Abstract
Background
Olfactory dysfunction (OD) is common, affecting an estimated 13 million adults in the United States. Prior studies may underestimate OD prevalence due to use of brief smell identification tests or age-adjusted cutoff values, which concede that it is acceptable for older people to have a decreased sense of smell.
Objective
To determine OD prevalence in the healthy community when the goal and expectation is ideal olfactory function, rather than age-based population norms. Secondary goals were to explore factors associated with OD.
Methods
Subjects without otolaryngic complaints were recruited from the community surrounding the Medical University of South Carolina. Olfactory-specific information was collected, and olfactory function was assessed using the Sniffin’ Sticks test (Burghardt, Wedel, Germany) to measure threshold, discrimination, and identification (TDI). OD was defined as a TDI score < 31. Bivariate analysis and linear regression were used to determine factors associated with OD.
Results
In total, 176 subjects were included with mean age of 52 years (range: 20–93), 111 (63%) female, and 127 (72%) white. Mean TDI score was 28.8 (6.9) and OD was present in 94 (53%) subjects. Multivariate linear regression revealed that TDI decreased an average of 1 point every 5 years. TDI was also associated with Mini-Mental Status Examination (MMSE) score, asthma, and gastroesophageal reflux disease. Threshold was associated with age, heart problems, and gastroesophageal reflux disease. Discrimination was associated with age and MMSE scores. Identification was associated with age, heart problems, and anxiety.
Conclusions
In a community-based sample, OD affects greater than 50% of subjects. Aging impacts all aspects of olfaction, while the effects of factors such as asthma, MMSE scores, gastroesophageal reflux disease, heart problems, and anxiety may only be evident in specific olfactory subtests.
Keywords: olfaction, smell, anosmia, hyposmia, dysosmia
Introduction
Olfactory dysfunction (OD) is a common ailment affecting an estimated 13.3 million adults in the United States.1 Prior studies evaluating self-reported olfactory problems demonstrated a decrease in olfactory function with age, but underestimate prevalence rates significantly due to lack of awareness.2 Prior population-based studies have used brief odor identification tests to identify impaired sense of smell with reported rates of OD from 3.8% to 62.5% depending upon the population studied.3,4 Moreover, in a study evaluating over 3000 adults aged 57 to 85 years, olfactory function as measured by a 5-item identification test was found to be one of the strongest independent predictors of 5-year mortality surpassing that of heart failure, lung disease, and cancer, thus demonstrating the importance of OD in the general population.5
In multiple large population studies, older age and male sex have been associated with higher OD prevalence.2,3,6 Various other factors such as nasal polyposis,6 current smoking,3 peripheral vascular disease,4 cognitive decline, racial/ethnic minority,7 coronary artery disease,8 diabetes mellitus,6 higher level of education,8 and moderate-to-vigorous physical activity have each been associated with OD prevalence.1
Prior epidemiologic studies have used brief smell identification tests to estimate OD prevalence with limited evaluation of potential etiologic/risk factors and inability to control for potential confounding/coexisting factors. Investigations utilizing detailed olfactory testing have been limited to specific populations, and those seeking care for OD or associated disorders. Age-specific cutoff values concede that it is acceptable for older people to have a decreased sense of smell. This is in contrast to vision and hearing, which use ideal norms for the entire age spectrum in order to identify the impact of aging upon ideal sensory function. Thus, OD is likely more prevalent when the goal and expectation for olfaction is based on the ideal level of function.
The aim of this study was to prospectively investigate the prevalence of OD in a healthy population without self-perceived olfactory disturbance, using detailed olfactory testing and ideal cutoff values based on peak olfactory function. Secondary goals were to explore factors associated with OD using multivariate linear regression.
Materials and Methods
Enrollment
Adult subjects from the local community were recruited to an ambulatory clinic at the Medical University of South Carolina (MUSC) as part of institutional review board approved study HR E-607R. Subjects were referred through word of mouth and flyers posted in buildings and clinics surrounding MUSC, Included subjects were not seeking medical care or presenting for sinonasal complaints. Referrals were obtained from family and friends of clinic patients, and represented a variety of socioeconomic backgrounds consistent with the local community. Enrollment was not influenced by perceived olfactory ability. Subjects were not examined or obtained through the MUSC rhinology clinic. Ability to complete written forms in English was required. Subjects with active upper respiratory infections, autoimmune disease or taking immunosuppressive medications including systemic steroids and chemotherapy were excluded, as were those with dementia of any form including Parkinson’s disease and Alzheimer’s disease. Subjects with overt or uncontrolled chronic rhinosinusitis (CRS) were excluded, as were those who reported active management of CRS. Subjects who reported a history of endoscopic sinus surgery that was not secondary to a history of CRS were included. Written informed consent was obtained and the subject data deidentified.
