Abstract
Coronavirus disease 2019 (COVID-19) is an infective disease generated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Given the pandemic urgency and lack of an effective cure for this disease, drug repurposing could open the way for finding a solution. Lots of investigations are ongoing to test the compounds already identified as antivirals. On the other hand, induction of type I interferons are found to play an important role in the generation of immune responses against SARS-CoV-2. Therefore, it was opined that the antivirals capable of triggering the interferons and their signaling pathway, could rationally be beneficial for treating COVID-19. On this basis, using a database of antivirals, called drugvirus, some antiviral agents were derived, followed by searches on their relevance to interferon induction. The examined list included drugs from different categories such as antibiotics, immunosuppressants, anti-cancers, non-steroidal anti-inflammatory drugs (NSAID), calcium channel blocker compounds, and some others. The results as briefed here, could help in finding potential drug candidates for COVID-19 treatment. However, their advantages and risks should be taken into account through precise studies, considering a systemic approach. Even though the adverse effects of some of these drugs may overweight their benefits, considering their mechanisms and structures may give a clue for designing novel drugs in the future. Furthermore, the antiviral effect and IFN-modifying mechanisms possessed by some of these drugs might lead to a synergistic effect against SARS-CoV-2, which deserve to be evaluated in further investigations.
Keywords: COVID-19, Pandemic, Severe acute respiratory syndrome coronavirus 2, Antiviral, Interferon, Immune system
1. Introduction
Coronavirus disease 2019 (COVID-19) pandemic has aroused a worldwide threat and resulted in a growing need for preventive and therapeutic strategies. The infective agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a member of coronaviruses family, a group of positive-sense, single-stranded RNA viruses surrounded by a lipid bilayer envelope. The envelope consists of spike glycoproteins, responsible for the virulence and cell receptor recognition mechanisms, which ultimately enables virus entry into the host cells [1]. Coronaviruses primarily cause zoonotic infections and are categorized into four genera: alphacoronavirus, betacoronavirus, deltacoronavirus, and gammacoronavirus [2]. SARS-CoV-2 is a novel betacoronavirus responsible for clinical manifestations similar to that of severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV), which are also from the genus betacoronavirus [3].
Multiple studies have uncovered the importance of interferons (IFNs) in effective adaptive immune responses against viral infections at various stages. The key role of IFNs in the induction of antiviral immune responses in coronavirus infections is also well-established [4]. Type I interferons (IFN-II) significantly contribute to the induction of immune cells, particularly antigen presenting cells (APCs), natural killer cells (NKs), T cells, and B cells [5]. While IFN-II are imperative in restricting viral replication through the signaling mechanisms integrated in type I interferon receptors (IFNAR), low levels of IFNs are detected in blood and lung tissue samples of COVID-19 patients [6], [7], implicating the inhibitory effect of SARS-CoV-2 on IFN-II induction [8]. Additionally, the cell count of regulatory T cells (Treg) was shown to correlate inversely with the infection severity in SARS-CoV-2 patients [9].
While the early induction of IFN-I inhibits viral replication, it seems that coronaviruses delay IFN-I production through various evasion mechanisms, resulting in a high viral load. This could consequently bring about the late induction of IFN-mediated antiviral responses, which not only is useless in the control and suppression of the viral load, but also would cause tissue damage and inflammatory responses [10]. In this line, in a retrospective cohort study in china, early administration of inhaled aerosolized IFN-α2b was associated with reduced mortality and shorter hospital stay in patients. On the other hand, late administration of inhaled IFN-α2b, resulted in longer hospital stay as well as increased patient mortality [11]. Besides, in a study by Monk et al. at nine sites in UK, inhaled nebulized IFN-β1a (SNG001) was administered in 48 COVID-19 patients, which resulted in a greater improvement on day 15 or 16 than placebo group according to OSCI (ordinal scale for clinical improvement) scale [12].
Additionally, a clear concordance between the pathogenesis of the coronaviruses and their ability to antagonize IFN-mediated antiviral responses was found, which seems to have a relation with the rapid replication of the virus and the subsequent proinflammatory overreactions and dysregulation of the immune system [13], [14]. Though, the probable reason for the lower mortality rate of COVID-19 than that of MERS and SARS might also be due to the weaker IFN-antagonizing effect imposed by SARS-CoV-2 [15]. Notably, various studies discovered the impaired IFN-mediated antiviral responses in the elderlies [16], which might be the cause of poor patient prognosis in this group. Moreover, it was suggested that the higher susceptibility of African-American populations to SARS-CoV-2 might be correlated with lower IFN-I production in response to the viral RNA [17].
The canonical signaling pathway of JAK/STAT induction by type I and II IFNs was well-established in previous studies [18]. Briefly, in the IFN-I mediated signaling pathway, IFNARs bind to IFN-I resulting in Janus kinase 1 (JAK1) and Tyrosine kinase 2 (TYK2) induction, which then phosphorylate the transcription factors signal transducer and activator of transcription 1 (STAT1) and 2 (STAT2). These phosphorylated factors are then relocated inside the nucleus, where they assemble with interferon -regulatory factor 9 (IRF9), constituting interferon -stimulated gene factor 3 (ISGF3). It ultimately induces the transcription of interferon-stimulated genes (ISGs), which plays a significant role in antiviral immune responses [19].
It is suggested that the pathogenicity of coronavirus infections highly depends on the virus ability to evade IFN mediated immune responses [20]. Coronaviruses have developed multiple evasion mechanisms to avoid IFN-I induction [19]. SARS-CoV-2, also might have developed similar evasion mechanisms, as suggested by the lack of adequate IFN-I/3 production in the primary bronchial cells, infected cell lines, and a ferret model [7]. Coronaviruses avoid pattern-recognition proteins (PRPs), which are responsible for the induction of proinflammatory reactions and IFN mediated antiviral responses. SARS-CoV-1 form a double membrane vesicle, hiding the dsRNA of the virus [21]. Additionally, several viral non-structural proteins (namely, nsp10, nsp13, nsp14, and nsp16) modify the viral RNA by adding a guanosine cap and methylation at the 5′ end, stunting the receptor sensing mechanisms [22], [23]. Coronaviruses have also developed mechanisms to interfere with IRF3 and membrane protein (M protein). Besides, they can interfere with accessory proteins (open reading frame 4a (ORF4a), ORF4b, and ORF5), which cause suppression of phosphorylation and translocation of IRF3 in MERS-CoV [24]. SARS-CoV-1 interrupts the functions of IRF3 by ORF3b and ORF6 and nucleocapsid protein (N protein) [25], [26]. Human coronaviruses are also able to block IFNAR and interferon-lambda receptor (IFNLR) signaling pathways and suppress ISG effector functions as part of their immune system evasion mechanisms [27], [28]. These mechanisms ultimately antagonize IFN production and might play a similar role in the IFN mediated response delay caused by SARS-CoV-2. In a recently published global study on critical SARS-CoV-2 patients hospitalized due to life-threatening pneumonia caused by SARS-CoV-2, 13 mutated loci were identified in patients with life-threatening influenza or directly connected to influenza susceptibility, all of which play a role in TLR3-, TLR7-, and TLR9-dependent IFN-I induction and amplification pathways. Results from this study identified at least 3.5% of patients aged 17–77 with life-threatening SARS-CoV-2 patients had known or novel genetic deficiencies in eight out of 13 candidate loci involved in the TLR3 and TLR7 dependent IFN-I induction and amplification pathways. These mutations consisted of autonomic recessive (AR) IRF7 and IFNAR1 deficiencies or autonomic dominant (AD) TLR3, TICAM1, TBK1, IRF3 deficiencies as known mutations and AD UNC93B1, IRF7, IFNAR1, IFNAR2 deficiencies as novel mutations. Ultimately, these results indicate the role of these genes in IFN-I inductions and amplification pathways, thus highlighting the role of IFN-I in controlling SARS-CoV-2 [29]. Another defect in critically ill SARS-CoV-2 patients was also reported in a study regarding neutralizing auto-antibodies against IFN-I, demonstrating that at least 10 percent of patients with life-threatening COVID-19 pneumonia possessed auto-antibodies against IFN-I. This study also concluded that early IFN-α therapy might not serve as an effective solution and proposed injected or nebulized IFN-β therapy as potential treatment candidates in these patients, due to rare auto-antibodies against IFN-β in this patient groups [30].
Although IFN administration is a globally accepted treatment strategy to excite the antiviral immune responses, IFN therapy in the coronavirus-infected cases appears to generate rather controversial results. It seems that IFN therapy in rather early stages of the infection produce effective outcomes, while this strategy shows detrimental outcomes in the severe or later stages of the infection [31], [32]. A recent study reported the effectiveness of using of IFN-I, as a prophylactic agent against SARS-CoV-2 [15].
