Abstract
Background
Lipodystrophy has been reported as a common complication in insulin-treated patients, which could lead to unexplained hypoglycemia and suboptimal glycemic control. This study aimed to determine the prevalence, associated risk factors, and clinical characteristics of insulin-induced lipodystrophy in Thai patients.
Patients and Methods
This was a cross-sectional study involving insulin-treated patients at Theptarin Hospital, one of the largest diabetes centers in Thailand.
Results
A total of 400 patients were studied (female 53.5%, T2DM 86%, mean age 65.6±15.4 years, duration of diabetes 23.0±10.2 years, median insulin treatment 10 years, usage of insulin analog 72.1%, A1C 7.9±1.6%) . The prevalence of lipohypertrophy (LH) in overall patients was 37.3% (T1DM 46.4% and T2DM 35.8%). The highest prevalence (57.5%) was observed in long-standing (≥10 years) T1DM patients. Multivariate analysis revealed that the duration of insulin use (≥10 years), use of human insulin, and incorrect rotation of injection sites were associated with LH. Patients with LH were found to have 7-times greater risk of unexplained hypoglycemia when compared with patients without LH. Lipoatrophy (LA) was found in only four cases (1.0%). All LA cases had a concurrence palpable area of LH.
Conclusion
Insulin-induced lipodystrophy is still an overlooked complication in the conundrum of diabetes care. The presence of lipodystrophy was significantly associated with the occurrence of unexplained hypoglycemia. It should be emphasized to recognize this condition by inspecting and palpating insulin injecting sites regularly, and educate patients to avoid the development of lipodystrophy.
Keywords: lipodystrophy, lipohypertrophy, lipoatrophy, insulin, Thai
Introduction
Since the discovery of insulin in the last century, a variety of insulin analogs are available to mimic the action of endogeneous insulin. While insulin therapy is the choice of treatment for people with type 1 diabetes mellitus (T1DM), most people with long-standing type 2 diabetes mellitus (T2DM) will eventually require insulin therapy in their lifetimes as their diabetes progresses. Currently, it is estimated that 20–25% of all people with T2DM use insulin therapy.1 Unfortunately, the importance of insulin injection technique and awareness of insulin-induced lipodystrophy is still an overlooked complication in the conundrum of diabetes care. Previous data from all over the world consistently demonstrated that there is a wide gap between the current practices of insulin injection technique and the guideline recommendations.2–5 Moreover, the awareness of insulin-induced lipodystrophy is inadequate among the healthcare personnel.6
Insulin-induced lipodystrophy has been classified into two major subtypes – lipohypertrophy and lipoatrophy. Insulin-induced lipohypertrophy (LH) remains a frequent complication of insulin injection, which has been reported as 49% in people with insulin-treated T2DM and 34% in people with T1DM in a recent meta-analysis.7 Nevertheless, some previous data suggested that LH is more common in people with T1DM.8 LH does not only cause cosmetic problems but also impairs the absorption of insulin which could lead to unexplained hypoglycemia and suboptimal glycemic control.9,10 At the other end of the spectrum of insulin-induced lipodystrophy is lipoatrophy (LA) which is related with immune reaction.11 It has rarely been found since the introduction of recombinant insulin in the 1980s.12 However, concurrent occurrence of LH and LA in the same patient had been occasionally reported even in patients with the use of insulin analogs.13,14 Therefore, healthcare professions should examine insulin injection sites in their insulin-treated patients periodically to early detect these cutaneous complications.
Though insulin-induced lipodystrophy is a well-recognized side-effect from insulin injection technique, there is still a dearth of information in Southeast Asia. Previous studies from various countries revealed that errors in insulin injections among insulin-treated patients were frequent; especially failure in the rotation insulin injection site which is one of the major causes of LH.3–5,15 The present cross-sectional study aimed to determine the prevalence, associated risk factors, clinical characteristics of insulin-induced lipodystrophy, and awareness of this condition from their treating physicians in a series of insulin-treated patients from a tertiary diabetes center in Thailand.
Patients and Methods
All consecutive cases of insulin-treated patients attending diabetes clinic for a routine visit were recruited from June 2018 to March 2019 at Theptarin Hospital which is one of the largest diabetes centers in Thailand. Consecutive patients with T1DM or T2DM, attending the diabetes clinic for a routine visit were interviewed by using a structured questionnaire (Supplementary material) focusing on key insulin injection parameters and asked about the occurrence of unexplained hypoglycemic episodes in the previous 3 months. Severe hypoglycemia was defined according to a joint position statement of the American Diabetes Association (ADA) and International Hypoglycemia Study Group (IHSG) as an episode leading to unconsciousness or requiring assistance by a third person.16 Only clinically important hypoglycemia (Level 2 hypoglycemia, a glucose level of <54 mg/dL with typical hypoglycemic symptoms) was collected. The awareness of this condition by receiving annual injection sites checked from their healthcare professionals and performing self-monitoring of injection site was inquired. Unexplained hypoglycemia was defined as the occurrence of hypoglycemia not related to a mismatch of meal and activities. Lack of insulin rotation was defined as injecting insulin in the same area or less than a fingerbreadth space between injections. Reused insulin needle was defined as using a needle more than 2-times. The frequency of reused needle was further classified as 3-times/needle, 4–5-times/needle, and more than 5-times/needle. Patients who use insulin less than 6 months, follow-up at our diabetes center less than 3-times in the past 12 months, and those who declined participation were excluded from the study.