Demographics and Comorbidities
Olfactory-specific information pertaining to demographics, medical comorbidities, potential olfactory exposures, and risk factors was collected from the subject at the time of the visit using a written questionnaire. Demographic information included subject age at enrollment, sex, race, and ethnicity. Self-reported medical comorbidities, including allergic rhinitis, asthma, diabetes, and depression were collected from each subject, and subjects were further instructed to include all existing, physician-diagnosed conditions. Olfactory-specific information was collected including prior sinus surgery, head trauma, tobacco use and military service, as well as patient-reported exposure to chemical odors, exhaust fumes, ionizing radiation, and silica. At the initial visit, a trained research coordinator performed a Mini-Mental Status Examination (MMSE).
Quantitative Olfactory Testing
Objective olfactory testing was performed using “Sniffin’ Sticks” testing (Burghardt, Wedel, Germany). The testing consisted of threshold, discrimination, and identification (TDI) subtests, which form a composite TDI summed score. Subjects were asked to close their eyes during the threshold and discrimination portions of testing, while the identification portion required their eyes remain open. Using dilutions of n-butanol, the threshold portion of the test was performed using a single-staircase triple-forced choice procedure. Identification of the pen with the highest concentration in 2 subsequent attempts constituted a correct response, whereas incorrectly identifying a pen even once would be considered incorrect. This was conducted 7 times, and the final 4 scores were averaged to produce the resultant threshold score. The discrimination subtest uses triplet sets of odorant pens, of which 2 contain the same odorant and the third, a different odorant. Sixteen sets of 3 are presented in random order, and subject earned 1 point for identifying the pen containing the odorant that differs from the other 2 of the set. Discrimination score was reported as the sum of correct responses. The identification subtest presents 16 suprathreshold odorants individually, along with 4 answer choices for each pen. The sum of correct responses was reported as the identification score. The points earned for TDI were then summed to produce a composite TDI score. In total, the TDI evaluation took around 60 minutes to complete.
Olfactory Function
Subjects were stratified by olfactory performance in relation to what was defined as “ideal” olfaction. Updated in 2019, the normative data for the “Sniffin’ Sticks” test presents olfactory data for subjects grouped by decade of life, and gender.9 The 10th percentile has been previously defined as the point separating normosmia from hyposmia.9 In order to establish ideal olfactory cutoff values, data from female subjects aged 31 to 40 years were used and represented the group in which olfaction is at its peak. This group demonstrated peak olfactory function, determined using 10th-percentile values for TDI. Specific cutoff values were set based on the 10th-percentile, with those falling below being considered abnormal. Olfactory function was classified based on composite TDI score. Subjects with TDI scores greater than or equal to 31 were considered normosmic, and OD was defined as a TDI less than 31. Subjects with TDI scores less than 16 were considered functionally anosmic, while those scoring between 16 and 31 were considered hyposmic. Olfactory subtest scores of 5.75, 11, and 12 or greater were considered normal for threshold, discrimination, and identification, respectively.
Statistical Analysis
Analyses were conducted using version 25 of SPSS (IBM Corporation, Armonk, New York). An initial bivariate analysis was performed to assess factors associated with TDI scores, including demographics, comorbidities, exposures, and other risk factors. Assumptions of normality were met for all continuous variables, and Pearson correlations were performed between olfactory outcomes and continuous variables such as age and MMSE score. A comparison of means was performed between TDI and dichotomous categorical factors within demographics, comorbidities, exposures, and risk factors using an Independent samples t test. Bivariate analysis was then repeated to assess factors associated with olfactory TDI subtest scores independently. For TDI, multivariate linear regression was performed on all factors with a P value <.075 in the initial bivariate analysis using TDI. This was done using forward selection, adding variables in a stepwise fashion. Variables that became insignificant were removed from the model, and those that remained significant were carried over. The process was repeated until only significant correlates were left. Significance was established with a P value of ≤.05. After ensuring the assumptions of multivariate linear regression had been satisfied, factors that remained in the model were considered independently associated with the olfactory test measure TDI and reported using unstandardized B. Multivariate analysis was repeated for TDI using variables with P value <.075 in bivariate analysis of the correspondent subtest domain using the same feed-forward process. Potential curvature in the association between age and TDI was determined by comparing r-squared measures with and without a quadratic term in the model. The r-squared value was slightly higher for both TDI and threshold with a quadratic term.