Drug repositioning has recently become an increasingly attractive subject due to the substantially decreased development costs and shorter timeline possibly achieved in this approach than those of conventional drug discovery methods, because of the need for undergoing many years of intensive investigations and phase I-III studies in the latter approach [33]. Since the outbreak of COVID-19, lots of attempts have taken place to find a treatment for this disease through drug repurposing of the licensed or well-studied drugs with antiviral activity.
The aim of this study is to briefly review some antiviral drugs and discuss their mechanism of action and impact on IFNs, as potential drug repositioning candidates for COVID-19 treatment.
2. Methods
The drugvirus web server (http://drugvirus.info/) was utilized in this study (the updated database at 23 May 2020), to find the relation between the antiviral effects of these conventional drugs and their effect on IFN signaling. This website provides data focusing on broad-spectrum antiviral agents (BSAA) and their target viruses as the main resource for an interactive exploration [34]. The name of each agent along with IFN related keywords including “interferon”, “ISG”, “STAT3”, “mTOR”, and “SARS” were searched in the PubMed database to find the relevance of the drug’ activity with IFN signaling pathway and its probable effect against SARS-CoV.
3. An overview of the findings
Several various categories of drugs present on drugvirus server were shown correlated with IFN signaling pathway, according to the results of our searches. These agents include antibiotic, anticancer, immunosuppressant, non-steroidal anti-inflammatory drugs (NSAID), and calcium channel blocker compounds (some are presented in Table 1 ), whose advantages and risks should be taken into account through precise studies, considering a systemic approach [35]. Multiple compounds of this list have already entered COVID-19 clinical trials, though some have been the subject of controversy [36]. The studied compounds will be discussed in the following in two main classes based on their origin: synthetic and natural compounds.
Table 1.
An overview on IFN-inducing effects of the antiviral agents. The clinical trial data was gathered on 17 September 2020. Molecular structures of the following drugs are shown as a representative of their classes; for instance, neomycin for aminoglycosides, idarubicin for anthracyclines, and simvastatin for statins. All structures are adapted from PubChem [37].
| Drug/Drug class | Molecular structure | Therapeutic category | Antiviral activity | COVID-19-related studies | Ref. | |
|---|---|---|---|---|---|---|
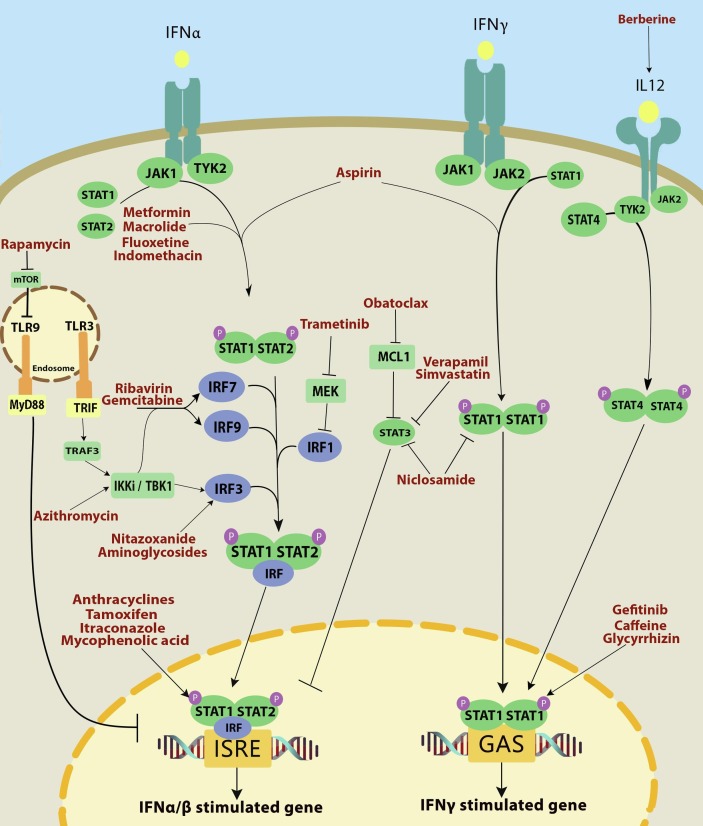
| 1 | Azithromycin | 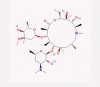 |
Antibiotic | Binding to IFNAR1 complex and ISGF3 upregulation/potentiating IFN type I signaling | 112 clinical trials specifically on azithromycin/Amplifying the effect of hydroxychloroquine | [38], [39], [40], [41], [42] |
| 2 | Aminoglycosides | 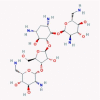 |
Antibiotic | ISG induction, suppress replication via binding to promotor RNA | No data found | [38], [43], [44] |
| 3 | Nitazoxanide | 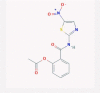 |
Amebicide | IFN signaling, autophagy promotion | 20 clinical trials in combination with azithromycin or hydroxychloroquine/Inhibits SARS-CoV-2 in cell culture | [38], [45], [46], [47], [48], [49], [50] |
| 4 | Niclosamide | 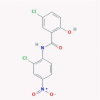 |
Anthelmintic | Inhibition of SARS-CoV 3CL protease, prevention of viral entry | Six clinical trials are enrolling./Suggested as an inhibitor of autophagy and viral replication | [45], [51], [52], [53] |
| 5 | Itraconazole |  |
Antifungal | Lanosterol 14α-demethylase inhibition, IFN A/B increase | No data found. | [38], [54] |
| 6 | Ribavirin | 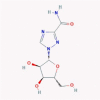 |
Antiviral | Inhibition of inosine monophosphate dehydrogenase/RdRp antagonism/causing error catastrophe | Suggested as a COVID-19 therapy/Suggested RdRp antagonist in an in silico study/Twelve clinical trials are enrolling | [45], [52], [55], [56] |
| 7 | Anthracyclines | 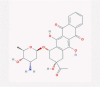 |
Antibiotic | Enhancement in ISRE activity, inhibition of RNA and DNA synthesis | Idarubicin was suggested as a potent inhibitor of SARS-CoV-2 endoribonuclease by an in silico study | [38], [57], [58], [59], [60] |
| 8 | Tamoxifen | 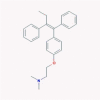 |
Anti-neoplastic | Multistep inflammation inhibitor | One clinical trial in combination with isotretinoin | [45], [52], [61] |
| 9 | Gefitinib | 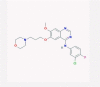 |
EGFR inhibitor | Inhibition of the NF-kB pathway and viral replication | No data found | [38], [62] |
| 10 | Trametinib | 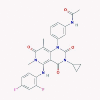 |
Multi-kinase inhibitor | Induction of IRF1 and IFN-κ | No data found | [45], [63] |
| 11 | Gemcitabine | 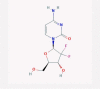 |
Anticancer | DNA synthesis inhibition | Predicted to have positive effects through AI analysis | [38], [64], [65] |
| 12 | Obatoclax | 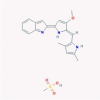 |
Anticancer | Suppression of viral endocytic uptake | No data found | [38], [66] |
| 13 | Sirolimus |  |
Immunosuppressant | Entry inhibitor, downregulation of CCR5 | Suggested in an interactome-based bioinformatics study/Six clinical trials are enrolling | [38], [52], [67], [68] |
| 14 | Mycophenolic acid |  |
Immunosuppressant | ISGs upregulation | Recognized as the most potent compound against the envelope protein and nucleocapsid phosphoprotein of SARS-CoV-2 by a docking study | [45], [69], [70] |
| 15 | Aspirin |  |
NSAID | COX-2 inhibition, p38 MAPK and MEK1/2 activation | Fourteen clinical trials are enrolling | [38], [52], [71] |
| 16 | Indomethacin |  |
NSAID | Production of 2′,5′-OAS, Th1 response enhancement, viral RNA synthesis block | Two clinical trials are enrolling. Opined for use in positive SARS-CoV-2 patients who have no cytokine storm |
[38], [52], [72], [73] |
| 17 | Metformin | 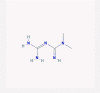 |
Anti-hyperglycemic | Promoting cells’ insulin sensitivity/JAK/STAT pathway activation | In silico suggestion as an interferer with Acetyl-CoA Carboxylase α gene | [38], [74], [75], [76] |
| 18 | Fluoxetine | 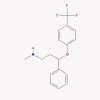 |
Antidepressant | JAK/STAT1 activation | Two clinical trials are enrolling | [38], [52], [77] |
| 19 | Verapamil | 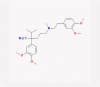 |
Calcium channel blocker | Cell entry inhibition | Three clinical trials are enrolling | [38], [52], [78] |
| 20 | Statins | 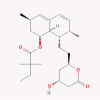 |
Anti-hypercholesterolemia | Prenylation inhibition, reduction of cell-to-cell fusion | Eleven clinical trials are enrolling./Hypothesized to be effective because of immunomodulatory effect and cardiovascular damage.Recommended for use in patients with cardiovascular disease history | [45], [52], [79], [80], [81] |
| 21 | Caffeine | 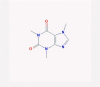 |
CNS stimulant | COX-2, HSP90, and Ras-ERK inhibition | Four clinical trials are enrolling | [45], [52], [82] |
| 22 | Glycyrrhizin | 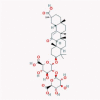 |
Anti-inflammatory | Downregulation of ROS formation, NF-κB, JNK, and p38 MAPK | Suggested as a potential drug because of ACE2 binding, proinflammatory cytokine downregulation, and ROS accumulation inhibition | [45], [83], [84] |
| 23 | Berberine | 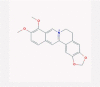 |
Antiseptic | Stimulation of Th1 response and IFN-γ | One clinical trial is enrolling | [45], [85], [86] |
2′, 5′-OAS: 2′-5′-oligoadenylate synthase/ACE: angiotensin converting enzyme/CoA: co-enzyme A/COVID-19: coronavirus disease 2019/COX: cyclooxygenase/ERK: Extracellular signal-regulated kinase/HSP90: heat shock protein 90/IFN: interferon/IFNAR: type I interferon receptor/IRF: interferon-regulatory factor/ISG: interferon-stimulated gene/ISGF: interferon-stimulated gene factor/ISRE: interferon-stimulated response element/JAK: Janus kinase/MEK: mitogen-activated protein kinase/NF-κB: nuclear factor-κB/RdRp: RNA-dependent RNA polymerase/ROS: reactive oxygen species/SARS-CoV: severe acute respiratory syndrome coronavirus disease/STAT: transducer and activator of transcription/Th: T helper cell.
