Abstract
Background/Aims
The proportions of intestinal and peripheral regulatory T cells (Tregs) in pediatric inflammatory bowel disease (IBD) were poorly investigated, as well as different subsets of these cells. Helios and Neuropilin-1 were proposed as markers differentiating between thymic and peripheral Tregs. Therefore, the aim of current work was to investigate the proportions of Tregs and expression of Helios and Neuropilin-1 in Tregs in peripheral blood and intestinal mucosa of children with inflammatory bowel disease.
Materials and methods
Fifteen patients newly diagnosed with inflammatory bowel disease: ulcerative colitis (n=7) and Crohn’s disease (n=8) were included in the study. Nine children who presented with no abnormalities in colonoscopy served as a control group. Quantification of regulatory T cells of the CD4+CD25highFOXP3+ phenotype, as well as Helios+ and Neuropilin-1+ in peripheral blood and bowel mucosa was based on multicolor flow cytometry.
Results
The rates of circulating and intestinal Tregs were significantly higher in the studied group than in the control group. The rate of intestinal T regulatory lymphocytes was significantly higher than circulating Tregs in patients with IBD, but not in the control group. The median proportion of circulating FOXP3+Helios+ cells amounted to 24.83% in IBD patients and 15.93% in the controls. The median proportion of circulating FOXP3+Nrp-1+ cells was 34.23% in IBD and 21.01% in the control group. No statistically significant differences were noted for the circulating FOXP3+Helios+ cells and FOXP3+Nrp-1+ cells between the studied and the control group.
Conclusion
The rates of circulating and intestinal T regulatory cells are increased in naïve pediatric patients with IBD. The rate of Tregs is higher in intestinal mucosa than in peripheral blood in patients with IBD. Flow cytometry is a valuable method assessing the composition of infiltrates in inflamed tissue. Helios and Neuropilin-1 likely cannot serve as markers to differentiate between natural and adaptive Tregs.
Keywords: T regulatory cells, circulating Tregs, intestinal Tregs, Helios, Neuropilin-1, IBD, inflammatory bowel disease, children
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), constitutes a growing problem in pediatrics due to its increasing incidence and not fully effective, burdensome treatment. IBD is a chronic condition of not entirely clear pathogenesis. Impaired immune regulation has been hypothesized as the mechanism responsible for the abnormal inflammatory response to enteric bacterial antigens. Regulatory T cells (Tregs) have been regarded as the crucial element of immune regulation, since the discovery that humans lacking Tregs due to mutation of FOXP3 develop severe bowel inflammation and autoimmune disorders.1 From that moment scientific interest has turned towards the assessment of these cells, identified as CD4+CD25highFOXP3+, in many inflammatory conditions.2–6 Soon studies concerning the frequency of circulating Tregs in IBD produced conflicting results showing the number increased,7 decreased,8 or unchanged,9 when compared to healthy controls. Moreover the site of inflammation was found to be enriched in Tregs, which does not support the hypothesis of a deficit of these cells in inflammatory bowel disease.10,11 Further research focused on the function of Tregs in inflammation, but the results have also been inconclusive.12,13 Thus, the postulated role of impaired immune regulation in the pathogenesis of IBD was neither definitely denied nor confirmed. It has been widely recognized that the population of Tregs is not homogenous, and there are subsets characterized by different molecule and cytokine patterns. It was also shown that Tregs originate not only from the thymus, as it was initially believed, but lymphocytes of CD4+CD25high FOXP3+ phenotype can also be induced in the periphery from naïve T cells upon antigen exposure.14,15 This gave the beginning to classification of Tregs in two subsets: natural (thymus derived, tTregs) and peripheral (adaptive, induced) iTregs. The distinction between the two subtypes has not been yet elucidated, but Helios and Neuropilin-1 (Nrp-1- CD304) characterizing tTregs have been proposed among the differentiating markers.16,17 The publications investigating the frequencies of these two subtypes of Tregs in humans are rather sparse and only a few of them refer to children.18–20 Single studies describe the phenotype of Tregs in gut mucosa of patients in IBD, but none of them refers to pediatric patients.21–23
The aim of the study was to determine the rates of Tregs in peripheral blood and intestinal mucosa, and to investigate the frequencies of Tregs expressing Helios and Neuropilin-1 in pediatric patients with IBD.
Patients and Methods
Patients and Controls
Fifteen children (11.2–17.5 years of age, eight girls and seven boys), hospitalized in the Department of Pediatrics, Pediatric Gastroenterology, Hepatology, Allergology, and Nutrition of Medical University of Gdańsk, newly diagnosed with IBD, were enrolled to the study prior to any treatment introduction. The diagnosis was based on colonoscopy and confirmed by histopathology. The group was divided into two subgroups: with CD (n=8) and UC (n=7). Clinical activity of the disease was expressed with Pediatric Ulcerative Colitis Activity Index (PUCAI) for UC and with Pediatric Crohn’s Disease Activity Index (PCDAI) for CD.24,25 Endoscopic evaluation of children with CD and UC was based on SES-CD scoring and Mayo criteria, respectively.26,27 Histopathologic activity was scored according to the system described by Geboes et al.28,29
Peripheral venous blood samples and biopsy specimens obtained during the first diagnostic colonoscopy were subjected to cytometric analysis. The bioptates were taken from the most inflamed areas of the patients’ colon, also in the CD subgroup. Nine patients (aged 10.5–17.5 years, six girls, three boys) qualified for the colonoscopy, in whom neither endoscopic nor histologic abnormalities were found, served as a control group. The biopsy specimens in the children with no IBD were also taken from the colon.
