Frailty was associated with delayed extubation on protocol‐based weaning from mechanical ventilation.

Keywords: Aged, airway extubation, intensive care units, respiratory insufficiency, ventilator weaning
Abstract
Aim
Frailty has been shown to be associated with prolonged mechanical ventilation (MV). However, due to limited physiological data, it has been unclear how frailty affects weaning from MV in septic patients subjected to a specific weaning protocol.
Methods
This was a single‐center retrospective cohort study. The study included patients with sepsis on MV who underwent protocol‐based weaning between August 2015 and December 2018. Frailty was defined as a Clinical Frailty Scale score 4 or more. The association between frailty and weaning was evaluated.
Results
Ninety‐nine eligible patients were identified and categorized as frail (n = 67) or not frail (n = 32). The duration of MV was significantly longer in the frail group (8 days versus 5 days, P < 0.01). In multivariate analysis, frailty was independently associated with duration of MV (regression coefficient 17.97, 95% confidence interval 1.77–34.17) and successful weaning (hazard ratio 0.60, 95% confidence interval 0.36–1.00). There was no significant between‐group difference in duration until the first separation attempt or reintubation rate. Respiratory failure was significantly more common in the frail group as a cause of weaning failure, whereas airway failure was common in both groups.
Conclusion
Frailty was independently associated with a longer duration of MV in patients with sepsis who underwent protocol‐based weaning. Frail patients were more likely to fail spontaneous breathing trials than nonfrail patients during the weaning process, although the risk after extubation was similar.
Introduction
Sepsis often requires mechanical ventilation (MV) and is more common among the elderly. 1 Thus, age may have a negative effect on the judgment of the intensivist on admissions to the intensive care unit (ICU). 2 However, a recent study has shown that frailty may affect the prognosis of patients in the ICU independently of chronological age. 3 Therefore, decision making for frail patients with sepsis, such as a new intervention strategy or treatment restriction, will become increasingly important.
Large‐scale observational studies in ICUs have shown that frailty is associated with short‐term mortality, 4 , 5 , 6 long‐term mortality, 6 and duration of MV. 7 , 8 Furthermore, Fernando et al. showed associations between the need for MV and an increased likelihood of extubation failure and tracheostomy in frail patients with a wide range of diseases. 3
Such big data trials have the common problem of reliance on data from large registries in which limited physiological data are available. The quality of treatment received by the patients enrolled in these studies is not known; therefore, it is unclear what treatments had problems or what interventions might have been effective for frail patients. We consider that the weaning process needs to be evaluated according to frailty status only in patients who have received protocol‐based treatment. We hypothesized that frail patients who develop sepsis require MV for longer periods even under protocol‐based weaning. The aim of this study was to determine how frailty affects weaning from MV in patients with sepsis.
Methods
Ethical approval and consent
This study was approved by the Ethics Committee of our hospital (Approval Number: NCGM‐G‐003271‐00). Based on the opt‐out approach, we disclosed information about this study and excluded data when the patient declined to participate directly or via proxy.
Study design, setting, and patients
This study had a single‐center retrospective cohort design and was performed in an emergency and general ICU in Japan. Mechanically ventilated patients over 15 years of age in the emergency department were screened for enrollment. Patients were included in the study if they received MV to support organ failure induced by sepsis. The exclusion criteria were cardiac arrest before or during resuscitation and tracheostomy before screening.
We evaluated each patient for frailty using the Clinical Frailty Scale (CFS), 9 which classifies patients ranging from very fit to terminally ill. In a previous study of CFS, 9 frailty was defined as a CFS score of 5 or more, and patients with a score of 4 were considered vulnerable or prefrail. However, we thought it was possible that the lower scale was chosen due to insufficient information or based on the absence of living assistance, such as in cases of homeless or living alone. Therefore, we included prefrailty in our definition of frailty and used a CFS score of 4 or more.
Basic information
We collected data on age, sex, body mass index (BMI), Sequential Organ Failure Assessment (SOFA) score, vasopressor use for resuscitation, the main site of infection, CFS score before admission, and medical history.
Primary and secondary outcomes
We collected information on duration of MV, whether weaning was successful or not, in‐hospital mortality, discharge to home, tracheostomy, nonweaning, and reintubation rates. We also identified the type of weaning failure. Successful weaning was defined as no requirement for reintubation and lack of mortality within 7 days of attempting extubation. When tracheostomy or reintubation were performed, the patient was considered liberated from MV if there was no requirement for continuous MV for 48 h or more. Nonweaning was defined as no separation attempt (SA) because of death or transfer.