4. Synthetic compounds
In this section, the chemically synthesized drugs will be reviewed, which are further classified based on their pharmacologic categories.
4.1. Anti-infective agents
4.1.1. Azithromycin
Antibiotics have been used for treating bacterial infections for many years. However, some of them also possess antiviral effects, such as macrolides, which are a class of antibiotics composed of a large lactone ring (12–16 atoms). Macrolides exhibit their antibacterial effect through interfering with protein synthesis in bacteria via binding to the 50S subunit of the bacterial ribosome. Many studies have reported the antiviral activity of macrolides against various viruses [87]. Although the antiviral modus operandi of macrolides are not clear, a possible mechanism is through binding to IFNAR1 complex and inducing STAT1/2, IRF7, IRF9, and finally, production of ISGF3 (Fig. 1 ) [40]. There is some evidence that different macrolides may be useful for viral respiratory infections. For instance, clarithromycin was found promising in the treatment of the influenza virus infection. Leucomycin A3 was also active against the influenza virus and enhanced IFN-α production [88].
Fig. 1.
The mechanism of action of possible anti-SARS-CoV-2 drugs on the interferon signaling pathway.
Azithromycin, as a macrolide, could inhibit rhinoviruses (rhinovirus 1B and rhinovirus 16) through potentiating IFN-I signaling. Moreover, azithromycin blocked the cell entry of Ebola virus through an unknown mechanism. It could inhibit the Zika virus infection as well [39], [40]. Both azithromycin and spiramycin showed antiviral effects against enterovirus A71 [89].
The most severe cases of influenza A H1N1 are usually associated with a secondary bacterial infection. In a cohort study, Ishaqui et al. showed that a combination of azithromycin and oseltamivir was more beneficial than oseltamivir alone in treating influenza A H1N1 [90]. In vitro studies demonstrated that azithromycin could block internalization of the H1N1 virus into host cells and inactivated the endocytic activity of the newly budded viruses. A single dose of nasal azithromycin reduced the virus load in mice lungs [7].
Azithromycin antiviral mechanism, theoretically, could be either through activation of IκB-kinase (IKK), IKK-ι/ε, and TANK-binding kinase 1 (TBK-1) signaling pathway, which leads to IRF stimulation (Fig. 1) or acting on interleukin (IL)-28 and IL-29 receptor complexes [40]. Azithromycin can induce type I and III IFNs (IFN-β and IFNλ1), melanoma differentiation-associated protein 5 (MDA5), toll-like receptor 3 (TLR3), retinoic inducible gene I (RIG-I), and RIG-I-like helicase in bronchial epithelial cells as the antiviral proteins thereby reducing viral load [39], [87], [91]. Moreover, azithromycin increased expression of IL-28 and IL-29. Interestingly, azithromycin can inhibit autophagy in macrophages. It was shown that azithromycin upregulated pathogen recognition receptors (PRRs) including IFIH1, DDX58, and ISGs, such as IFITM3, MX1, and RASD2, which suggests that azithromycin may improve the cell sensitivity to viral infections [39]. Azithromycin could increase rhinovirus-induced IFN and ISG expression as well [40]. It is believed that the IFN-β induction by azithromycin is mediated via the MDA5 receptor [92].
In addition to ACE2, SARS-CoV-2 seems to have other specific binding receptors. CD147, also called extracellular matrix metalloproteinase inducer (EMMPRIN), might be another route for internalization of SARS-CoV-2. It is suggested that azithromycin may interfere with the ligand/receptor interaction of the virus and CD147 [93].
Furthermore, there are some shreds of evidence that macrolides exert anti-inflammatory effects through inhibiting cytokine release [87]. Lin et al. proved that azithromycin could reduce production of proinflammatory cytokines, such as IL-12, IL-10, IL-6, IFN-γ, and tumor necrosis factor alpha (TNF-α). Therefore, azithromycin was proposed as an immunomodulatory agent for the treatment of inflammatory diseases [94]. In another study, azithromycin was shown to alleviate inflammation through suppressing IFN-γ-induced protein 10 (IP-10) and myeloid dendritic cell (mDC), which are the T helper cell 1 (Th1) and Th2 chemokines in monocytes, respectively. Such suppression is caused by inhibition of mitogen-activated protein kinase (MAPK)-JNK/Extracellular signal-regulated kinase (ERK) and nuclear factor κB (NF-κB) p65 signaling pathways [95]. In an experiment on mice, the prophylactic administration of azithromycin reduced airway inflammation, cytokine levels (IFN-γ, IL-5, and IL-6), and mortality rate after respiratory syncytial virus infection (RSV) [96].
The in vitro antiviral function of azithromycin and its pharmacological effect on COVID-19 in combination with hydroxychloroquine was proved [97]. In vitro experiments in Vero cells showed that these two drugs had a synergistic effect on SARS-CoV-2 [98].
In a clinical trial on 80 COVID-19 patients, in cases treated with a combination of hydroxychloroquine and azithromycin, the clinical condition was improved and the disease contagiousness was reduced [42]. The synergistic effect of azithromycin and hydroxychloroquine was also shown in another COVID-19 trial [41]. Though, Molina et al. claimed that no improvement in patients were observed by using this combination in their study [99].
Until September 17, 2020, 112 trials on the efficacy of azithromycin or its combination with other drugs on COVID-19 were registered on clinicaltrials.gov (Table 1), which could hopefully provide insight on using azithromycin in treating COVID-19.
4.1.2. Aminoglycosides
Another class of antibiotics with antiviral activity is aminoglycosides, which are amino-modified sugars. Their antibacterial effect is mediated through binding to the 30S subunit of prokaryotic ribosome and causing mistranslation and premature termination. The antiviral activity of different aminoglycosides was shown against the influenza A virus, Zika virus, and herpes simplex virus-2 (HSV-2) [43], [44].
Kim et al. showed that aminoglycosides, especially neomycin B, bound to influenza virus promotor RNA and suppressed its replication [44]. It is proved that topical administration of five aminoglycosides including neomycin, neamine A, kanamycin, sisomicin, and kasugamycin had anti-HSV-2 and anti-influenza A effects in the vaginal and nasal mucosa through upregulation of IFN-I pathway genes and ISGs. The aminoglycoside-induced ISG expression can cause significant protection against both RNA and DNA viruses. Although the effect of neomycin was restricted to the site of administration, a single intranasal dose of this drug was enough to make an expressive enhancement in ISGs. Kasugamycin could significantly reduce the influenza A virus replication in human monocytes and increase the expression of ISGs similar to polyI:C [43].