The study was conducted according to the Declaration of Helsinki. The protocol of the study was approved by the Independent Bioethical Committee of Medical University of Gdansk. Informed consent was obtained from legal guardians and additionally from patients older than 15 years.
Peripheral Blood Mononuclear Cell Separation
Peripheral blood mononuclear cells (PBMC) were routinely isolated from 2 mL of peripheral venous blood collected on EDTA, diluted 1:1 with PBS, by centrifugation over Histopaque (Sigma Chemical Co., St. Louis, Missouri, USA) as described previously.23
Tissue Specimen Processing
Single cell suspension was prepared from mucosa bioptates according to the method described by Lord et al.23 Briefly, single cells were released from intestine mucosa bioptates suspended in cold RPMI by delicate homogenization with a hand-held glass Potter homogenizer, the suspension was then filtered (BD Falcon) to remove cell clumps and larger tissue debris.23 No further enrichment of lymphoid cells was done due to the small volume of the bioptates on one hand and the need to acquire more than 100,000 events per sample (due to expected relatively low frequency of Treg cells).
Staining
PBMC and mucosal mononuclear cells were then stained on ice for 30 minutes in the dark with the following fluorochrome-labeled antibodies: FITC-conjugated anti-human CD3 (clone OKT3), APC-Cy7-anti-human CD4- (clone OKT4), PE-Cy5-anti-human CD25 (clone 150D), and APC-conjugated anti-human CD304 (clone 12C2) (all from BioLegend, San Diego, USA). Unbound antibodies were then washed-out by two cycles of dilution of cell suspension in cold PBS and centrifugation. The surface-stained cells were then permeabilized using True-Nuclear™ Transcription Factor Buffer Set (BioLegend, San Diego, USA) according to the manufacturer’s protocol, incubated with 5% human immunoglobulins (Sandoglobulin, IMED, Poland) to prevent nonspecific binding of fluorescent antibodies to intracellular proteins, and subsequently stained for intracellular markers FoxP3 and Helios with: PE-anti-human FOXP3 (clone 206D)- and Pacific Blue-conjugated anti-human Helios (clone 22F6) (all from BioLegend, San Diego, USA). All antibodies used were mouse monoclonal Abs, with the exception of anti-Helios which was Armenian hamster monoclonal IgG. Isotype control samples were stained with APC-conjugated mouse IgG2a or with Pacific Blue-conjugated Armenian hamster IgG (also from BioLegend, San Diego, CA) instead of anti-CD304 and anti-Helios, respectively. After 30-minutes staining on ice in the dark, the unbound antibodies were washed-out with two rounds of cold PBS dilution and centrifugation, and finally the cells were suspended in cold PBS for cytometric analysis. Cytometric data were acquired using a FACSVerse™ cytometer (Becton-Dickinson, USA). At least 100,000 (105) events were acquired from each sample in order to obtain sufficient numbers of Treg cells for credible analysis. The proportions of Helios+ and Nrp-1+ (CD304+) Treg cells were determined using FlowJo™ software.
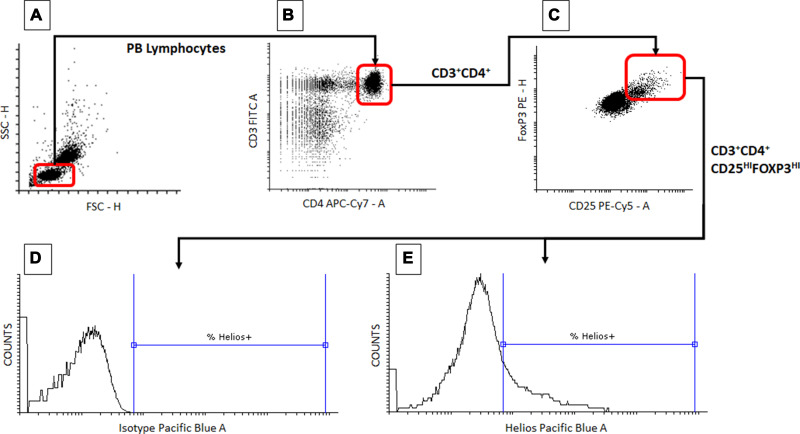
The proportions of Tregs defined as the CD3+CD4+CD25highFOXP3+ phenotype were expressed in relation to CD3+CD4+ T cells. Then, proportions of Helios- and Nrp-1 (CD304)- positive Tregs were determined. The representative gating strategy for estimation of the proportion of Tregs among CD3+CD4+ T cells and of Helios-positive lymphocytes among the Treg cells is shown in Figure 1. The same strategy was used for both peripheral blood and intestine samples, and also for estimation of the proportion of Nrp-1+ (CD304+) cells among the Tregs.
Figure 1.
Representative gating strategy – determination of the proportion of peripheral blood Treg cells expressing Helios. Peripheral blood mononuclear cells were obtained and processed as in the Patients and Methods section. During flow cytometry data analysis, lymphocytes were first identified based on their forward (FSC-H) and side scatter (SSC-H) (A). Contents of the lymphocyte gate were then analyzed for the presence of CD3 and CD4 markers and CD3+CD4+ T cells were gated (B). Then the contents of the CD3+CD4+ T cell gate were analyzed for the expression of CD25 and FoxP3 antigens and the cells displaying the CD3+CD4+CD25highFoxP3+ phenotype (Tregs) were delineated (C). Finally, the cells displaying the Treg phenotype were analyzed for the proportion of Helios+ cells ((D) sample stained with isotype control, (E) sample stained with anti-Helios antibody). Rounded rectangles mirror the actual gates used for analysis.