Results
Cohort Characteristics
A total of 176 patients were enrolled, with cohort characteristics detailed in Table 1. The average age of the cohort was 52.7 years (range: 20–93), and 111 (63.1%) females made up the majority. Allergic rhinitis was reported in 31.5% of subjects, and 7.9% of the cohort reported asthma. Prior sinus surgery had been reported in 6.8% of subjects and 10.1% endorsed diabetes. A significant number also had potential olfactory exposures and risk factors, including smoke exposure, prior military service and chemical odor exposure. The mean MMSE score for the cohort was 28.7 (SD = 1.6).
Table 1.
Baseline Characteristics and Olfactory Function of the Study Cohort.
| Overall cohort (n = 176) | Mean (SD) |
|---|---|
| Age in years | 52.7 (17.4) |
| Cognitive function | Mean (SD) |
| MMSE total score | 28.7 (1.6) |
| Demographic group | N (%) |
| Gender | |
| Male | 65 (36.9) |
| Female | 111 (63.1) |
| Race | |
| White | 127 (72.2) |
| Non-white | 49 (27.8) |
| Ethnicity | |
| Hispanic/Latino | 6 (3.4) |
| Non-Hispanic/Latino | 170 (96.6) |
| Comorbidities | N (%) |
| Allergic rhinitis | 56 (31.5) |
| Allergy testing | 43 (24.2) |
| Asthma | 14 (7.9) |
| Previous sinus surgery | 12 (6.8) |
| Diabetes | 18 (10.1) |
| Heart problems | 19 (10.8) |
| Hyperlipidemia | 38 (21.8) |
| Hypertension | 53 (29.8) |
| Obstructive sleep apnea | 20 (11.2) |
| Depression | 33 (18.5) |
| Anxiety | 25 (14.2) |
| Memory deficit | 5 (2.8) |
| Arthritis | 39 (22.2) |
| Cancer (any) | 21 (11.9) |
| GERD | 25 (14.2) |
| Exposures/risk factors | N (%) |
| Environmental (any) | 39 (21.9) |
| Chemical odors | 20 (11.2) |
| Dust storms | 7 (3.9) |
| Exhaust fumes | 15 (8.4) |
| Pollution/smog | 13 (7.4) |
| Wood dust | 14 (7.9) |
| Ionizing radiation | 18 (10.1) |
| Silica | 6 (3.4) |
| Toxic materials | 14 (7.9) |
| Head injury (previous) | 19 (10.8) |
| Military service | 23 (12.9) |
| Smoking: current | 20 (11.2) |
| Smoking: ever | 68 (38.2) |
| Second-hand smoke (current) | 13 (7.4) |
| Second-hand smoke (previous) | 99 (56.5) |
| Alcohol use: current | 106 (59.6) |
| Olfactory measure | Mean (SD) |
| TDI | 28.8 (6.9) |
| Threshold | 6.1 (2.6) |
| Discrimination | 10.9 (2.7) |
| Identification | 11.8 (2.8) |
| Olfactory measure classification | N (%) |
| TDI | |
| Normosmic | 82 (46.6) |
| Hyposmic | 84 (47.7) |
| Anosmic | 10 (5.7) |
| Threshold | |
| Normal | 103 (57.9) |
| Abnormal | 74 (41.6) |
| Discrimination | |
| Normal | 79 (44.4)) |
| Abnormal | 97 (54.5) |
| Identification | |
| Normal | 81 (45.5) |
| Abnormal | 95 (53.4) |
Abbreviations: GERD, gastroesophageal reflux disease; MMSE, Mini-Mental Status Examination; n, sample size; N, number of subjects; SD, standard deviation; TDI, composite of threshold (T), discrimination (D), identification (I) score; Y, years; %, percentage of subjects.
Olfactory Function
Olfactory function of the entire cohort is detailed in Table 1. The overall mean TDI score was 28.8 (SD = 6.9). OD was present in 94 (53.4%) subjects, and 82 (46.6%) were considered normosmic. OD was considered hyposmia in 84 (47.7%), while 10 (5.7%) were functionally anosmic. There were 103 (57.9%) subjects, which scored in the normal range for threshold, whereas 79 (44.4%) and 81 (45.5%) subjects had normal scores in discrimination and identification subtests, respectively.