Studies proved that TLR3, TIR-domain-containing adapter-inducing interferon-ß (TRIF), and IRF3/7 were the most important signaling molecules that mediated ISG-induction following administration of aminoglycosides (Fig. 1). The phagocytosis of aminoglycoside-containing epithelial cells caused accumulation of RNA-bound aminoglycosides in the endosome, which led to TLR3 activation in the vaginal dendritic cells [43].
The immunostimulatory effects of current aminoglycosides were found lower than needed, so their derivatives were investigated. Some aminoglycoside derivatives showed immunostimulatory effects through induction of ISGs, interferon-inducible proteins, and proinflammatory cytokines by activation of phosphatidylinositol phospholipase C [100], [101].
Considering all the above, the prophylactic use of aminoglycosides increases host resistance to a wide range of viruses. Though, administration of aminoglycosides, particularly in a prophylactic manner, could not be recommended and requires further investigations because of their side effects [43].
4.1.3. Nitazoxanide
Nitazoxanide (NTZ) (of the thiazolide family) is a FDA (food and drug administration) approved broad-spectrum non-mutagenic drug, which is highly available and was traditionally used against protozoa and anaerobic intestinal parasites, such as Cryptosporidium parvum and Giardia lamblia. Recent studies on protozoa and anaerobic bacteria demonstrated that NTZ successfully inhibited pyruvate-ferredoxin oxidoreductase (PFOR), which is an integral enzyme in anaerobic energy metabolism [102]. Apart from its anti-parasitic activities, its potential for elimination of hepaciviruses and astroviruses through IFN signaling and promoting autophagy was proved [47], [48].
Regarding its effects on the immune system, it reversibly inhibits mTORC1 but not mTORC2 signaling. Given the presence of mTOR catalytic subunit in both mTORC1 and mTORC2, it could possibly block the kinase activity of mTOR indirectly, and might probably affect the upstream mTORC1 regulatory pathway [103]. One study showed that it exerted inhibitory activity against IL-6 production from murine macrophages both in vitro and in vivo [104]. NTZ also enhanced the IRF3-induced IFNβ reporter activity in a dose-dependent way, and in the RIG-I overexpressing cells triggered with 1AB. Furthermore, it enhanced the IFN-Iinduced transcription of the ISGs, IFI27, Mx1, IFITM3, and IFN-β LUC activity in MAVS-overexpressing cells even when no other cellular stimulation exists [105].
Moreover, it inhibited production of proinflammatory cytokines, including TNF-α, IL-2, IL-4, IL-5, IL-6, IL-8, and IL-10, in the peripheral blood mononuclear cells (PBMCs) [106]. Recent evidence revealed that NTZ potentiated secretion of IFN-Is (alpha and beta) by the host’s fibroblasts in the PBMCs triggered by influenza virus [107].
NTZ was previously reported to represent an in vitro activity against MERS-CoV and other coronaviruses [106]. Additionally, a regimen of two oral daily doses of NTZ 600 mg for five days in patients with acute uncomplicated influenza reduced the symptoms with minor adverse effects [108]. To date, about twenty NTZ clinical trials are undergoing worldwide (Table 1), evaluating its probable effectiveness against SARS-CoV2, where it is used in combination with azithromycin, hydroxychloroquine (NCT04341493), and some other drugs [46], [49].
4.1.4. Niclosamide
In 1960, scientists at Bayer found the niclosamide efficacy against human tapeworm (cestoda) infection, which was introduced into market for human use as Yomesan® outside the United States in 1962 [109], [110]. The FDA approved niclosamide to treat tapeworm infection in humans in 1982. It is also mentioned in the World Health Organization list of essential medicines [111]. Millions of people have been treated with niclosamide safely. Despite its vast usage, the mechanism of action has not been clearly established, and was stated to entail uncoupling of oxidative phosphorylation [112]. Recently, increasing evidence was found that niclosamide is a multifunctional drug and able to regulate or prevent several biological processes or signaling pathways, proposing its potentiality as a novel therapeutic agent for conditions other than just helminthic diseases. The candidate usages include its firmly and broadly proven anticancer effects [113].
To be more detailed, this drug strongly impeded the activation, nuclear translocation, and transactivation of STAT3, while no apparent impact was found on the closely related proteins such as STAT1 and STAT5, Src kinases, and the upstream JAK1, JAK2, or other receptor tyrosine kinases. Additionally, niclosamide suppressed transcription of STAT3 target genes, inhibited cell growth, and induced apoptosis and cell cycle arrest of cancer cells with essentially active STAT3 [114]. An in vitro study of human rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS), showed that niclosamide also suppressed production of IFN-c, IL-1b, IL-6, IL-8, and IL-17A induced by TNF-α in the cultured RA-FLS, which possibly might be moderated by NF-κB signaling pathway, suggesting that phosphorylation of ERK and the JNK anti-inflammatory effect on RA-FLS is affected by niclosamide. Niclosamide strongly inhibited ecretion of the proinflammatory cytokines (IL-6, IL-12, TNF-α) and chemokines (RANTES, MIP-1a, MCP-1) induced by lipopolysaccharide (LPS) in cultured DC cells. In this study, niclosamide was found to downregulate generation of IFN-c, IL-1b, IL-6, IL-8, and IL-17A in a dose-dependent way by TNF-α-stimulated RA-FLS [115]. It showed strong STAT3 inhibition through downregulating STAT3 transcriptional activity (Fig. 1) via inhibiting its phosphorylation and nuclear translocation in prostate cancer DU145 cells [116]. Another study suggested that suppression of mTORC1 by niclosamide is caused through lysosomal dysfunction. The lysosomal degradative function is blocked by niclosamide probably via changing lysosomal permeability and the pH gradient [114].
Wu et al. discovered that a certain concentration of niclosamide could suppress SARS-CoV replication and totally destroyed the synthesis of viral antigens [117]. The cytopathic effect of SARS-CoV was stopped at a low concentration of niclosamide (1 μM). Moreover, it blocked SARS-CoV replication in Vero E6 cells with an EC50 value of less than 0.1 μM [118]. SARS-CoV 3CL protease is identified with a vital role in the processing of replicase polyprotein, and could be a major target for development of anti-SARS agents [119], [120]. In one study on concentrations up to 50 μM, no clear inhibitory effect was found against SARS-CoV 3CL protease, therefore, they may be other explanations for its effects [51].
Gassen et al. reported that E3 ligase S-phase kinase-associated protein 2 (SKP2) executed lysine-48-linked poly-ubiquitination of Beclin 1 (BECN1), which caused its proteasomal degradation. Suppression of SKP2 raised the BECN1 level, enhanced autophagy, and effectively decreased MERS-CoV replication. Niclosamide blocked MERS-CoV replication by up to 1000-fold at 48 h post-infection. At a concentration of 10 μM, niclosamide increased the BECN1 level and autophagy related 14 (ATG14) oligomerization, and raised the number of autolysosomes by more than double and influenced autophagic flux in the cells infected with MERS-CoV [121]. Given the multi-potency of niclosamide, its plausible further mechanisms could not be denied (Table 1).
4.1.5. Itraconazole
Itraconazole is a member of triazole antifungal agents, which is useful against blastomycosis, histoplasmosis, cryptococcal meningitis, and aspergillosis. The main mechanism of action of itraconazole includes interacting with 14α-demethylase, a cytochrome P450-dependent enzyme, which leads to disruption of ergosterol synthesis [38].
According to a recent study, itraconazole was found effective against the influenza A virus infection by priming the innate immune response and inhibiting lanosterol 14α-demethylase, a cytochrome P450-dependent enzyme, which is crucial for ergosterol biosynthesis (both in mammalian and fungal cells). As a result of this interruption, the levels of both IFN-α and IFN-β were risen in response to itraconazole [54].
Based on the above information, itraconazole could be considered as a potential IFN inducer and probably effective against SARS-CoV-2.
4.1.6. Ribavirin
Ribavirin is a guanosine nucleoside analog, which serves as a broad-spectrum antiviral agent. HCV, hepatitis E virus, RSV, and hemorrhagic fever virus are some of the viruses that can be suppressed by ribavirin [122]. Its antiviral activity is because of the following features: 1) competitive inhibition of inosine monophosphate dehydrogenase that works in the de novo pathway of guanosine synthesis, 2) acting as a mutagen to cause error catastrophe, 3) specifically for HCV, acts as a substrate of HCV-RNA-dependent RNA polymerase (RdRp) and leads to premature termination, and 4) modulating the immune system by regulating macrophage and T helper cytokine production, Th1/Th2 balance, and an increase in ISGs expression [55], [56].