Statistical Analysis
The significance of intergroup differences was verified with Mann–Whitney U-test. Wilcoxon test was used for comparison of rate differences between blood and bowel mucosa.
The relationships between variables were tested with Spearman’s rank correlation coefficient. The value of P<0.05 was considered significant.
Results
The mean age was 14.4 years for the IBD group and 13.4 years for the control group. The mean PUCAI score for UC patients was 47.14, and the mean PCDAI for CD patients amounted to 29.37. The clinical and endoscopic characteristics of the study subjects are summarized in Table 1.
Table 1.
Endoscopic and Clinical Findings in the Study Subjects
| Endoscopic Activity Score | Localization of Endoscopic Findings | Activity Index | Severity Based on Activity Index | |
|---|---|---|---|---|
| UC (n=7) | Mayo score (1–3): Mild (n=1) Moderate (n=5) Severe (n=1) |
Pancolitis (n=5) Left-side colitis (n=2) |
PUCAI Mean: 47.14 Range: 15–75 |
Mild (n=2) Moderate (n=2) Severe (n=3) |
| CD (n=8) | SES-CD: Mean:10,2 Range: 5.6–34 |
L3 (n=6) L2 (n=1) L4 (n=1) Ileum (n=7) Right colon (n=3) Transverse colon (n=3) Left and sigmoid colon (n=3) Rectum (n= 1) |
PCDAI Mean: 29.37 Range: 17.5–52,5 |
Mild (n=5) Moderate (n=2) Severe (n=1) |
Notes: PUCAI: 10–34=mild, 35–64=moderate, >65=severe; PCDAI: 11–30=mild, 31–50=moderate, >51=severe. L2, involvement of the colon, L3, involvement of the terminal bowel and colon, L4, involvement of the upper GI and colon or terminal bowel.
Abbreviations: UC, patients with ulcerative colitis; CD, patients with Crohn’s Disease; SES-CD, Simplified endoscopic scoring for Crohn’s disease.
Circulating and Intestinal Tregs
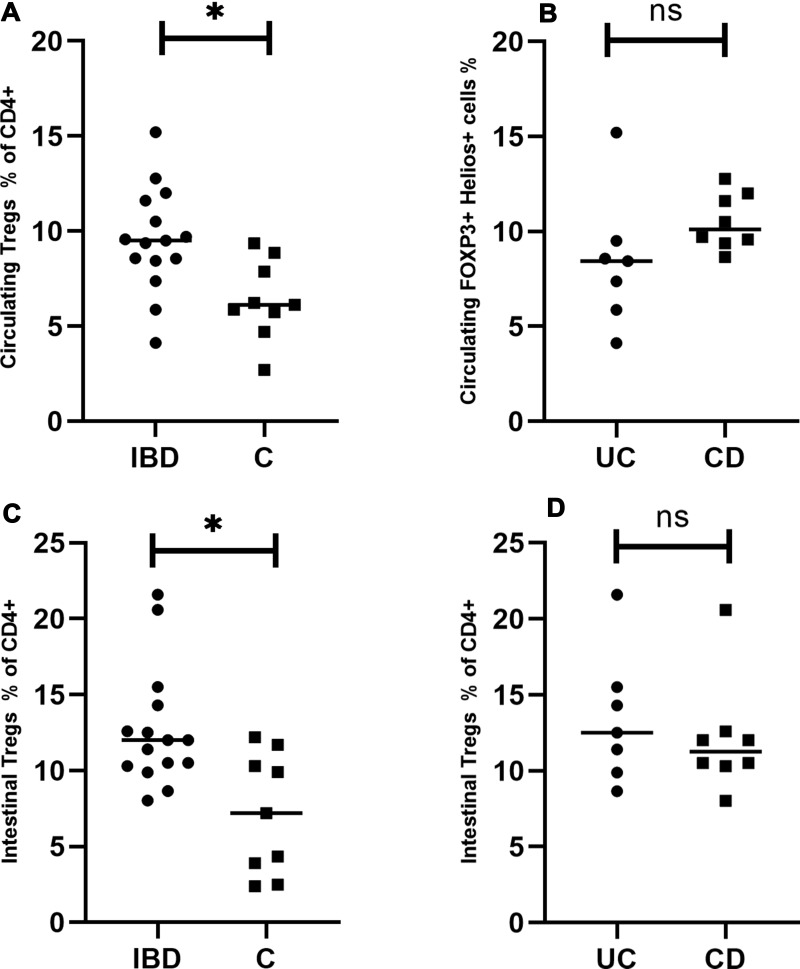
The rates of circulating and intestinal Tregs expressed as CD4+CD25high FOXP3+ cells were significantly higher in the studied (IBD) than in the control group (P=0.008; P=0.007, respectively) (Figure 2A and C). There was no significant difference between the rates of circulating and intestinal Tregs between the patients with CD and UC (P=0.14, P=0.52, respectively) (Figure 2B and D). The rate of intestinal T regulatory lymphocytes was significantly higher than circulating Tregs in patients with IBD, but not in the control group (P=0.01, P=0.35, respectively) (Figure 3A, left panel).
Figure 2.
Circulating and intestinal Tregs in patients with inflammatory bowel disease. (A) Comparison of circulating Tregs between the studied and the control group. (B) Comparison of circulating Tregs between UC and CD patients. (C) Comparison of intestinal Tregs between the studied and the control group. (D) Comparison of intestinal Tregs between UC and CD patients. *Statistically significant difference. Individual results are shown as dots (●)/squares (■). Horizontal lines indicate median values. Circulating and intestinal Tregs are expressed as a percentage of CD4+ lymphocytes.
