Abstract
Coccidioidomycosis a fungal infection endemic to southwestern United States. It is caused by inhalation of spores of Coccidioides immitis. Sixty percent of infections are asymptomatic; the remaining 40% are primarily pulmonary disease. In <1% of infections, dissemination can occur. Dissemination usually affects those with impaired cellular immunity and pregnant women, and can involve bones, joints, meninges, and skin. We present the case of a 29-year-old Hispanic male who presented to the emergency department (ED) complaining of pain and swelling of right wrist and ankle as well as left knee for 2 months. He was referred to rheumatology clinic but returned to the ED as he developed spontaneous purulent drainage from his wrist. In the ED, an arthrocentesis of 2 of the joints showed total nucleated cells of 520 000/cm2 and 90 000/cm2 with 61% and 93% neutrophils, respectively. Fungal culture eventually grew Coccidioides immitis from his wrist and knee. Coccidioidomycosis complement fixation titer came back >1:512. Bone scan showed uptake of adjacent bones in the affected joints. Superimposed bacterial infection of the wrist complicated the treatment course and delayed the start of liposomal amphotericin B. Eventually patient received 12 weeks of intravenous liposomal amphotericin-B with slow clinical improvement and then switched to oral isavuconazonium for maintenance therapy. This case shows that although disseminated polyarthritis coccidioidomycosis is very rare, clinicians should keep the diagnosis of disseminated synovial coccidioidomycosis in mind in patients with risk factors.
Keywords: coccidioidomycosis, polyarthritis, Coccidioides, osteomyelitis, arthritis, tenosynovitis, reactive arthritis
Introduction
Coccidioidomycosis is a fungal infection that is endemic to the southwestern region of the United States. It is caused by inhalation of spores of Coccidioides immitis and Coccidioides posadasii. Coccidioides species are dimorphic fungi found in desert soils; they produce arthroconidia from branching septate hyphae, which detach and remain stable in the environment. Once aerosolized and inhaled, a primary pulmonary infection may occur.1,2 The pulmonary infection is usually self-limiting in most immunocompetent hosts. Only 1% of those with pulmonary coccidioidomycosis advance to disseminated disease, and when dissemination occurs, it can involve the bones, joints, meninges, and/or skin.2 In this case report, we present a rare case of disseminated polyarticular coccidioidomycosis involving the right wrist, left elbow, and left knee in a 29-year-old male, complicated with a superimposed methicillin-resistant Staphylococcus aureus (MRSA) infection.
Case Presentation
The patient is a 29-year-old Hispanic male with no previous medical history presented to the emergency department (ED) complaining of worsening pain and swelling in multiple joints. He had previously been seen in the ED as well as in rheumatology clinic for similar symptoms. The 6 months leading up to the presentation are summarized in the diagram below:

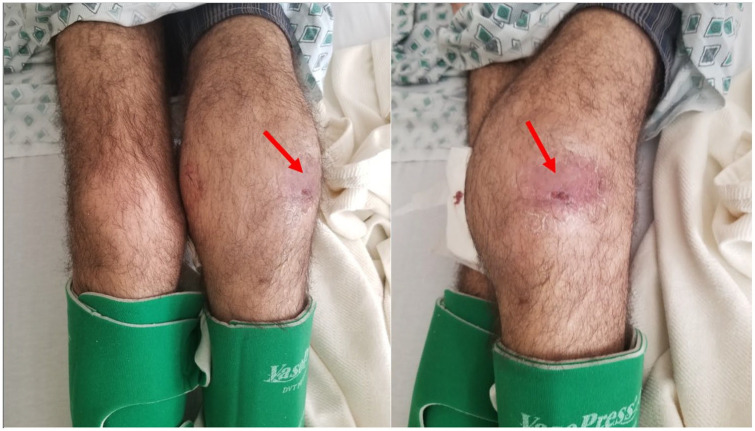
On the day of presentation, the patient presented to the ED complaining of worsening left knee pain and swelling, a new onset of left elbow swelling, and a new onset of purulent discharge from his right wrist. The previously clear discharge from his right wrist had progressively worsened and became purulent. He also developed a scaly plaque-like lesion with skin breakdown in the same area (Figure 1). Furthermore, he had developed similar scaly plaque-like lesions over the anterolateral aspect of his left knee; however, without any skin breakdown or discharge (Figure 2). He endorsed 35 lbs unintentional weight loss over the past 2 months. He denied any recent travel; however, he lives in the endemic area of Bakersfield, California.
