Abstract
Background:
Jiedu Sangen Decoction (JSD), a traditional Chinese medicine formula, has been widely applied in the treatment of gastrointestinal cancer, especially in colorectal cancer. Our study mainly aimed to assess the combined efficacy of Jiedu Sangen aqueous extract (JSAE) and a PD-L1 inhibitor (PI) in colon cancer cells migration and invasion, along with epithelial-mesenchymal transition, and then provide deep insights into the potential mechanism.
Methods:
We explored the inhibitory effects on invasion and metastasis and the reverse effect on EMT process in CT-26 colon cancer cell via Transwell migration assay, Matrigel invasion assay and confocal laser scanning microscopy. Furthermore, regulation in expression of EMT-related proteins and molecular biomarkers and underlying signal pathway proteins were detected through Western blotting and IHC.
Results:
The combination of JSD and PD-L1 inhibitor could inhibit migration, invasive ability and EMT of CT-26 cells in a concentration-dependent manner. Meanwhile, JSD combined with PD-L1 inhibitor could also remarkably reverse EMT and metastasis in vivo. In addition, the protein expression of N-cadherin, Slug, Snail, Vimentin was down-regulated along with E-cadherin s up-regulation with the combination of JSD and PD-L1 inhibitor, while that of PI3K/AKT was notably down-regulated.
Conclusions:
These findings indicated that JSAE and a PD-L1 inhibitor could drastically inhibit the migration and invasion of colorectal cancer by reversing EMT through the PI3K/AKT signaling pathway.
Keywords: Jiedu Sangen decoction, colorectal cancer, epithelial-mesenchymal transition, metastasis, PI3K/AKT
Background
Colorectal cancer (CRC) is the world’s third deadliest cancer and kills about 700,000 people every year.1 The metastasis of colorectal cancer has been supposed to mainly lead to treatment failure.2 Now it is believed that CRC metastasis is closely related to epithelial-mesenchymal transition (EMT).3,4 EMT is a biological process in which epithelial cells lose their polarity and are converted into a mesenchymal phenotype. Cancer cells with EMT will lose their polarity and increase their motility, which facilitates their invasion and metastasis.5 Notably, the PI3K/AKT signaling pathway plays a key role in the EMT process,6,7 and suppression of EMT contributes to inhibition of cancer metastasis.8,9
Programmed cell death 1 ligand 1 (PD-L1) is the ligand of programmed cell death protein 1 (PD-1), which is known as an immune-inhibitory receptor mainly expressed by activated T cells, B cells, and myeloid cells. The interaction between PD-1 and PD-L1 activates a critical immune checkpoint leading to T cell dysfunction, exhaustion, and tolerance.10 Thus, PD-L1 is highly related to cancer progression and invasion.11,12 Numerous studies have revealed that PD-L1, as expressed by tumor cells, can promote EMT13–17; one study reported that PD-L1 may induce EMT via the PI3K/AKT pathway.17
Jiedu Sangen Decoction (JSD) is a Chinese herbal formulation exploited by us as a supplementary treatment of CRC,18,19 comprising 3 Chinese traditional herbs: Teng Li Gen (Radix Actinidiae chinensis), Shui Yang Mei Gen (Root of Thinleaf Adina) and Hu Zhang Gen (Polygoni Cuspidati Radix). The known constituents of this concoction, including ursolic Acid, 12-en-28-oic acids of oleanane-type (from Radix Actinidiae chinensis) and emodin (from Polygoni Cuspidati Radix), have been proved to have antitumor potential via the PI3K/AKT signaling pathway.20–23 In previous research, we found that JSD could upregulate PD-L1 expression in the SW480 cell line by using Western blot assay. And several studies had suggested that more benefit from PD-L1 inhibitory agents comes along with the high level of PD-L1 expression.24,25 Thus, from the above points, we hypothesized that JSD plus PD-L1 inhibitor may take effect synergistically in suppressing EMT and migration and invasion of CRC cells. In this report, we demonstrated that, in vivo and in vitro, the combination of JSD and PD-L1 inhibitor inhibited and reversed the EMT and repressed the migration and invasion of CT-26 murine colon cancer cells via inhibiting the PI3K/AKT pathway.
Methods
Preparation of JSAE and PD-L1 Inhibitor
The 3 herbs of JSD formula were sourced from The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China. Two hundred grams of Teng Li Gen (Radix Actinidiae chinensis), Shui Yang Mei Gen (Root of Thinleaf Adina), and Hu Zhang Gen (Polygoni Cuspidati Radix) was pulverized to rough powder, followed by decoction with 8 and 6 times volume of distilled water for 2 hour, respectively. The aqueous extracts were merged and vacuum evaporated to 2g/ml as Jiedu Sangen aqueous extract (JSAE) and stored at 4°C for further use. The PD-L1 inhibitor was purchased from Selleck (S7911, Shanghai, China).