Abbreviations: IBD, patients with inflammatory bowel disease; C, control group; UC, patients with ulcerative colitis; CD, patients with Crohn’s disease; ns, not significant.
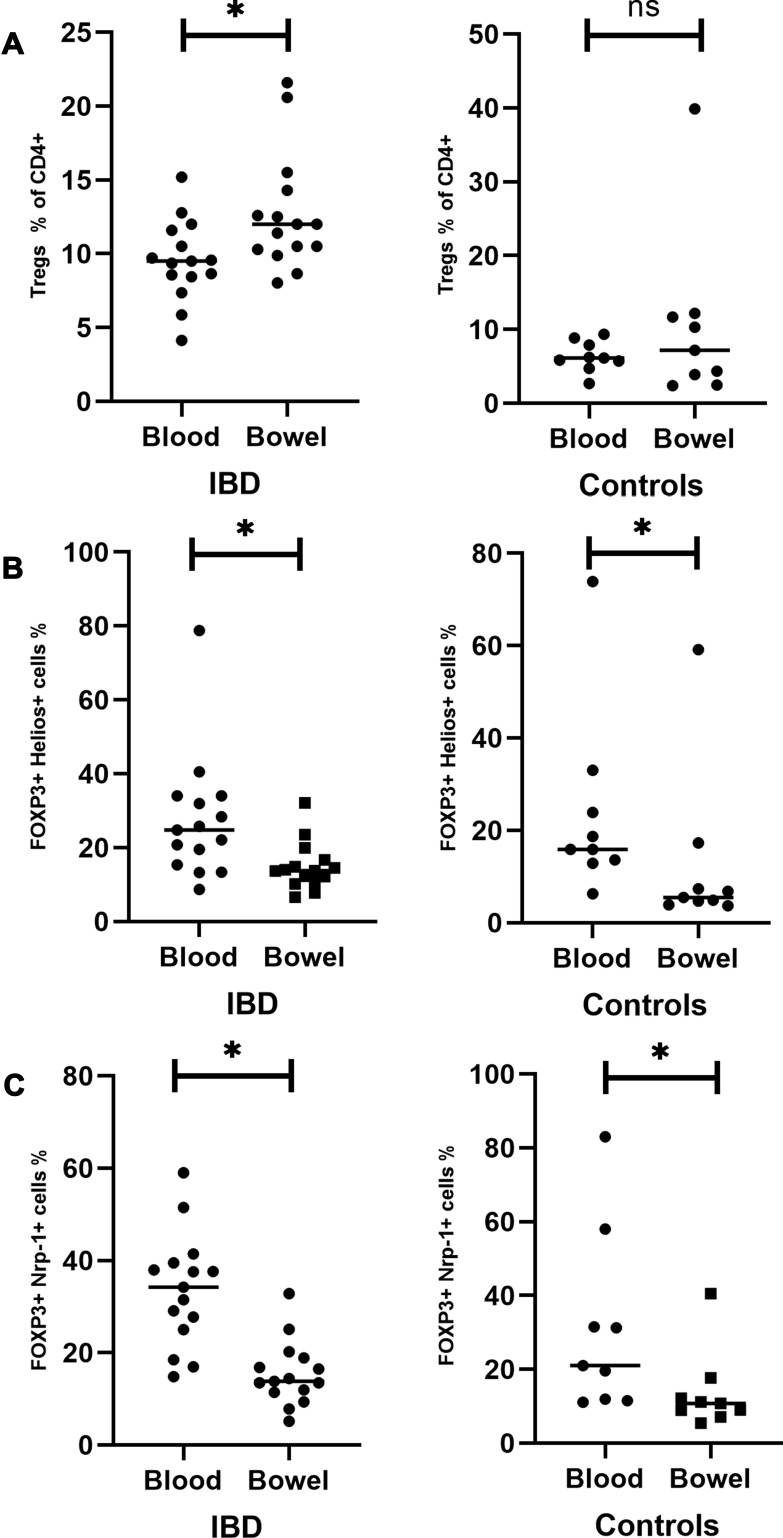
Figure 3.
Comparison of circulating and intestinal Tregs in the studied group (left panel) and the control group (right panel). (A) Circulating and intestinal Tregs expressed as a percentage of CD4+ lymphocytes in the IBD group (left side) and the controls (right side). (B) Circulating and intestinal Helios+ Tregs expressed as a percentage of all Tregs in the IBD group (left side) and the controls (right side). (C) Circulating and intestinal Nrp-1+ Tregs expressed as a percentage of all Tregs in the IBD group (left side) and the controls (right side). *Statistically significant difference. Individual results are shown as dots (●)/squares (■). Horizontal lines indicate median values.
Abbreviations: IBD, patients with inflammatory bowel disease; C, control group; ns, not significant.