Figure 1.
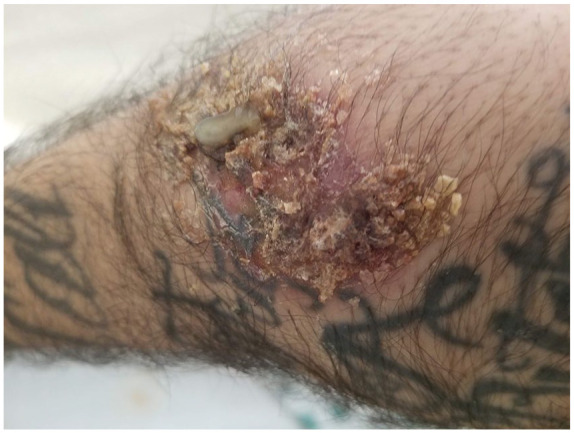
Purulent lesion on the dorsal aspect of the right wrist.
Figure 2.
Swelling and skin changes (red arrow) involving the right knee.
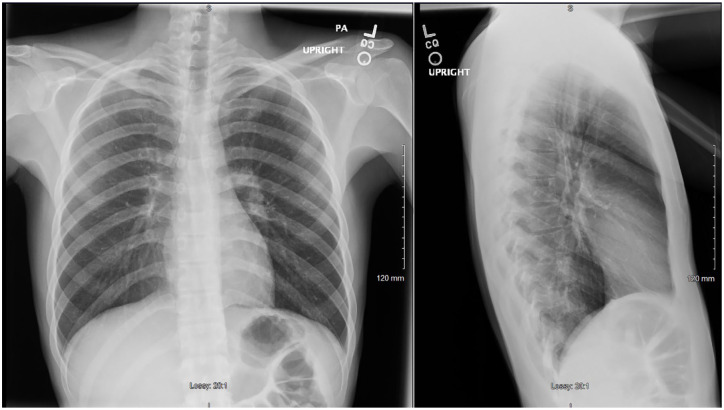
The initial investigation was primarily for reactive arthritis, rheumatoid arthritis, and gout. However, laboratory analyses including HLA-B27, uric acid, rheumatoid factor, and anti-CCP IgG were all within normal limits. Further investigation including X-ray imaging of the affected joints showed interval progression of the osteopenia along with erosive changes (Figures 3-8). Laboratory studies were significant for erythrocyte sedimentation rate >100 mm/h (similar to that 2 months prior), C-reactive protein 19 mg/dL (as compared with 17 previously), and lactate dehydrogenase 319 (H). A complete blood count was significant for hemoglobin of 9.7 g/dL and platelet count of 438 000/µL. Gonorrhea and chlamydia RNA in the urine were negative.
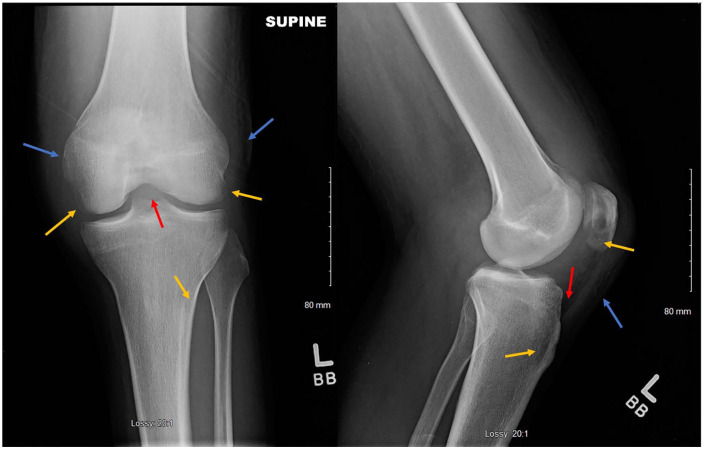
Figure 3.
X-ray of left knee showing osteopenia with loss of cortical margination involving the medial and lateral epicondyles, medial and lateral tibial plateau, and patella (yellow arrows). Large joint effusion (red arrow), and soft tissue swelling (blue arrow).
Figure 4.