Weaning: protocols and definitions
First step: separation attempt
SA from MV was defined as a spontaneous breathing trial (SBT) with or without extubation or extubation performed directly without an identified SBT according to a previous large‐scale epidemiological study of weaning outcome. 10 We performed an SBT if the patient met the standard readiness‐to‐wean criteria. 11
Second step: SBT
The SBT was implemented with appropriate ventilator settings (FiO2 ≤ 0.4, pressure support ≤5 cmH2O, and positive end‐expiratory pressure ≤5 cmH2O) or a T‐piece. The patient was observed for 30–120 min. Our SBT criteria were based on the international consensus conference on weaning. 11
Third step: cuff leak test
We performed the cuff leak test following SBT. We considered the cuff leak test to be positive if the tidal volume was reduced by 110 mL or less when the cuff was deflated. 12 If the variation in tidal volume was difficult to measure because of spontaneous breathing, we determined whether or not the leak sound disappeared completely.
Fourth step: extubation or tracheostomy
We performed extubation if the SBT was successful and the cuff leak test was negative. Noninvasive ventilation (NIV) was not used after scheduled extubation. We used a large‐volume nebulization‐based humidifier or high‐flow nasal cannula first, followed by NIV as needed. Of note, we had previously found no significant difference in the reintubation rate according to whether or not a large‐volume nebulization‐based humidifier or high‐flow nasal cannula was used. 13
We did not perform early tracheostomy. The enrolled patients underwent tracheostomy only when the duration of MV was actually prolonged.
Types of weaning failure
We classified weaning failure as airway failure, respiratory failure, or other. Airway failure was also classified as poor airway clearance, laryngeal edema, or a positive cuff leak test. Poor airway clearance was defined as obstruction of the tracheal tube by excessive trachea‐bronchial secretions or sudden exacerbation of respiratory failure in the inability to clear secretions. Respiratory failure included oxygenation failure and ventilatory failure not due to airway failure.
Sample size and statistical analysis
Based on previous studies, 7 , 8 we estimated that the difference in the duration of MV between frail and nonfrail patients would be 2 ± 3 days. We speculated that the number of patients in each group differed by up to threefold. Therefore, we calculated a required sample size of 96 patients using R version 3.4.1 and SAS version 9.4 software. We defined the study period as 41 months from August 1, 2015, because we started extubation according to the recommended protocol 11 from that date.
We used Fisher’s exact test for categorical variables, the Mann–Whitney U test for continuous variables, and Gray’s test for cumulative incidence of successful weaning or death when performing between‐group comparisons. We also used a multiple regression analysis or the Fine and Gray multivariate regression model for multivariate analysis of the duration of MV and successful weaning. We selected age, sex, BMI, SOFA score, and use of a vasopressor as covariables in these analyses. In addition, because this study was retrospective, BMI data were missing in some patients. The values for missing data were imputed using multiple regression with age, sex, and presence/absence of frailty.
Results
In total, 99 patients (frail, n = 67; not frail, n = 32) were enrolled in the study (Fig. 1). There were more patients with a medical history of neurologic disease, such as stroke or Parkinson’s disease, in the frail group. In four patients, the initially administered antibiotics were ineffective against the pathogenic microorganism. There was no significant between‐group difference in the distribution of BMI (Table 1). None of the patients had missing data except for BMI.
Fig. 1.
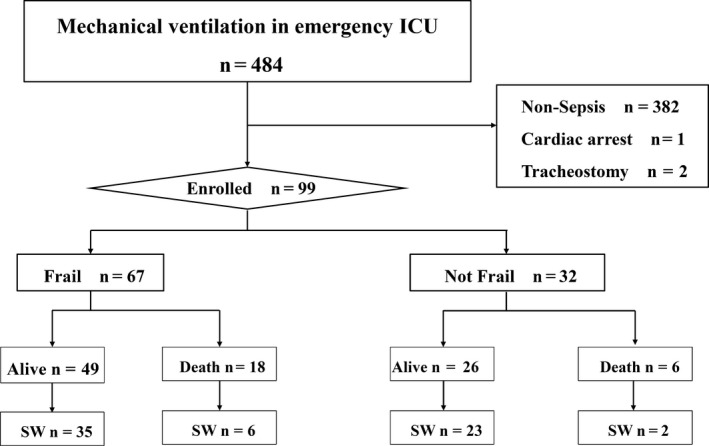
Flowchart of participants. Successful weaning was defined as no requirement for reintubation and no mortality within 7 days of attempted extubation. Mechanical ventilation excluded noninvasive mechanical ventilation. Sepsis was defined as infection with an acute change in sequential organ failure assessment score of 2 or more. ICU, intensive care unit; SW, successful weaning.
