Abstract
Objective
Beclin1 plays a central role in the activation of the autophagy signaling pathway. Beclin1 and LC3-related proteins are involved in the initial steps of autophagy, which are closely related to the occurrence and development of tumors. The current meta-analysis aimed to clarify the correlation between expression of Beclin1 and LC3 and prognosis of ovarian cancer.
Methods
We searched PubMed, Embase, The Cochrane Library, Web of Science, and CNKI using predefined selection criteria. Pooled hazard ratios and relative risks with 95% confidence intervals were used to evaluate the correlation between autophagy-related genes Beclin1 and LC3 and overall survival (OS), progression-free survival (PFS), and International Federation of Gynecology and Obstetrics (FIGO) stage.
Results
In total, 1497 patients from 10 articles were enrolled in this meta-analysis. Expression of Beclin1 was significantly correlated with improved OS and PFS, and increased expression of Beclin1 was correlated with early FIGO stage, but not with lymph node metastasis or histological grade. No association was found between LC3 expression and prognosis in patients with ovarian cancer.
Conclusions
Expression of Beclin1 is an independent risk factor for the progression of ovarian cancer. Thus, Beclin1 is a promising indicator in predicting prognosis in patients with ovarian cancer.
Keywords: Ovarian neoplasm, autophagy, Beclin-1, LC3, meta-analysis, prognosis
Introduction
Ovarian cancer is the third most common gynecological malignant tumor in the female reproductive system, with about 21,750 new cases worldwide every year. The 5-year survival rate of ovarian cancer patients is only 48%.1 According to statistics, 59% of patients have distant metastasis when diagnosed with the disease. Despite tumor cell cytoreductive surgery and first-line chemotherapy, about 70% to 80% of stage III–IV patients relapse within 5 years.2,3 Ovarian cancer is prone to be resistant to chemotherapy drugs, which greatly affects the prognosis and life quality of patients.
Autophagy is a highly conserved degradation mechanism. The autophagy of eukaryotic cells is effected via three pathways: macroautophagy, chaperone-mediated autophagy, and microautophagy.4,5 The correlation between autophagy and ovarian cancer has gained increasing attention in recent years. Our previous study demonstrated a critical role of the autophagy-related gene Beclin1 in the process of killing ovarian cancer cells by chemotherapy, whereas regulation of autophagy of abnormal cell autophagy regulation was closely related to chemotherapy resistance.6–8 Therefore, with the present meta-analysis, we aimed to assess the correlation between the expression of Beclin1 and LC3 in ovarian cancer patients and prognosis. This would aid in exploring the role of autophagy in the occurrence and development of ovarian cancer and provide a reference for the diagnosis, treatment, and prognosis of this disease.
Material and methods
This study was conducted according to the guidelines outlined in Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement. Ethical approval and informed consent were deemed unnecessary for this meta-analysis.
Search strategy
Publications were retrieved from the PubMed, Embase, The Cochrane Library, Web of Science, and CNKI databases up to 31 October 2019. The keywords in our investigation were (ovarian cancer or ovarian neoplasm or ovarian tumor or ovarian carcinoma) and (autophagy-related protein or Beclin1 or Beclin-1 or BECN1 or LC3). The search was performed independently in duplicate by two investigators (Xinbei Chen and Yang Sun).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) any human studies that estimated the level of Beclin1 and LC3 expression and prognosis and were published in English and Chinese; (2) the patients in the original research were definitively diagnosed with ovarian cancer by histopathology; (3) the correlation between Beclin1 and LC3 expression and the clinicopathological parameters or prognosis of ovarian cancer was reported; (4) hazard ratios (HRs) and 95% confidence intervals (CIs) could be extracted directly from the article or calculated indirectly by the Kaplan–Meier curve. Studies were excluded if they met one of the following criteria: (1) reviews and case reports; (2) studies carried out on cell or animal specimens; (3) in a language other than English or Chinese; or (4) full-text of the article was not available.
Data extraction and quality assessment
The data were independently extracted by two investigators (Xinbei Chen and Yang Sun) from the published literature, according to the prescribed standards. The results were carefully checked by a third investigator (Bingrong Wang). The specific characteristics were extracted from each study: first author, year, nation, number of patients, average age, International Federation of Gynecology and Obstetrics (FIGO) stage, lymph node metastasis, and HR. The quality of retrieved papers was evaluated using the Newcastle–Ottawa scale (NOS); the scores varied from 0 to 9, and a score of >6 indicated a high-quality study.