Cell Culture
The murine colon cancer cell line CT-26 (Catalog number: TCM37) was purchased from the cell resource center of Shanghai Institutes for Biological Sciences (Shanghai, China) and was cultured in RPMI Medium 1640 (GIBCO, US) supplemented with 10% fetal bovine serum (GIBCO, US), 100 U/ml penicillin and 100 mg/ml streptomycin, incubated at 37°C in a humidified atmosphere with 5% CO2. The cells were subcultured using Trypsin-EDTA digestion and divided into 5 groups: Control, EGF, JSAE, PD-L1 inhibitor (PI) and JSAE plus PD-L1 inhibitor (JP). Cells were treated with 50 ng/ml EGF for 48 hours for EMT induction to establish the CT-26 cell EMT model,26,27 except for the Control group. Subsequently, the cells in groups of Control and EMT were cultured with normal RPMI Medium 1640, while JSAE, PI and JP groups were intervened with 6 mg/ml JSAE, 10 μg/ml PD-L1 inhibitor (dissolved in PBS) and 6 mg/ml JSAE plus 10 μg/ml PD-L1 inhibitor for 48h, respectively.
Animals
After completing all the experiments in vitro, we continued to culture the murine colon cancer cell line CT-26 and performed remaining experiments in vivo. All animal experiments were approved by Zhejiang Chinese Medicine University Laboratory Animal Research Center with the approval number of SYXK (Zhejiang) 2013-0184, and conducted according to the Animal Research Act, 1985 (New South Wales, Australia) and the Australian Code of Practice for Care and Use of Animals for Scientific Purposes (2013). 52 healthy male specific pathogen free BALB/c mice at weight of 120-200g were obtained from the experimental animal center of Zhejiang Chinese Medical University and maintained for one-week environment accustomization. To establish the mouse model of hepatic metastatic CRC, 50 μl CT-26 cells in a concentration of 2 × 106/mL were intrasplenically inoculated. The 52 BALB/c mice were randomly allocated to 4 groups after 1 week from the inoculation: JSAE group, normal saline group, PI group, and JP group. The mice in JSAE and JP group were intragastrically administrated with 0.4 ml/20g JSAE of 1.2 g/ml for 14 days. The mice in PI and JP group were treated with intraperitoneal injection of 100 μg PD-L1 inhibitor (dissolved in normal saline) for fourteen days. The mice in NS group received oral treatment of 0.4 ml/20 g normal saline for 14 days. All mice were sacrificed by cervical dislocation with intraperitoneal anesthesia of 0.1 ml 1% Pentobarbital Sodium in the fifteenth day from the initiation of treatment. The liver tissues, after removal from the body of mice, were split into 2 parts, then fixed in 4% paraformaldehyde for immunohistochemistry and stored in −80°C for further research. After animal experiments done, all mice were sacrificed by cervical dislocation with intraperitoneal anesthesia of 0.1 ml 1% Pentobarbital Sodium.
Immunohistochemistry (IHC)
The liver tissues excised from the mice were fixed in 4% paraformaldehyde buffer for 24 h and then embedded in paraffin. Subsequently, the paraffin sections were stained with E-cadherin, N-cadherin antibody (1:100, Abcam, UK) for immunohistochemistry to detect the expression in the liver of hepatic metastatic colon cancer mice.
Transwell Migration Assay
3×105 CT-26 cells per well were seeded in the upper chamber of a polycarbonate transwell filter (8 µm, 24well; Corning, USA) with 200 μL of serum-free medium, while 500 μL of fetal bovine serum (FBS) was added to the lower chamber as a chemoattractant simultaneously. After 24 hours incubation at 37°C, the cells remaining at the upper side of the well membrane were erased with cotton swabs. Then, the migrated cells on the lower surface were fixed in 4% paraformaldehyde, stained with 0.1% crystal violet solution, and counted for 9 random×400 fields per well. These experiments were repeated for 3 times.
Matrigel Invasion Assay
40 μL of 1:8 RPMI Medium 1640-diluted Matrigel (Corning, USA) was added to each well. Then 7×105 CT-26 cells per well were plated onto the Matrigel surface. The following process was carried out as Transwell migration assay. The experiments were independently repeated thrice.
Confocal Laser Scanning Microscopy
Cell slide was fixed in 4% paraformaldehyde for 30 minutes and incubated with diluted primary antibodies (E-cadherin 1:100, N-cadherin 1:100, F-cadherin 1:100) overnight at 4°C, and followed by secondary antibody incubation (1:400) at room-temperature in darkness for 50 minutes. Then, the cells were further incubated in DAPI staining solution at atmospheric temperature for 10 minutes in dark environment, and observed at magnification×400 under the confocal microscope and photographed. These experiments were repeated 3 times.
