Abstract
Background:
Restless legs syndrome (RLS) affects approximately 30% of patients with end-stage kidney disease and is associated with impaired sleep and health-related quality of life. Medications used to treat RLS in patients receiving dialysis may have an increased risk of adverse events with dose titration, and residual RLS symptoms are common despite the use of effective treatments. Randomized controlled trials of monotherapy and combination pharmacologic therapy for RLS in hemodialysis are needed.
Objective:
To perform a randomized, crossover, placebo-controlled blinded trial of pharmacologic therapy for RLS in hemodialysis.
Design/setting:
The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) trial is a randomized, crossover, placebo-controlled blinded trial of fixed low-dose pharmacologic therapy in patients receiving hemodialysis in 10 centers across Canada. It uses patient partners in its design, conduct, and reporting.
Participants:
Adults receiving thrice-weekly hemodialysis for at least 3 months with RLS of at least mild symptoms defined International Restless Legs Syndrome Study Group Rating Scale (IRLS) of 10 or more will enter a double placebo run-in period to exclude nonadherent participants and those unable to tolerate double placebo. Seventy-two participants who completed the run-in period will be randomized to 1 of 8 treatment sequences based on modeling with 4 treatment periods.
Methods:
Each treatment period lasts 4 weeks and consists of ropinirole 0.5 mg daily and gabapentin 100 mg daily, both together or neither with a double dummy placebo control for each treatment. The primary outcome is the difference in change scores of the IRLS between study treatments. Secondary outcomes are the differences in change scores of the Restless Legs Syndrome-6 Scale, patient global impression, 5-level EQ-5D version, and safety outcomes.
Results:
This randomized, crossover, placebo-controlled blinded trial will evaluate the efficacy and safety of fixed low-dose combination of ropinirole and gabapentin in patients receiving hemodialysis with RLS.
Limitations:
Patients with chronic kidney disease not on dialysis, kidney transplant recipients and those receiving peritoneal dialysis or home hemodialysis are not included. The intervention’s long term safety and efficacy including the risk of augmentation is not captured.
Conclusion:
This randomized crossover placebo controlled blinded trial will evaluate the efficacy and safety of fixed low-dose combination ropinirole and gabapentin in patients receiving hemodialysis with RLS.
Trial Registration:
ClinicalTrials.gov (NCT03806530)
Keywords: restless legs syndrome, hemodialysis, randomized, crossover, controlled trial
Abrégé
Contexte:
Le syndrome des jambes sans repos (SJSR) touche environ 30 % des patients atteints d’insuffisance rénale terminale (IRT) et est associé à des troubles du sommeil et à des altérations de la qualité de vie. Les médicaments employés pour traiter le SJSR chez les patients dialysés pourraient présenter un risque accru d’effets indésirables avec un ajustement de la dose, et les symptômes résiduels du SJSR sont fréquents malgré l’utilisation de traitements efficaces. Des essais contrôlés à répartition aléatoire examinant des traitements pharmacologiques en monothérapie ou en combinaison chez les patients hémodialysés sont nécessaires.
Objectif:
Procéder à un essai croisé, en aveugle, réparti aléatoirement et contrôlé par placébo examinant un traitement pharmacologique contre le SJSR en contexte d’hémodialyse.
Conception:
DISCO-RLS (DIalysis Symptom COntrol-Restless Legs Syndrome) est un essai croisé, en aveugle, réparti aléatoirement et contrôlé par placébo examinant une faible dose fixe d’un médicament administré à des patients hémodialysés. L’essai fait appel à des patients-partenaires pour sa conception, sa conduite et ses rapports.
Cadre:
Dix centres à travers le Canada.
Sujets:
Des adultes hémodialysés trois fois par semaine depuis plus de trois mois et présentant au moins des symptômes légers de SJSR, tels que définis par un score de 10 (score IRLS) ou plus sur l’échelle d’évaluation du groupe international d’étude sur le SJSR seront examinés lors d’une période de rodage à double placébo. Cette dernière permettra d’exclure les patients non adhérents et ceux qui ne tolèrent pas le double placébo. Parmi les patients qui complèteront la période de rodage, soixante-douze seront répartis aléatoirement dans une des huit séquences de traitement en fonction d’une modélisation avec quatre périodes de traitement.