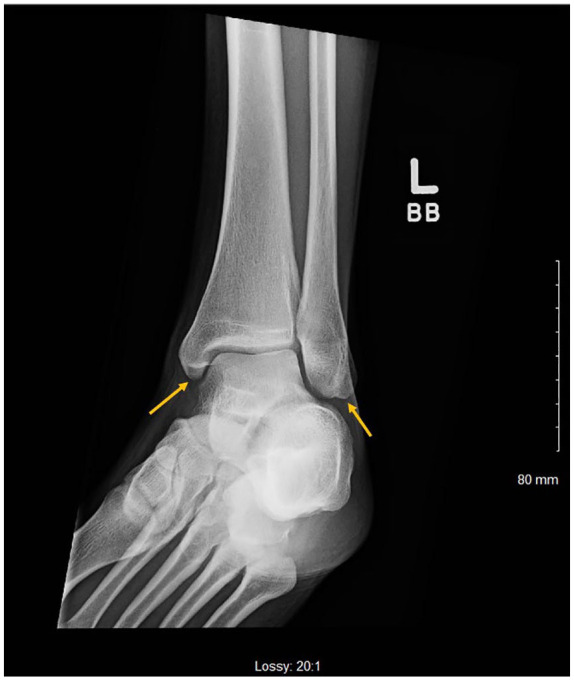
X-ray of left ankle showing lucency involving the medial and lateral malleoli.
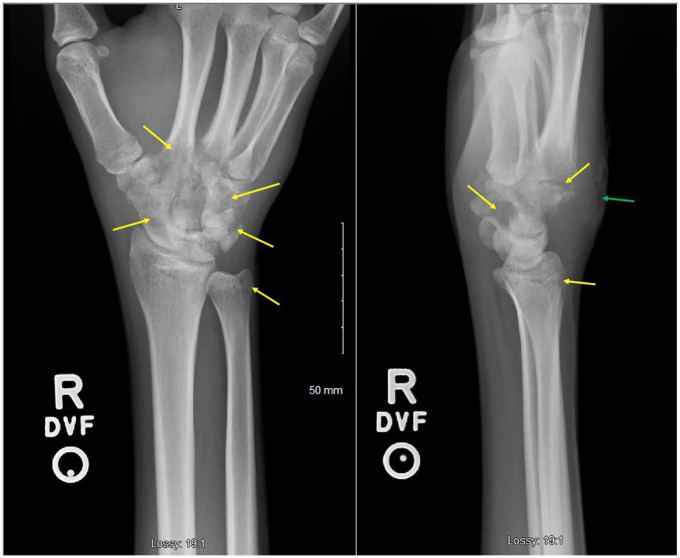
Figure 5.
X-ray of right wrist showing pronounced osteopenia with areas of cortical irregularity involving the first through fourth metatarsal bases and several carpal bones with surrounding soft tissue swelling.
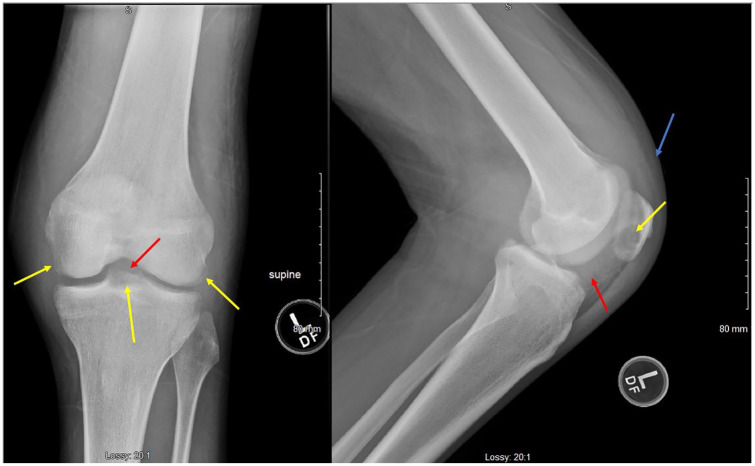
Figure 6.