Western Blotting
The protein variation in CT-26 cells and liver tissues from the sacrificed mice were determined by Western blotting. Briefly, the protein concentration was determined using the BCA assay kit. Equal quantities of proteins (30 μg) were electrophoresed by 10% sodium dodecyl sulfate-polyacrylamide (SDS–PAGE) and transferred onto polyvinylidene difluoride membranes. Then, membranes were blocked in 5% non-fat milk in TBS buffer containing 0.1% tween (TBST) and followed by incubated with the primary antibody (1:1000, Abcam, UK) at 4°C overnight. After additional TBST washes, membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, USA) at 1:5000 dilutions for 2 hours at room temperature and visualized by adding ECL detection reagent (Bio-Rad, USA). These experiments were repeated thrice.
Statistical Analysis
All values are presented as the mean ± SD. The groups were compared using ANOVA with SPSS (version 22.0; SPSS, Inc., USA). P < .05 was considered to be statistically significant difference.
Results
JSD Combined with PD-L1 Inhibitor Reverse EMT in vitro
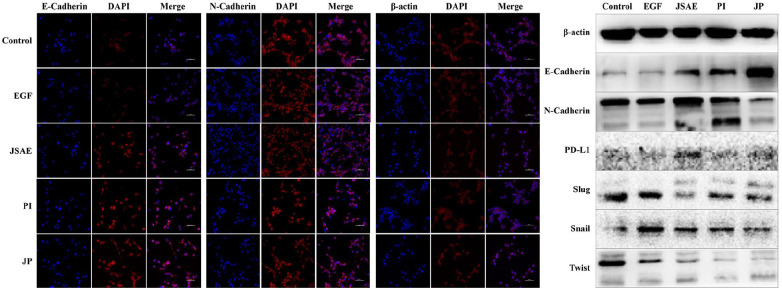
EMT plays a pivotal role involved in the metastasis of CRC,28–30 mainly featured by the decrease of E-cadherin accompanied by elevated N-cadherin and β-actin.31,32 Using confocal laser scanning microscopy (CLSM), we found that E-cadherin expression increased in JSAE, PI and JP group, but N-cadherin expression in PI and JP group decreased, when compared with the control and EGF group. Meanwhile, β-actin expression was decreased in JP and JSAE group, compared with control and EGF group (Figure 1a). In addition, Western blotting assay demonstrated that E-cadherin expression significantly increased while N-cadherin, Snail, Slug, Twist were attenuated in JP group, compared with control and EGF group (Figure 1b). The alteration in EMT-related molecules hints that the joint use of JSD and PD-L1 inhibitor might reverse the EMT process in CT-26 cells.
Figure 1.
JSD combined with PD-L1 inhibitor reverse EMT in vitro. (a) The expression of E-cadherin, N-cadherin and β-actin were measured by CLSM. (b) The expression of EMT-related proteins in CT-26 cell were measured by Western blotting assay.
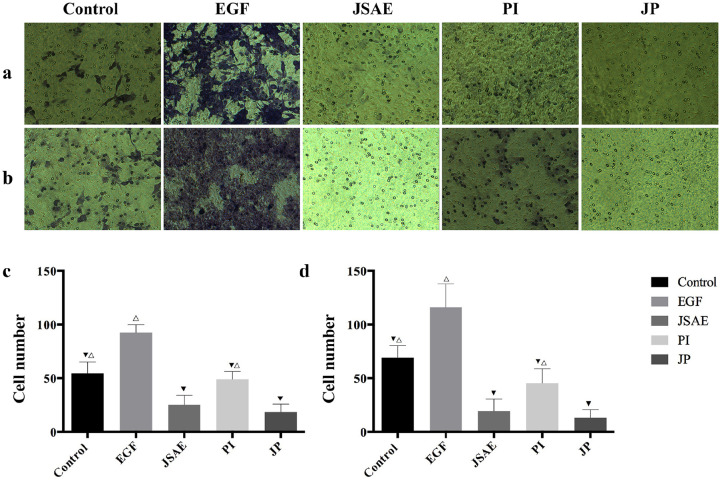
JSD Combined with PD-L1 Inhibitor Inhibits Migratory and Invasive Ability of CT-26 Cells
To further examine the effect of united application of JSD and PD-L1 inhibitor on migration ability of colon cancer cells, we used Transwell Assay to monitor the changes in CT-26 cells migration behavior. As can be seen from the figure, EGF group has the best migration ability among other groups, especially when compared to control group, which indicated that the CT-26 cell EMT model was successfully established (Figure 2c and d). Furthermore, the results demonstrated that JP group significantly decreased the migration ability of CT-26 cells when compared with control, EGF and PI group (Figure 2a and c). In the invasion experiment, the Matrigel Assay displayed significant reduction of CT-26 cells invasive ability in JP group compared with control, EGF, JSAE and PI group (Figure 2b and d). These data indicate that the combination of JSD and PD-L1 inhibitor dampens the migratory and invasive ability of CT-26 cells.