Mesures:
Le principal résultat est l’observation d’une différence entre les traitements à l’étude dans les variations du score IRLS par rapport aux valeurs initiales. Les résultats secondaires incluent des différences dans les variations de scores sur l’échelle du SJSR (Restless Legs Syndrome-6 Scale), dans l’impression générale du patient, dans les résultats de la version à 5 niveaux du EQ-5D et dans les résultats d’innocuité.
Méthodologie:
Chaque période de traitement dure quatre semaines et consiste en l’administration quotidienne de 0,5 mg de ropinirole et de 100 mg de gabapentine, les deux ensembles ou aucun des deux, avec un double placébo comme témoin pour chaque traitement.
Limites:
Les patients non dialysés atteints de néphropathies chroniques, les receveurs d’une greffe rénale et les patients traités par dialyse péritonéale ou par hémodialyse à domicile sont exclus. L’efficacité et l’innocuité de l’intervention à long terme ne sont pas prises en compte.
Conclusion:
Cet essai croisé, en aveugle, réparti aléatoirement et contrôlé par placébo évaluera l’efficacité et l’innocuité d’une combinaison de ropinirole et de gabapentine à faible dose fixe chez les patients hémodialysés atteints du SJSR.
Enregistrement de l’essai:
ClinicalTrials.gov (NCT03806530)
Introduction
The symptom burden of patients with end-stage kidney disease (ESKD) is significant1 but underrecognized by many nephrologists and other health care providers.2 Restless legs syndrome (RLS) is a neurological sensorimotor disease that affects approximately 30% of patients with ESKD3 and is associated with impaired sleep quality and health-related quality of life.4-6 It may also increase morbidity and mortality.6-9 Restless legs syndrome is an urge to move the legs that begins or worsens during periods of rest or inactivity, is partially or totally relieved by movement, only occurs or is worse in the evening or night, and is not solely accounted for as symptoms to another condition.10 The importance of RLS is underscored by its identification as a top research priority by patients with or nearing ESKD.11
The pathophysiology of RLS in the general population is poorly understood but may involve genetic predisposition, abnormal dopamine nervous system pathways, iron deficiency, and peripheral neuropathy. The causes of RLS in patients with ESKD are even less well understood and treatments are less well studied.12 Although some therapies may reduce the symptoms of RLS in patients with ESKD, there remains a large burden of symptoms.13 Among pharmacologic agents, alpha-2-delta ligands (gabapentin, pregabalin) and dopamine agonists14 (ropinirole, pramipexole, rotigotine) appear the most promising both in the general population with RLS15 and in patients with ESKD.16-19 Alpha-2-delta ligands target the sensory peripheral neuropathy, and dopamine agonists target the dopamine deficiency–induced motor abnormalities implicated in the development of RLS. However, the evidence supporting the use of these agents in patients with ESKD is limited by small sample sizes, heterogeneity in patient-reported outcome measures (PROMs), and short-term follow-up.12
The use of alpha-2-delta ligands and dopamine agonists is complicated by the risk of augmentation20 (the development of worsening, refractory symptoms) and safety concerns in the ESKD population. In 2011, 19% and 4% of US Renal Data System patients on hemodialysis were prescribed gabapentin and pregabalin, respectively, only 4% of which was for RLS. These alpha-2-delta ligands were associated with an increased risk of emergency department visits and hospitalizations for altered mental status, falls, and fractures, with evidence of higher doses associated with an increased risk of these adverse events.21 Sides effects of alpha-2-delta ligands and dopamine agonists such as nausea, vomiting, and dizziness are also common, especially with dose escalation to adequately control RLS symptoms which may limit their clinical use.