Regression Analysis
Threshold, discrimination, and identification
Bivariate analysis of factors associated with TDI was performed for all demographics, comorbidities, exposures, and other risk factors listed in Table 1. Factors with a P value < .075 on bivariate testing were included in the multivariate analysis for TDI; results of bivariate and multivariate analysis are outlined in Table 2. Multivariate regression revealed factors with statistically significant independent association with TDI were age (unstandardized B = −0.2, P < .001), asthma (unstandardized B = −5.3, P = .001), gastroesophageal reflux disease (GERD; unstandardized B = 4.5, P < .001), and MMSE score (unstandardized B = 0.6, P = .043). Associations were reported based on 1-year or 1-point units for age and MMSE, respectively. Associations of dichotomous variables, such as asthma or GERD, were reported based on yes/no response. For example, a 5-year increase in age was associated with TDI scores 1 point lower, whereas asthma was associated with a TDI 5.3 points lower than those without asthma.
Table 2.
Bivariate Followed by Multivariate Analysis of Demographic Factors and Comorbidities Associated With Total TDI Score.
| TDI Bivariate Analysis | |||
|---|---|---|---|
| Demographic Variable | N | Correlation Coefficient (r) | P |
| Age | 176 | −0.567 | <.001 |
| MMSE | 176 | 0.282 | <.001 |
| Comorbidities | N | Mean (SD) | P |
| Allergic rhinitis | |||
| Yes | 56 | 26.99 (6.9) | .026 |
| No | 118 | 29.49 (6.7) | |
| Asthma | |||
| Yes | 14 | 23.80 (8.1) | .028 |
| No | 162 | 29.21 (6.6) | |
| Previous sinus surgery | |||
| Yes | 12 | 29.19 (5.6) | .796 |
| No | 163 | 28.73 (6.9) | |
| Diabetes | |||
| Yes | 18 | 26.57 (5.6) | .096 |
| No | 158 | 29.04 (7) | |
| Heart problems | |||
| Yes | 19 | 21.68 (6.7) | <.001 |
| No | 157 | 29.65 (6.4) | |
| Hyperlipidemia | |||
| Yes | 38 | 27.89 (6.3) | .382 |
| No | 136 | 28.94 (7) | |
| Hypertension | |||
| Yes | 53 | 26.64 (6.2) | .005 |
| No | 123 | 29.71 (6.9) | |
| Obstructive sleep apnea | |||
| Yes | 20 | 26.26 (6.8) | .089 |
| No | 156 | 29.11 (6.8) | |
| Depression | |||
| Yes | 33 | 29.19 (6.5) | .698 |
| No | 143 | 28.69 (7) | |
| Anxiety | |||
| Yes | 25 | 31.24 (5.7) | .031 |
| No | 151 | 28.38 (6.9) | |
| Arthritis | |||
| Yes | 39 | 27.11 (6.3) | .071 |
| No | 137 | 29.26 (7) | |
| Cancer (any) | |||
| Yes | 21 | 23.97 (8.1) | .007 |
| No | 155 | 29.44 (6.5) | |
| GERD | |||
| Yes | 25 | 30.77 (5.1) | .054 |
| No | 151 | 28.46 (7.1) | |
| Exposures/risk factors | N | Mean (SD) | P |
| Military service | |||
| Yes | 23 | 25.67 (8.2) | .058 |
| No | 152 | 29.22 (6.6) | |
| Smoking: ever | |||
| Yes | 68 | 27.11 (7.8) | .015 |
| No | 108 | 29.84 (5.9) | |
| TDI Multivariate Analysis | |||
| Variable | Unstandardized B | t | P |
| TDI (constant) | 22.884 | 2.726 | |
| Age | −0.2 | −7.693 | <.001 |
| Asthma | −5.294 | −3.396 | .001 |
| GERD | 4.498 | 3.64 | <.001 |
| MMSE | 0.561 | 2.043 | .043 |
Abbreviations: GERD, gastroesophageal reflux disease; MMSE, Mini-Mental Status Examination; N, number of subjects; r, correlation coefficient; SD, standard deviation; t, t statistic; TDI, composite of threshold (T), discrimination (D), identification (I) score.
Threshold
All factors were tested for associations with the threshold subtest, and those with a P value <.075 on bivariate testing were incorporated into multivariate analysis and the results are reported in Table 3. Multivariate regression revealed factors with statistically significant independent association with threshold were age (unstandardized B= −0.05, P < .001), heart problems (unstandardized B = −1.6, P = .012), and GERD (unstandardized B = 1.5, P = .004).