Patients also underwent a careful clinical examination of the injection site by well-trained nurses. A training workshop for all six experienced diabetes nurse educators to standardize the detection method of lipodystrophy had been conducted by a senior nurse educator who had been working for more than 25 years as a diabetes nurse educator. Experienced diabetes nurse educators, skilled in performing observation and palpation techniques, evaluated the presence of LH or LA in all patients. The inspection of injection site was done carefully with using direct and tangential light and then a gentle palpation technique involving fingertip movements followed by pinching maneuver in the suspected LH area. A clinical grading of LH was applied (Grade 1: lipohypertrophy without visible skin lesion but increased palpable density of subcutaneous tissue; Grade 2: severe hypertrophy with increased density of the injection site).8 Lipoatrophy at any injection site was noted separately. Clinical parameters including the most recent glycated hemoglobin (A1C) value in the previous 3 months, were all recorded and analyzed. Ultrasonographic studies were performed by an experienced radiologist in some patients with equivocal area of LH or some patients who had concurrent LH and LA. If ultrasound findings suggest other subcutaneous lesions (such as lipoma, hematoma, cyst, etc.), the participant will be excluded from the study. All procedures followed were in accordance with the Declaration of Helsinki. All participants provided informed consent and the Ethics Committee of Theptarin Hospital approved the study (EC 09/2018). Any data intended for sharing were de-identified.
Statistical Analysis
Based on a previous meta-analysis which demonstrated the prevalence of LH at 38%,7 this study would require a sample size of 361 subjects for estimating the expected proportion with 5% absolute precision and 95% confidence.17 All statistical analyses were conducted using the Statistical Package for the Social Sciences (version 22.0; SPSS, Armonk, NY, USA). Data is presented as mean±standard deviation (SD) when Gaussian distribution of the continuous data was observed, and as median (Interquartile range, IQR) when the distribution was not normal. The categorical data are presented as percentages. Descriptive statistics for the categorical variables were assessed using the χ2 and for the continuous variables using Student’s T-test, paired and unpaired, or the Mann Whitney U-test and Wilcoxon signed-ranks test when applicable. Variables with established association with insulin-induced lipohypertrophy were selected for univariate logistic regression analysis, and those with a P-value<0.05 were included in the multivariate models with forward variable selection to determine associated clinical factors and the presence of lipohypertrophy. Results are expressed as odds ratios (ORs) with their 95% confidence intervals (CI). The frequency of unexplained hypoglycemia was also compared between patients with and without LH. P-value<0.05 was considered statistically significant. The study was registered retrospectively with www.clinicaltrials.in.th (TCTR20190707003), registered 6 July 2019.
Results
Baseline Demographic and Clinical Characteristics
A total of 400 patients were recruited into the study (female=53.5%, T2DM=86%, mean age=65.6±15.4 years, duration of diabetes=23.0±10.2 years, median of insulin treatment=10 (IQR=4–16), years, usage of insulin analog=72.1%, A1C=7.9±1.6%) were studied. Only 28.5% of all participants had optimal glycemic control (A1C<7.0%). The demographic and clinical characteristics of the participants are presented in Table 1. The details of insulin regimens and injection techniques are presented in Table 2. In people with T2DM, human premixed insulin was the most commonly used insulin (43.0%). Only 12.8% of T2DM participants received a basal-bolus insulin regimen. Syringes were used only in 6.2% of all participants. Needles with a 6 mm length were most commonly used (50.0%), followed by 8 mm (23.3%), and 5 mm (20.5%). The abdomen was the most frequent site of injection (92.8%). Most of the study subjects (74.5%) rotated the insulin injection sites. Reuse of needles was very common (94.5%), with the majority reusing a needle more than 5-times. In the past 3 months, severe hypoglycemia was found in only three patients (0.8%), two of whom had T1DM. Mismatch between insulin and meal caused severe hypoglycemia in all patients. Clinically important hypoglycemia (level 2 hypoglycemia) was found in 10 patients (2.5%), with fiveof these patients having the definition of unexplained hypoglycemia in our present study. The frequency of unexplained hypoglycemia had been reported 1–2-times over the previous 3 months in 80% of patients and 3–6-times over the previous 3 months in 20% of patients.
Table 1.
