI retreatment for patients with initial disease control after previous immunotherapy, and the ORR was 11.4–53%. However, the Italian EAP and CA180-002 studies showed that improved response could be achieved on retreatment compared with the best objective response during the initial therapy.11,12 Moreover, patients with primary resistance to prior immunotherapy could also benefit from ICI retreatment, as they showed similar ORR (11–29%) in some included studies when ICIs were switched to another type or combined with other agents.18,30 Therefore, further randomized controlled trials for a non-selective population are required to identify patients who could benefit most from ICI retreatment, and more diverse treatment regimens should also be explored to identify the most effective strategy.
Currently, there are no well-established biomarkers for predicting the efficacy of ICI retreatment. Peripheral blood biomarkers including GEP and MDSCs were investigated in this review.15,18 In the KEYNOTE-001 trial, the GEP was significantly different between ICI-responsive patients and ICI-resistant patients irrespective of previous ipilimumab exposure.15,52 Another potential biomarker for retreatment was the PD-L1 expression status. For anti-PD-(L)1 retreatment in NSCLC, patients obtained relatively high ORRs in the KEYNOTE-010 (43%) and KEYNOTE-024 trials (33%).20,21 This high efficacy may be attributed to the fact that the KEYNOTE-010 and KEYNOTE-024 trials only included PD-L1-positive patients. GEPs, peripheral MDSCs and PD-L1 expression status are predictive biomarkers for ICI treatment in unselected patients.53,54 Other general biomarkers including tumor mutational burden (TMB) and neoantigen load might also be applicable in the specific population receiving ICI retreatment, which requires further investigation.
Safety
When considering retreatment with ICIs, grade ⩾3 toxicities generally warrant suspension or even permanent discontinuation of ICIs.55,56 In most included studies of this systematic review, patients were not permitted to receive ICI retreatment if they had grade ⩾3 toxicities during the previous course of ICIs, and the incidence of grade ⩾3 irAEs during retreatment was summarized in Figure 3. Considering the types of ICIs for retreatment, caution should be paid to the use of CTLA-4 inhibitors for retreatment, as the incidence of grade ⩾3 irAEs could be as high as 61%, especially in anti-PD-1/anti-CTLA-4 retreatment. The safety profiles of retreatment with a PD-(L)1 single agent were generally acceptable, as the incidence of grade ⩾3 irAEs was 0–15% among the included studies (Table 1), which was similar to that observed in treatment-naive patients.35,41,57 Fewer grade ⩾3 TRAEs were also observed after PD-1 retreatment than after chemotherapy.16,17 However, strategies combining ICIs with other potentially synergistic agents should be administered carefully. The safety profile was scarcely investigated in these trials, and a much higher incidence of severe toxicity was observed considering the available evidence. For example, in a phase I trial, researchers reported that the incidence of grade ⩾3 TRAEs was 64% in patients treated with PD-1 inhibitors combined with dsRNA.30 Thus, more evidence is required to assess the risk–benefit profile in this clinical setting.
Beyond the safety restriction mentioned above, Weber et al. reported acceptable safety outcomes of nivolumab retreatment in 21 patients with grade ⩾3 irAEs during previous ipilimumab treatment.18 Consistently, another retrospective study showed that anti-PD-1 therapy could be safely administered after severe ipilimumab-related adverse events for patients with melanoma.58 Therefore, safety restrictions for retreatment with anti-PD-1 or anti-PD-L1 inhibitors may be eased in ipilimumab-refractory patients, as toxicities due to ipilimumab were not indicative for anti-PD-(L)1 therapy.
Limitations
This systematic review has several limitations. First, the included studies exhibited study design heterogeneity, which allows a generalizable conclusion but limits in-depth analysis considering specific strategies or populations. Second, although the methodological quality of most included studies was moderate, several studies still exhibited a high risk of bias. Third, several included studies were preliminary results with relatively short follow-up and small sample sizes; they may lack robustness and require further investigation.