Table 1.
Background of participants
| Participant demographics | Frail, n = 67 | Not frail, n = 32 | P value † |
|---|---|---|---|
| Age (years), median [25%, 75%] | 78 [72, 86] | 75 [63, 84] | 0.06 |
| Male, n (%) | 42 (66) | 25 (78) | 0.17 |
| BMI (kg/m2), median [25%, 75%] | 20.4 [18.2, 23.4] | 21.4 [18.4, 24.2] | 0.62 |
| BMI ≥ 25 kg/m2, n (%) | 7 (10) | 6 (19) | 0.34 |
| BMI ≤ 18.5 kg/m2, n (%) | 20 (30) | 8 (25) | 0.81 |
| SOFA score, median [25%, 75%] | 10 [8, 12] | 9 [7, 12] | 0.78 |
| Vasopressor use, n (%) | 54 (81) | 24 (75) | 0.60 |
| Septic shock, n (%) | 46 (69) | 21 (66) | 0.82 |
| Serum lactate (mmol/L median) [25%, 75%] | 4.4 [2.5, 7.2] | 4.6 [2.5, 5.9] | 0.53 |
| Site of infection, n (%) | |||
| Respiratory | 51 (76) | 18 (56) | 0.23 |
| Urinary | 8 (12) | 6 (19) | |
| Abdomen | 1 (1) | 0 (0) | |
| Soft tissue | 2 (3) | 2 (6) | |
| Other | 5 (7) | 6 (19) | |
| Medical history, n (%) | |||
| Chronic respiratory disease | 13 (19) | 2 (6) | 0.13 |
| Chronic heart disease | 17 (25) | 6 (19) | 0.61 |
| Neurologic disease | 19 (28) | 3 (9) | 0.04 |
| Collagen disease | 4 (6) | 1 (3) | >0.99 |
| Kidney disease | 6 (9) | 2 (6) | >0.99 |
| Diabetes | 11 (16) | 6 (19) | 0.78 |
| Hypertension | 19 (28) | 10 (31) | 0.82 |
| Malignancy | 13 (19) | 4 (13) | 0.57 |
| Pathogenic microorganisms, n (%) | |||
| Gram positive | 12 (18) | 12 (38) | 0.053 |
| Gram negative | 35 (52) | 13 (41) | |
| Multiple | 14 (21) | 2 (6) | |
| Other | 6 (9) | 5 (16) | |
| Bacteremia, n (%) | 23 (34) | 16 (50) | 0.19 |
| Respiratory status | |||
| PaO2/FiO2 (mmHg), median [25%, 75%] | 140 [101, 202] | 147 [111, 182] | 0.97 |
| pH, median [25%, 75%] | 7.28 [7.17, 7.37] | 7.33 [7.28, 7.39] | 0.09 |
| Lowest PaCO2 (mmHg), median [25%, 75%] | 31 [27, 43] | 30 [27, 36] | 0.35 |
| Highest PaCO2 (mmHg), median [25%, 75%] | 43 [35, 61] | 42 [37, 49] | 0.15 |
| PaCO2 > 45 mmHg, n (%) | 30 (45) | 9 (28) | 0.13 |
| PEEP (cmH2O) median [25%, 75%] | 8 [6, 12] | 8 [7, 10] | 0.53 |
| Treatments | |||
| Fluid volume within the first 24 h (mL), median [25%, 75%] | 6,940 [3,910, 9,880] | 8,475 [5,230, 10,988] | 0.29 |
| Adequate use of antibiotics, n (%) | 65 (97) | 30 (94) | 0.59 |
| Use of corticosteroids for any reason, n (%) | 32 (48) | 17 (53) | 0.67 |
| Use for distributive shock, n (%) | 17 (25) | 10 (31) | 0.63 |
| Use for ARDS, n (%) | 6 (9) | 4 (13) | >0.72 |
| Use for exacerbation of COPD, n (%) | 1 (1) | 0 (0) | >0.99 |
| Use for suspected laryngeal edema, n (%) | 12 (18) | 4 (13) | 0.57 |
ARDS, acute respiratory distress syndrome; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FiO2, fraction of inspiratory oxygen; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; PEEP, positive end‐expiratory pressure; SOFA, Sequential Organ Failure Assessment.