Demographic Data of Studied Participants (N=400)
| Total (N=400) | T1DM (N=56) | T2DM (N=344) | |
|---|---|---|---|
| Age | 65.6±15.4 | 45.2±13.7 | 68.9±12.9 |
| Female | 53.5% | 48.2% | 54.4% |
| Education | |||
| -Less than high school | 156 (39.0%) | 2 (3.6%) | 154 (44.8%) |
| -High school | 86 (21.5%) | 9 (16.1%) | 77 (22.4%) |
| -Bachelor degree or colledge | 116 (29.0%) | 32 (57.1%) | 84 (24.4%) |
| -Higher than bachelor degree | 42 (10.5%) | 13 (23.2%) | 29 (8.4%) |
| Duration of DM | 23.0±10.2 | 20.1±11.6 | 23.5±9.8 |
| Duration of insulin (years) | 11.4±8.7 | 18.9±11.0 | 10.2±7.6 |
| BMI (kg/m2) | 26.2±4.8 | 23.8±3.6 | 26.5±4.8 |
| A1C (%NGSP) | 7.9±1.6 | 7.8±1.5 | 7.9±1.6 |
| Daily insulin dose (units/day) | 41.9±24.9 | 46.5±17.8 | 41.1±25.8 |
| Daily insulin dose (unit/kg/day) | 0.6±0.3 | 0.7±0.3 | 0.6±0.3 |
| Type of insulin* | |||
| Human insulin | 150 (27.9%) | 16 (24.6%) | 134 (36.8%) |
| -Regular insulin | 21 | 8 | 13 |
| -NPH | 15 | 4 | 11 |
| -Pre-mixed human insulin | 114 | 4 | 110 |
| Insulin analog | 387 (72.1%) | 49 (75.4%) | 230 (63.2%) |
| -Aspart | 81 | 19 | 62 |
| -Lispro | 84 | 25 | 59 |
| -Glulisine | 18 | 4 | 14 |
| -Glargine U100 | 96 | 19 | 77 |
| -Glargine U300 | 31 | 5 | 26 |
| -Detemir | 9 | 2 | 7 |
| -Degludec | 68 | 17 | 51 |
| Insulin device | |||
| -Insulin pen | 375 (93.8%) | 48 (85.7%) | 327 (95.0%) |
| -Insulin syringe | 20 (5.0%) | 6 (10.7%) | 14 (4.1%) |
| -Mixed (pen and syringe) | 5 (1.2%) | 2 (3.6%) | 3 (0.9%) |
| Patients with concurrent anti-diabetic medications (%) | 218 (54.5%) | 11 (19.6%) | 207 (60.2%) |
| Specified type of anti-diabetic medications# | |||
| -Sulfonylurea | 28 | 0 | 28 |
| -Metformin | 162 | 5 | 157 |
| -DPP4 inhibitor | 82 | 3 | 79 |
| -Thiazolidinedione | 58 | 2 | 56 |
| -SGLT2 inhibitor | 48 | 4 | 44 |
| -GLP1 receptor agonist | 12 | 0 | 12 |
Notes: *The denominator was 537, due to some patients using more than one type of insulin. #Some patients received more than one type of anti-diabetic medication.
Table 2.
The Details of Insulin Regimens and Injection Techniques in Studied Participants
| Total (N=400) | T1DM (N=56) | T2DM (N=344) | |
|---|---|---|---|
| Insulin regimen | |||
|
155 (38.8%) | 7 (12.5%) | 148 (43.0%) |
|
87 (21.8%) | 43 (76.8%) | 44 (12.8%) |
|
60 (15.0%) | 5 (8.9%) | 55 (16.0%) |
|
86 (21.4%) | – | 86 (25.0%) |
|
12 (3.0%) | 1 (1.8%) | 11 (3.2%) |
| Location of injection site | |||
|
371 (92.8%) | 43 (76.8%) | 328 (95.3%) |
|
4 (1.0%) | – | 4 (1.2%) |
|
1 (0.2%) | 1 (1.8%) | – |
|
23 (5.8%) | 11 (19.6%) | 12(3.5%) |
|
1 (0.2%) | 1 (1.8%) | – |
| Rotation injection site | |||
|
298 (74.5%) | 41 (73.2%) | 257 (74.7%) |
|
102 (25.5%) | 15 (26.8%) | 87 (25.3%) |
| Needle length | |||
|
20 (5.0%) | 2 (3.6%) | 18(5.2%) |
|
82 (20.5%) | 13 (23.2%) | 69 (20.1%) |
|
200 (50.0%) | 27 (48.2%) | 173 (50.3%) |
|
93 (23.2%) | 11 (19.6%) | 82 (23.8%) |
|
5 (1.3%) | 3 (5.4%) | 2 (0.6%) |
| Reused insulin needles | |||
|
378 (94.5%) | 53 (94.6%) | 325 (94.5%) |
| 3 times | 88 (23.3%) | 13 (24.5%) | 75 (23.1%) |
| 4–5 times | 87 (23.0%) | 10 (18.9%) | 77 (23.7%) |
| >5 times | 203 (53.7%) | 30 (56.6%) | 173 (53.2%) |
|
22 (5.5%) | 3 (5.4%) | 19 (5.5%) |
The Prevalence and Associated Risk Factors of Insulin-Induced Lipohypertrophy
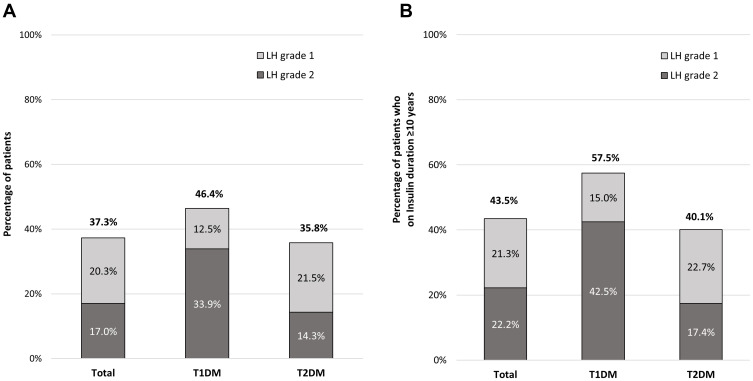
The overall prevalence of insulin-induced LH was 37.2% (T1DM=46.4% and T2DM=35.5%) with grade 2 (severe LH) in 45.9% of all patients with LH, as shown in Figure 1. Compared to patients without LH, those with LH had a longer diabetes duration and insulin therapy duration, were treated with higher insulin dose, incorrect rotation of injection sites, and reusing insulin needles (≥2-times) as shown in Table 3. The highest prevalence (57.5%) was observed in long-standing (≥10 years) T1DM patients. Multivariate analysis revealed the duration of insulin use (≥10 years), use of human insulin, and incorrect rotation of injection sites were independently associated with LH, as demonstrated in Table 4. The strongest factor associated with LH was incorrect rotation of injection sites (OR=26.14; 95% CI=13.68–49.95). The incidence of unexplained hypoglycemia in the past 3 months was reported in 1.3% of all participants. The patients with LH were found to increase the risk of unexplained hypoglycemia by 7-times when compared with patients without LH (2.7% vs 0.4%, P-value=0.045)
Figure 1.