Statistical analysis
Results were pooled and expressed as HR and 95% CI using RevMan5.3 (The Nordic Cochrane Centre, www.cochrane.org) to evaluate the correlation between autophagy-related genes Beclin1 and LC3 and prognosis. For studies that only provided the Kaplan–Meier curves, we estimated the HR and 95% CI from the curves. Heterogeneity was assessed by the inconsistency index I2, which was considered significant at >50%. If the heterogeneity was significant, the random-effects model was adopted; otherwise, the fixed-effects model was used. A sensitivity analysis was performed to estimate the reliability of the pooled result.
Results
Inclusion criteria
A total of 1410 studies were identified according to the search strategy. Of these, 163 studies were removed because of duplication. Then, the abstracts were screened, and 1352 studies were excluded due to duplication or because they were case reports, reviews, or laboratory research reports. After screening the full-text of the articles, 48 studies were excluded. Finally, 10 studies were included for further analysis (Figure 1). These studies were published from 2012 to 2017. One article was in Chinese and the others were in English. The studies encompassed 1497 patients. The expression of Beclin1 and LC3 expression in ovarian cancer specimens was evaluated by immunohistochemistry (IHC). The HR of five studies was obtained directly from the articles or, in three studies, indirectly calculated from the Kaplan–Meier curves. The NOS score of the enrolled studies ranged from 5 to 8, and the studies were mostly high quality (Figure 2). The baseline characteristics of the included studies9–18 are shown in Table 1.
Figure 1.
Schematic of the study search and the selection process.
Figure 2.
Risk of bias graph, including (A) risk of bias items for each included study, and (B) judgments about each risk of bias item.
Table 1.
Baseline characteristics of the included studies.
| Author | Region | Sample size | Age (years) | Assay method | TNM stage (I–II/III–IV) | Lymph node metastasis (yes or no) | Histological grade (G1+G2/G3) | Research object | HR statistic |
|---|---|---|---|---|---|---|---|---|---|
| Valente et al., 201414 | Italy | 61 | 50.8 (19–84) | IHC | 34/27 | NA | 23/38 | Beclin1, LC3 | NA |
| Katagiri et al., 201517 | Japan | 60 | NA | IHC | 45/15 | 51/9 | NA | Beclin1 | Survival curve |
| Lin et al., 201310 | China | 169 | 61 (46–76) | IHC | 50/119 | 83/86 | 129/40 | Beclin1 | Data in paper |
| Spowart et al., 201213 | Canada | 485 | 51.2 (20–81) | IHC | NA | NA | NA | LC3 | Data in paper |
| Jin et al., 201716 | China | 63 | 48 (25–72) | IHC | 20/43 | 47/16 | 17/46 | Beclin1, LC3 | Survival curve |
| Ju et al., 201618 | China | 39 | 57 (29–89) | IHC | 19/20 | NA | NA | Beclin1 | NA |
| Cai et al., 20149 | China | 148 | NA | IHC | 65/83 | 90/24 | 94/54 | Beclin1 | Data in paper |
| Miyamoto et al., 201712 | Japan | 100 | 48 (35–58) | IHC | 63/37 | 55/15 | NA | LC3 | Data in paper |
| Minamoto et al., 201711 | Japan | 141 | 50.3 (19–79) | IHC | 80/61 | NA | NA | Beclin1 | Data in paper |
| Zhao et al., 201315 | China | 231 | NA | IHC | 86/145 | NA | NA | Beclin1 | Survival curve |
IHC, immunohistochemistry; TNM, tumor node metastases; HR, hazard ratio.
Correlation between Beclin1 and prognosis of ovarian cancer
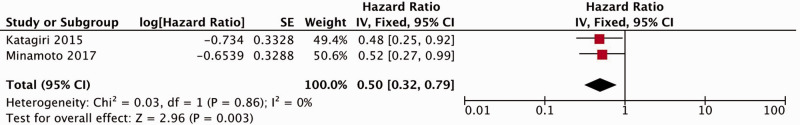
Six studies reported a correlation between Beclin1 expression and overall survival (OS).9–11,15–17 Because the samples were not heterogeneous (I2 = 0%), the fixed-effect model was applied to calculate the accumulated HR and 95% CI. The results indicated that patients with increased Beclin1 expression showed improved OS (HR = 0.56, 95% CI: 0.44–0.71, P < 0.00001) (Figure 3). Two studies evaluated the correlation between Beclin1 expression and progression-free survival (PFS),11,17 and because no heterogeneity was observed (I2 = 0%), we used the fixed-effects model. The pooled results suggested that the PFS was improved in patients with high Beclin1 expression (HR = 0.50, 95% CI: 0.32–0.79, P = 0.003) (Figure 4).