We used Fisher’s exact test as categorical variables and Mann–Whitney U test as continuous variables.
The duration of MV was significantly longer (P < 0.01) in the frail patients than in the nonfrail patients (Table 2). In multivariate analysis, frailty was independently associated with duration of MV (regression coefficient 17.97, 95% confidence interval [CI] 1.77–34.17; Table 3). Similar results were shown in post‐hoc analysis of the original definition of frail (CFS ≥ 5; Table S1) and in that with the addition of appropriate antibiotic use as covariates (Table [Link], [Link], [Link]).
Table 2.
Univariate analysis
| Main outcome |
Frail n = 67 |
Not frail n = 32 |
P value † | |
|---|---|---|---|---|
| Duration of MV (days), median [25%, 75%] | <0.01 | |||
| In all patients | 8 [6, 19] | 5 [3, 9] | 0.10 | |
| In patients who tracheostomy performed | 7 [4, 8] | 5 [3, 7] | 0.90 | |
| In patients who tracheostomy did not performed | 23 [18, 46] | 22 [21, 31] |
| Secondary outcomes |
Frail n = 67 |
Not frail n = 32 |
Odds ratio (95% CI) | P value † |
|---|---|---|---|---|
| In‐hospital death, n (%) | 18 (27) | 6 (19) | 1.58 (0.52‐5.49) | 0.46 |
| CFS 1–3 | 6/32 (19) | |||
| CFS 4 | 8/29 (28) | |||
| CFS 5–6 | 7/30 (23) | |||
| CFS 7–8 | 3/8 (38) | |||
| Discharge to home, n (%) | 12 (18) | 13 (41) | 0.32 (0.11‐0.91) | 0.02 |
| Successful weaning, n (%) | 40 (60) | 25 (78) | 0.42 (0.13‐1.18) | 0.11 |
| Nonweaning, n (%) | 12 (18) | 5 (16) | 1.18 (0.34‐4.71) | >0.99 |
| Duration until the first SA (days), median [25%, 75%] | 6 [4, 8] | 5 [3, 7] | 0.27 | |
| Reintubation rate in attempted extubation only, n (%) | 11 (22) | 6 (22) | 1.01 (0.29‐3.84) | >0.99 |
| Tracheostomy, n (%) | 22 (33) | 3 (9) | 4.66 (1.23‐26.51) | 0.01 |
CFS, Clinical Frailty Scale; CI, confidence interval; MV, mechanical ventilation; SA, separation attempt.
We used Fisher’s exact test as categorical variables and Mann–Whitney U test as continuous variables.
Table 3.
Multivariate analysis of duration of mechanical ventilation and successful weaning
| Variables | Regression coefficient | 95% confidence interval | t value | P value † |
|---|---|---|---|---|
| Duration of mechanical ventilation | ||||
| Age | −0.80 | −1.31 to −0.28 | −3.09 | <0.01 |
| Female | 5.98 | −10.06 to 22.02 | 0.74 | 0.46 |
| BMI | −0.26 | ‐2.30 to 1.78 | −0.26 | 0.80 |
| Frailty | 17.97 | 1.77 to 34.17 | 2.20 | 0.03 |
| SOFA score | −1.16 | −4.08 to 1.76 | −0.79 | 0.43 |
| Vasopressor use | 16.60 | −9.25 to 42.46 | 1.28 | 0.21 |
| Variables | Hazard ratio | 95% confidence interval | P value ‡ |
|---|---|---|---|
| Successful weaning | |||
| Age | 1.00 | 0.98 to 1.01 | 0.69 |
| Female | 1.00 | 0.58 to 1.71 | 0.99 |
| BMI | 1.01 | 0.94 to 1.07 | 0.89 |
| Frailty | 0.60 | 0.36 to 1.00 | 0.048 |
| SOFA score | 0.97 | 0.89 to 1.06 | 0.50 |
| Vasopressor use | 1.00 | 0.42 to 2.37 | 0.99 |
Multivariate results were adjusted by age, sex, SOFA score, and vasopressor use. The value of missing data was imputed by the multiple regression model using age, sex, and presence/absence of frailty.