Circulating and Intestinal Helios+ Tregs
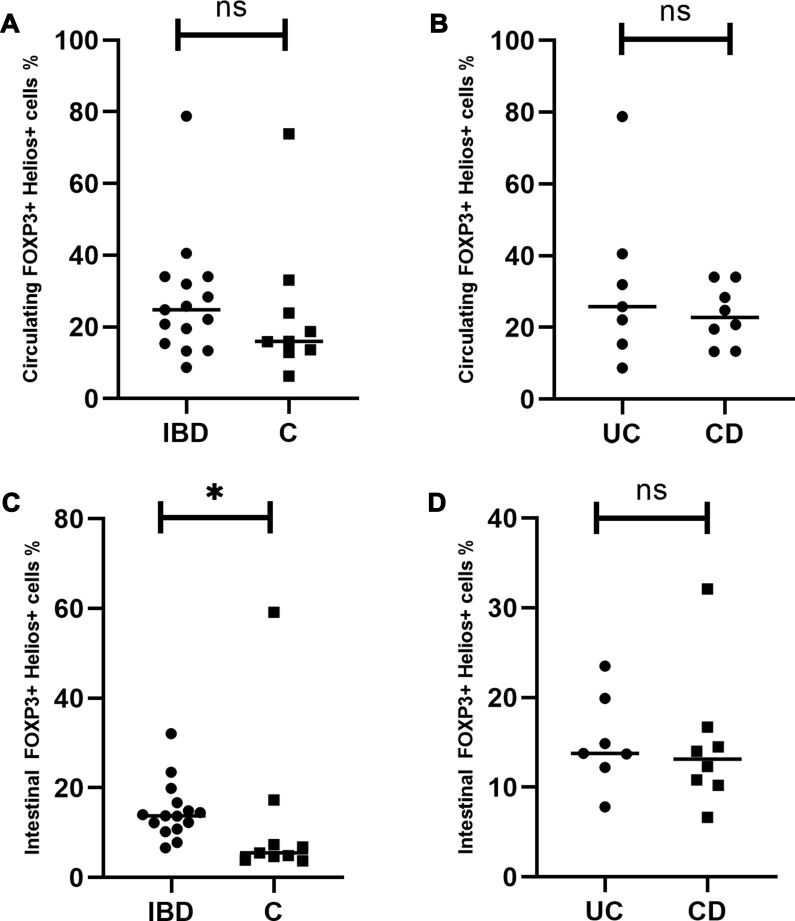
The median proportion of circulating Helios+ Treg cells amounted to 24.83% (range=8.7–78.77%) in IBD and 15.93% (range=6.32–73.89%) in the controls. The difference was not statistically significant (P=0.26) (Figure 4A). The median proportion of these cells in the intestinal mucosa of IBD patients was significantly higher than in the controls and amounted to 13.89% (range=6.65–32.11) (P=0.02) (Figure 4C). The median proportion noted for the control group was 5.49% (range=3.7–59.17). Patients with CD and UC did not differ significantly in terms of circulating and intestinal Helios+ Tregs percentages (P=0.61, P=0.69, respectively) (Figure 4B and D).
Figure 4.
Circulating and intestinal Helios+ Tregs in patients with inflammatory bowel disease. (A) Comparison of circulating Helios+Tregs between the studied and the control group. (B) Comparison of circulating Helios+ Tregs between UC and CD patients. (C) Comparison of intestinal Helios+ Tregs between the studied and the control group. (D) Comparison of intestinal Helios+ Tregs between UC and CD patients. *Statistically significant difference. Individual results are shown as dots (●)/squares (■). Horizontal lines indicate median values. Helios+ Tregs are expressed as the percentage of all Tregs.
Abbreviations: IBD, patients with inflammatory bowel disease; C, control group; UC, patients with ulcerative colitis; CD, patients with Crohn’s disease; ns, not significant.
The frequency of Helios+ Tregs was significantly higher in peripheral blood compared to intestinal mucosa both in the studied (IBD) and the control group (P=0.0001, P=0.0039,respectively) (Figure 3B).
Circulating and Intestinal Nrp-1+ Tregs
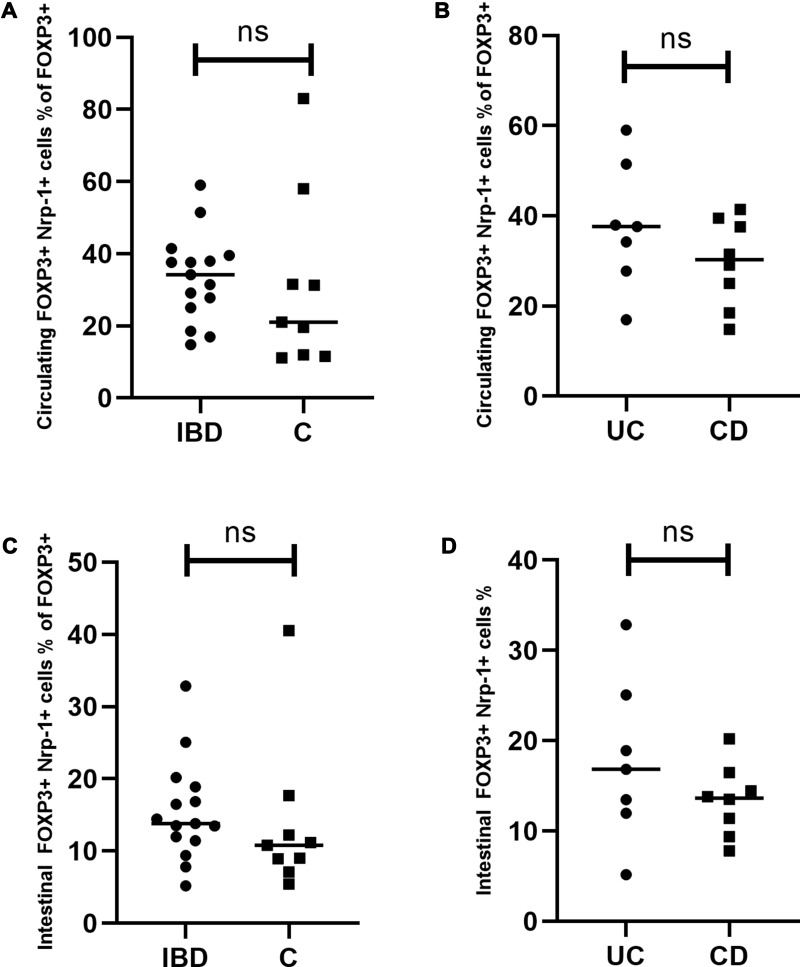
The median proportion of circulating Nrp-1 (CD304)+ Treg cells reached 34.23% (range=14.81–59.00%) in the IBD group and 21.01% (range=11.11–83.05%) in the controls (Figure 5A). The median rates of these cells in the intestinal mucosa were 13.8% (range=5.18–32.86%) in the studied group and 10.8% (range=5.43–40.52%) in the control group (Figure 5C). Thus, the rates of circulating and intestinal Nrp-1+ Tregs did not differ significantly in IBD patients compared to the control group (P=0.26, P=0.15, respectively) (Figure 5A and C).