Figure 3.
Forest plot for the association between Beclin1 expression and overall survival. CI, confidence interval.
Figure 4.
Forest plot for the association between Beclin1 expression and progression-free survival. CI, confidence interval.
Correlation between Beclin1 and FIGO stage of ovarian cancer
Eight studies included FIGO stage (III/IV vs. I/II) associated with different expression levels of Beclin1.9–11,14–18 Because of the heterogeneity among the included studies (I2 = 74%), the random-effect model was used to generate cumulative relative risk (RR), together with the corresponding 95% CI. The results demonstrated that ovarian cancer with elevated Beclin1 expression was predisposed to early (I/II) FIGO stage (RR = 0.78, 95% CI: 0.62–0.99, P = 0.04) (Figure 5).
Figure 5.
Forest plot for the association between Beclin1 expression and International Federation of Gynecology and Obstetrics (FIGO) stage. CI, confidence interval; M-H, Mantel-Haenszel.
Correlation between Beclin1 and lymph node metastasis
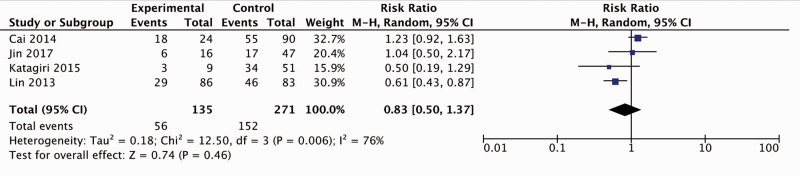
Four studies elucidated the link between Beclin1 expression and lymph node metastasis.9,10,14,16 With obvious heterogeneity among the included studies (I2 = 76%), the random-effects model was used to generate the cumulative RR and corresponding 95% CI, which showed statistical significance (RR = 0.83, 95% CI: 0.50–1.37) (Figure 6). Although ovarian cancer patients with upregulated Beclin1 expression had a lower incidence of lymph node metastasis, the difference was not statistically significant.
Figure 6.
Forest plot for the association between Beclin1 expression and lymph node metastasis. CI, confidence interval; M-H, Mantel-Haenszel.
Correlation between Beclin1 and histological grade
Herein, four studies investigated the association between Beclin1 expression and histological grade.9,10,14,16 Because the samples were heterogeneous (I2 = 87%), the random-effects model was applied to calculate the accumulated RR and 95% CI (Figure 7). No significant association was detected between Beclin1 expression and histological grade.
Figure 7.
Forest plot for the association between Beclin1 expression and histological grade. CI, confidence interval; M-H, Mantel-Haenszel.
Correlation between LC3 and prognosis of ovarian cancer
To further understand the prognosis of LC3, we investigated the correlation between LC3 expression and OS.11–13,16 As shown in Figure 8, heterogeneity was observed (I2 = 72%) and the random-effects model was applied. The pooled results showed no association between LC3 expression and prognosis of ovarian cancer patients.
Figure 8.
Forest plot for the association between LC3 expression and overall survival. CI, confidence interval.
Subgroup analysis
Subgroup analyses were stratified by region, sample size, and method of extraction (direct extraction of HRs and 95% CI from the published paper versus indirect extraction from Kaplan–Meier curves) for OS. As shown in Table 2, the combined HRs for China and other regions were 0.59 (95% CI: 0.45–0.79, P = 0.0004) and 0.48 (95% CI: 0.30–0.76, P = 0.002). The combined HRs for small and large sample sizes were 0.56 (95% CI: 0.39–0.81, P = 0.002) and 0.56 (95% CI: 0.40–0.77, P = 0.0005), respectively, when 100 patients were used as the threshold. In addition, the summary HR was 0.52 for direct extraction group (95% CI: 0.37–0.73, P = 0.0001) and 0.56 for the indirect extraction group (95% CI: 0.40–0.77, P = 0.006). The heterogeneity of the indirect extraction group was larger than that of the direct extraction group, albeit not significantly.
Table 2.