BMI, body mass index; SOFA, Sequential Organ Failure Assessment.
Multiple regression analysis.
Fine and Gray multivariate regression model. Death before attempting extubation was a competitive event for successful weaning.
In terms of secondary outcomes, there was no significant between‐group difference in the rate of successful weaning (60% versus 78%, odds ratio 0.42, 95% CI 0.13–1.18). However, successful weaning took significantly longer in the frail group (Fig. 2) and frailty was an independent risk factor for successful weaning in the Fine and Gray multivariate regression model (adjusted hazard ratio 0.60, 95% CI 0.36–1.00; Table 3). Because an early tracheostomy was not performed, there was no difference in the cumulative incidence of successful weaning in subgroups of patients who did not undergo tracheostomy in both groups (Fig. S1). Although tracheostomy was performed more often in the frail group (33% versus 9%, P = 0.01), there was no difference in the reintubation rate in patients with attempted extubation (22% versus 22%, P > 0.99; Table 2).
Fig. 2.
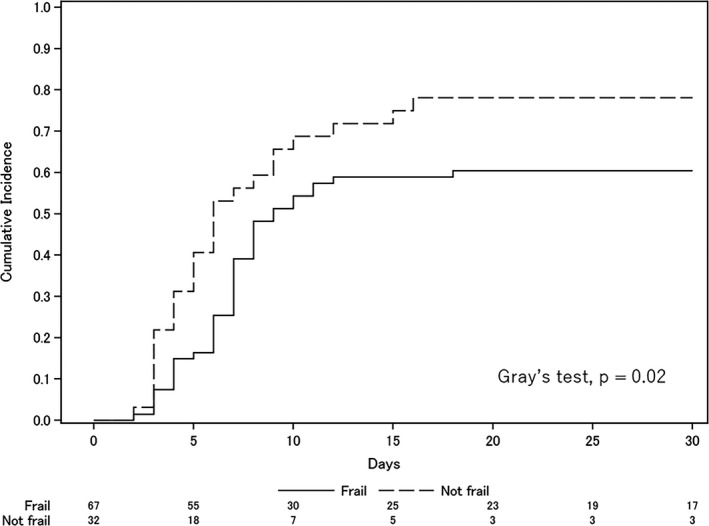
Cumulative incidence of successful weaning or death before attempted extubation. The two lines represent the cumulative incidence of successful weaning competing with death before attempted extubation. Successful weaning was defined as no requirement for reintubation and no mortality within 7 days of attempting extubation.
Respiratory failure was the most frequent type of weaning failure in the frail group. Ventilatory failure tended to be common in frail patients, while airway failure was common in both groups (Table 4).
Table 4.
Types of weaning failure
| Type of weaning failure | Frail, n = 67 | Not frail, n = 32 | Odds ratio (95% confidence interval) | P value † |
|---|---|---|---|---|
| Airway failure, n (%) | 14 (21) | 7 (22) | 0.94 (0.31 to 3.12) | >0.99 |
| Airway clearance, n (%) | 8 (12) | 5 (16) | 0.73 (0.19 to 3.13) | 0.75 |
| Laryngeal edema or cuff‐leak test positive, n (%) | 6 (9) | 2 (6) | 1.47 (0.24 to 15.74) | >0.99 |
| Respiratory failure, n (%) | 19 (28) | 3 (9) | 3.78 (0.98 to 21.68) | 0.04 |
| Oxygenation failure, n (%) | 8 (12) | 2 (6) | 2.02 (0.37 to 20.70) | 0.49 |
| Ventilatory failure, n (%) | 11 (16) | 1 (3) | 6.01 (0.80 to 270.08) | 0.10 |
| Cardiac failure, n (%) | 4 (6) | 4 (13) | 0.45 (0.08 to 2.59) | 0.27 |
| Shock, n (%) | 0 (0) | 2 (6) | 0.00 (0.00 to 2.51) | 0.10 |
| Arrhythmia, n (%) | 0 (0) | 1 (3) | 0.00 (0.00 to 18.63) | 0.32 |
| Multiple organ failure, n (%) | 3 (4) | 1 (3) | 1.45 (0.11 to 78.68) | >0.99 |
| Cardiac arrest, n (%) | 1 (1) | 0 (0) | Inf (0.01 to Inf) | >0.99 |
| Coma, n (%) | 1 (1) | 0 (0) | Inf (0.01 to Inf) | >0.99 |
| Transfer, n (%) | 1 (1) | 0 (0) | Inf (0.01 to Inf) | >0.99 |
| Unknown, n (%) | 1 (1) | 0 (0) | Inf (0.01 to Inf) | >0.99 |
Weaning failure included the failure in all phase of separation attempt, spontaneous breathing trial, and postextubation.