Figure 2.
JSD combined with PD-L1 inhibitor inhibits migratory and invasive ability of CT-26 cells. (a, c) The migration ability of CT-26 cells in relevant groups was detected by Transwell Assay. (b, d) The invasive ability of CT-26 cells in relevant groups was detected by Matrigel assay. Compared to EGF group, ▼P < .05; Compared to JP group, △P < .05.
JSD Combined with PD-L1 Inhibitor is Correlated with Improved Overall Survival (OS) of Mice with Hepatic Metastatic Colorectal Cancer
We used CT-26 cells for the hepatic metastatic colorectal cancer mice models. To evaluate the overall survival of each group, a Kaplan-Meier survival curve (Figure 3) was generated, which showed that JSD plus PD-L1 inhibitor significantly prolonged mice survival compared with other groups. Concretely, the OS appeared as NS < PI < JSAE < JP.
Figure 3.
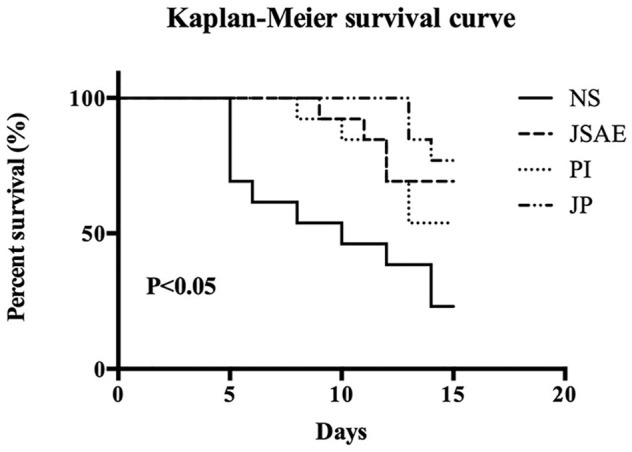
Survival curves of mice (12 mice per group) with metastatic tumor formed by CT-26 cells in relevant groups.
JSD Combined with PD-L1 Inhibitor Inhibits EMT and Metastasis in vivo
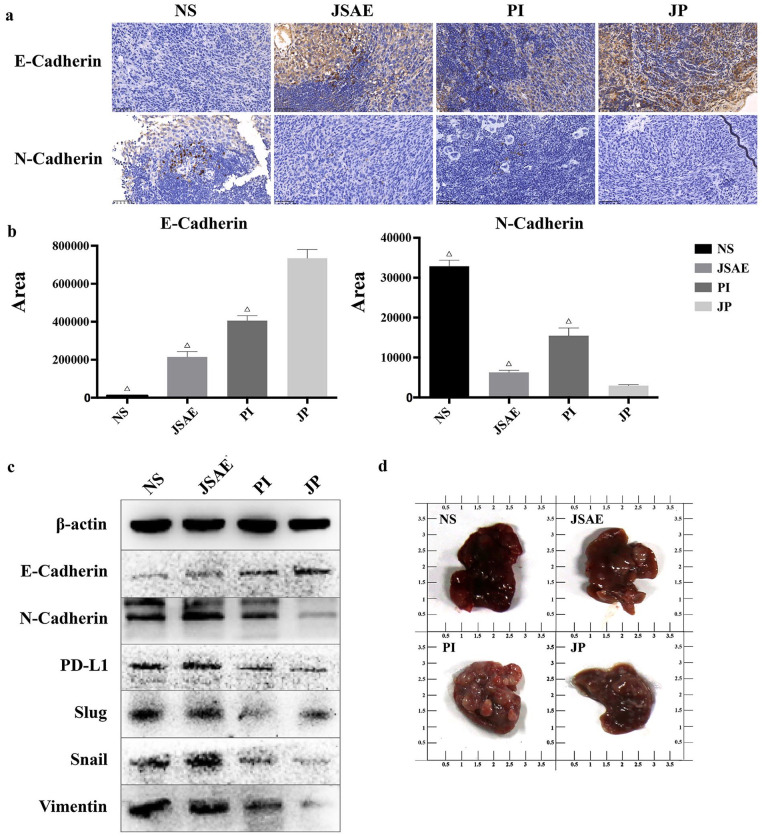
Having conducted the survival analysis, we tried to determine if the EMT level is associated with the survival data, and to test the inhibitory effect of JSD plus PD-L1 inhibitor on EMT and metastasis in vivo. Using IHC to detect the biomarkers of E-cadherin and N-cadherin in livers from mice model, we found that the joint use of JSAE and PD-L1 inhibitor upregulated the expression of E-cadherin but reduced the N-cadherin expression, compared with the NS, JSAE and PD-L1 groups (Figure 4a and b). We also used Western blotting assay to examine the relevant protein levels of EMT, and the results were presented as elevated expression of E-cadherin in the JP group compared with the NS and JSAE groups, and reciprocally significant reduction of the mesenchymal markers expression (N-cadherin and Vimentin). Meanwhile, the EMT transcriptional regulators (Snail and Slug) were correspondingly downregulated in the JP group compared with the NS group (Figure 4c). And notably, the PD-L1 expression was upregulated in the JSAE group compared with the NS, PI and JP groups, which is consistent with our previous study outcomes. Moreover, we found that the liver size of the JP group is smaller than that of the NS group, indicating that the combination of JSD and PD-L1 inhibitor may exert more effect on suppressing hepatic metastases of colorectal cancer (Figure 4d). Taken together, these results suggest that the joint use of JSD and a PD-L1 inhibitor have the potential of suppressing EMT and distant liver metastasis in mice.