There is a paucity of adequately powered randomized controlled trials using valid, reliable, and responsive PROMs evaluating pharmacologic and nonpharmacologic therapies for RLS in ESKD.12 Fixed low-dose combination pharmacologic therapy that targets the multiple pathways implicated in the pathophysiology of RLS might control symptoms while avoiding the exposure to higher doses of individual medications and their side effects and adverse events. We designed the DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) trial to test this hypothesis.
Methodology
Design
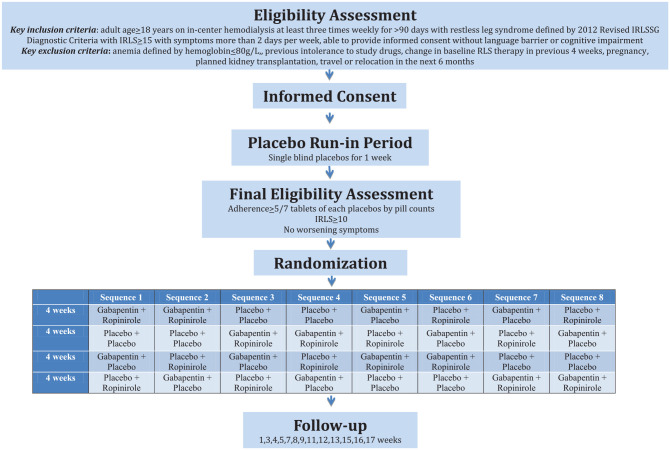
The DISCO-RLS trial is a multicenter randomized, 4× crossover, placebo-controlled trial of 2 fixed low-dose active study medications (gabapentin 100 mg orally daily, an alpha-2-delta ligand and ropinirole 0.5 mg orally daily, a dopamine agonist) in combination, each alone with a placebo, or as double placebo (Figure 1). Participants, healthcare providers, outcome assessors, and analysts will be blinded. The sponsor for trial is the Population Research Health Institute in Hamilton, Ontario, Canada. The trial is funded by the Strategy for Patient-Oriented Research (SPOR) network for kidney disease: Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD)22 and uses patient partners for its design, conduct, and reporting. The trial will be conducted in accordance with ICH E6 (R2): Guideline for Good Clinical Practice. Regulatory approval has been obtained by Health Canada, and the trial is registered at ClinicalTrials.gov (NCT03806530).
Figure 1.
Study flowchart.
Objectives
The primary objective of this trial is to assess the efficacy of pharmacologic therapy on RLS severity as measured by the International Restless Legs Syndrome Study Group Rating Scale (IRLS) in patients with ESKD on hemodialysis. The 4 following medication combinations will be compared: (1) gabapentin + ropinirole, (2) gabapentin + ropinirole placebo, (3) ropinirole + gabapentin placebo, and (4) gabapentin placebo + ropinirole placebo. Secondary objectives include the evaluation of the efficacy of the different treatment regimens on other PROMs, including the Restless Legs Syndrome-6 scale23 (RLS-6), patient global impression (PGI), 5-level EQ-5D version (EQ-5D-5L) and safety outcomes.
Study Population
The inclusion criteria are (1) age ≥18 years, (2) received at least 90 days of in-center hemodialysis at a frequency of at least 3 times weekly, (3) RLS defined by 2012 Revised International Restless Legs Syndrome Study Group (IRLSSG) Diagnostic Criteria for RLS (criteria 1, 2, 3, and 4 but not 5 which allows for conditions that mimic RLS; see Table 1) and RLS of at least mild severity defined by an IRLS score ≥10 with symptoms more than 2 days per week, and (4) provided informed consent. The exclusion criteria are (1) hemoglobin ≤80g/L in the previous 4 weeks (anemia is associated with worsening symptoms of RLS),3 (2) previous intolerance to a dopamine agonist or alpha-2-delta ligand, (3) change in medication to treat RLS in the previous 4 weeks (suggestive of augmentation),24 (4) current pregnancy or breastfeeding, (5) planned kidney transplantation, travel, or relocation in the next 6 months, and (6) unable to complete any PROM in English or French due to language barrier or cognitive impairment. There is no exclusion for any RLS mimickers including peripheral neuropathy, cramping, peripheral vascular disease, edema due to its prevalence in the hemodialysis population, and the challenge of distinguishing whether RLS or a mimicker is mostly responsible for a patient’s symptoms in dialysis.13 Participants who fail screening can be rescreened at a later date if appropriate. The study will recruit participants from 10 sites across Canada, including tertiary hemodialysis units in Hamilton, Halifax, Ottawa, Calgary, Montreal, Winnipeg, Toronto, and Kingston.