We used Fisher’s exact test.
Discussion
In this study, we evaluated only patients with sepsis requiring MV for whom protocol‐based weaning was performed. Furthermore, because we defined and described each step of the weaning process as well as the type of weaning failure, we could highlight the problems encountered with MV in frail patients with sepsis even among the small number of patients enrolled.
We found an association between frailty and difficult liberation from MV in patients with sepsis. There was no significant difference in the duration of the first SA between the groups. Our experience was that frail patients tended to take longer to wean in the SBT phase. Interestingly, the reintubation rate was almost the same in the two groups, suggesting that our SBT criteria need not be determined by frailty. We believe that the cut‐off values for physiological parameters in terms of SBT are reliable, even in frail patients. There was no significant between‐group difference in terms of in‐hospital mortality; however, the relative risk of in‐hospital mortality and the likelihood of discharge to home in the frail patients were similar to the findings of a previous study. 4
Our findings indicate that the main problem during weaning in frail patients may be at the SBT stage and that we should focus on pre‐extubation rather than postextubation treatments. We also found that ventilatory failure was more common in frail patients. Ventilatory failure is caused not only by pulmonary pathology but also by systemic factors, such as respiratory muscle atrophy or respiratory depression at a neural level. For example, an association has been demonstrated between diaphragmatic dysfunction and delayed weaning. 14 Frailty is the result of muscle weakness and sarcopenia and therefore frail patients are more likely to have diaphragmatic weakness. Accordingly, we believe that there should be more emphasis on systemic interventions in frail patients, including nutritional support and early mobilization. NIV‐based extubation methods may also be useful. In this study, there was no increase in airway problems among frail patients at high risk for NIV. Thille et al. recently reported that a combination of NIV and a high‐flow nasal cannula was beneficial after extubation in high‐risk patients, namely, those who were elderly or those who had chronic respiratory or heart disease. 15 Vaschetto et al. also reported that an aggressive weaning protocol that included NIV reduced the duration of invasive MV. 16 However, another group of investigators reported negative results using a similar treatment approach. 17 Therefore, the benefits of this strategy remain controversial. Undoubtedly, treatment options should be carefully considered for patients suspected to be approaching end of life. Nevertheless, 60% of the frail patients in our study were successfully extubated. Not all frail patients with sepsis have a poor outcome, such as permanent MV.
Our study has several limitations. First, the duration of MV may be affected by high mortality or timing of tracheostomy. In our hospital, tracheostomy was performed at the discretion of the attending physician. Therefore, we evaluated not only the duration of MV but also the timing of successful weaning to determine if there was an association between frailty and prolonged MV. Second, because of the small number of patients enrolled, we included prefrail patients (CFS score = 4) in the frail group. In this study, prefrail patients had a mortality rate more similar to that of frail patients than to that of fit patients. Multivariate analysis with prefrail patients included in the fit group also showed that frailty tended to be associated with prolonged MV, but the hazard ratio was not significant. We believe this classification was reasonable, but it needs to be validated. Third, treatment during resuscitation, especially the volume of fluid replacement, may have affected the duration of MV. However, due to the retrospective nature of this study, we could not collect enough data to include it as a covariate in multivariate analysis. Finally, it was not possible to precisely define the type of weaning failure. In particular, it was often difficult to evaluate the specific abnormality in airway failure. This may have been influenced by the judgement of the attending physician or medical records.
Conclusions
In this study, frailty was independently associated with a longer duration of MV in patients with sepsis who underwent protocol‐based weaning. During the weaning process, frail patients were more likely to fail the SBT than nonfrail patients, but the risk after extubation was similar. This study also suggests that frail patients are more difficult to wean from MV than nonfrail patients, not because of increased airway failure, but because of increased ventilatory failure.