Figure 5.
Circulating and intestinal Nrp-1+ Tregs in patients with inflammatory bowel disease. (A) Comparison of circulating Nrp-1+ Tregs between the studied and the control group. (B) Comparison of circulating Nrp-1+ Tregs between UC and CD patients. (C) Comparison of intestinal Nrp-1+ Tregs between the studied and the control group. (D) Comparison of intestinal Nrp-1+ Tregs between UC and CD patients. Individual results are shown as dots (●)/squares (■). Horizontal lines indicate median values. Neuropilin-1+ Tregs are expressed as the percentage of all Tregs.
Abbreviations: IBD, patients with inflammatory bowel disease; C, control group; UC, patients with ulcerative colitis; CD, patients with Crohn’s disease; ns, not significant.
Similarly, no statistically significant difference in the percentages of circulating and intestinal Nrp-1+ Tregs were noted between UC and CD patients (P=0.33, P=0.39, respectively) (Figure 5B and D). The frequency of Nrp-1+ Tregs was significantly higher in peripheral blood compared to intestinal mucosa in the studied and the control group (P=0.0001, P=0.0039) (Figure 3C)
The numbers of circulating and intestinal Tregs, Helios+ Tregs, and Nrp-1+ Tregs did not correlate with the clinical, histopathologic, and endoscopic activity of UC and CD.
Discussion
The results of this study confirm our previous observation, that the proportion of T regulatory cells in peripheral blood of patients with IBD is higher than in healthy controls.7 These results stay in opposition to some publications concerning IBD, which report decreased or unchanged rates of circulating Tregs compared to healthy controls.8,12,30 Contrary to the mentioned studies, we quantified these cells at the time of diagnosis, prior to the treatment, which provides better insight into the immunological background of the disease. Only one paper demonstrating increased proportions of circulating Tregs in children with IBD also concerned naïve patients, what supports our previous and present observations.31
Our investigation also revealed increased rates of intestinal Tregs in IBD. This finding stays in agreement with other studies, which demonstrated the accumulation of T regulatory cells in inflamed bowel mucosa of both adult and pediatric patients.11,32,33 It is worthwhile to mention here that these studies quantified mucosal Tregs using immunochemistry for visualization of FoxP3 positive cells, which were then calculated by the investigating pathologist.
One should, however, realize that inflamed mucosa is generally more infiltrated with many types on immune cells, and absolute number of FoxP3 cells does not reflect the real involvement of immune regulation. Only the use of flow cytometry showing the rates of Tregs among CD4+ T cells illustrates better the recruitment of immunoregulatory cells. To the best of our knowledge, our study was the first to quantify intestinal regulatory T cells based on cytometry in pediatric IBD. Still, in our opinion, obtaining similar results using different methods supports the notion that the finding of the increased rate of intestinal Tregs in IBD children is genuine.
The existing studies addressing this issue, which were based on cytometry, referred to adults, and the authors also presented similar results, showing increased rates of Tregs in IBD.21–23 Thus, cytometry can be regarded as a useful tool for investigating the composition of inflammatory infiltrates in inflamed tissue.
Another important observation of the presented study was the higher percentage of intestinal Tregs compared to circulating Tregs in the IBD group. This difference was not found in healthy controls, what may indicate the involvement of immune regulation in the site of inflammation in IBD. This increase is likely associated with the presence of induced (adaptive) regulatory cells (iTregs), which arise at the site of inflammation from naïve T lymphocytes.
All these mentioned findings do not seem to confirm the hypothesis of a quantitative deficiency of regulatory cells in pediatric patients with IBD, but they are not so strong to negate it. According to some authors, the expansion of Tregs is not numerically adequate for the situation. Maul et al13 showed that the number of intestinal FOXP3+ cells in IBD is lower than in acute diverticulitis, a condition expected to exhibit undisturbed immunoregulatory function. He has however based on absolute counts of Tregs seen upon immunochemistry. Thus, comparing the rates of intestinal Tregs in these two conditions could support the conception of inadequate immune regulatory response in IBD.
Looking at the increasing number of studies showing upregulation of intestinal Tregs, the question arises as to why they are not effective to suppress inflammation. The only possible answer is either that the function of these cells in IBD is decreased or inflammation escapes somehow from immune regulation. The issue has not been clarified yet, although an increasing number of publications demonstrate proper function of Tregs in IBD in vitro.16,17,34–36 It is, however, worth noting that the population of Tregs is also functionally heterogenous. The latest data revealed accumulation of Tregs coexpressing the Th17-related transcription factor RORγt in lamina propria in IBD patients.37 Detection of the subsets of cells exerting proinflammatory effects among T regulatory cells is a challenge for future research on immunoregulatory mechanisms in inflammatory bowel disease.