(A) The overall prevalence of insulin-induced lipohypertrophy and prevalence stratified by type of diabetes and duration of insulin treatment. (B) The prevalence of insulin-induced lipohypertrophy in people with long-standing (≥10 years) DM and prevalence stratified by type of diabetes.
Table 3.
Comparison of Clinical Parameters and Glycemic Control in Patients with and without Insulin-Induced Lipohypertrophy
| Patients with LH (N=149) | Patients without LH (N=251) | P-value | |
|---|---|---|---|
| Age | 64.8±14.2 | 66.0±16.1 | 0.420 |
| Female | 71 (47.7%) | 143 (57.0%) | 0.071 |
| Education | 0.338 | ||
|
55 (37.2%) | 101 (40.1%) | |
|
31 (20.9%) | 55 (21.8%) | |
|
50 (33.8%) | 66 (26.2%) | |
|
12 (8.1%) | 30 (11.9%) | |
| Durationof DM | 24.1 ± 8.9 | 22.4 ± 10.8 | 0.103 |
| Duration of insulin (years) | 13.4 ± 9.2 | 10.3 ± 8.1 | 0.001 |
| BMI (kg/m2) | 25.9 ± 4.8 | 26.3 ± 4.8 | 0.437 |
| A1C (%NGSP) | 7.8 ± 1.5 | 7.9 ± 1.7 | 0.545 |
| Daily insulin dose (units/day) | 43 ± 24 | 41 ± 25 | 0.439 |
| Daily insulin dose (unit/kg/day) | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.441 |
| Type of insulin | 0.021 | ||
|
54 (36.2%) | 59 (23.5%) | |
|
88 (59.1%) | 181 (72.1%) | |
|
7 (4.7%) | 11 (4.4%) | |
| Insulin device | 0.265 | ||
|
136 (91.3%) | 239 (95.2%) | |
|
10 (6.7%) | 10 (4.0%) | |
|
3 (2.0%) | 2 (0.8%) | |
| Insulin regimen | 0.074 | ||
|
61 (41.2%) | 94 (37.3%) | |
|
27 (18.2%) | 60 (23.8%) | |
|
29 (19.6%) | 31 (12.3%) | |
|
29 (19.6%) | 57 (22.6%) | |
|
2 (1.4%) | 10 (4.0%) | |
| Location of injection site | 0.282 | ||
|
143 (96.0%) | 228 (90.8%) | |
|
- | 4 (1.6%) | |
|
- | 1 (0.4%) | |
|
6 (4.0%) | 17 (6.8%) | |
|
- | 1 (0.4%) | |
| Rotation injection site | <0.001 | ||
|
60 (40.3%) | 238 (94.8%) | |
|
88 (59.7%) | 13 (5.2%) | |
| Needle length | 0.652 | ||
|
5 (3.4%) | 15 (6.0%) | |
|
30 (20.1%) | 52 (20.7%) | |
|
75 (50.3%) | 125 (49.8%) | |
|
38 (25.5%) | 55 (21.9%) | |
|
1 (0.7%) | 4 (1.6%) | |
| Reused insulin needles | 0.057 | ||
|
145 (97.3%) | 233 (92.8%) | |
|
4 (2.7%) | 18 (7.2%) |
Table 4.