Disclosure
Approval of the research protocol: This study was approved by the Ethics Committee of the Center Hospital of the National Center for Global Health and Medicine (Approval Number: NCGM‐G‐003271‐00).
Informed Consent: Based on the opt‐out approach, we disclosed information about this study and excluded data when the patient declined to participate directly or via proxy.
Registry and the Registration No. of the study: N/A.
Animal Studies: N/A.
Conflict of Interest statement: All authors declare no conflict of interests.
Author contributions
WM: literature search, data collection, study design, data analysis, manuscript preparation; MY, TU, AK: study design, manuscript preparation; YU: data analysis, manuscript preparation.
Supporting information
Fig. S1. Cumulative incidence of successful weaning or death before attempted extubation in a subgroup of patients who did not undergo tracheostomy. The two lines represent the cumulative incidence of successful weaning competing with death before attempted extubation. Successful weaning was defined as no requirement for reintubation and no mortality within 7 days of attempting extubation. Enrolled patients underwent tracheostomy only when the duration of mechanical ventilation was actually prolonged.
Table S1. Multivariate analysis of duration of mechanical ventilation and successful weaning. Multivariate results were adjusted by age, sex, SOFA score and vasopressor use. The value of missing data was imputed by multiple regression model using age, sex, and CFS ≥ 5. SOFA, Sequential Organ Failure Assessment; BMI, body mass index. *Multiple regression analysis. **Fine and Gray multivariate regression model. Death before attempting extubation was a competitive event for successful weaning.
Table S2. Univariate analysis of duration of mechanical ventilation. BMI, body mass index; SOFA, Sequential Organ Failure Assessment; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspiratory oxygen; PaCO2, partial pressure of arterial carbon dioxide; PEEP, Positive end‐expiratory pressure. * We used simple regression analysis.
Table S3. Univariate analysis of duration of successful weaning. BMI, body mass index; SOFA, Sequential Organ Failure Assessment; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspiratory oxygen; PaCO2, partial pressure of arterial carbon dioxide; PEEP, Positive end‐expiratory pressure. * we used Fine and Gray multivariate regression model. Death before attempting extubation was a competitive event for successful weaning.
Table S4. Post‐hoc analysis of duration of mechanical ventilation and successful weaning. Multivariate results were adjusted by age, sex, SOFA score and vasopressor use. The value of missing data was imputed by multiple regression model using age, sex, and presence/absence of frailty. SOFA, Sequential Organ Failure Assessment; BMI, body mass index. *Multiple regression analysis. **Fine and Gray multivariate regression model. Death before attempting extubation was a competitive event for successful weaning.
Acknowledgements
We thank Atsushi Imaizumi (Department of Clinical Research, National Center for Global Health and Medicine, Tokyo, Japan) for data analysis. This study was supported in part by a Grant‐in‐Aid for Research from the National Center for Global Health and Medicine (20A‐3002).
Funding information
No funding information provided.
References
- 1. Rudd KE, Johnson SC, Agesa KM et al Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 2020; 395: 200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bassford CR, Krucien N, Ryan M, et al Preferences for patient admission to ICU: evidence from a choice experiment. Crit. Care Med. 2019; 47: 1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernando SM, McIsaac DI, Rochwerg B, et al Frailty and invasive mechanical ventilation: association with outcomes, extubation failure, and tracheostomy. Intensive Care Med. 2019; 45: 1742–52. [DOI] [PubMed] [Google Scholar]
- 4. Muscedere J, Waters B, Varambally A, et al The impact of frailty on intensive care unit outcomes: a systematic review and meta‐analysis. Intensive Care Med. 2017; 43: 1105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guidet B, de Lange DW, Boumendil A, et al The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020; 46: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva‐Obregon JA, Quintana‐Diaz M, Saboya‐Sanchez S, et al Frailty as a predictor of short‐ and long‐term mortality in critically ill older medical patients. J. Crit. Care 2020; 55: 79–85. [DOI] [PubMed] [Google Scholar]