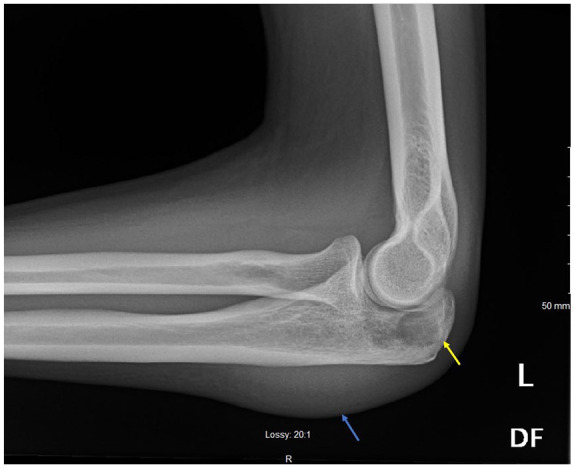
X-ray of the left elbow showing lucency of the olecranon process (yellow arrow) with moderate joint effusion and a large amount of left elbow soft tissue swelling (blue arrow).
Figure 7.
X-ray of the left knee showing interval progression of osteopenia and erosive changes involving the medial and lateral femoral condyles as well as tibial plateau and patella (yellow arrows). It also shows an increased size of the joint effusion (red arrows) as well as surrounding soft tissue swelling (blue arrow).
Figure 8.
X-ray of right wrist showing interval progression of osteopenia and erosive change involving the bases of the second through fifth metacarpals, carpal bones, and radial styloid (yellow arrows), with surrounding soft tissue swelling. Skin irregularity involving the dorsal wrist soft tissues at the site of the purulent skin lesion is also noted (green arrow).
Arthrocentesis of the left elbow produced brown-colored synovial fluid that was significant for total nucleated cells of 520 000/cmm with a slight neutrophilic predominance (61% neutrophils, 20% macrophages, 10% monocytes, 9% lymphocytes), 39 000 RBC/cmm, and no crystals. Similarly, synovial fluid from the left knee produced light orange-colored synovial fluid with total nucleated cells of 90 000/cmm with a large neutrophilic predominance (93% neutrophils, 4% monocytes, 3% lymphocytes), 15 000 RBC/cmm, and no crystals. Gram stain and KOH wet mount of both synovial fluids plus the draining purulent fluid from the right wrist showed evidence for spherules resembling Coccidioides sp (Figure 9). Initial culture of the purulent material from the right wrist joint grew MRSA as well. Fungal culture eventually grew C immitis from all 3 joints.
Figure 9.
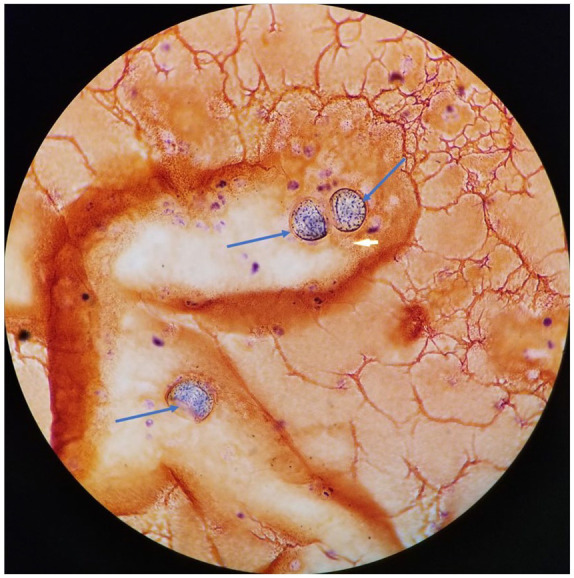
Gram stain of synovial fluid from the left elbow showing spherules (blue arrows) resembling Coccidioides sp under light microscopy.