Table 3.
Bivariate Followed by Multivariate Analysis of Demographic Factors and Comorbidities Associated With Threshold (T), Discrimination (D), and Identification (I).
| Threshold Bivariate Analysis | |||
|---|---|---|---|
| Demographic Variable | N | Correlation Coefficient (r) | P |
| Age | 176 | −0.37 | <.001 |
| MMSE | 176 | 0.159 | .034 |
| Comorbidities | N | Mean (SD) | P |
| Allergic rhinitis | |||
| Yes | 56 | 5.48 (2.6) | .048 |
| No | 119 | 6.32 (2.7) | |
| Asthma | |||
| Yes | 14 | 4.16 (2.8) | .018 |
| No | 163 | 6.25 (2.6) | |
| Heart problems | |||
| Yes | 19 | 3.52 (2.3) | <.001 |
| No | 158 | 6.39 (2.5) | |
| Hypertension | |||
| Yes | 53 | 5.53 (2.6) | .074 |
| No | 123 | 6.31 (2.6) | |
| Arthritis | |||
| Yes | 39 | 5.37 (2.5) | .052 |
| No | 138 | 6.28 (2.7) | |
| Cancer | |||
| Yes | 21 | 4.69 (2.8) | .021 |
| No | 156 | 6.27 (2.6) | |
| GERD | |||
| Yes | 25 | 7.01 (2.5) | .051 |
| No | 152 | 5.93 (2.7) | |
| Exposures/risk factors | N | Mean (SD) | P |
| Military service | |||
| Yes | 23 | 4.85 (2.8) | .03 |
| No | 153 | 6.25 (2.6) | |
| Smoking: ever | |||
| Yes | 68 | 5.34 (2.8) | .004 |
| No | 109 | 6.54 (2.5) | |
| Threshold Multivariate Analysis | |||
| Variable | Unstandardized B | t | P |
| Threshold (constant) | 8.752 | 14.769 | |
| Age | −0.052 | −4.503 | <.001 |
| Heart problems | −1.601 | −2.524 | .012 |
| GERD | 1.54 | 2.94 | .004 |
| Discrimination Bivariate Analysis | |||
| Demographic Variable | N | Correlation Coefficient (r) | P |
| Age | 176 | −0.481 | <.001 |
| MMSE | 176 | 0.347 | <.001 |
| Comorbidities | N | Mean (SD) | P |
| Allergic rhinitis | |||
| Yes | 56 | 10.3 (2.5) | .043 |
| No | 118 | 11.16 (2.7) | |
| Heart problems | |||
| Yes | 19 | 9.21 (2.9) | .012 |
| No | 157 | 11.12 (2.6) | |
| Hypertension | |||
| Yes | 53 | 10.32 (2.5) | .045 |
| No | 123 | 11.17 (2.8) | |
| Cancer | |||
| Yes | 21 | 9.24 (2.9) | .009 |
| No | 155 | 11.14 (2.6) | |
| Exposures/risk factors | N | Mean (SD) | P |
| Smoking: ever | |||
| Yes | 68 | 10.37 (3) | .042 |
| No | 108 | 11.26 (2.4) | |
| Discrimination Multivariate Analysis | |||
| Variable | Unstandardized B | t | P |
| Discrimination (constant) | 4.427 | 1.254 | |
| Age | −0.061 | −5.627 | <.001 |
| MMSE | 0.336 | 2.906 | .004 |
| Identification Bivariate Analysis | |||
| Demographic Variable | N | Correlation coefficient (r) | P |
| Age | 176 | −0.482 | <.001 |
| MMSE | 176 | 0.344 | <.001 |
| Comorbidities | N | Mean (SD) | P |
| Asthma | |||
| Yes | 14 | 9.93 (3.5) | .056 |
| No | 162 | 11.92 (2.7) | |
| Heart problems | |||
| Yes | 19 | 8.95 (2.9) | <.001 |
| No | 157 | 12.1 (2.8) | |
| Hypertension | |||
| Yes | 53 | 10.79 (2.6) | .002 |
| No | 123 | 12.18 (2.8) | |
| Anxiety | |||
| Yes | 25 | 13.08 (2.5) | .009 |
| No | 151 | 11.54 (2.8) | |
| Cancer | |||
| Yes | 21 | 10.05 (3.2) | .015 |
| No | 155 | 11.99 (2.6) | |
| Exposures/risk factors | N | Mean (SD) | P |
| Military service | |||
| Yes | 23 | 10.22 (3.4) | .024 |
| No | 152 | 11.97 (2.6) | |
| Identification Multivariate Analysis | |||
| Variable | Unstandardized B | t | P |
| Identification (constant) | 14.912 | 24.341 | |
| Age | −0.6 | −5.218 | <.001 |
| Heart problems | −1.665 | −2.602 | .01 |
| Anxiety | 1.147 | 2.205 | .029 |
Abbreviations: GERD, gastroesophageal reflux disease; MMSE, Mini-Mental Status Examination; N, number of subjects; r, correlation coefficient; SD, standard deviation; t, t statistic.