Our next discussion point concerns the expression of Helios and Neuropilin-1 (Nrp-1, CD304) in T regulatory cells. Initially we aimed to assess the proportion of thymic-natural and peripheral-induced Tregs in the blood and bowel of investigated patients, based on the above-mentioned markers, which are supposed to differentiate between these two subsets of regulatory T cells.36,38,39 Unexpectedly the expression of Helios- and Nrp-1 in peripheral blood of our patients appeared to be lower than presented in other publications, and surely not sufficient to distinguish between these two types of Tregs.18–20,22 However, it is worth noting, that some investigators did not confirm the importance of Nrp-1 and Helios to indicate natural Tregs.39–41 Moreover, although the rates of circulating Helios+ cells reported by other authors were higher than noted in our cohort, they do not seem to reflect the real frequencies of natural Tregs, considering that majority of T regulatory cells in blood originates from the thymus.42 We cannot compare the rates of Nrp-1 positivity in our cohort with other results, as to our best knowledge all the available studies concern animal models. Thus, our results confirm that Helios and Neuropilin-1 cannot serve as markers of thymus derived Tregs in children with IBD.
Finally, we also examined the relationship between the frequencies of circulating and intestinal FOXP3+ cells and the clinical, endoscopic, and histopathologic activity of IBD. The presence of positive correlations could be an argument negating the hypothesis on inadequate response of regulatory cells in IBD. The fact that we did not find any significant correlations might be associated with the small size of our sample; also, the clinical and endoscopic activity of UC and CD was determined using different scoring systems. Thus, the correlations with FOXP3+ rates were calculated separately for individuals with either condition, and therefore the analysis was likely underpowered.
Conclusions
The rates of circulating and intestinal T regulatory cells are increased in naïve pediatric patients with IBD.
The rate of intestinal Tregs is higher than circulating Tregs of patients with IBD.
Cytometry seems to be a valuable method assessing the composition of infiltrates in inflamed intestinal mucosal tissue.
Helios and Neuropilin-1 likely cannot serve as markers to differentiate between natural and adaptive Tregs in pediatric patients.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- 2.Han GM, O’Neil-Andersen NJ, Zurier RB, Lawrence DA. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell Immunol. 2008;253(1–2):92–101. doi: 10.1016/j.cellimm.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sznurkowska K, Boćkowska M, Zieliński M, et al. Peripheral regulatory T cells and anti-inflammatory cytokines in children with juvenile idiopathic arthritis. Acta Biochim Pol. 2018;65(1):119–123. doi: 10.18388/abp.2017_2308 [DOI] [PubMed] [Google Scholar]
- 4.Lin S-C, Chen K-H, Lin C-H, et al. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest. 2010;16(11):987–996. doi: 10.1111/j.1365-2362.2007.01882.x [DOI] [PubMed] [Google Scholar]
- 5.Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol. 2005;62(3):312–317. [DOI] [PubMed] [Google Scholar]
- 6.Yan B. Liu Y: the nature of increased circulating CD4CD25Foxp3 T cells in patients with systemic lupus erythematosus: a novel hypothesis. Open Rheumatol J. 2009;3:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sznurkowska K, Żawrocki A, Sznurkowski J, et al. Peripheral and Intestinal T-regulatory Cells are Upregulated in Children with Inflammatory Bowel Disease at Onset of Disease. Immunol Invest. 2016;45(8):787–796. [DOI] [PubMed] [Google Scholar]
- 8.Boschetti G, Nancey S, Sardi F, Roblin X, Flourie B, Kaiserlian D. Therapy with anti-TNFalpha antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):160–170. [DOI] [PubMed] [Google Scholar]
- 9.Guidi L, Felice C, Procoli A, et al. T regulatory cell modifications in inflammatory bowel disease patients treated with anti-TNFalpha agents. Biomed Res Int. 2013;286368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sznurkowska K, Żawrocki A, Sznurkowski J, et al. Indoleamine 2,3-dioxygenase and regulatory T cells in intestinal mucosa in children with inflammatory bowel disease. J Biol Regul Homeostat Agents. 2017;31(1):125–131. [PubMed] [Google Scholar]
- 11.Holmén N, Lundgren A, Lundin S, et al. Functional <sup>CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12(6):447–456. doi: 10.1097/00054725-200606000-00003 [DOI] [PubMed] [Google Scholar]
- 12.Di Sabatino A, Biancheri P, Piconese S, et al. Peripheral regulatory T cells and serum transforming growth factor-β: relationship with clinical response to infliximab in Crohnʼs disease. Inflamm Bowel Dis. 2010;16(11):1891–1897. doi: 10.1002/ibd.21271 [DOI] [PubMed] [Google Scholar]
- 13.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–257. doi: 10.1053/j.gastro.2005.03.043 [DOI] [PubMed] [Google Scholar]