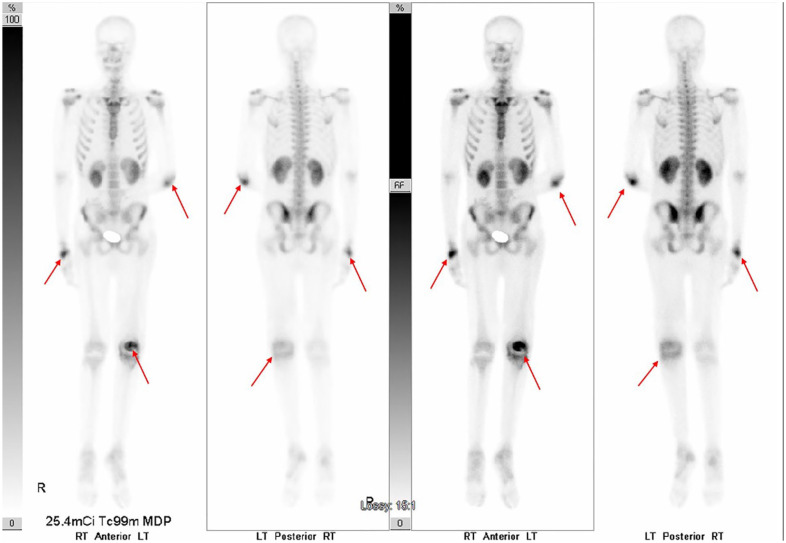
The patient was started on vancomycin to treat superimposed MRSA septic joint infection in his right wrist, while he underwent investigation for infective endocarditis that came back negative. One day following the vancomycin treatment was started, the patient quickly developed acute kidney injury. Therefore, he was placed on 800 mg of fluconazole instead of amphotericin and his antibiotic was changed to linezolid to complete his course. A chest X-ray was performed and showed subsegmental right lower lung zone patchy airspace disease (Figure 10). Serum Cocci serology showed reactivity for both IgM and IgG immunodiffusion assay with complement fixation titers of >1:512. A whole-body bone scan was performed to investigate for osseous involvement and showed abnormal uptake in the right wrist, left elbow, and left knee consistent with disseminated osseous coccidioidomycosis (Figure 11). HIV testing was negative, but he was found to have chronic hepatitis C infection. Other laboratory tests were significant for iron 23 (L), TIBC (total iron binding capacity) 127 (L), ferritin 1965.7 (H), and haptoglobin of 511 (H).
Figure 10.
Chest X-ray showing subsegmental right lower lung zone patchy airspace disease.
Figure 11.
Bone scan showing abnormal uptake seen in the right wrist, left elbow, and left knee (red arrows).
After the improvement in the patient’s kidney function, he was switched from fluconazole to the originally planned intravenous liposomal amphotericin B with daily induction dose for 14 days followed by 3 times a week for another 12 weeks. While on the amphotericin treatment, his complement fixation titers remained high (> 1:512); however, he showed significant clinical improvement. Therefore, at the end of his 12-week therapy, he was switched to oral isavuconazonium 372 mg daily. Unfortunately, the patient stopped following up and thus, his prognosis is unclear.
Discussion
As mentioned, only about 1% of those with pulmonary coccidioidomycosis advance to disseminated disease. Furthermore, of those who develop dissemination, 10% to 50% have musculoskeletal involvement.3 In patients presenting with dimorphic fungal osteoarticular infections, C immitis is the second most frequent pathogen (following Sporothrix schenckii). In a study of all dimorphic fungal infections over a 42-year period, 58% of infections presented as arthritis and 42% as osteomyelitis.4
One of the initial manifestations of coccidioidomycosis along with constitutional symptoms and erythema nodosum is a symmetric polyarticular disease known as “desert rheumatism,” which is an inflammatory process that usually involves the ankles and knees bilaterally. One third of patients develop polyarticular and migratory arthritis; with remission within 4 weeks.5
In contrast, the patient in our case did not have symmetric involvement of joints, nor did he have erythema nodosum. Furthermore, synovial fluid analysis of the joints involved were in the range of septic arthritis rather than an inflammatory process. This was solidified when the cultures grew C immitis.
Similar finding distinguishes this case from reactive arthritis, which was the main consideration based on initial laboratory analysis when it was found that he was positive for chlamydial infection. Reactive arthritis is a painful inflammation of joints in reaction to infection by Chlamydia trachomatis, Campylobacter, Salmonella, Shigella, or Yersinia.6 Of all the sexually transmitted pathogens, Chlamydia trachomatis has the greatest implication. Once called “Reiter’s syndrome,” it is now classified in the family of spondyloarthropathies. As per the American College of Rheumatology, reactive arthritis is mostly seen in males 20 to 50 years of age, with 30% to 50% having positive HLA-B27 genetics.7 Those with the HLA-B27 gene often have more severe and acute symptoms. Those with persistent arthropathy have a greater incidence of being HLA-B27 positive.8 Patients typically report painful asymmetric oligoarthritis, with or without migratory polyarthritis and fever.7,9
Patients with coccidioidal septic arthritis, similar to the one in our case, might not have other organ involvement.10 Therefore, when a patient presents with chronic progressive monoarticular or polyarticular arthritis and exposure to an endemic zone, coccidioidal arthritis should be strongly suspected. When faced with a clinical picture of inflammatory versus septic arthritis, synovial fluid aspirate and analysis may provide additional insight into the presenting pathology. Synovial fluid cell count does not usually lead to a change in the differential diagnosis if the gross analysis suggests a noninflammatory process. It is still standard to consider both cell counts, and gross analysis when determining a potential septic joint. Gross synovial fluid analysis has a higher sensitivity than cell count and is often regarded with higher confidence.11
The patient in our case was determined, through a multifactorial approach, to have septic arthritis secondary to dissemination of a coccidioidal infection. From our experience, extended and close follow-up to ensure the therapeutic response is pivotal for successful treatment of patients with bone and joint dissemination.