Discrimination
All factors were tested for associations with the discrimination subtest, and those with a P value <.075 on bivariate testing were incorporated into multivariate analysis and the results are reported in Table 3. Multivariate regression revealed factors with statistically significant independent associations with discrimination were age (unstandardized B = −0.6, P < .001) and MMSE (unstandardized B = 0.3, P = .004).
Identification
All factors were tested for associations with the identification subtest, and those with a P value <.075 on bivariate testing were incorporated into multivariate analysis, and the results are reported in Table 3. Multivariate regression revealed factors with statistically significant independent associations with identification were age (unstandardized B = −0.6, P < .001), heart problems (unstandardized B = −1.7, P = .01), and anxiety (unstandardized B = 1.2, P = .029).
Change in olfaction in older adults
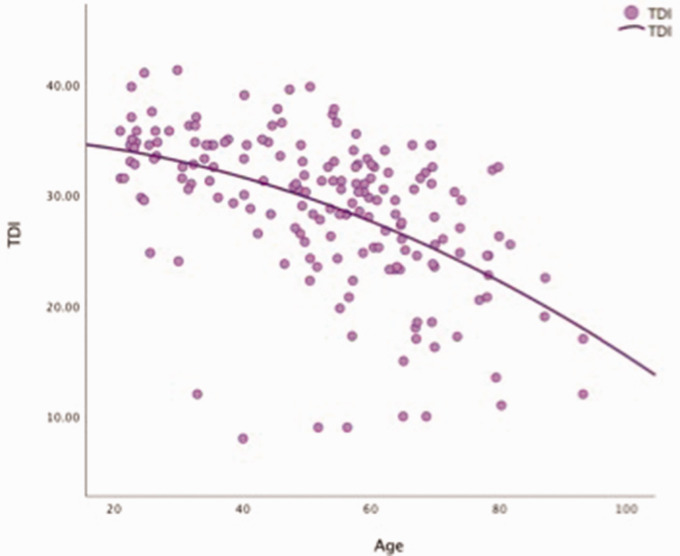
Given that age was a significant factor in all aspects of olfaction, we examined the relationship of age and olfaction across the lifespan of our adult cohort (Figure 1). Starting around age 20 years, the composite TDI begins to decline gradually, but then continues to decline more precipitously around age 60 years. Changes in specific subtests with age are demonstrated in Figure 2. The decline in discrimination and identification starts as early as 20 years of age and they continue to decline in a gradual linear fashion throughout life. Threshold in contrast is relatively flat until about age 50 years when it decreases more precipitously in a nonlinear fashion. Based on this crude analysis, it appears that the gradual linear decline in olfactory function from age 20 to 50 years is likely due to declines in olfactory discrimination and identification, as threshold is relatively flat during those decades. Around age 50 years, the precipitous decline appears to be a combination of the linear decline in discrimination and identification coupled with a nonlinear loss of threshold.
Figure 1.
TDI plotted as a function of age (years). Total TDI score versus age. TDI indicates composite of threshold (T), discrimination (D), and identification (I) score.
Figure 2.
Correlations between threshold (T), discrimination (D), and identification (I) score and age (years). Threshold, discrimination, and identification score versus age.
Discussion
Prior studies using brief identification tests across the adult lifespan have found a prevalence of OD in the general population ranging from 3.8% to 62.5% depending on the type of test and the age of the population.3,4 Our study, using detailed Sniffin’ Sticks testing and ideal olfactory cutoff, found that approximately half of a nonselected adult population has some degree of OD. Two prior studies focused on subjects aged 40 years and older and reported prevalence rates of 13.5% and 12.4%.1,10 In subjects aged 40 years and older, we report a prevalence rate of 65.1%. Our study using ideal olfactory cutoffs reports a prevalence rate greater than 4 times higher and indicates that OD may be a much more common problem than previously reported using age-specific population norms.