Table 1.
2012 Revised International RLS Study Group Diagnostic Criteria for RLS.
| Diagnostic criteria | Description |
|---|---|
| 1 | An urge to move the legs usually but not always accompanied by or felt to be caused by uncomfortable and unpleasant sensations in the legs |
| 2 | The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting |
| 3 | The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues |
| 4 | The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are get worse in the evening or night than during the day |
| 5a | The occurrence of the above features are not solely accounted for as symptoms primary to another medical or a behavioral condition (eg, myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping) |
Note. RLS = restless legs syndrome; DISCO-RLS = DIalysis Symptom COntrol-Restless Legs Syndrome.
Not required for eligibility in DISCO-RLS.
Run-in Period
Eligible participants will stop any dopamine-based drugs, dopamine agonists, and alpha-2-delta ligands and will be treated with 2 placebo tablets (gabapentin and ropinirole) to be taken orally daily at night for 1 week during a single-blind, double-placebo run-in period to identify patients who are nonadherent (<5/7 tablets by self-report for each placebo) and have worsening RLS symptoms requiring study withdrawal due to the washout of baseline RLS medications. Self-management strategies for RLS including nonpharmacologic therapies (exercise, stretching, massage) will be reviewed and encouraged at the start of the run-in period to minimize differential use after randomization. A run-in period is included in DISCO-RLS’s design to maximize the likelihood of detecting treatment effects of the interventions by selecting an adherent population that tolerates double placebo which all participants are exposed to during one crossover period.
Randomization
Participants who remain eligible after the run-in period will be randomly allocated to 1 of 8 treatment sequences. The 8 potential treatment sequences given in Figure 1 were chosen from among all permutations of treatment sequences to minimize the likelihood of carryover effects by prioritizing active agents prior to double placebos or placing the double active agent treatment period as the last crossover period. Stratified block randomization with the site as stratum will be performed using computer-generated sequences. Each treatment sequence is composed of 4 periods of 4 weeks each for a postrandomization treatment time of 16 weeks with an additional study visit 1 week after permanently stopping study treatments. Each study participant will receive the following low fixed-dose study medications for 4 weeks, each supplied as capsules to be taken with food or water at the same time every evening:
Gabapentin 100 mg + ropinirole 0.5 mg
Gabapentin placebo + ropinirole 0.5 mg
Gabapentin 100 mg + ropinirole placebo
Gabapentin placebo + ropinirole placebo
Cointerventions
Standard therapy for RLS other than the study interventions and dopamine will be permitted throughout the trial. Nonpharmacologic interventions including exercise, stretching, and massage will be prioritized. The use of benzodiazepines, vitamins C and E, opioids, and alpha-2 agonists (ie, clonidine) will be permitted, and clinical targets for iron stores and hemoglobin through the use of intravenous iron and erythroid-stimulating agents will be maintained as per usual clinical practice and left at the discretion of local physicians.
Adherence
Participants’ self-reported adherence and adherence by pills counts will be monitored and reinforced at all study visits.
Follow-up
Follow-up visits will occur at regularly scheduled hemodialysis sessions. If a participant experiences any intolerable side effects thought to be due to either of the study drugs and is not willing to continue taking both study medications, they must be discontinued together. A participant will not be rechallenged with study drug during that treatment period and will immediately crossover to the next treatment period after completing a study visit if it is in a study visit window. If RLS symptoms are not tolerated during a period, patients can also be crossed over to the next treatment period after completing a study visit if appropriate.