Increasing evidence demonstrated ribavirin may enhance IFN-α signaling [122], thereby augmenting STAT1/3 phosphorylation. It also activates the IFN-α-JAK/STAT signaling pathway leading to increased expression of MxA, an antiviral protein. Mx proteins are involved in the translocation of vesicles. It is assumed that this activity is responsible for trafficking the viral nucleocapsids to the degradation locations of cells. Thus, MxA is believed to inhibit replication of RNA viruses [55].
Since ribavirin is the main HCV therapy, most studies have focused on their correlation. Ribavirin was shown to upregulate expression of IFN-α receptor on hepatocytes [55]. Furthermore, a combinational therapy with ribavirin and IFN-α exerted a stronger antiviral response [123]. Interestingly, Testoni et al. claimed that ribavirin downregulated the abnormal levels of preactivated ISGs, such as RSAD, CXCL10, and IFI27. This epigenetic downregulation raised the liver susceptibility to IFN-α [122].
Some researchers proposed ribavirin for controlling the COVID-19 pandemic because of its broad activity, multiple direct antiviral mechanisms, T helper1 polarization, and induction of random mutagenesis in the virus to promote T cell response against it (Table 1). Although ribavirin cannot diminish SARS-CoV completely even in high doses, its usage can be helpful in decreasing the viral load. It is notable that ribavirin plus IFN-ß could inhibit the replication of SARS-CoV. Besides, ribavirin plus IFN-α showed positive outcomes during the MERS-CoV pandemic in Saudi Arabia [124], [125], [126]. Ribavirin was also shown to bind tightly to SARS-CoV-2 RdRp in a docking study, so it could be a promising drug [127].
4.2. Anti-neoplastic and immunomodulatory agents
4.2.1. Anthracyclines
Anthracyclines are a class of antibiotic and chemotherapeutic agents extracted from certain types of Streptomyces bacteria, which are used to treat various types of cancer. Anthracyclines have various general mechanisms for eradicating tumor cells. These mechanisms include production of free radicals, intercalation between adjacent DNA or RNA base pairs, topoisomerase II inhibition, and alteration of the cell membranes [128].
Several studies have reported antiviral effects for anthracyclines. Holtzman et al. screened many approved compounds and drugs in two studies with a novel screening technique, named high-throughput screening (HTS) [58] to recognize the components that induce IFN signaling pathway. In one of those studies, some anthracyclines including idarubicin, doxorubicin, epirubicin, and daunorubicin were tested [129], which showed similar effects and a remarkable enhancement in the interferon-stimulated response element (ISRE) activity. Moreover, no inhibitory effect on topoisomerase and no enhancement of cytotoxicity were reported by idarubicin due to the low concentration of idarubicin needed for activating the ISRE and IFN signaling pathway; however, cytotoxic effects would appear with higher doses of this drug. Anthracyclines inhibit cell replication by preventing RNA and DNA synthesis. Moreover, they activate the type I IFN signaling pathway, which is known as an antiviral mechanism for idarubicin. It improves the immune system of the host against viral infections (Table 1). According to these information about idarubicin and other anthracyclines, these drugs could be used as a prophylaxis for viral infections [57], [58], [59].
4.2.2. Tamoxifen
Tamoxifen, is a small molecule approved by the FDA in 1977 as a selective estrogen receptor modulator (SERM). It promotes apoptosis in tumor cells and prevents their growth. Tamoxifen is used for the treatment of early-stage breast cancer as well as reducing the risk of recurring cancer after surgery or other cancer treatments [130], [131].
Based on some in vitro studies, tamoxifen has an antiviral effect against HSV, hepatitis C virus (HCV), human immunodeficiency virus (HIV), and Ebola virus. These studies indicated that tamoxifen inhibited the replication of those viruses through different mechanisms. In HIV, tamoxifen could prevent the interaction of protein kinase C (PKC) and other targets in the NF-κB pathway. However presently, more efficient drugs are available to treat HIV. Additionally, based on a study about the effect of tamoxifen on HIV and HCV, tamoxifen could prevent the virus in all steps of inflammation such as attachment, infusion, replication, and exhausting of the virus. This multistep inflammation-inhibiting activity of tamoxifen was shown against HSV-1 as well [61]. In another study, a pretreatment with tamoxifen had a considerable inhibitory effect on vesicular stomatitis virus (VSV). Tamoxifen was also shown to induce IFN-Is [132]. Based on a study on breast cancer, tamoxifen treatment could also stimulate expression of ISGs (Fig. 1) [133].
4.2.3. Gefitinib
Gefitinib, a selective inhibitor of the epidermal growth factor receptor (EGFR), was approved in the US and some other countries for treating non-small cell lung cancer (NSCLC). Gefitinib inhibits tyrosine kinase activity by competing with adenosine triphosphate (ATP) and prevents ATP binding to this enzyme [134], [135].
Mosquera et al. reported the antiviral effect of gefitinib against dengue virus (DENV) infection. They showed that both gefitinib and EGFR could inhibit the NF-κB pathway. Gefitinib decreased viral replication and also the level of antiviral cytokines produced in response to DENV-infected monocytes [62].
On the other hand, it was demonstrated that gefitinib could increase the level of INF-γ and IL-6 circulation in viral small cell lung cancer. Moreover, it increased NKs, which have an important role in induction of innate immunity by gefitinib against viral infections [136], [137].
4.2.4. Trametinib
Trametinib is an anti-cancer drug, which acts through inhibition of mitogen-activated protein kinase 1 (MEK1) and MEK2. Mekinist® or trametinib was approved by FDA in 2013. This drug is used for the treatment of metastatic melanomas created by deficiency in the BRAF gene [138].
The antiviral activity of trametinib is apparent. It is shown that the administration of trametinib and cobimetinib (another MEK inhibitor) activates the EGFR-ERK pathway. This causes a reduction in IRF1 followed by a decrease in IFN-I expression. Considering the role of MEK in the EGFR-ERK pathway, MEK inhibitors can induce IRF1 and IFN-κ through preventing this pathway, which leads to STAT1 activation. Consequently, induction of inflammatory chemokines and upregulation of anti-viral genes leads to type I IFN expression [63].
In addition to gefitinib and trametinib, a clinical trial held by European Research Initiative on CLL (chronic lymphocytic leukemia), demonstrated that the hospitalization rate for COVID-19 was lower than other therapies in CLL patients who were on ibrutinib, another tyrosine kinase inhibitor [139]. Thus, ibrutinib could be suggested as a potential treatment for modulation of the overactivated immune system in COVID-19 patients with severe disease state.
4.2.5. Gemcitabine
Gemcitabine (2′, 2′-difluorodeoxycytidine, dFdC) is a chemotherapeutic drug indicated in lung [140] or pancreatic cancers [141]. Its anticancer effect is mostly because of interrupting the natural DNA synthesis procedure through the involvement of gemcitabine’s metabolites into DNA. Moreover, it prevents the thymidine de novo synthesis by blocking its main enzyme, thymidylate synthase (TS) [142], [143].
Gemcitabine induces the production of multiple ISGs namely IFIT1, IRF7, IRF9CXCL10, and DDX58 (Fig. 1). The inactivation of metabolic enzymes directly or thorough altered nucleotide pools could generate a signal, which finally would reach some cis-acting elements on the promoters of a group of ISGs, probably via the transfer of kinases and transcription factors. According to some recent studies, this signal depends less likely on STAT1/2-IRF9 (ISGF3), at least for gemcitabine, as the main transcriptional complex in the JAK/STAT pathway, which is induced by IFN. Upon gemcitabine therapy, the phosphorylation of STAT1 at Tyr701, which was mostly induced by IFN-α, did not happen. Furthermore, IRF9 knockdown did not alter the gemcitabine-induced upregulation of DDX58 mRNAs. It was against the findings about the obvious suppression of the upregulation of DDX58 mRNAs induced by IFN-α under the same conditions. Further studies are needed to determine whether ISG activation is mediated by the inhibition of pyrimidine biosynthesis [144], [145], [146]. All taken into account, there are some reports showing the induction of ISGs while JAK1 or STAT1 were not activated [147]. According to one study, the identified DNA synthesis inhibitors such as gemcitabine, were active against at least one coronavirus, suggesting that these drugs could show antiviral effects against coronaviruses [64]. However, more studies are needed to evaluate gemcitabine impacts on coronaviruses and especially SARS-CoV-2.
4.2.6. Obatoclax
Obatoclax is a drug from the BH3 mimetic compounds, a class which can enhance apoptosis through activation of the intrinsic apoptosis pathway. It has been investigated extensively in preclinical and clinical evaluations as an anti-cancer treatment and is now on phase II clinical trial for leukemia, lymphoma, myelofibrosis, and mastocytosis. Obatoclax antagonizes the proteins of Bcl-2 family (Bcl-2, Bcl-w, Bcl-XL, Mcl-1, and A1/Bfl-1 [148]), which suppresses apoptosis.