Conclusion
In conclusion, retreatment with ICIs for patients with solid tumors exhibits encouraging efficacy and acceptable safety. Nevertheless, further prospective trials are needed to explore more promising retreatment strategies and identify the most suitable population for retreatment.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_1758835920975353 for Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review by Kaili Yang, Jiarui Li, Zhao Sun, Lin Zhao and Chunmei Bai in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Conceptualization: JRL, KLY, LZ, CMB
Data curation: KLY, JRL
Original draft writing: KLY, JRL
Manuscript review and editing: JRL, LZ, CMB, ZS
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (no. 61435001) and the CAMS Innovation Fund for Medical Sciences (no. 2016-I2M-1-001, no. 2017-I2M-4-003)
ORCID iD: Kaili Yang  https://orcid.org/0000-0002-2894-4965
https://orcid.org/0000-0002-2894-4965
Jiarui Li  https://orcid.org/0000-0002-9340-3962
https://orcid.org/0000-0002-9340-3962
Chunmei Bai  https://orcid.org/0000-0003-1333-9145
https://orcid.org/0000-0003-1333-9145
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kaili Yang, Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing China.
Jiarui Li, Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing China.
Zhao Sun, Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing China.
Lin Zhao, Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1 Shuai Fu Yuan, Dongcheng District, Beijing 100032, China.
Chunmei Bai, Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1 Shuai Fu Yuan, Dongcheng District, Beijing 100032, China.
References
- 1. Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 2020; 12: 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell 2020; 37: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 2019; 20: 1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balar AV, Dreicer R, Loriot Y, et al. Atezolizumab (atezo) in first-line cisplatin-ineligible or platinum-treated locally advanced or metastatic urothelial cancer (mUC): long-term efficacy from phase 2 study IMvigor210. J Clin Oncol 2018; 36: 4523. [Google Scholar]
- 5. Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 2019; 30: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 7. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15: 504–535. [DOI] [PubMed] [Google Scholar]
- 8. Daniel GC, John AT, Mark RA, et al. Cutaneous melanoma, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019; 17: 367–402. [DOI] [PubMed] [Google Scholar]
- 9. Patrinely JR, Jr, Baker LX, Davis EJ, et al. Outcomes after progression of disease with anti-PD-1/PD-L1 therapy for patients with advanced melanoma. Cancer 2020; 126: 3448–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giaj Levra M, Cotté F-E, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer 2020; 140: 99–106. [DOI] [PubMed] [Google Scholar]
- 11. Chiarion-Sileni V, Pigozzo J, Ascierto PA, et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: the expanded access programme in Italy. Br J Cancer 2014; 110: 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robert C, Schadendorf D, Messina M, et al. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res 2013; 19: 2232–2239. [DOI] [PubMed] [Google Scholar]
- 13. Lebbé C, Weber JS, Maio M, et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol 2014; 25: 2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019; 30: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer 2017; 86: 37–45. [DOI] [PubMed] [Google Scholar]
- 17. Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018; 36: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber J, Gibney G, Kudchadkar R, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res 2016; 4: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long GV, Schachter J, Arance A, et al. Long-term survival from pembrolizumab (pembro) completion and pembro retreatment: phase III KEYNOTE-006 in advanced melanoma. J Clin Oncol 2020; 38: 10013. [Google Scholar]
- 20. Herbst RS, Garon EB, Kim D-W, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol 2020; 38: 1580–1590. [DOI] [PubMed] [Google Scholar]
- 21. Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. LBA51 – KEYNOTE-024 5-year OS update: first-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ⩾50%. Ann Oncol 2020; 31: S1181–S1182. [Google Scholar]
- 22. Diaz LA, Le DT, Kim TW, et al. Pembrolizumab monotherapy for patients with advanced MSI-H colorectal cancer: longer-term follow-up of the phase II, KEYNOTE-164 study. J Clin Oncol 2020; 38: 4032. [Google Scholar]
- 23. Sheth S, Gao C, Mueller N, et al. Durvalumab activity in previously treated patients who stopped durvalumab without disease progression. Ann Oncol 2019; 30: v475–v476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garassino M, Cho BC, Kim J, et al. Safety of durvalumab retreatment in advanced NSCLC patients who progressed following initial disease control in ATLANTIC. J Thorac Oncol 2018; 13: S467–S468. [Google Scholar]