- 7. Zampieri FG, Iwashyna TJ, Viglianti EM, et al Association of frailty with short‐term outcomes, organ support and resource use in critically ill patients. Intensive Care Med. 2018; 44: 1512–20. [DOI] [PubMed] [Google Scholar]
- 8. Hope AA, Adeoye O, Chuang EH, Hsieh SJ, Gershengorn HB, Gong MN. Pre‐hospital frailty and hospital outcomes in adults with acute respiratory failure requiring mechanical ventilation. J. Crit. Care 2018; 44: 212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shears M, Takaoka A, Rochwerg B, et al Assessing frailty in the intensive care unit: A reliability and validity study. J. Crit. Care 2018; 45: 197–203. [DOI] [PubMed] [Google Scholar]
- 10. Beduneau G, Pham T, Schortgen F, et al Epidemiology of Weaning Outcome according to a New Definition. The WIND Study. Am. J. Respir. Crit. Care Med. 2017; 195: 772–83. [DOI] [PubMed] [Google Scholar]
- 11. Boles JM, Bion J, Connors A, et al Weaning from mechanical ventilation. Eur. Respir. J. 2007; 29: 1033–56. [DOI] [PubMed] [Google Scholar]
- 12. Miller RL, Cole RP. Association between reduced cuff leak volume and postextubation stridor. Chest 1996; 110: 1035–40. [DOI] [PubMed] [Google Scholar]
- 13. Matsuda W, Hagiwara A, Uemura T, et al High‐flow nasal cannula may not reduce the re‐intubation rate after extubation in respiratory failure compared with a large‐volume nebulization‐based humidifier. Respir. Care 2020; 65: 610–617. 10.4187/respcare.07095 [DOI] [PubMed] [Google Scholar]
- 14. Goligher EC, Dres M, Fan E, et al Mechanical ventilation‐induced diaphragm atrophy strongly impacts clinical outcomes. Am. J. Respir. Crit. Care Med. 2018; 197: 204–13. [DOI] [PubMed] [Google Scholar]
- 15. Thille AW, Muller G, Gacouin A, et al Effect of postextubation high‐flow nasal oxygen with noninvasive ventilation vs high‐flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA 2019; 322: 1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaschetto R, Longhini F, Persona P, et al Early extubation followed by immediate noninvasive ventilation vs. standard extubation in hypoxemic patients: a randomized clinical trial. Intensive Care Med. 2019; 45: 62–71. [DOI] [PubMed] [Google Scholar]
- 17. Perkins GD, Mistry D, Gates S, et al Effect of protocolized weaning with early extubation to noninvasive ventilation vs invasive weaning on time to liberation from mechanical ventilation among patients with respiratory failure: the breathe randomized clinical trial. JAMA 2018; 320: 1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cumulative incidence of successful weaning or death before attempted extubation in a subgroup of patients who did not undergo tracheostomy. The two lines represent the cumulative incidence of successful weaning competing with death before attempted extubation. Successful weaning was defined as no requirement for reintubation and no mortality within 7 days of attempting extubation. Enrolled patients underwent tracheostomy only when the duration of mechanical ventilation was actually prolonged.
Table S1. Multivariate analysis of duration of mechanical ventilation and successful weaning. Multivariate results were adjusted by age, sex, SOFA score and vasopressor use. The value of missing data was imputed by multiple regression model using age, sex, and CFS ≥ 5. SOFA, Sequential Organ Failure Assessment; BMI, body mass index. *Multiple regression analysis. **Fine and Gray multivariate regression model. Death before attempting extubation was a competitive event for successful weaning.
Table S2. Univariate analysis of duration of mechanical ventilation. BMI, body mass index; SOFA, Sequential Organ Failure Assessment; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspiratory oxygen; PaCO2, partial pressure of arterial carbon dioxide; PEEP, Positive end‐expiratory pressure. * We used simple regression analysis.
Table S3. Univariate analysis of duration of successful weaning. BMI, body mass index; SOFA, Sequential Organ Failure Assessment; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspiratory oxygen; PaCO2, partial pressure of arterial carbon dioxide; PEEP, Positive end‐expiratory pressure. * we used Fine and Gray multivariate regression model. Death before attempting extubation was a competitive event for successful weaning.
Table S4. Post‐hoc analysis of duration of mechanical ventilation and successful weaning. Multivariate results were adjusted by age, sex, SOFA score and vasopressor use. The value of missing data was imputed by multiple regression model using age, sex, and presence/absence of frailty. SOFA, Sequential Organ Failure Assessment; BMI, body mass index. *Multiple regression analysis. **Fine and Gray multivariate regression model. Death before attempting extubation was a competitive event for successful weaning.