Apart from its BH3 mimetic effect, it maintains substantial functionality in the cells void of Bak and/or Bax, key effectors in the intrinsic apoptosis pathway [149], [150], [151]. In some studies on obatoclax, the successful blocking of the transcription of IFIT1, IFIT2, IFIT3, and other cellular genes involved in the antiviral responses (innate immune system, IFN α/β signaling, RIG-I, and MDA5 pathways, RNA binding, DNA metabolic processes, chromatin remodeling, and cell cycle, according to gene set enrichment analysis) mediated by ZIKV was shown [19].
Obatoclax also inhibited production of antiviral IFN-β and -γ. It suppressed activation of cellular antiviral responses, probably, via inhibition of the myeloid leukemia cell differentiation 1 protein (Mcl-1) -type I IFN signaling axis. This is possible via the Mcl-1-mediated regulation of STAT3 signaling pathway, which regulates IFN-I responses (Fig. 1). It also suppressed the WSN-mediated production of proinflammatory IP10, IL6, IL8, CXCL1, CCL2, and CCL5 in retinal pigment epithelium (RPE) cells [152]. This drug was effective in vitro against influenza, Zika virus, Yellow fever virus (YFV), West Nile virus (WNV), Junin virus (JUNV), Sindbis virus (SINV), Lassa mammarenavirus (LASV), and lymphatic choriomeningitis virus (LCMV) [66], [153], [154], [155]. Obatoclax suppressed the viral endocytic uptake by targeting cellular induced Mcl-1 [66]. Considering its immunomodulatory and antiviral effects, further studies seem essential to determine the possible effects of obatoclax on SARS-CoV-2.
4.2.7. Mycophenolic acid
Mycophenolate mofetil (MMF), the ester prodrug of mycophenolic acid (MPA), is mainly considered an effective immunosuppressant in some of the standard immunosuppressive regimens [156]. When administered orally, it is hydrolyzed by intestinal and blood esterases and releases MPA, which is an efficient selective noncompetitive inhibitor of inosine monophosphate dehydrogenase (IMPDH) [157].
MPA showed some effects on ISG expression (IRF1, 2, 3, 4, 5, 7, interferon-induced protein 35 (IFI35), interferon-induced transmembrane protein 1 (IFITM1), and interferon-stimulated exonuclease gene 20 kDa (ISG20)) in a T-lymphocyte and Huh7 cell line. The ISRE promoter element is the regulator of expression of most ISGs by IFNs. Therefore, this MPA effect on ISGs expression stems from their ability to dampen IL-17 production by CD4 + T cells. In one study on the immunomodulatory effects of MPA and tacrolimus, their capability in inhibiting Th17-related response was observed. MPA seems to suppress IL-17 more strongly than tacrolimus [69], [158].
MPA was investigated as a candidate treatment for MERS-CoV because of its antiviral activities in several studies including six in vitro, two in vivo, and one clinical observational study. The in vitro studies revealed some evidences that MPA could target the papain-like proteases of both MERS-CoV and SARS-CoV. Moreover, MPA inhibited the virus potently, with a low IC50 [159], [160], [161], [162], [163], [164]. MPA was also administered in combination with IFN-β. This regimen was tested in marmosets showing a severe disease similar to human MERS, which resulted in high virus titers with more severe or even fatal outcomes [165]. Unsatisfactorily, as the study researchers also stated, it seems that MPA was more harmful than beneficial to MERS patients. MPA monotherapy was not evaluated in clinical setting in this regard [166]. In one study, MPA was given to eight MERS-CoV patients, in seven of which IFN-β was also indicated [167]. All patients survived, though they showed lower acute physiology and chronic health evaluation II (APACHE II) scores than the patients who received different antiviral agents namely ribavirin and IFN-α, steroids, and antibiotics. Thus, interpretation of these findings must be done with caution.
To sum, while promising results were obtained in the in vitro studies of MPA against MERS, the in vivo studies suggested that its usage might be more harmful than advantageous and so was not likely to be clinically favorable in coronavirus infections. Besides, the clinical trials were too limited to verify the beneficial outcome of MPA in MERS-CoV patients.
4.2.8. Sirolimus
Rapamycin® or sirolimus is a macrolide approved for prophylaxis of renal transplant rejection. It is used in various diseases such as nephrotoxicity, ischemia, autoimmune, and inflammatory diseases. It acts through suppressing T cell activation and proliferation and cytokine production. Moreover, sirolimus is the main inhibitor of the mammalian target of rapamycin (mTOR) signaling pathway [38], [168]. In a study by Cao et al., it was proved that inhibition of mTOR signaling would block interaction of TLR9 and myeloid differentiation primary response 88 (MyD88) and subsequently repress IRF7 followed by impaired production of IFN-α/β (Fig. 1). Therefore, sirolimus suppresses expression of antiviral and anti-inflammatory genes [169]. PI3K-PKB-mTOR pathway limits hepatitis E virus infection, while mTOR inhibition exacerbates the infection [170]. Huang et al. reported no antiviral activity for sirolimus against H1N1 and H3N2 influenza viruses in vitro, although they suggested that it can work as a salvage therapy for severe influenza, if its therapeutic window would be determined. On the other hand, some studies found sirolimus as an anti-H1N1 agent, when used as an adjuvant in addition to oseltamivir [171].
There is some positive evidence showing that sirolimus may serve as an immunomodulatory agent. It is indicated that sirolimus can regulate Th1/T cytotoxic cell 1 (Tc1) and Th2/Tc2 cell balance by downregulation of Th1 and Tc1 cells after bone marrow transplantation. Therefore, rapamycin is presented as a modulator of T cell cytokines through a decrease in type I cytokine secretion [172]. Sirolimus also reduced production of IFN-γ and IL-4 [173]. A combination of sirolimus and mycophenolic acid synergistically inhibited proinflammatory cytokines, including intercellular adhesion molecule 1 (ICAM-1) and inducible nitric oxide synthase (iNOS), via suppression of the ERK/p38 MAPK pathway [168].
Sirolimus modulated both ERK/MAPK and PI3K/AKT/mTOR signaling pathways, which are believed to play a critical role in the host defense against MERS-CoV, a similar virus to SARS-CoV-2. Different sirolimus concentrations suppressed MERS-CoV infection in vitro through mTOR inhibition. Though, mTOR signaling might have a central role in MERS-CoV infection [174].
It is hypothesized that due to the higher expression of aging-related proteins such as angiotensin converting enzyme 2 (ACE2) and CD26, which are known as SARS-CoV-2 receptors on the host cells, there would be more receptors for SARS-CoV-2 on the surface of senescent lung cells. Besides, since their ability in protein synthesis is enhanced, they produce more SASP (senescence-associated secretory phenotype) inflammatory mediators such as IL-6. This might be the reason of higher susceptibility of elderlies to COVID-19 and their increased related deaths. It is opined that the drugs with anti-aging activities, such as sirolimus, may serve as a treatment for COVID-19, considering that both azithromycin and doxycycline, which showed positive effects against COVID-19, have anti-aging activities as well [175].
Some studies demonstrated that the treatment of old mice with mTOR inhibitors, such as sirolimus or its analogs, could rejuvenate their immune system and strengthen their response to influenza vaccination [176]. In human studies, sirolimus usage with steroids in severe H1N1 influenza were shown to improve the condition. But, their systemic administration had adverse effects; for instance, lung toxicity was caused by using sirolimus inhalation. Thus, biguanides could be considered as mTOR inhibitors without lung toxicity. Interestingly, metformin is a member of this drug class [177]. Based on the virus-host interactome, a bioinformatics research suggested a list of 16 drugs that might serve as an anti-SARS-CoV-2 agent, among which sirolimus and its combination with dactinomycin were also found [68].
4.3. Non-steroidal anti-inflammatory drugs
4.3.1. Acetylsalicylic acid (aspirin)
Acetylsalicylic acid (also known as aspirin) has been used over 115 years for the treatment of pain and fever as an anti-inflammatory and antipyretic agent. It can also prevent myocardial infarction and blood clot stroke by inhibiting platelet aggregation.
Aspirin is categorized as a non-selective cyclooxygenase inhibitor. According to some studies, its long-term use might reduce the risk of different types of cancer, including breast, lung, colorectal, liver, prostate, esophageal, and skin cancers.
Aspirin blocks prostaglandin secretion by targeting COX-1 and COX-2 non-selectively. The acetyl group of aspirin binds with a serine residue of the COX-1 enzyme, causing its permanent inhibition. As a result, the level of prostaglandin decreases and the pain is alleviated. The same process can be explained for the antipyretic effect of aspirin in which the production of prostaglandin E1 is inhibited [38].