Current guidelines for the treatment of severe osseous and/or joint coccidioidomycosis is to initially start with intravenous ambisome therapy daily in the acute setting, then transitioning to thrice weekly up to 3 months on an outpatient setting. Typically, after the commencement of the ambisome therapy, patients are then treated with an azole for a protracted period of 3 years to lifetime depending on the severity of disease and the immunocompetence of the patient.12 Although initiation of the ambisome therapy was delayed due to the development of an acute kidney injury in our case; our overall approach was consistent with the guidelines.
Conclusion
We report a case of a 29-year-old male, with a history of chlamydial infection and positive coccidioidal serum titers, presenting with polyarthritis. This case report illustrates the challenges in approaching a presentation of multiple possible etiologies, and the challenges in the treatment of coccidioidomycosis in the setting of renal impairment and a superimposed MRSA soft tissue infection. C immitis and C posadsii are unusual causes of septic arthritis, but should always be considered in patients with epidemiological risk factors.
Footnotes
Authors’ Note: An abstract of this case report was previously presented at the AFMR’s Western Medical Research Conference in Carmel, California, January 25th 2020.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval to report this case was obtained from Kern Medical Institutional Review Board (ID# 19042).
Informed Consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Faisal Nasrawi  https://orcid.org/0000-0002-6792-7666
https://orcid.org/0000-0002-6792-7666
References
- 1. Galgiani JN. Coccidioidomycosis. West J Med. 1993;159:153-171. [PMC free article] [PubMed] [Google Scholar]
- 2. Longo DL, Harrison TR. Fungal infections. In: Harrison’s Manual of Medicine. McGraw-Hill Medical; 2013. [Google Scholar]
- 3. Liu YW, Johnston M, Lee H, Varma R. Coccidioidomycosis with musculoskeletal involvement. Appl Radiol. 2019;48:44-45. [Google Scholar]
- 4. Rammaert B, Gamaletsou MN, Zeller V, et al. Dimorphic fungal osteoarticular infections. Eur J Clin Microbiol Infect Dis. 2014;33:2131-2140. [DOI] [PubMed] [Google Scholar]
- 5. Cuellar ML, Silveira LH, Espinoza LR. Fungal arthritis. Ann Rheum Dis. 1992;51:690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell RN, Kumar V, Abbas AK, Aster JC. Pocket Companion to Robbins and Cotran Pathologic Basis of Disease. Elsevier; 2017. [Google Scholar]
- 7. Cheeti A, Chakraborty RK, Ramphul K. Reactive Arthritis (Reiter Syndrome) In: StatPearls. StatPearls; 2020. [PubMed] [Google Scholar]
- 8. Gaston JSH. Immunological basis of chlamydia induced reactive arthritis. Sex Transm Infect. 2000;76:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madoff LC. Infectious disease: infectious arthritis. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Internal Medicine. McGraw Hill; 2015:833. [Google Scholar]
- 10. Weisenberg SA. Coccidioides immitis septic knee arthritis. BMJ Case Rep. 2018;2018:bcr2017222585. doi: 10.1136/bcr-2017-222585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdullah S, Young-Min SA, Hudson SJ, Kelly CA, Heycock CR, Hamilton JD. Gross synovial fluid analysis in the differential diagnosis of joint effusion. J Clin Pathol. 2007;60:1144-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63:e112-e146. doi: 10.1093/cid/ciw360 [DOI] [PubMed] [Google Scholar]