Figure 4.
JSD combined with PD-L1 inhibitor inhibits EMT and metastasis in vivo. (a, b) The expression of E-cadherin and N-cadherin in mouse liver tissues was measured by IHC. (c) The expression of EMT-related proteins in mouse liver tissues was measured by Western blotting assay. (d) The difference of mouse liver size in relevant groups. Compared to EGF group, ▼P < .05; Compared to JP group, △P < .05.
JSD Combined with PD-L1 Inhibitor may Inhibit EMT via PI3K/AKT Signaling Pathway
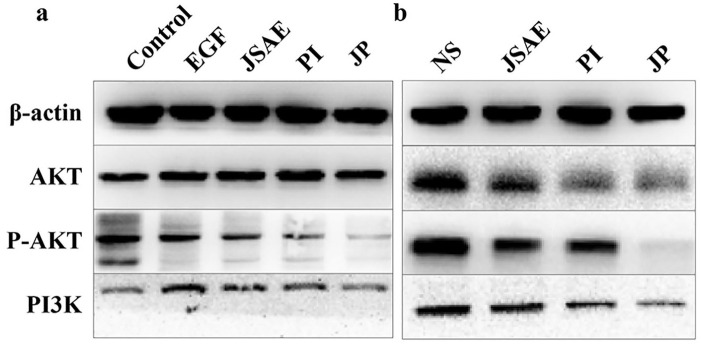
Since the PI3K/AKT pathway is essential in regulating EMT,8,9 Western blotting was performed to determine whether the PI3K/AKT pathway was involved in the inhibitory effect of the combination of JSD and PD-L1 inhibitor on EMT. The results, in vitro, demonstrated that PI3K expression was downregulated significantly in JP group compared with EGF group. Although no significant difference in AKT was observed between the JP and EGF groups, the expression of P-AKT was significantly decreased in the JP group (Figure 5a). As for liver tissues, the expression of PI3K, AKT and P-AKT was significantly decreased in the JP group compared with the NS and JSAE groups (Figure 5b). Our results indicated that the combination of JSD and PD-L1 inhibitor may inhibit EMT via the PI3K/AKT signaling pathway.
Figure 5.
JSD combined with PD-L1 inhibitor may inhibit EMT via PI3K/AKT signaling pathway. (a) The discrepant expression of proteins related to PI3K/AKT signaling pathway in CT-26 cells. (b) The discrepant expression of proteins related to PI3K/AKT signaling pathway in mouse liver tissues.
Discussion
CRC is one of the most frequent types of cancer worldwide.1 Distant metastasis, mainly liver and lung, is the main cause of death for patients with colorectal cancer.33 Unfortunately, the mechanisms underlying metastasis remain elusive. Tumor metastasis comprises sequential, interlinked, and selective steps,34 many of which are favored by conversions between the epithelial and mesenchymal phenotypes, namely, the epithelial-mesenchymal transition. Several studies have reported that EMT is of key importance in infiltration and metastasis of cancer.35,36 Hallmarks of EMT include the loss of expression or function of E-cadherin, known as one of the cell adhesion molecules, and reduced tight junction proteins (zona occludens 1 and occludin), along with the concomitant increase of mesenchymal markers, such as Vimentin and N-cadherin.36 Among these, multiple invasive and metastatic cancers have been relevant to high level of E-cadherin and low level of N-cadherin, E-cadherin is essential to keep the epithelial phenotype and modulate the balance of the microenvironment through numerous signal pathways,37,38 including Akt/mTOR,39 Wnt/β-catenin pathways. N-cadherin acts as an indicator of EMT and its expression has been related to the promotion of angiogenesis and vascular homeostasis by stabilizing micro-vessels,40 and thus suggesting that the loss of E-cadherin and the growthe of N-cadherin have been widely recognized to be EMT-activation molecules. We thus recommend E-cadherin and N-cadherin as key biomarkers to evaluate EMT progress.