Factors Associated with the Presence of Insulin-Induced Lipohypertrophy
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | P-value | 95% CI | OR | P-value | 95% CI | |
| T1DM | 1.58 | 0.117 | 0.89–2.79 | |||
| Female | 0.67 | 0.057 | 0.45–1.01 | |||
| Education less than high school | 0.88 | 0.564 | 0.58–1.34 | |||
| BMI <25 kg/m2 | 1.24 | 0.293 | 0.83–1.87 | |||
| Duration of insulin ≥10 years | 1.79 | 0.006 | 1.19–2.70 | 1.83 | 0.025 | 1.08–3.09 |
| Number of daily insulin injection | 0.98 | 0.936 | 0.64–1.52 | |||
| Use of human insulin | 1.82 | 0.006 | 1.19–2.80 | 1.78 | 0.037 | 1.04–3.05 |
| Insulin dose ≥0.6 units/kg/day | 1.03 | 0.872 | 0.69–1.55 | |||
| Incorrect insulin rotation | 24.93 | <0.001 | 13.27–46.86 | 26.14 | <0.001 | 13.68–49.95 |
| Use of needle length ≥8 mm | 1.17 | 0.510 | 0.733–1.87 | |||
| Reuse insulin needles | 2.769 | 0.070 | 0.92–8.35 | |||
| 3 times | 2.972 | 0.067 | 0.93–9.52 | |||
| 4–5 times | 2.491 | 0.126 | 0.77–8.02 | |||
| >5 times | 2.808 | 0.071 | 0.92–8.60 | |||
The Prevalence and Clinical Characteristics of Insulin-Induced Lipoatrophy
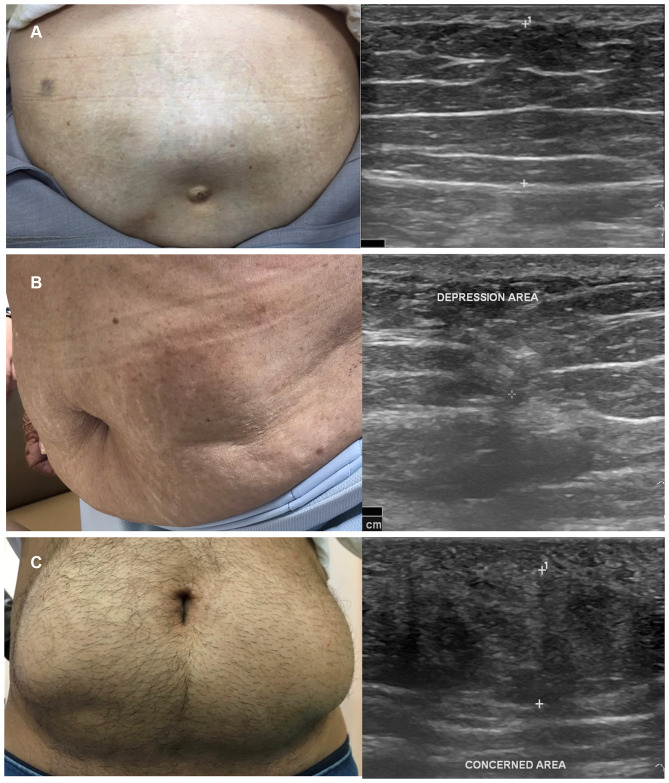
A total of four cases (male=50%, mean age=60.5±17.1 years, duration of diabetes=27.0±11.4 years, median of insulin treatment=16 years, A1C=8.5±1.1%, median total daily dose of insulin=40 units per day) of insulin-induced LA were found, which accounted for only 1.0% of all participants. Three patients had T2DM and one patient had T1DM. All LA cases had a concurrence palpable area of lipohypertrophy. Lipoatrophic areas were developed in the abdominal area, both on the same side and the opposite side of lipohypertrophic areas. Ultrasonographic findings of lipoatrophy revealed a focal area of decreased thickness and increased heterogeneous echogenicity of subcutaneous fat texture, as demonstrated in Figure 2. One patient had suspected insulin-derived localized amyloidosis based on palpable subcutaneous mass at sub-umbilical region and homogeneous hypoechoic fat interspersed with hyperechoic linear echogenic area. However, the patient refused to have excision for confirmed diagnosis.
Figure 2.
(A) A typical insulin-induced lipohypertrophy in T2DM patient with ultrasound characteristics of thickening heterogeneous echogenicity of subcutaneous fat. (B) Insulin-induced lipoatrophy in a patient with long-standing T2DM with ultrasonographic findings of lipoatrophy revealed a focal area of decreased thickness and increased heterogeneous echogenicity of subcutaneous fat texture. (C) A T1DM patient with suspected insulin-derived localized amyloidosis based on palpable subcutaneous mass at subumbilical region and homogeneous hypoechoic fat interspersed from ultrasound.
All LA patients, except for one, used various recombinant insulin analogs. Repeated insulin injections at the same sites were reported in all patients, and unexplained hypoglycemia was found in 80% of the patients. None of the patients received their injection sites checked at least annually from their healthcare professionals.
Awareness of Insulin-Induced Lipodystrophy
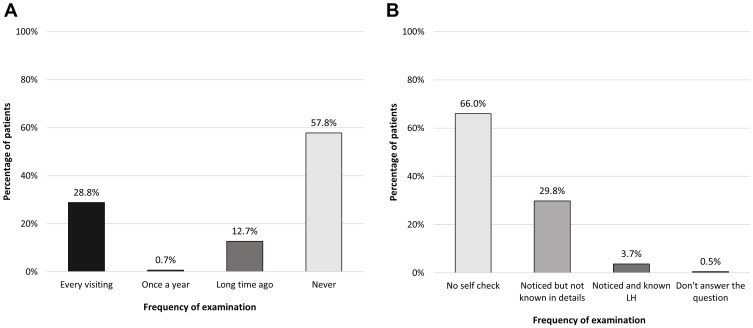
As revealed in Figure 3, only about 30% of patients had their injection sites checked at least annually by their healthcare professionals and more than half of the patients had received no injection checking in the last 1 year. Almost two-thirds of all participants never performed self-monitoring of the presence of insulin-induced lipodystrophy.
Figure 3.
(A) Frequency of lipodystrophy checking from healthcare professions. (B) Self-checking the presence of lipodystrophy by patients.