Outcomes
The primary outcome is the difference in IRLS scores from baseline for each of the treatment regimens. The IRLS is a validated, reliable, and responsive PROM for RLS that is typically the primary outcome for most RLS trials.25,26 It consists of 10 questions rated from 0 to 4 with a range from 0 to 40, with higher scores indicating a greater severity of RLS. Its minimal important difference is 3.27 Secondary outcomes include the difference in RLS-6, which is another PROM that measures RLS severity under a variety of circumstances and times of day; the difference in PGI; the difference in EQ-5D-5L; and safety events, including the incidence of hospitalizations or emergency department visits due to altered level of consciousness, falls, and fractures.
Statistical Considerations
Sample Size
Seventy-two randomized participants are required for this design to detect a clinically meaningful reduction in change in IRLS of 3,27 assuming a standard deviation of 6 in change in IRLS15 with repeated measures at 3 and 4 weeks of every crossover period with a correlation in repeated measures of 0.88,13 an interperiod correlation of 0.2, a 2-sided type I error of 2.5%, and a power of 80%, assuming a 10% loss to follow-up. The interperiod correlation of 0.2 was assumed based on experiences with previous crossover trials28 and a 10% loss to follow-up based on previous trials of RLS in ESKD.12 Participants who do not complete more than 2 crossover periods will be replaced (but their data retained) until a maximum sample size of 80 patients is met. The sample size was powered to test the interaction between gabapentin treatment and ropinirole treatment by setting a type I error at 2.5%.
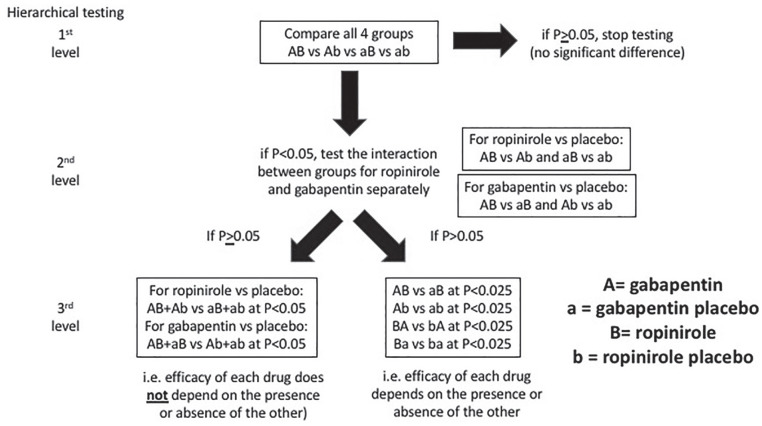
The 2 × 2 factorial design will be tested hierarchically to compare all treatment groups and any interaction effect using a gate-keeping approach to account for multiplicity testing,29 as shown in Figure 2.
Figure 2.
Hierarchical testing statistical analysis.
Analysis
Baseline variables will be presented as means with standard deviations for continuous normally distributed variables, medians with interquartile ranges for skewed variables, and frequency or percentages for categorical variables.
All primary and secondary analyses will analyze participants in the group to which they were assigned irrespective of the therapy received (ie, according to the intention-to-treat principle).
The primary outcome of the difference in IRLS between study treatments and secondary outcomes of the differences in RLS-6, PGI, and EQ-5D-5L will be compared among the 4 groups using a mixed linear model with repeated measures. The interaction between gabapentin vs gabapentin placebo and ropinirole vs ropinirole placebo will be tested if there is a difference in 4 groups. If the interaction between 2 interventions is significant, each pair of the 4 treatment groups will be tested at a significance level of 2.5% (refer to Figure 2). The presence of a carryover period will be assessed using a treatment-by-period interaction term in the model which will be excluded if not statistically significant, although the limited power to detect carryover is acknowledged. EQ-5D-5L subdomains will be compared but will be adjusted for multiple treatment comparisons. Adverse events will be presented using descriptive statistics. The primary analysis will be a complete case analysis that includes only participants who complete all 4 crossover periods. Sensitivity analyses accounting for missing data using the last observation carried forward technique as well as multiple imputation will be performed. A sensitivity analysis using a modified intention-to-treat analysis will be performed including participants with at least 80% or more adherence to therapy. Planned subgroup analyses include participants naïve to pharmacologic therapy, participants with severe RLS at baseline, participants previously treated with a dopamine agonist or alpha-2-delta ligand, participants with RLS mimickers, and participants with intradialytic RLS symptoms. A statistical analysis plan will be finalized prior to unlocking the trial’s database.