The use of ideal cutoffs is an important detail in this study. Similar to evaluating the other sensory systems, the goal and expectation for olfactory function should be based on the ideal function and not in accordance to age-based population norms. Age is a substantial driver of olfactory function,1–8,10–16 and using age-specific norms to classify dysosmia concedes that it is acceptable for older people to have a decreased sense of smell. Patients with age-related hearing loss are not considered to have age-appropriate hearing. Likewise, although near vision decreases with age, normative values are not age-adjusted for vision testing. When evaluating the senses, the expectation is ideal functioning. Decreased sense of smell should not be accepted in an older patient as a normal part of aging. Instead, efforts should be made to understand why these declines occur and how best to prevent and treat OD when present.
Not surprisingly, age was a primary driver across all aspects of olfaction, whereas we found no impact of sex or race. Interestingly, though, age appears to affect different aspects of olfaction in varying ways. Discrimination and identification appear to decline as early as 20 years of age and then continue a slow linear decline. Threshold in contrast is relatively flat until about age 50 years when it decreases rapidly in a nonlinear fashion. Subsets of olfaction were differentially impacted by various demographic and comorbidity factors. Some items, like older age and heart problems may have broader effects due to multifactorial mechanisms. The impact of MMSE scores on the discrimination subtest is likely related to it being a higher order aspect of olfaction and a more complex task. Our study failed to show any impact of our hypothesized olfactory risk factors such as prior sinus surgery and prolonged or repeated olfactory exposures. It is possible that most olfactory toxins require prolonged, repetitive exposures and OD only manifests itself as a factor of aging that we were unable to independently identify. In addition, it may be that a certain cumulative level of toxin exposure is needed. This may explain the relatively flat threshold curve until age 50 years or so, and then a rapid decline once a critical level of cumulative toxin exposure is reached or natural reparative processes are impaired.
Similar to older age, the effects of cognition upon olfaction have been previously reported.7,16 We excluded subjects with known dementia and used the MMSE as a general marker of cognitive function, which demonstrated significant associations with olfaction. Our study was able to control for age, a known cognition cofounder, indicating an independent impact. Interestingly, MMSE was associated with discrimination, but when multivariate modeling controlled for confounders, its impact upon threshold and identification dropped out. It may be that mild subclinical cognitive impairment is present and impacting olfactory discrimination as a more complex task requiring higher order function. This is supported by a prior study that reported olfactory discrimination deficits to be a predictor of subsequent cognitive decline.16 Prior studies have also reported mental health conditions, especially depression, as being associated with OD.2,13 While we inquired regarding history of depression, we did not find an association; however, anxiety demonstrated a positive impact, which has not been previously reported.
Asthma demonstrated a negative impact on TDI scores. Although this has been reported previously in the CRS population, it has not been reported in the general population using detailed olfactory testing.12,13 A study published in 2016 found asthma to be independently associated with increased prevalence of smell dysfunction using an 8-item identification test.10 When examining the potential of asthma to be associated with upper airway conditions, we specifically excluded subjects with active/current acute or CRS and only 6% of subjects had prior sinus surgery. In addition, neither positive allergy testing nor allergic rhinitis was associated with olfactory loss. Thus, the impact of asthma was independent of overt CRS or atopic conditions. This suggests some mechanism in asthmatics that is independent of upper airway inflammation playing a key role in olfactory function, or possibly that subclinical olfactory inflammation is present. Previously, asthma has demonstrated independent associations with prevalence of hearing loss, tinnitus and visual impairment.17–19 Perhaps, there is a mechanistic effect of broad systemic inflammation linking these neurosensory impairments to asthma.
Surprisingly, GERD demonstrated a positive impact on olfaction, which has not been reported previously. Reasons for this are unclear. It is possible that patients with GERD alter their diet or take medications that have a positive impact upon olfaction and this remains an interesting area for further study. A history of heart problems had a negative impact on olfactory function. Prior studies have reported the association of atherosclerosis and increased OD prevalence.15 In another study in 2009, carotid artery atherosclerosis was associated with magnetic resonance imaging indices of subclinical brain ischemia.14 Similar changes to the vasculature supply of the olfactory epithelium or central olfactory structures may affect olfactory function as well. Prior studies have also reported that stroke is associated with increases in OD, lending support to the possibility that subclinical infarcts may be responsible for the association demonstrated in our data.3 Hyperlipidemia is a risk factor for cardiovascular disease, and a study published in 2017 reported an association between chemosensory dysfunction and total serum cholesterol levels.11 In addition, drug-induced dysosmia is an established side effect for various cardiovascular medications. This includes selecting angiotensin-converting enzyme inhibitors, beta-adrenergic blockers, and calcium channel blockers, all of which are commonly for a variety of cardiovascular diseases. Although it is unlikely that medications are entirely responsible for the association demonstrated in our study, the concept of drug-induced dysosmia deserve consideration, along with the former potential mechanisms.