Additionally, the EMT process is mainly initiated by 3 core groups of transcriptional regulators recognized as Snail zinc-finger family (SNAI1 and SNAIL2 (also known as SLUG))41–43 zinc-finger E-box-binding homeobox family proteins (ZEB1and ZEB2 (SIP1))44,45 and basic helix–loop–helix family (TWIST1, TWIST2),46,47 which can suppress E-cadherin transcription directly or indirectly. For instance, Snail1 and Snail2 are able to repress E-cadherin transcription by binding to the promoter of CDH1, which encodes E-cadherin.41,42 Expression of Slug and ZEB1 is highly correlated with decreased E-cadherin.48,49 Further, it was reported that Twist cooperates with BMI1 to repress E-cadherin and the cell cycle inhibitor p16INK4α.50 Generally, the cells gaining the EMT phenotype lose their junctions and apical–basal polarity, reorganize their cytoskeleton and reprogram gene expression, which increases their motility and invasiveness.35,51 On the other hand, numerous studies have shown that PD-L1, an important negative immune regulation molecule, plays a vital role in regulating the biological functions of cancer cells, increasing their malignancy and aggressiveness and conferring on them the EMT phenotype.16,52–54 Therefore, anti-PD-L1 therapy would be very promising for the treatment of human carcinomas.55,56
In this study, we found that the joint use of JSD and PD-L1 inhibitor leads to lower potential of migration and invasion of CT-26 cell lines by Transwell and Matrigel Assay. Further research by means of Confocal Laser Scanning Microscopy displayed enhanced E-cadherin expression but oppositely reduced N-cadherin and β-actin expression in EMT cell model treated with JSAE combined with PD-L1 inhibitor. The EMT pathway-related proteins test demonstrated that, while treated with JSD combined with PD-L1 inhibitor, E-cadherin were significantly upregulated, yet positive EMT markers such as N-cadherin, Snail, Slug and Twist in CT-26 cells, and N-cadherin, Vimentin, Snail and Slug in mouse liver tissues were dampened, respectively. These results were then corroborated by immunohistochemistry exhibiting increased E-cadherin and decreased N-cadherin expression in the JP group, compared with the NS and EGF groups. It is worth noting that Western blotting assay indicated that JSD may upregulate PD-L1 expression, which remain consistent with our previous results. Jointly considered with literature search, we assume that JSD increases the expression level of PD-L1 on the tumor cell surface, which makes the PD-L1 inhibitors play a positive role in targeting PD-L1 in order to improve the anti-tumor effects. Subsequently, it is important for us to develop specific mechanisms of synergetic effects between Chinese medicinal formulae and PD-L1 inhibitors, and we need further research to understand better how this combination works.
Up to now, EMT has been considered as a complex process orchestrated by several signaling pathways, in which the PI3K/AKT pathway counts a great deal.8,28,52,57 And interestingly, a recent study carried out by Fei et al.17 reported that PD-L1 is able to induce EMT in nasopharyngeal carcinoma cells through the PI3K/AKT pathway, which result is echoed by our study in the significant reduction of PI3K and P-AKT expression in CT-26 cells, and PI3K, AKT and P-AKT expression in mouse liver tissues when treated with a PD-L1 inhibitor or JSD plus a PD-L1 inhibitor (Figure 5).
Conclusion
Our results have given evidence of the inhibitory effect of the combination of JSD and PD-L1 inhibitor on migration and invasion of CT-26 murine colon cancer cells in vitro and in vivo by inhibiting and reversing EMT, and implied that this regulatory activity may function via the PI3K/AKT signaling pathway.
Footnotes
Authors’ contributions: FYS and LTS conceived and designed the experiments; FYS, LTS, QYY and GF performed the experiments; LYZ and YZ analyzed the data; FYS, LTS and QYY contributed analysis tools; GF, LYZ YZ and KBG prepared figures and tables; MHS reviewed drafts of the paper; SMR approved the final draft. All authors have read and approved the manuscript.