Interim Analyses
There is no plan for any interim analyses for safety, efficacy, or futility due to the short duration of the study and the rapidly changing sequence of treatments for participants.
Discussion
Restless legs syndrome is a top research priority for patients with ESKD because of its prevalence and negative impact on sleep and quality of life.4-6 Few high-quality randomized controlled trials have been performed in the hemodialysis population, and effective treatments are limited by their side effects and risk of adverse events commonly associated with dose escalation. Residual symptoms of RLS often remain despite monotherapy, so novel approaches such as combination pharmacologic therapy are needed but need to be evaluated in randomized controlled trials.
The DISCO-RLS trial will determine whether fixed low-dose combination therapy with gabapentin and ropinirole is safe and effective for the treatment of RLS in patients on hemodialysis compared with the individual study drugs and their respective placebo. It includes patients with mild, moderate, and severe forms of RLS without excluding individuals with RLS mimickers given its pragmatic nature and the challenge of differentiating RLS from its mimickers in clinical practice. However, given its run-in period with double placebo, it is unlikely to include participants with the most severe forms of RLS. Its crossover design is justified from a sample size perspective and will facilitate recruitment given its pragmatic nature and that all participants can receive other treatments during the trial. Our patient partners support this approach.
The cross over design of DISCO-RLS does not include washout periods to exclude carryover effects. This was done for several reasons. Both ropinirole and gabapentin have rapid onsets of action. Furthermore, the pharmacokinetics of ropinirole30 (half-life, 2-10 hours) and gabapentin31,32 (mean half-life, 132 hours in the absence of hemodialysis with 35% removed at each dialysis treatment) in hemodialysis are well known. The 3-week interval between efficacy assessments at crossover periods allows the effects of both drugs to wash out from the previous treatment period while allowing their full effect for the current period. Including a washout period in the trial would have resulted in 6 to 9 additional weeks of participants not being on any study medication with the potential for worsening symptoms of RLS. These periods would have risked lower participant consent rates, recruitment, and retention and were considered an unnecessary risk by patient partners.
A novel aspect of the DISCO-RLS trial is the use of patient partners with ESKD and RLS in its design, conduct, and reporting. The trial originates from a research priority setting exercise and uses patient engagement in several ways, including the acceptability of its run-in period and crossover design to reduce the trial’s sample size, the selection of the intervention and outcomes, and the feasibility of follow-up with repeated measures during each crossover period without overburdening participants with too many study visits and PROMs. Patient partners also helped develop informed consent forms, patient information sheets, and other study-specific materials (see Supplemental Material). We look forward to our patient partners’ assistance regarding knowledge translation at the trial’s completion.
The trial has obtained regulatory approval and local ethics approval from 10 sites across Canada. The first visit of the first patient occurred in May 2019, and 15 participants have completed follow-up at 5 sites across Canada as of June 2020. A protocol amendment was completed in January 2020 to lower the IRLS severity for inclusion from 15 to 10 to include mild forms of RLS, shorten the run-in period from 2 weeks to 1 week, and to remove an improvement of IRLS ≥3 (placebo response) as a reason to exclude participants during the run-in period. The last visit of the last patient is expected to be completed in December 2020.
Supplemental Material
Supplemental material, sj-jpg-1-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-jpg-2-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-pdf-1-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-pdf-2-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease
Acknowledgments
To the authors thank Lucy Delgado, Elizabeth Wallace, Paul Duperron (deceased), and Roger Hillier (deceased) for their involvement as patient partners.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval was obtained by all centers and all participants will provide informed consent prior to enrollment.