Results of subgroup analyses for Beclin1 expression and overall survival.
| Stratified analysis | Number of studies | HR (95% CI) | P-value | Heterogeneity | |
|---|---|---|---|---|---|
| I² (%) | P-value | ||||
| Region | |||||
| China | 4 | 0.59 (0.4–0.79) | 0.0004 | 0 | 0.47 |
| Other | 2 | 0.48 (0.30–0.76) | 0.002 | 0 | 0.83 |
| Sample size | |||||
| <100 | 2 | 0.56 (0.39–0.81) | 0.002 | 0 | 0.43 |
| ≥100 | 4 | 0.56 (0.40–0.77) | 0.0005 | 0 | 0.46 |
| Extracted method | |||||
| Direct | 3 | 0.52 (0.37–073) | 0.0001 | 0 | 0.94 |
| Indirect | 3 | 0.61 (0.43–0.86) | 0.006 | 26 | 0.26 |
HR, hazard ratio; CI, confidence interval.
Sensitivity analysis
Sensitivity analysis was performed to determine the reliability of the pooled results. The pooled HR for OS and FIGO stage was not influenced by the sequential exclusion of studies, indicating the consistency of the results.
Discussion
Autophagy is a highly conserved process of programmed cell death. Under stress, autophagy can also degrade proteins, glycogen, and damaged organelles in lysosomes via a series of pathways and provide energy for cell survival.19 Abnormal autophagy regulation may lead to tumors. Intriguingly, autophagy plays a dual role in malignant tumors. In early stages, it often inhibits the progression of tumors, whereas in later stages, it resists stress environments such as hypoxia and nutritional deficiency and induces drug resistance to promote tumor development.20
Recent studies have demonstrated that the autophagy-related genes Beclin1 and LC3 could be used to predict the prognosis of malignant tumors. Beclin1 was found to be associated with the clinical prognosis of patients (breast cancer and gastric cancer), and patients with high expression of Beclin1 exhibited a satisfactory outcome.21,22 Researchers are paying more attention to the correlation between autophagy and gynecological malignancies. Our previous study discovered that the upregulation of Beclin1 was related to the downregulation of vascular endothelial growth factor (VEGF) and matrix metalloproteinase 9 (MMP-9); both proteins inhibit cell proliferation, invasion, and metastasis. In addition, cervical cancer patients with high expression of Beclin1 show a higher 3-year OS.23 Studies concerning autophagy and ovarian cancer in the SKOV3/DDP cell line demonstrated that Beclin1 regulates antitumor activity through a mitochondrial-dependent pathway. Cells with high expression of Beclin1 showed an increasing number of apoptotic cells under a specific concentration of cisplatin.8 Recent studies have found that autophagy may be related to targeted therapy of ovarian cancer. Wen et al.24 proposed a human-derived monoclonal antibody MORAB-003 (farletuzumab) against folate receptor-α (FRα) by regulating autophagy-related genes Beclin1 and LC3-II, which can promote the expression of LC3-II and the formation of autophagic vacuoles. We found a meta-analysis that evaluated the prognostic role of autophagy-related proteins in epithelial ovarian cancer in 2017,25 but the number of studies included was smaller and the quality was average compared with those of the current meta-analysis. It found that the expression of Beclin1 and LC3 was not related to the prognosis of ovarian cancer, a finding that is inconsistent with recent clinical studies. In our meta-analysis, we included a larger number of recent clinical studies of higher quality to better reflect the relationship between the expression of Beclin1 and LC3 and the prognosis of ovarian cancer. Our results showed that ovarian cancer patients with high expression of Beclin1 had better prognosis, longer OS and PFS, and earlier FIGO stage than those with low expression. However, expression of LC3 was not significantly correlated with prognosis of ovarian cancer. In our study, Beclin1 expression was not associated with lymph node metastasis or histopathological grade. Thus, additional studies focusing on the correlation between autophagy and ovarian cancer are essential. Zhou et al.26 conducted cell experiments and found that tanshinone I promotes Beclin1 expression and increases autophagy to suppress the proliferation of the ovarian cancer cell lines A2780 and ID-8 by inhibiting the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway. Liang et al.27 proposed that the transfection of STAT-DN (dominant negative signal transducer and activator of transcription) in SKOV3 ovarian cancer cells inhibits the mitogen-activated protein kinase (MAPK) and PI3K/AKT/mTOR signaling pathways, activates the expression of autophagy-related molecules, reduces the resistance of cells to cisplatin therapy, and promotes cell death. These studies suggested a putative mechanism for the prognosis of ovarian cancer with the highly expressed Beclin1. Bhattacharjee et al.28 demonstrated that ormeloxifene promotes the expression of Beclin1 and LC3-II by inhibiting the PI3K/AKT/mTOR signaling pathway, which in turn, significantly reduces the size of ovarian cancer tumors in mice. As described earlier, several studies have found that the drug-elevated expression of Beclin1 promotes the death of ovarian cancer cells, reduces tumor volume, and improves the prognosis of ovarian cancer patients, which is consistent with the results of the current meta-analysis. Beclin1, a protein that interacts with either BCL-2 or PI3k class III, plays a critical role in the regulation of both autophagy and cell death, and LC3 is an autophagy-related protein considered to be a sign of ongoing autophagy.14 Usually, the expression of Beclin1 and LC3 is highly consistent. However, in the current meta-analysis, expression of LC3 was not significantly correlated with the prognosis of ovarian cancer. This might be due to the high expression of BCL-2, which may cause Beclin1 to lose the effect of autophagy and decrease autophagy, which might show that the expression of LC3 was negative.14 Minamoto et al.11 noted that cancers that are Beclin1-positive but LC3-negative may be associated with a function of Beclin1 outside its involvement in autophagy. In a previous study, Rohatgi et al.29 reported that Beclin1 regulates growth factor signaling, including AKT and the extracellular signal-regulated kinase (ERK) pathway. More in-depth clinical research is required to explore this issue.