Discussion
Our present study confirms previous findings from a recent meta-analysis that the prevalence of lipohypertrophy was a common complication in insulin-treated people with diabetes and was associated with an increased risk of unexplained hypoglycemia. Unfortunately, it received little attention from healthcare professionals, and many patients still have inadequate instructions on insulin injection technique. Poor injection technique, especially incorrect rotation of insulin injection sites, leads to the occurrence of LH in our study. Since the advent of recombinant human insulin and insulin analogs, insulin-induced lipoatrophy, which is believed to be related with immune complex-mediated inflammatory reaction, is rarely seen today.18 Interestingly, concurrent occurrence of lipoatrophy and lipohypertrophy in the same patient had been found up to 1.0% in our series.
Subcutaneous adipose tissue has been known to be a local target of exogeneous insulin action since 1950.19 The lipodystrophic reactions from insulin could roughly be divided into hypertrophic or atrophic lesions. While LH is most commonly seen in those with poor injection technique since the discovery of insulin, LA was commonly found before the 1970s when impure animal insulin was used. With the availability of recombinant insulin, the prevalence of LA was reported at only 0.2–1.4%.12 These skin complications occurring at insulin injection sites not only cause cosmetic concerns but also are known to cause impaired absorption of insulin. In a recent combined glucose-clamp/meal test study to quantify the impact of insulin lispro injection into LH area,20 the researchers found that the LH area injection increased the variability of both insulin absorption and insulin action in euglycemic clamps. As a result, postprandial hyperglycemia (≥26% higher in plasma glucose concentrations) was observed in people with T1DM with LH area injection. Therefore, it should be emphasized that suboptimal glycemic control and in patients with unexplained hypoglycemia, treating physicians should inspect and examine the presence of LH.21
Repeated trauma to the same injection site when patients fail to rotate injections and/or repeated use of the same needle was reported worldwide.5 The patient usually finds less painful injection when injected into LH area, and this could further aggravate the enlargement of LH area. In the extreme cases, insulin-derived localized amyloidosis or “insulin ball” could develop, and cosmetic surgery is required to manage this cutaneous complication.22–24 In our series, one case of suspected insulin-derived amyloidosis in a patient with long-standing T1DM who repeated injected insulin glargine and insulin aspart into the same abdominal area over 15 years was found. The patient denied further investigations with abdominal computed tomography (CT) and excision. In vitro studies reported that toxicity of insulin amyloid fibrils cause fat necrosis in the surrounding tissue, and recombinant insulin or human insulin could lead to this uncommon problem.25 Therefore, the role of imaging including ultrasound and CT scan could differentiate insulin-derived localized amyloidosis from the more common insulin-induced LH.
Consistent to other cross-sectional studies from Caucasian patients,2,8 we also found that the prevalence of LH was more common in people with T1DM, especially in long-standing T1DM patients. However, a milder form of LH (or LH grade 1) was detected more frequently in people with T2DM. Specific training in inspection and palpation techniques should be emphasized to healthcare professionals to identify smaller and flatter LH area.26 Sometimes, ultrasound scans are needed in equivocal lesions to identify culprit lesions in an early phase.27 Ideally, treating physicians should perform a thorough injection sites inspection in all insulin-treated patients in every visit. However, time constraint is one of the barriers physicians face in a routine diabetes clinic. Therefore, focusing on higher risk patients (such as people with T1DM, all patients with duration of insulin use ≥10 years, patients who reusing insulin needles ≥3 times, etc.) would help busy physicians to triage the higher risk patients. Moreover, training diabetes nurse educators would also facilitate screening and educating patients in the primary care setting.28
Apart from poor injection techniques, insulin devices and needles might also play a role in the development of LH from the possible greater tissue injury from mismatch devices and repetitive uses.6 Theoretically, needle lengths should be as short as possible to minimize tissue trauma and to avoid inadvertent intramuscular administration, especially in skinny people.29 Most insulin pen needles range from 4–12 mm in length and 29–32 gauge in diameter. Based on the results of our study, which was conducted in the private setting, the majority of patients used insulin pens, with half of them using a 6-mm needle length. However, if it is possible, the smallest 4 mm needles would carry the least risk of tissue trauma and avoid intramuscular injection.30 Regarding reusing insulin needles (≥2-times), the results revealed that it was a very common practice in our participants. Possible explanations could be economic reasons or convenience for patients. Even though our present study revealed no association between reuse of insulin needle and the occurrence of LH, patients should be educated to not reuse needles if and needle tip deformity or increased pain were observed.31,32 Moreover, the dose of insulin which needed to be consumed more in LH patients might outweigh the economic concern for reused needles.33
Another spectrum of insulin-induced lipodystrophy is insulin-induced lipoatrophy which the prevalence decreased sharply from 10–55% of patients using animal-derived insulin to less than 2% in the present day.12 However, our study was also consistent with previous reports that insulin analogs did not prevent patients from developing this complication and concurrence of LH and LA in the same patient could be seen.13,14 Recognizing this rare insulin reaction and timely detection of lipoatrophy with ultrasound as a non-invasive simple imaging modality is necessary to avoid further injection in the skin lesion. Even though specific treatment of LA is still unavailable, therapeutic trials of dexamethasone and cromolyn sodium had been reported successfully in the anecdotal cases.34,35
This study has several limitations that should be acknowledged. First, this was a cross-sectional study from a private tertiary diabetes center in Bangkok. The results may not be applicable to other populations. Further multi-center studies are required to confirm these findings. Second, it was not possible to use ultrasound which is the gold standard to detect lipodystrophy in all participants in this study so the prevalence of lipodystrophy could be underestimated. However, all experienced diabetes nurse educators who conducted this study had been trained to minimize inter-observer variation in observation and palpation techniques. Moreover, the clinical significance of “subclinical lipohypertrophy”, which was identified by ultrasonographic features of hyperechogenicity in non-palpable injection area, remains unknown. Third, the objective data of documented hypoglycemic episodes and responses after avoiding injection into LH area could not be examined. Nevertheless, this study is represented by the relatively large sample size with a comprehensive set of risk factors assessment. To the best of our knowledge, this study is also the first study in a Southeast Asian population. Future prospective studies should be conducted to improve insulin injection techniques among LH patients and determine whether effects of interventions to the impact of glycemic control and risk of hypoglycemia.36
Conclusion
Insulin-induced lipodystrophy is still an overlooked complication in the modern era of diabetes care. While the presence of lipohypertrophy is very common and significantly associated with the occurrence of erratic glucose control and unexplained hypoglycemia, the presence of lipoatrophy is rare but is still seen in some exceptional patients. Insulin injection technique continues to be suboptimal in many insulin-treated patients, and our study also highlights the need for improved awareness of physicians to recognize these insulin-related skin complications.