Consent for Publication: All authors approved the manuscript for publication.
Availability of Data and Materials: Not applicable.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: D.C. is supported by a Kidney Research Scientist Core Education and National Training Program (KRESCENT) Post-doctoral Fellowship Award. S.A.S. is supported by a Kidney Research Scientist Core Education and National Training Program (KRESCENT) New Investigator Award. M.W. is supported by a Mid Career Investigator Award from McMaster University.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is funded by the Canadian Institutes for Health Research (CIHR) Strategy for Patient-Oriented Research (SPOR) for kidney disease: Canadians Seeking Innovations and Solutions to Chronic Kidney Disease (Can-SOLVE CKD).
ORCID iDs: David Collister  https://orcid.org/0000-0002-2323-6521
https://orcid.org/0000-0002-2323-6521
Braden Manns  https://orcid.org/0000-0002-8823-6127
https://orcid.org/0000-0002-8823-6127
Rita S. Suri  https://orcid.org/0000-0002-0519-3927
https://orcid.org/0000-0002-0519-3927
Samuel A. Silver  https://orcid.org/0000-0002-1843-6131
https://orcid.org/0000-0002-1843-6131
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82-99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2. Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960-967. [DOI] [PubMed] [Google Scholar]
- 3. Mao S, Shen H, Huang S, Zhang A. Restless legs syndrome in dialysis patients: a meta-analysis. Sleep Med. 2014;15(12):1532-1538. doi: 10.1016/j.sleep.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 4. Mucsi I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20(3):571-577. doi: 10.1093/ndt/gfh654. [DOI] [PubMed] [Google Scholar]
- 5. Takaki J, Nishi T, Nangaku M, et al. Clinical and psychological aspects of restless legs syndrome in uremic patients on hemodialysis. Am J Kidney Dis. 2003;41(4):833-839. doi: 10.1016/S0272-6386(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 6. Unruh ML, Levey AS, D’Ambrosio C, et al. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43(5):900-909. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 7. La Manna G, Pizza F, Persici E, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26(6):1976-1983. doi: 10.1093/ndt/gfq681. [DOI] [PubMed] [Google Scholar]
- 8. Molnar MZ, Szentkiralyi A, Lindner A, et al. Restless legs syndrome and mortality in kidney transplant recipients. Am J Kidney Dis. 2007;50(5):813-820. doi: 10.1053/j.ajkd.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 9. Stefanidis I, Vainas A, Giannaki CD, et al. Restless legs syndrome does not affect 3-year mortality in hemodialysis patients. Sleep Medicine. 2015;16:1131-1138. doi: 10.1016/j.sleep.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 10. Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—history, rationale, description, and significance. Sleep Med. 2014;15(8):860-873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 11. Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9:1813-1821. doi: 10.2215/CJN.01610214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gopaluni S, Sherif M, Ahmadouk NA. Interventions for chronic kidney disease-associated restless legs syndrome. The Cochrane Database of Systematic Reviews. 2016;11:CD010690. doi: 10.1002/14651858.CD010690.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collister D, Rodrigues JC, Mazzetti A, et al. Screening questions for the diagnosis of restless legs syndrome in hemodialysis. Clin Kidney J. 2018;12:559-563. doi: 10.1093/ckj/sfy129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hornyak M, Trenkwalder C, Kohnen R, Scholz H. Efficacy and safety of dopamine agonists in restless legs syndrome. Sleep Med. 2012;13(3):228-236. doi: 10.1016/j.sleep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 15. Allen RP, Chen C, Garcia-Borreguero D, et al. Comparison of pregabalin with pramipexole for restless legs syndrome. New Engl J Med. 2014;370:621-631. doi: 10.1056/NEJMoa1303646. [DOI] [PubMed] [Google Scholar]