Although this study recorded data relating to demographics, comorbidities, olfactory exposures, and other risk factors as well as detailed olfactory testing, it does have several limitations. The study relied on self-reporting olfactory-specific exposures. This was necessary as specific testing for exposure to substances like silica is not logistically feasible; however, self-reporting may be unreliable. Another limitation is the sample size. Although large for a prospective cohort study focused solely on OD, it is likely that the impact of some factors, specifically exposures such as silica or toxic materials, may be quite small and thus only evident in massive population studies. Therefore, an absence of effect should not necessarily be interpreted as proving no impact. The cohort was recruited from South Carolina, and the results may not be applicable to populations in other areas. Participants in this study were not seeking medical care, and thus the results may not apply to OD in patients seeking care for specific conditions, such as CRS, trauma, or viral-induced anosmia. In addition, this study utilized a cross-sectional design, and thus, conclusions about disease mechanisms or temporal changes in individual subjects cannot be made, emphasizing the need for longitudinal cohort studies.
Author Contributions
All authors made substantial contributions to the analysis and interpretation of the work, revising the drafts, final approval of the manuscript, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Zachary M. Soler is a consultant for Olympus, Optinose, Novartis, Regeneron, and Healthy Humming, which are not affiliated with this study. Rodney J. Schlosser is a consultant for Olympus, Arrinex, Optinose, Sanofi, and Healthy Humming, which are not affiliated with this study.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Vincent M. Desiato https://orcid.org/0000-0001-6261-1921
Nicholas R. Rowan https://orcid.org/0000-0003-1296-2648
Judy R. Dubno https://orcid.org/0000-0003-2340-4721
References
- 1.Hoffman HJ, Rawal S, Li CM, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Dis. 2016; 17(2):221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman HJ, Ishii EK, MacTurk RH. Age-related changes in the prevalence of smell/taste problems among the United States adult population. Results of the 1994 disability supplement to the National Health Interview Survey (NHIS). Ann N Y Acad Sci. 1998; 855:716–722. [DOI] [PubMed] [Google Scholar]
- 3.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002; 288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- 4.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses. 2012; 37(4):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014; 9(10):e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the Skövde population-based study. Laryngoscope. 2004; 114(4):733–737. [DOI] [PubMed] [Google Scholar]
- 7.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2014; 69(3):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seubert J, Laukka EJ, Rizzuto D, et al. Prevalence and correlates of olfactory dysfunction in old age: a population-based study. J Gerontol A Biol Sci Med Sci. 2017; 72(8):1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oleszkiewicz A, Schriever VA, Croy I, Hahner A, Hummel T. Updated Sniffin’ sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019; 276(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G, Zong G, Doty RL, Sun Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open. 2016; 6(11):e013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z, Huang S, Cong H, et al. Smell and taste dysfunction is associated with higher serum total cholesterol concentrations in Chinese adults. J Nutr. 2017; 147(8):1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009; 23(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattos JL, Schlosser RJ, Storck KA, Soler ZM. Understanding the relationship between olfactory-specific quality of life, objective olfactory loss, and patient factors in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7(7):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009; 40(5):1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Carotid intima media thickness, atherosclerosis, and 5-year decline in odor identification: the Beaver Dam Offspring Study. J Gerontol A Biol Sci Med Sci. 2015; 70(7):879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohrabi HR, Bates KA, Weinborn MG, et al. Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl Psychiatry. 2012; 2:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HJ, Lee HJ, An SY, et al. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One. 2015; 10(5):e0127578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilic T, Karatas E, Toplu Y, Koc A, Bulam N, Kaya O. Evaluation of auditory functions in patients with asthma. Eur Rev Med Pharmacol Sci. 2014; 18(18):2615–2620. [PubMed] [Google Scholar]
- 19.Crews JE, Chou CF, Sekar S, Saaddine JB. The prevalence of chronic conditions and poor health among people with and without vision impairment, aged >/=65 years, 2010-2014. Am J Ophthalmol. 2017; 182:18–30. [DOI] [PubMed] [Google Scholar]