The present study has some limitations. First, the studies included were all cohort-based, which represents a medium quality of clinical evidence; the meta-analysis lacked non-public published literature. Second, the IHC methods and scoring criteria adopted in each study were slightly different. The evaluation methods were qualitative and not quantitative. Moreover, the samples investigated in this meta-analysis were obtained intraoperatively, making it impossible to investigate longitudinal changes in expression of Beclin1 and LC3 before and after chemotherapy. Furthermore, for some studies, we extracted HR values using the Kaplan–Meier curve, which might have introduced some errors in the results. Our study was not registered in PROSPERO.
Conclusion
The expression of the autophagy-related gene Beclin1 is an independent risk factor that affects the prognosis of ovarian cancer patients. It can be applied to screen patients for poor prognosis and would provide a basis for clinicians to develop personalized treatment plans. prospective and large-scale studies are essential to confirm the correlation between autophagy and the prognosis of ovarian cancer. We hope to explore the correlation between autophagy and chemotherapy resistance through clinical trials in the future.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This project was funded by grant from the National Natural Science Foundation of China (no. 81873045).
ORCID iDs: Xinbei Chen https://orcid.org/0000-0002-4247-1148
Yang Sun https://orcid.org/0000-0003-3271-1491
Bingrong Wang https://orcid.org/0000-0003-2597-1033
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Bookman MA, Okamoto A, Stuart G. Harmonising clinical trials within the Gynecologic Cancer InterGroup: consensus and unmet needs from the Fifth Ovarian Cancer Consensus Conference. Ann Oncol 2017; 28: 30–35. doi: 10.1093/annonc/mdx449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson K, Pujade LE, Aoki MR. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol 2017; 28: 727–732. doi: 10.1093/annonc/mdw663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 2018; 19: 365–381. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol 2018; 20: 521–527. doi: 10.1038/s41556-018-0092-5. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Jin L, Sui YX, et al. Circadian gene CLOCK affects drug-resistant gene expression and cell proliferation in ovarian cancer SKOV3/DDP cell lines through autophagy. Cancer Biother Radiopharm 2017; 32: 139–146. doi: 10.1089/cbr.2016.2153. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Liu JH, Jin L, et al. Effect of autophagy-related Beclin1 on sensitivity of cisplatin- resistant ovarian cancer cells to chemotherapeutic agents. Asian Pac J Cancer Prev 2015; 16: 2785–2791. doi: 10.7314/apjcp.2015.16.7.2785. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Liu JH, Jin L, et al. Inhibition of Beclin 1 expression enhances cisplatin-induced apoptosis through a mitochondrial-dependent pathway in human ovarian cancer SKOV3/DDP cells. Oncology Res 2014; 21: 261–269. doi: 10.3727/096504014X13946388748992. [DOI] [PubMed] [Google Scholar]
- 9.Cai M, Hu Z, Liu JH, et al. Beclin 1 expression in ovarian tissues and its effects on ovarian cancer prognosis. Int J Mol Sci 2014; 15: 5292–5303. doi: 10.3390/ijms15045292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin HX, Qiu HJ, Zeng F, et al. Decreased expression of Beclin 1 correlates closely with Bcl-xL expression and poor prognosis of ovarian carcinoma. PLoS One 2013; 8: e60516. doi: 10.1371/journal.pone.0060516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minamoto T, Nakayama K, Nakamura K, et al. Loss of beclin 1 expression in ovarian cancer: A potential biomarker for predicting unfavorable outcomes. Oncol Lett 2017; 15: 1170–1176. doi: 10.3892/ol.2017.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto M, Takano M, Aoyama T, et al. Inhibition of autophagy protein LC3A as a therapeutic target in ovarian clear cell carcinomas. J Gynecol Oncol 2017; 28: e33. doi: 10.3802/jgo.2017.28.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spowart JE, Townsend KN, Huwait H, et al. The autophagy protein LC3A correlates with hypoxia and is a prognostic marker of patient survival in clear cell ovarian cancer. J Pathol 2012; 228: 437–447. doi: 10.1002/path.4090. [DOI] [PubMed] [Google Scholar]