- 16. Giannaki CD, Sakkas GK, Karatzaferi C, et al. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 2013;14:194. doi: 10.1186/1471-2369-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miranda M, Kagi M, Fabres L, et al. Pramipexole for the treatment of uremic restless legs in patients undergoing hemodialysis. Neurology. 2004;62:831-832. [DOI] [PubMed] [Google Scholar]
- 18. Pieta J, Millar T, Zacharias J, et al. Effect of pergolide on restless legs and leg movements in sleep in uremic patients. Sleep. 1998;21:617-622. [DOI] [PubMed] [Google Scholar]
- 19. Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis. 2001;38(1):104-108. doi: 10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- 20. Liu GJ, Wu L, Wang SL, et al. Incidence of augmentation in primary restless legs syndrome patients may not be that high: evidence from a systematic review and meta-analysis. Medicine. 2016;95:e2504. doi: 10.1097/MD.0000000000002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. 2018;29(7):1970-1978. doi: 10.1681/asn.2018010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levin A, Adams E, Barrett BJ, et al. Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD): form and function. Can J Kidney Health Dis. 2018;5. doi: 10.1177/2054358117749530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohnen R, Martinez-Martin P, Benes H, et al. Rating of daytime and nighttime symptoms in RLS: validation of the RLS-6 scale of restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2016;20:116-122. doi: 10.1016/j.sleep.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 24. GarcÃ-a-Borreguero D, Allen RP, Kohnen R, et al. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine-International Restless Legs Syndrome Study Group consensus conference at the Max Planck Institute. Sleep Med 2007;8(5):520-530. doi: 10.1016/j.sleep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 25. Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Medicine. 2003;4:121-132. doi:S1389945702002587. [DOI] [PubMed] [Google Scholar]
- 26. Allen R, Oertel W, Walters A, et al. Relation of the International Restless Legs Syndrome Study Group rating scale with the Clinical Global Impression severity scale, the restless legs syndrome 6-item questionnaire, and the restless legs syndrome-quality of life questionnaire. Sleep Med. 2013;14(12):1375-1380. doi: 10.1016/j.sleep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 27. Allen RP. Minimal clinically significant change for the International Restless Legs Syndrome Study Group rating scale in clinical trials is a score of 3. Sleep Med. 2013;14(11):1229. doi: 10.1016/j.sleep.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 28. Lee SF, Bangdiwala SI, Spence J, Connolly S. When and how should we cluster and cross over: methodological and ethical issues (letter 2). Can J Anaesth. 2019;66(2):237-238. [DOI] [PubMed] [Google Scholar]
- 29. Dmitrienko A, Offen WW, Westfall PH. Gatekeeping strategies for clinical trials that do not require all primary effects to be significant. Stat Med. 2003;22:2387-2400. doi: 10.1002/sim.1526. [DOI] [PubMed] [Google Scholar]
- 30. Kaye CM, Nicholls B. Clinical pharmacokinetics of ropinirole. Clin Pharmacokinet. 2000;39:243-254. doi: 10.2165/00003088-200039040. [DOI] [PubMed] [Google Scholar]
- 31. Blum RA, Comstock TJ, Sica DA, et al. Pharmacokinetics of gabapentin in subjects with various degrees of renal function. Clin Pharmacol Ther. 1994;56(2):154-159. [DOI] [PubMed] [Google Scholar]
- 32. Wong MO, Eldon MA, Keane WF, et al. Disposition of gabapentin in anuric subjects on hemodialysis. J Clin Pharmacol. 1995;35(6):622-626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-jpg-2-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-pdf-1-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-pdf-2-cjk-10.1177_2054358120968959 for The DIalysis Symptom COntrol-Restless Legs Syndrome (DISCO-RLS) Trial: A Protocol for a Randomized, Crossover, Placebo-Controlled Blinded Trial by David Collister, Kayla Pohl, Gwen Herrington, Shun Fu Lee, Christian Rabbat, Karthik Tennankore, Deborah Zimmermann, Navdeep Tangri, Ron Wald, Braden Manns, Rita S. Suri, Annie-Claire Nadeau-Fredette, Remi Goupil, Samuel A. Silver and Michael Walsh in Canadian Journal of Kidney Health and Disease