- 14.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–257. doi: 10.1038/nri1032 [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Yamaguchi T, Nomura T, Ono OM. Regulatory T Cells and Immune Tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 16.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros Transcription Factor Family Member, Differentiates Thymic-Derived from Peripherally Induced Foxp3 + T Regulatory Cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav M, Louvet C, Davini D, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(10):1713–1722. doi: 10.1084/jem.20120822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golding A, Hasni S, Gabor Illei G, Shevach EM. Shevach The Percentage of FoxP3+Helios+ Treg Cells Correlates Positively With Disease Activity in Systemic Lupus Erythematosus Arthritis Rheum. Arthritis Rheum. 2013;65(11):2898–2906. doi: 10.1002/art.38119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Zhang L, Chen H, et al. Analysis of Regulatory T Cell Subsets and Their Expression of Helios and PD-1 in Patients with Hashimoto Thyroiditis. Int J Endocrinol. 2019;2019:5368473. doi: 10.1155/2019/5368473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Černý V, Hrdý J, Novotná O, et al. Distinct characteristics of Tregs of newborns of healthy and allergic mothers. PLoS One. 2018;13(11):e0207998. doi: 10.1371/journal.pone.0207998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X, He S, Lv C, et al. Analysis of murine and human Treg subsets in inflammatory bowel disease. Mol Med Rep. 2017;16(3):2893–2898. doi: 10.3892/mmr.2017.6912 [DOI] [PubMed] [Google Scholar]
- 22.Lord JD, Shows DM. Thiopurine use associated with reduced B and natural killer cells in inflammatory bowel disease. World J Gastroenterol. 2017;23(18):3240–3251. doi: 10.3748/wjg.v23.i18.3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord JD, Shows DM, Chen J, Thirlby RC, Blood H, Unutmaz D. Human Blood and Mucosal Regulatory T Cells Express Activation Markers and Inhibitory Receptors in Inflammatory Bowel Disease. PLoS One. 2015;10(8):e0136485. doi: 10.1371/journal.pone.0136485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otley AR, Loonen H, Parekh N, Corey M, Sherman PM, Griffith AM. Assessing activity of pediatric Crohn’s disease: which index to use? Gastroenterology. 1999;116(3):527–531. doi: 10.1016/S0016-5085(99)70173-3 [DOI] [PubMed] [Google Scholar]
- 25.Turner D, Otley AR, Mack D, et al. Development, Validation, and Evaluation of a Pediatric Ulcerative Colitis Activity Index: A Prospective Multicenter Study. Gastroenterology. 2007;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029 [DOI] [PubMed] [Google Scholar]
- 26.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505–512. doi: 10.1016/S0016-5107(04)01878-4 [DOI] [PubMed] [Google Scholar]
- 27.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. New England Journal of Medicine. 1987;317(26):1625–1629. doi: 10.1056/NEJM198712243172603 [DOI] [PubMed] [Google Scholar]
- 28.Geboes K, Riddell R, Ӧst A, Jensfelt B, Persson T, Lӧfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geboes K, Dalle I. Influence of treatment on morphological features of mucosal inflammation. Gut. 2002;50(Suppl Supplement 3):III47. doi: 10.1136/gut.50.suppl_3.iii37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Liu XP, Zhao ZB, Chen JH, Yu CG. Expression of CD4+ forkhead box P3 (FOXP3)+ regulatory T cells in inflammatory bowel disease. Journal of Digestive Diseases. 2011;12(4):286–294. doi: 10.1111/j.1751-2980.2011.00505.x [DOI] [PubMed] [Google Scholar]
- 31.Vitale A, Strisciuglio C, Vitale S, et al. Increased frequency of regulatory T cells in pediatric inflammatory bowel disease at diagnosis: a compensative role? Pediatr Res. 2020;87(5):853–861. doi: 10.1038/s41390-019-0662-7 [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Arijs I, De Hertogh G, et al. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab†. Inflamm Bowel Dis. 2010;16(8):1299–1310. doi: 10.1002/ibd.21229 [DOI] [PubMed] [Google Scholar]
- 33.Cho J, Kim S, Yang DH, et al. Mucosal Immunity Related to Regulatory T Cells, Th17 Cells and Cytokines in Pediatric Inflammatory Bowel Disease. J Korean Med Sci. 2018;33(52):e336. doi: 10.3346/jkms.2018.33.e336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saruta M, Yu QT, Fleshner PR, et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125(3):281–290. doi: 10.1016/j.clim.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 35.Eastaff-Leung N, Mabarrack N, Barbour A, et al. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30(1):80–89. doi: 10.1007/s10875-009-9345-1 [DOI] [PubMed] [Google Scholar]
- 36.Yadav M, Stephan S, Bluestone JA. Peripherally Induced Tregs – role in Immune Homeostasis and Autoimmunity. Front Immunol. 2013;4:232. doi: 10.3389/fimmu.2013.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo A, Di Giovangiulio M, Stolfi C, et al. RORγt-Expressing Tregs Drive the Growth of Colitis-Associated Colorectal Cancer by Controlling IL6 in Dendritic Cells. Cancer Immunol Res. 2018;6(9):1082–1092. doi: 10.1158/2326-6066.CIR-17-0698 [DOI] [PubMed] [Google Scholar]
- 38.Mayne CG, Williams CB. Induced and Natural Regulatory T Cells in the Development of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2013;19(8):1772–1788. doi: 10.1097/MIB.0b013e318281f5a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szurek E, Cebula A, Wojciech L, et al. Differences in Expression Level of Helios and Neuropilin-1 Do Not Distinguish Thymus-Derived from Extrathymically-Induced CD4+Foxp3+ Regulatory T Cells. PLoS One. 2015;10(10):e0141161. doi: 10.1371/journal.pone.0141161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milpied P, Renand A, Bruneau J, et al. Neuropilin‐1 is not a marker of human Treg. Eur J Immunol. 2009;39(6):1466–1471. doi: 10.1002/eji.200839040 [DOI] [PubMed] [Google Scholar]
- 41.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–257. [DOI] [PubMed] [Google Scholar]
- 42.Mayne CG, Williams CB. Induced and Natural Regulatory T Cells in the Development of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2013;19(8):1772–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]