Acknowledgments
The authors wish to thank Dr. Tinapa Himathongkam for excellent language editing. Parts of this manuscript had previously been presented as a poster in International Diabetes Federation (IDF) meeting 2019, Busan, South Korea.
Funding Statement
This work was supported by the grant for promoting research in Theptarin hospital (Grant No. 2/2561). The funder had no role in the study design, data collection and analysis.
Abbreviations
A1C, glycated hemoglobin value; LA, Lipoatrophy; LH, Lipohypertrophy; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus.
Data Sharing Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Holden SE, Gale EA, Jenkins-Jones S, et al. How many people inject insulin? UK estimates from 1991 to 2010. Diabetes Obes Metab. 2014;16(6):553–559. doi: 10.1111/dom.12260 [DOI] [PubMed] [Google Scholar]
- 2.Vardar B, Kizilci S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract. 2007;77(2):231–236. doi: 10.1016/j.diabres.2006.12.023 [DOI] [PubMed] [Google Scholar]
- 3.De Coninck C, Frid A, Gaspar R, et al. Results and analysis of the 2008–2009 insulin injection technique questionnaire survey. J Diabetes. 2010;2(3):168–179. doi: 10.1111/j.1753-0407.2010.00077.x [DOI] [PubMed] [Google Scholar]
- 4.Strauss K, De Gols H, Hannet I, et al. A Pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diabetes Int. 2002;19(3):71–76. doi: 10.1002/pdi.314 [DOI] [Google Scholar]
- 5.Frid AH, Hirsch LJ, Menchior AR, et al. Worldwide injection technique questionnaire study: injecting complications and the role of the professional. Mayo Clin Proc. 2016;91(9):1224–1230. doi: 10.1016/j.mayocp.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 6.Baruah MP, Kalra S, Bose S, et al. An audit of insulin usage and insulin injection practices in a large Indian cohort. Indian J Endocrinol Metab. 2017;21(3):443–452. doi: 10.4103/ijem.IJEM_548_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng N, Zhang X, Zhao F, et al. Prevalence of lipohypertrophy in insulin-treated diabetes patients: a systematic review and meta-analysis. J Diabetes Investig. 2018;9(3):536–543. doi: 10.1111/jdi.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauner H, Stockamp B, Haastert B. Prevalence of lipohypertrophy in insulin-treated diabetic patients and predisposing factors. Exp Clin Endocrinol Diabetes. 2009;104(2):106–110. doi: 10.1055/s-0029-1211431 [DOI] [PubMed] [Google Scholar]
- 9.Johansson U-B, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025–2027. doi: 10.2337/diacare.28.8.2025 [DOI] [PubMed] [Google Scholar]
- 10.Gentile S, Agrusta M, Guarino G, et al. Metabolic consequence of incorrect insulin administration techniques in aging subjects with diabetes. Acta Diabetol. 2011;48(2):121–125. doi: 10.1007/s00592-009-0172-x [DOI] [PubMed] [Google Scholar]
- 11.Reeves WC, Allen BR, Tattersall RB. Insulin-induced lipoatrophy: evidence for an immune pathogenesis. Br Med J. 1980;280(6230):1500–1503. doi: 10.1136/bmj.280.6230.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajheydari Z, Kashi Z, Akha O, et al. Frequency of lipodysdrophy induced by recombinant human insulin. Eur Rev Med Pharmacol. 2011;15(10):1196–1201. [PubMed] [Google Scholar]
- 13.Holstein A, Stege H, Kovacs P. Lipoatrophy associated with the use of insulin analogues: a new case associated with the use of insulin glargine and review of the literature. Expert Opin Drug Saf. 2010;9(2):225–231. doi: 10.1517/14740330903496402 [DOI] [PubMed] [Google Scholar]
- 14.Singha A, Bhattarcharjee R, Ghosh S, et al. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51–53. doi: 10.2337/diaclin.34.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji J, Lou Q. Insulin pen injection technique survey in patients with type 2 diabetes in mainland China in 2010. Curr Med Res Opin. 2014;30(6):1087–1093. doi: 10.1185/03007995.2014.895711 [DOI] [PubMed] [Google Scholar]
- 16.International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes: table 1. Diabetes Care. 2017;40(1):155–157. doi: 10.2337/dc16-2215 [DOI] [PubMed] [Google Scholar]
- 17.Sample size calculator for estimating a single proportion [Internet]. Sydney. [cited March 28, 2019] Available from: http://statulator.com/SampleSize/ss1P.html. Accessed November4, 2020.