- 14.Valente G, Morani F, Nicotra G, et al. Expression and clinical significance of the autophagy proteins BECLIN 1 and LC3 in ovarian cancer. Biomed Res Int 2014; 2014: 462658. doi: 10.1155/2014/462658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Chen S, Gou WF, et al. Aberrant Beclin 1 expression is closely linked to carcinogenesis, differentiation, progression, and prognosis of ovarian epithelial carcinoma. Tumour Bio 2013; 35: 1955–1964. doi: 10.1007/s13277-013-1261-6. [DOI] [PubMed] [Google Scholar]
- 16.Jin L, Sui YX, Sun Y, et al. Expression of autophagy-related genes Beclinl and LC3 in ovarian serous carcinoma and their clinical significance. J Clin Exp Pathol 2017; 33: 1219–1224. [Google Scholar]
- 17.Katagiri H, Nakayama K, Razia S, et al. Loss of autophagy-related protein Beclin 1 may define poor prognosis in ovarian clear cell carcinomas. Int J Oncol 2015; 47: 2037–2044. doi: 10.3892/ijo.2015.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju LL, Zhao CY, Ye KF, et al. Expression and clinical implication of Beclin1, HMGB1, p62, survivin, BRCA1 and ERCC1 in epithelial ovarian tumor tissues. Eur Rev Med Pharmacol Sci 2016; 20: 1993–2003. [PubMed] [Google Scholar]
- 19.Yim WWY, Mizushima N. Lysosome biology in autophagy. Cell Discov 2020; 11: 6. doi:10.1038/s41421-020-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol 2017; 14: 170–184. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 21.Tang H, Sebti S, Titone R, et al. Decreased BECN1 mRNA expression in human breast cancer is associated with estrogen receptor-negative subtypes and poor prognosis. EBioMedicine 2015; 2: 255–263.doi: 10.1016/j.ebiom.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao QH, Liu F, Yang ZL, et al. Prognostic value of autophagy related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Transl Res 2016; 8: 3831–3847. [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Liu JH, Sui YX, et al. Beclin1 overexpression inhibits proliferation, invasion and migration of CaSki cervical cancer cells. Asian Pac J Cancer Prev 2011; 12: 1269–1273. [PubMed] [Google Scholar]
- 24.Wen Y, Grabill WS, Previs RA, et al. Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res 2015; 21:448–459. doi: 10.1158/1078-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Z, Xue J, Zhao XR, et al. Prognostic role of autophagy-related proteins in epithelial ovarian cancer: a meta-analysis of observational studies. Minerva Med 2017; 108: 277–286. doi: 10.23736/S0026-4806.16.04767-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Jiang YY, Chen H, et al. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif 2020; 53: e12739. doi: 10.1111/cpr.12739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Liang F, Ren C, Wang J, et al. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT- mediated regulation of EMT and autophagy. Oncogenesis 2019; 8: 59. doi: 10.1038/s41389-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharjee A, Hasanain M, Kathuria M, et al. Ormeloxifene-induced unfolded protein response contributes to autophagy-associated apoptosis via disruption of Akt/mTOR and activation of JNK. Sci Rep 2018; 8: 2303. doi: 10.1038/s41598-018-20541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohatgi RA, Janusis J, Leonard D, et al. Beclin 1 regulates growth factor receptor signaling in breast cancer. Oncogene 2015; 34: 5352–5362. doi: 10.1038/onc.2014.454. [DOI] [PMC free article] [PubMed] [Google Scholar]