According to Glatthaar‐Saalmüller et al., aspirin was significantly effective against Influenza A (H1N1), human rhinovirus 14 (HRV-14), and human rhinovirus 39 (HRV-39). It also showed remarkable antiviral activity against carbonic anhydrase 9 (CA9), human rhinovirus 1A (HRV-1A), and human rhinovirus 2 (HRV-2) in a dose- dependent manner. To the best of our knowledge, the precise mechanism has not yet been understood [178].
In another study, aspirin downregulated the HCV-induced mRNA and protein expression of COX-2, which was possibly independent of NF-κB mechanism. Aspirin also inhibited the viral replication, which was related to COX-2 inhibition and activation of p38, MEK1/2, and MAPKs [71].
Previous studies showed that aspirin boosted the production of both IFN-α and IFN-γ. The enhancement of IFN-γ was related to the blocking of prostaglandin synthesis. In contrast, the yield of IFN-α was not affected by prostaglandin [179], [180].
There are some other pieces of evidence mentioning the role of aspirin in the IFN-α-induced enhancing phosphorylation of STAT1, JAK1, and JAK2, which results in the improvement of the antitumor function of IFN-α (Fig. 1) [181].
As mentioned, aspirin inhibits prostaglandin production, which has become a concern whether it can worsen COVID-19 symptoms. According to FitzGerald, prostaglandins such as PGE2, PGD2, and prostacyclin (PGI2) can both boost and limit inflammation. Therefore, further investigations are needed on the possible roles of aspirin in COVID-19 treatment [182].
4.3.2. Indomethacin
Indomethacin (INDO), another famous member of NSAIDs, was discovered in 1963 and approved by FDA in 1965. INDO’s main indications can be categorized based on its route of administration. The oral form is prescribed for alleviating the symptoms of mild to severe rheumatoid arthritis and also migraine, but the intravenous form is indicated for treating patent ductus arteriosus (PDA) in the premature infants with certain characteristics.
INDO’s analgesic and anti-inflammatory effects can be defined by its mechanism of action, which includes reversible inhibition of both the isoforms of COX enzyme. COX-1 is available in most body tissues and causes secretion of prostaglandins and thromboxane A2, while COX-2 expression only occurs in response to inflammation or injury. Both of these isoforms are involved in catalyzing conversion of arachidonic acid to PGG2 and PGG2 to PGH2. PGE2, which is produced from PGH2 in COX-2-mediated pathway, plays a vital role in mediating inflammation, pain, and fever. The antipyretic effects might be a result of action on the hypothalamus, leading to increased peripheral blood flow and vasodilation.
Unlike other NSAIDs, INDO restrains phospholipase A2, which is involved in releasing arachidonic acid from phospholipids [38].
According to Andreone et al., INDO could boost production of 2′, 5′-OAS, an IFN-induced protein with remarkable antiviral effects, in chronic HCV and HBV infections. Moreover, INDO enhanced Th1-response, which is needed for HCV clearance, and suppressed Th2-response, which was related with HCV persistence. These effects of INDO might be related to a dramatic boost of ISRE-dependent transcription by inducing STAT 1 phosphorylation [72].
INDO’s antiviral activity was also reviewed against SARS-CoV with promising results. INDO was shown to impede coronavirus replication by blocking the viral RNA synthesis selectively. However, further investigations are required.
It was emphasized that this special antiviral activity was COX-independent, because: 1) aspirin, another member of NSAIDs, was not able to mimic this effect. 2) the concentration in which INDO showed antiviral activity was not compatible with the dose needed for COX blocking.
Finally, INDO as a potent inducer of IFN response, with positive effects on inhibiting SARS-CoV replication, might be considered as a potential candidate to strengthen the immune system against SARS-COV-2 [183].
4.4. Miscellaneous synthetic compounds
4.4.1. Metformin
Presently, metformin is the first line oral therapy for type 2 diabetes, due to its natural origin (derived from Galega officinalis [184]), wide experience of usage for more than 60 years, and its impressive safety. Besides, it seems to exert positive effects on cardiovascular diseases and cancer incidence [185].
This drug could inhibit the complex I of electron transport chain (NADH dehydrogenase), which subsequently increases the AMP (adenosine monophosphate) level leading to AMP-activated protein kinase (AMPK) induction. It is considered that metformin pharmacological effects is mainly through its effects on AMPK [186]. AMPK activation leads to inhibition of liver glucose output and reduces insulin resistance in the peripheral cells [74].
Despite a variation in metformin effect at the molecular level, which depends on the dose, duration, and acute or chronic administration [184], Marcucci et al. opined that metformin effect is relevant to the context of pathological condition. They suggested that in cancers, metformin works as an immunostimulatory agent, which reduces anti-inflammatory cytokines, shifts M2-macrophages to M1-macrophages, and stimulates the anti-tumor responses of T cells. On the other hand, in some other diseases, metformin suppresses the immune system through reduction of inflammatory mediators, increase of anti-inflammatory cytokines, upregulation of Th2 and regulatory T cells as anti-inflammatory and immunosuppressive T cells, and downregulation of Th1 and Th17 as inflammatory T cells [186]. Moreover, inflammation reduction may happen through suppression of NF-κB p65 pathway in monocytes and TNF-α and IL-6 production in macrophages. Furthermore, AMPK activation and STAT3 inhibition by metformin inhibit differentiation of monocytes to macrophages [187]. All of these anti-inflammatory effects and AMPK activation make metformin a caloric restriction mimetic, which rejuvenate the whole body and the immune system [188]. Additionally, metformin administration in healthy people showed to induce anti-inflammatory effects [186]. Considering all these data, metformin can be regarded as an immunomodulatory agent.
IFN-β stimulates antiviral mechanisms in cells including antiviral protein synthesis by regulating glucose metabolism to supply the energy needed for antiviral responses. Experimentally, after treating a cell with IFN-β, a robust uptake of glucose is observed. The antiviral effect of metformin is probably due to increasing the cell sensitivity to insulin, which enables cells to synthesize antiviral proteins. Clinical studies proved the synergistic effect of metformin on IFN-β antiviral activity as well [75].
Besides, there is a crosstalk between AMPK and IFN, which is still unclear. It was shown that metformin increased expression of STAT1 and STAT2 and their phosphorylation. It was concluded that metformin could activate the JAK-STAT signaling pathway of the host cell antiviral mechanism. Considering these data, Tsai et al. deduced that metformin activates the antiviral signaling of IFN-Is through the AMPK pathway [74].
Some studies showed that metformin use in asthmatic and diabetic patients reduced the rate of hazards and exacerbations. Therefore, it is opined that because of its effect on weight reduction and pneumonia, metformin can be used as an adjuvant therapy in the obese and diabetic patients who get COVID-19 [189]. Additionally, it is claimed that metformin had no interaction with ACE2–the main cell receptor of SARS-CoV-2– and its usage in diabetic patients was safe. Cava et al. made an in silico research to identify drugs that might be useful for COVID-19, through the interaction with important genes that are related to ACE2. Interestingly, metformin is a candidate drug for treatment of COVID-19, which interferes with fatty acid synthesis through interaction with acetyl-CoA carboxylase α gene [76], [190].
4.4.2. Fluoxetine
Fluoxetine, which is a selective serotonin reuptake inhibitor (SSRI), gained its FDA approval in 1987 as a 2nd generation antidepressant. It is indicated for the obsessive-compulsive disorder, in the treatment of both maintenance and acute phases of major depressive disorder and bulimia nervosa.
As an SSRI, its mechanism of action includes hindering the presynaptic reuptake of serotonin. Following this, the level of 5-hydroxytryptamine (5-HT) is boosted in different parts of the brain [38].
Fluoxetine has been proposed as an IFN-α regulator in the process of cell growth inhibition via increasing the activation of STAT-1 and PPAR-α [191]. According to Young et al., fluoxetine is a successful inhibitor of HCV, which functions by facilitation of the IFN-α-mediated antiviral actions through activating JNK and STAT-1 (Fig. 1) [77].
On the other hand, the therapeutic plasma concentrations of fluoxetine showed a negative immuno-modulatory effect through remarkable reduction of IFN-γ production [192], [193].
In contrast to its positive effect on IFN-α-mediated antiviral actions, Sacre et al. found that fluoxetine is an inhibitor of endosomal TLR-3 and -7, which are two main PRRs in recognition of ssRNA viruses (such as SARS-CoV-2). As a result, further investigation is required on the possible role of fluoxetine against SARS-CoV-2 [20].