- 18.Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol. 2003;4(10):661–667. doi: 10.2165/00128071-200304100-00001 [DOI] [PubMed] [Google Scholar]
- 19.Renold AE, Marble A, Fawcett DW. Action of insulin on deposition of glycogen and storage of fat in adipose tissue. Endocrinology. 1950;46(1):55–66. doi: 10.1210/endo-46-1-55 [DOI] [PubMed] [Google Scholar]
- 20.Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486–1492. doi: 10.2337/dc16-0610 [DOI] [PubMed] [Google Scholar]
- 21.Strollo F, Guarino G, Armentano V, et al. on behalf of AMD-OSDI Italian. Study Group on injection techniques. Unexplained hypoglycaemia and large glycaemic variability: skin lipohypertrophy as a predictive sign. Diabetes Res Open J. 2016;2(1):24–32. doi: 10.17140/DROJ-2-126 [DOI] [Google Scholar]
- 22.Swift B, Hawkins PN, Richards C, Gregory R. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881–882. doi: 10.1046/j.1464-5491.2002.07581.x [DOI] [PubMed] [Google Scholar]
- 23.Nagase T, Katsura Y, Iwaki Y, et al. The insulin ball. Lancet. 2009;373(9658):184. doi: 10.1016/S0140-6736(09)60041-6 [DOI] [PubMed] [Google Scholar]
- 24.Ansari AM, Osmani L, Matsangos AE, et al. Current insight in the localized insulin-derived amyloidosis (LIDA): clinico-pathological characteristics and differential diagnosis. Pathol Res Pract. 2017;213(10):1237–1241. doi: 10.1016/j.prp.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 25.Iwaya K, Zako T, Fukunaga J, et al. Toxicity of insulin-derived amyloidosis: a case report. BMC Endocr Disord. 2019;19(1):61. doi: 10.1186/s12902-019-0385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentile S, Guarino G, Giancaterini A, et al. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. Springerplus. 2016;5(1):563. doi: 10.1186/s40064-016-1978-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapeluto JE, Paty BW, Chang SD, et al. Ultrasound detection of insulin-induced lipohypertrophy in type 1 and type 2 diabetes. Diabet Med. 2018;35(10):1383–1390. doi: 10.1111/dme.13764 [DOI] [PubMed] [Google Scholar]
- 28.Spollett G, Edelman SV, Mehner P, et al. Improvement of insulin injection technique: examination of current issues and recommendations. Diabetes Educ. 2016;42(4):379–394. doi: 10.1177/0145721716648017 [DOI] [PubMed] [Google Scholar]
- 29.Hirsch L, Byron K, Gibney M. Intramuscular risk at insulin injection sites—measurement of the distance from skin to muscle and rationale for shorter-length needles for subcutaneous insulin therapy. Diabetes Technol Ther. 2014;16(12):867–873. doi: 10.1089/dia.2014.0111 [DOI] [PubMed] [Google Scholar]
- 30.Bergenstal RM, Strock ES, Peremislov D, et al. Safety and efficacy of insulin therapy delivered via a 4mm pen needle in obese patients with diabetes. Mayo Clin Proc. 2015;90(3):329–338. doi: 10.1016/j.mayocp.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 31.Puder JJ, Atar M, Muller B, et al. Using insulin pen needles up to five times does not affect needle tip shape nor increase pain intensity. Diabetes Res Clin Pract. 2005;67(2):119–123. doi: 10.1016/j.diabres.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 32.Blanco M, Hernández MT, Strauss KW, et al. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445–453. doi: 10.1016/j.diabet.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 33.Ji L, Sun Z, Li Q, et al. Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther. 2017;19(1):61–67. doi: 10.1089/dia.2016.0334 [DOI] [PubMed] [Google Scholar]
- 34.Kumar D, Miller LV, Mehtalia SD. Use of dexamethasone in treatment of insulin lipoatrophy. Diabetes. 1977;26(4):296–299. doi: 10.2337/diab.26.4.296 [DOI] [PubMed] [Google Scholar]
- 35.Phua E-J, Lopez X, Ramus J, et al. Cromolyn sodium for insulin-induced lipoatrophy: old drug, new use. Diabetes Care. 2013;36(12):e204–e205. doi: 10.2337/dc13-1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misnikova IV, Gubkina VA, Lakeeva TS, et al. A randomized controlled trial to assess the impact of proper insulin injection technique training on glycemic control. Diabetes Ther. 2017;8(6):1309–1318. doi: 10.1007/s13300-017-0315-y [DOI] [PMC free article] [PubMed] [Google Scholar]