Availability of data and materials: The datasets generated and analyzed during the current study are not publicly available due to continuing the research, but are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by National Natural Science Foundation of China (Minhe Shen, no.81573902); Natural Science Foundation of Zhejiang Province (Shanming Ruan, no.LY17H270007); General Research Program for Education of Zhejiang Provincial (Leitao Sun, No. Y202045212); China Postdoctoral Science Foundation (Shanming Ruan, no.2017M612040/ 2018T110610); Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents (Shanming Ruan, no.2015-43); Program for the Cultivation of Youth talents in China Association of Chinese Medicine (Shanming Ruan, no.QNRC2-C08); Zhejiang Provincial Program for the Cultivation of the Young and Middle-Aged Academic Leaders in Colleges and Universities (Shanming Ruan, no.2017-248); The seventh batch of provincial famous traditional Chinese medicine studios in Zhejiang Province (Minhe Shen); Cultivation Program for Innovative Talent Graduate Students (Leyin Zhang; President office of Zhejiang Chinese Medical University[2020]no. 68). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics approval and consent to participate: All animal experiments were approved by Zhejiang Chinese Medicine University Laboratory Animal Research Center with the approval number of SYXK (Zhejiang) 2013-0184, and performed in line with U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU.
ORCID iDs: Leitao Sun  https://orcid.org/0000-0002-1441-3899
https://orcid.org/0000-0002-1441-3899
Kaibo Guo  https://orcid.org/0000-0002-6348-9785
https://orcid.org/0000-0002-6348-9785
Shanming Ruan  https://orcid.org/0000-0003-1061-5255
https://orcid.org/0000-0003-1061-5255
Reference
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490-1502. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Lin Z, Chen B, et al. Ezrin/NF-kB activation regulates epithelial-mesenchymal transition induced by EGF and promotes metastasis of colorectal cancer. Biomed Pharmacother. 2017;92:140-148. [DOI] [PubMed] [Google Scholar]
- 4. Xu K, Tao W, Su Z. Propofol prevents IL-13-induced epithelial-mesenchymal transition in human colorectal cancer cells. Cell Biol Int. 2018;42(8):985-993. [DOI] [PubMed] [Google Scholar]
- 5. Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21-45. [DOI] [PubMed] [Google Scholar]
- 6. Suman S, Kurisetty V, Das TP, et al. Activation of AKT signaling promotes epithelial-mesenchymal transition and tumor growth in colorectal cancer cells. Mol Carcinog. 2014;53(Suppl 1):E151-E160. [DOI] [PubMed] [Google Scholar]
- 7. Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19(11):1438-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen HN, Yuan K, Xie N, et al. PDLIM1 stabilizes the E-cadherin/beta-catenin complex to prevent epithelial-mesenchymal transition and metastatic potential of colorectal cancer cells. Cancer Res. 2016;76(5):1122-1134. [DOI] [PubMed] [Google Scholar]
- 9. Liu X, Ji Q, Deng W, et al. JianPi JieDu recipe inhibits epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta/smad mediated snail/E-cadherin expression. Biomed Res Int. 2017;2017:2613198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Z, Dong P, Ren M, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016;7(7): 784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen K, Cheng G, Zhang F, et al. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget. 2016;7(21):30772-30780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Xiong Y, Li J, et al. PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal cancer. Cell Physiol Biochem. 2017;42(6):2267-2280. [DOI] [PubMed] [Google Scholar]
- 14. Ock CY, Kim S, Keam B, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget. 2016;7(13):15901-15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Wang H, Zhao Q, Xia Y, Hu X, Guo J. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol. 2015;32(8): 212. [DOI] [PubMed] [Google Scholar]
- 16. Kim S, Koh J, Kim MY, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol. 2016;58:7-14. [DOI] [PubMed] [Google Scholar]
- 17. Fei Z, Deng Z, Zhou L, Li K, Xia X, Xie R. PD-L1 induces epithelial-mesenchymal transition in nasopharyngeal carcinoma cells through activation of the PI3K/AKT pathway. Oncol Res. 2019;27(7):801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang K, Peng T, Yan Q, et al. Jiedu Sangen decoction inhibits migration and invasion of colon cancer SW480 cells via suppressing epithelial mesenchymal transition. Evid Based Complement Alternat Med. 2018;2018:1495768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruan SM, Shen MH, Lin H, Zheng LP, Wang Y, Li MT. Jiedu Sangen decoction intervened carcinoma-associated fibroblasts and inhibited migration and invasion of colon cancer: an experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33(9):1242-1246. [PubMed] [Google Scholar]
- 20. Xu YX, Xiang ZB, Jin YS, Shen Y, Chen HS. Two new triterpenoids from the roots of Actinidia chinensis. Fitoterapia. 2010;81(7):920-924. [DOI] [PubMed] [Google Scholar]
- 21. Meng Y, Lin ZM, Ge N, Zhang DL, Huang J, Kong F. Ursolic acid induces apoptosis of prostate cancer cells via the PI3K/Akt/mTOR pathway. Am J Chin Med. 2015;43(7):1471-1486. [DOI] [PubMed] [Google Scholar]