- 25. Lee C-H, Shah AY, Hsieh JJ, et al. Phase II trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) for disease progression after PD-1/PD-L1 immune checkpoint inhibitor (ICI) in metastatic clear cell renal cell carcinoma (mccRCC). J Clin Oncol 2020; 38: 5008. [Google Scholar]
- 26. Fernandez AMA, O’Day SJ, de la Cruz Merino L, et al. LBA44 Lenvatinib (len) plus pembrolizumab (pembro) for advanced melanoma (MEL) that progressed on a PD-1 or PD-L1 inhibitor: initial results of LEAP-004. Ann Oncol 2020; 31: S1173. [Google Scholar]
- 27. Sandhu SK, Atkinson VG, Cao MG, et al. Interim analysis of a phase Ib study of cobimetinib plus atezolizumab in patients with advanced BRAFV600 wild type melanoma progressing on prior anti-PD-L1 therapy. Ann Oncol 2019; 30: v554. [DOI] [PubMed] [Google Scholar]
- 28. Robert C, Lejeune FJ, Lebbé C, et al. Combination of triptorelin with nivolumab in ICI resistant advanced melanoma. Ann Oncol 2019; 30: xi44. [Google Scholar]
- 29. Ahn M-J, Niu J, Kim D-W, et al. 1400P Vibostolimab, an anti-TIGIT antibody, as monotherapy and in combination with pembrolizumab in anti-PD-1/PD-L1-refractory NSCLC. Ann Oncol 2020; 31: S887. [Google Scholar]
- 30. Marquez-Rodas I, Longo F, Ponce Aix S, et al. Combination of intratumoural double-stranded RNA (dsRNA) BO-112 with systemic anti-PD-1 in patients with anti-PD-1 refractory cancer. Ann Oncol 2019; 30: xi37–xi38. [Google Scholar]
- 31. Pedrazzoli ABA, Moreno V, Gomez-Roca CA, et al. 560P Safety and efficacy of Debio 1143, an antagonist of inhibitor of apoptosis proteins (IAPs), in combination with nivolumab in a phase Ib/II trial in patients (pts) failing prior PD-1/PD-L1 treatment. Ann Oncol 2020; 31: S483. [Google Scholar]
- 32. Niglio SA. Ipilimumab challenge/re-challenge in metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors treated with cabozantinib+nivolumab (CaboNivo) or cabozantinib+nivolumab+ipilimumab (CaboNivoIpi). J Clin Oncol 2020; 38: 5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haymaker C, Andtbacka RHI, Johnson DB, et al. 1083MO Final results from ILLUMINATE-204, a phase I/II trial of intratumoral tilsotolimod in combination with ipilimumab in PD-1 inhibitor refractory advanced melanoma. Ann Oncol 2020; 31: S736. [Google Scholar]
- 34. Jochems A, Leeneman B, Franken MG, et al. Real-world use, safety, and survival of ipilimumab in metastatic cutaneous melanoma in The Netherlands. Anticancer Drugs 2018; 29: 572–578. [DOI] [PubMed] [Google Scholar]
- 35. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19: 1480–1492. [DOI] [PubMed] [Google Scholar]
- 36. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mohr P, Ascierto P, Arance A, et al. Real-world treatment patterns and outcomes among metastatic cutaneous melanoma patients treated with ipilimumab. J Eur Acad Dermatol Venereol 2018; 32: 962–971. [DOI] [PubMed] [Google Scholar]
- 38. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019; 20: 1239–1251. [DOI] [PubMed] [Google Scholar]
- 41. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 42. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: a randomised clinical trial. Eur J Cancer 2018; 101: 236–243. [DOI] [PubMed] [Google Scholar]
- 43. Chuk MK, Chang JT, Theoret MR, et al. FDA approval summary: accelerated approval of pembrolizumab for second-line treatment of metastatic melanoma. Clin Cancer Res 2017; 23: 5666–5670. [DOI] [PubMed] [Google Scholar]
- 44. Hazarika M, Chuk MK, Theoret MR, et al. US FDA approval summary: nivolumab for treatment of unresectable or metastatic melanoma following progression on ipilimumab. Clin Cancer Res 2017; 23: 3484–3488. [DOI] [PubMed] [Google Scholar]
- 45. Aznar MA, Planelles L, Perez-Olivares M, et al. Immunotherapeutic effects of intratumoral nanoplexed poly I:C. J Immunother Cancer 2019; 7: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 48. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- 49. Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol 2015; 33: 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 2010; 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 51. Shreders A, Joseph R, Peng C, et al. Prolonged benefit from ipilimumab correlates with improved outcomes from subsequent pembrolizumab. Cancer Immunol Res 2016; 4: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017; 127: 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17: e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019; 7: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36: 1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017; 390: 1853–1862. [DOI] [PubMed] [Google Scholar]
- 58. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017; 28: 368–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_1758835920975353 for Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review by Kaili Yang, Jiarui Li, Zhao Sun, Lin Zhao and Chunmei Bai in Therapeutic Advances in Medical Oncology