- 22. Wu B, Wang X, Chi ZF, et al. Ursolic acid-induced apoptosis in K562 cells involving upregulation of PTEN gene expression and inactivation of the PI3K/Akt pathway. Arch Pharm Res. 2012;35(3):543-548. [DOI] [PubMed] [Google Scholar]
- 23. Lu Y, Jeong YT, Li X, et al. Emodin isolated from polygoni cuspidati radix inhibits TNF-alpha and IL-6 release by blockading NF-kappaB and MAP kinase pathways in mast cells stimulated with PMA plus A23187. Biomol Ther (Seoul). 2013;21(6):435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gibson J. Anti-PD-L1 for metastatic triple-negative breast cancer. Lancet Oncol. 2015;16(6):e264. [DOI] [PubMed] [Google Scholar]
- 25. Forde PM, Reiss KA, Zeidan AM, Brahmer JR. What lies within: novel strategies in immunotherapy for non-small cell lung cancer. Oncologist. 2013;18(11):1203-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis FM, Parsonage MT, Cabot PJ, et al. Assessment of gene expression of intracellular calcium channels, pumps and exchangers with epidermal growth factor-induced epithelial-mesenchymal transition in a breast cancer cell line. Cancer Cell Int. 2013;13(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu R, Zhang Y, Gu L, et al. Arf6 regulates EGF-induced internalization of E-cadherin in breast cancer cells. Cancer Cell Int. 2015;15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ren Y, Yang M, Chen M, et al. Microcystin-LR promotes epithelial-mesenchymal transition in colorectal cancer cells through PI3-K/AKT and SMAD2. Toxicol Lett. 2017;265: 53-60. [DOI] [PubMed] [Google Scholar]
- 29. Schwab RHM, Amin N, Flanagan DJ, Johanson TM, Phesse TJ, Vincan E. Wnt is necessary for mesenchymal to epithelial transition in colorectal cancer cells. Dev Dyn. 2018;247(3):521-530. [DOI] [PubMed] [Google Scholar]
- 30. Peng M, Hu Y, Song W, et al. MIER3 suppresses colorectal cancer progression by down-regulating Sp1, inhibiting epithelial-mesenchymal transition. Sci Rep. 2017;7(1):11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tauriello DV, Calon A, Lonardo E, Batlle E. Determinants of metastatic competency in colorectal cancer. Mol Oncol. 2017;11(1):97-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6): 453-458. [DOI] [PubMed] [Google Scholar]
- 35. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871-890. [DOI] [PubMed] [Google Scholar]
- 36. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30(10):764-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loh CY, Chai JY, Tang TF, et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8(10):1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee JH, Chinnathambi A, Alharbi SA, Shair OHM, Sethi G, Ahn KS. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol Res. 2019;150:104504. [DOI] [PubMed] [Google Scholar]
- 40. D’Angelo E, Lindoso RS, Sensi F, et al. Intrinsic and extrinsic modulators of the epithelial to mesenchymal transition: Driving the fate of tumor microenvironment. Front Oncol. 2020;10:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84-89. [DOI] [PubMed] [Google Scholar]
- 42. Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2): 76-83. [DOI] [PubMed] [Google Scholar]
- 43. Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62(6):1613-1618. [PubMed] [Google Scholar]
- 44. Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7(6):1267-1278. [DOI] [PubMed] [Google Scholar]
- 45. Eger A, Aigner K, Sonderegger S, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24(14):2375-2385. [DOI] [PubMed] [Google Scholar]
- 46. Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927-939. [DOI] [PubMed] [Google Scholar]
- 47. Fang X, Cai Y, Liu J, et al. Twist2 contributes to breast cancer progression by promoting an epithelial-mesenchymal transition and cancer stem-like cell self-renewal. Oncogene. 2011;30(47):4707-4720. [DOI] [PubMed] [Google Scholar]
- 48. Shioiri M, Shida T, Koda K, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94(12):1816-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singh AB, Sharma A, Smith JJ, et al. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology. 2011;141(6):2140-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang MH, Hsu DS, Wang HW, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12(10):982-992. [DOI] [PubMed] [Google Scholar]
- 51. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131-142. [DOI] [PubMed] [Google Scholar]
- 52. Alsuliman A, Colak D, Al-Harazi O, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer. 2015;14:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cao Y, Zhang L, Kamimura Y, et al. B7-H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res. 2011;71(4):1235-1243. [DOI] [PubMed] [Google Scholar]
- 54. Hirai M, Kitahara H, Kobayashi Y, et al. Regulation of PD-L1 expression in a high-grade invasive human oral squamous cell carcinoma microenvironment. Int J Oncol. 2017;50(1):41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bagley SJ, Bauml JM, Langer CJ. PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. Clin Adv Hematol Oncol. 2015;13(10):676-683. [PubMed] [Google Scholar]
- 57. Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275(47):36803-36810. [DOI] [PubMed] [Google Scholar]