4.4.3. Verapamil
Verapamil belongs to the non-dihydropyridine class of calcium channel blockers, introduced in the early 60s as the first drug of its class. Its main indications include angina, high blood pressure, and heart arrhythmias.
Verapamil binds to the alpha-1 subunit of Cav1.2, an L-type calcium channel, which is found in the cardiovascular system. Its interaction is frequency- and voltage-dependent, and consequently, it inhibits the L-type calcium channels [38].
The common side effect of verapamil is sinus bradycardia. Other side effects include pulmonary edema, severe hypotension, and second-degree atrioventricular block [45].
Recent studies revealed that verapamil, as well as some other ion channel blockers, could be useful against filoviruses, such as Ebola, by inhibiting their cell entry [78].
According to Khakzad et al., the level of serum and bronchoalveolar lavage fluid (BALF) IFN-γ was elevated remarkably in the verapamil treated-mice compared to the sensitized mice [194]. In contrast, there are some other studies suggesting verapamil as a suppressor of IFN-γ production [195]. Moreover, verapamil was found to reduce IFN-γ-induced neurotoxicity by the repression of the STAT3 signaling pathway (Fig. 1) [196].
4.4.4. Statins
β-Hydroxy β-methlylglutaryl-coenzyme A reductase (HMGCR) inhibitors, also known as statins, are a therapeutic class that inhibit the conversion of HMG-CoA to mevalonic acid and the cholesterol biosynthesis in the liver. They are approved for the treatment of hypercholesterolemia. The antiviral activity of many statin drugs against different types of viruses has been reported. Statins also promote the antiviral activity of IFN-α [197], [198]. Moreover, some researchers proposed statins for the treatment and prophylaxis of the next pandemic influenza [81], [199].Some evidence showed that simvastatin could reduce the mortality rate and hospitalization of influenza patients [200]. It is suggested that statins could be administered prophylactically or therapeutically for influenza by interfering with the cell structure and the required environment for viral replication. Additionally, statins are proved to reduce the mortality risk of the influenza-caused pneumonia [201].
The antiviral activity of statins was proved against HCV (through prenylation inhibition), HIV-1 (via targeting Rho by simvastatin), RSV (through reduction of cell to cell fusion by lovastatin), and polioviruses [80], [81].
Statins are known to exhibit anti-inflammatory, endothelium-stabilizing, and antioxidant effects. They are proved to inhibit NF-κB, MAPK, and peroxisome proliferator activated receptor (PRAR)-dependent pathways, and can affect the activation and proliferation of many immune cells [202]. It is suggested that the anti-inflammatory effect of statins is due to inhibiting the adhesion of leucocytes and diminishing cytokine production [201], [203]. Moreover, inhibition of the intermediates of cholesterol biosynthesis, farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), can be another mechanism of statin anti-inflammatory effects. Reduction of these two intermediates declines “isoprenylated, small GTPase (Rho GTPase) signaling molecules”, which is pivotal in many signaling pathways, including immune responses [200], [204].
According to some in vitro data, the anti-inflammatory effect of statins on immune cell lines needs higher doses than their physiological blood levels. Though, some evidence showed that inhalation of statins as a route of administration increases their anti-inflammatory effects [200].
For example, it was shown that fluvastatin can inhibit expression of proinflammatory cytokines, including IFN-γ, TNF-α, and IL-8 [81]. Lovastatin could inhibit production of T cell cytokines, such as IL-2, IL-4, and IFN-γ, and downregulate the signaling pathways of NF-κB and activator protein 1 (AP-1). Lovastatin could also regulate the balance of Th1/Th2 [205]. Simvastatin also suppressed Th2 cytokines [205] and lymphocytes and inhibited TNFα, IL-2, and IFN-γ production [206].
The suppression of IFN-α release and phospho-STAT1 in monocytes by simvastatin suggests that it can affect IFNAR’s downstream and upstream signaling (Fig. 1) [200]. Besides, simvastatin can reduce IFN-γ-induced cyclo-oxygenase2 (COX-2) via the inhibition of STAT1/3 [207].
SARS-CoV infection induces MyD88 and NF-κB expression leading to ARDS (acute respiratory distress syndrome), which is the major cause of death in COVID-19. It is well known that statins regulate MyD88 concentration and diminish NF-κB activation. Hence, it is suggested that statin usage in patients with cardiovascular diseases or diabetes who get COVID-19, might be helpful [208]. Moreover, the advantages of statin therapy in improving the symptoms of influenza, H1N1, and Ebola virus infections are proved. Given that SARS-CoV-2 enters the cells through ACE2 and downregulates it, the ability of statins to upregulate ACE2 could be advantageous for COVID-19 treatment [79].
There are some conflicting reports as well. Gu et al. reported that simvastatin activated autophagy in the bronchial smooth muscle cells (BSMCs) through increasing the expression of ATG5, LC3B, and Beclin 1, which leads to autophagosome formation and reduced airways inflammation. This autophagy in BSMCs was related to increased IFN-γ and decreased IL-4, IL-5, and IL-13, and was parallel with the upregulation of Th1 and downregulation of Th2 cytokines [209].
Overall, it has been proved that statins can inhibit the proinflammatory factors responsible for hyperinflammatory responses and cytokine storm in the lungs. This suggests them as candidate for the prophylaxis or treatment of COVID-19 (Table 1) [80].
5. Natural compounds
5.1. Caffeine
Caffeine is derived from coffee and cacao beans as well as tea, and is a member of methylxanthine class drugs. Its main indications include certain respiratory conditions of premature newborns, pain alleviation, and restoring mental alertness during drowsiness.
Caffeine is presented with various functions in different parts of the body. Unfortunately, the clinical relevance of its general and cellular actions is poorly understood. Suggested mechanisms include inhibition of nucleotide phosphodiesterase enzymes, participation in adenosine receptor (A1, A2a, A2b, A3) antagonism, and regulation of calcium handling in cells [38].
Horrigan et al. investigated the immunomodulatory effect of caffeine. They concluded that caffeine’s role in the immune system is dose-dependent; and its intervention is mainly due to the antagonism of adenosine receptors and cAMP-phosphodiesterase [210].
Tej et al. found that caffeine treatment lowered the incidence and growth rate of tumors dramatically. The possible mechanism depends on the antagonism of the adenosine A2A receptor. When adenosine activates the A2A receptors of T cells, proliferation of CTLs, production of cytokines (TNF-α and IFN-γ), and expression of programmed cell death protein 1 (PD-1) are reduced [211].
Caffeine has been mentioned in several studies as an anti-HCV drug via preventing its replication, though its mechanism is unclear yet. Studies suggest that expression of specific proteins such as heat shock protein 90 (HSP90), Ras-ERK, and COX-2 is reduced in cells by caffeine, resulting in inhibition of HCV replication [82].
Some evidence suggest that long-term consumption of caffeine results in IFN-γ elevation [212]. Thus, caffeine can be considered as a target for further investigations in SARS-COV-2 interventions.
5.2. Glycyrrhizin
Glycyrrhiza glabra L. (Fam. Fabaceae) is known as licorice or mulethi [213], whose roots possess a significant content of glycyrrhizin (2–8% dry weight). Glycyrrhizin is an oleanane-type triterpenoid saponin [214] with various medicinal functions, such as antiulcer, anti-inflammatory, immunomodulatory, and antiallergic activities [215]. It is also effective against HIV [216], [217] and SARS [218]. Glycyrrhizin blocks accumulation of the intracellular reactive oxygen species (ROS) due to virus infection [83], [219]. Suppression of ROS formation by glycyrrhizin decreases the NF-kB, JNK, p38, and redox-sensitive signaling activities, which are believed to relate to virus replication [83], leading to preventing the virus replication.
The immunomodulatory effect of glycyrrhizin was shown to be exerted by reducing the capillary permeability, increasing IFN-γ, activating NK cells and T cells, and inhibiting the complement response [220], [221]. A study on ConA-induced mouse liver fibrosis models showed that glycyrrhizin administration significantly decreased infiltration of Th1, Tregs, Th2, and Th17. Besides, glycyrrhizin increased the Th1/Th2 and Treg/Th17 ratios in the liver and spleen [222]. A significantly decrease was observed in the expression level of Th17 cell and IL-22 when glycyrrhizin was added to the therapeutic protocols of psoriasis patients versus the group without glycyrrhizin [223]. In one study, glycyrrhizin could inhibit porcine epidemic diarrhea virus (PEDV) infection and decreased the proinflammatory cytokine secretion via the high mobility group box 1 (HMGB1)/TLR4 MAPK p38 pathway [224]. Glycyrrhizin reduced the mRNA levels of proinflammatory cytokines through the competitive inhibition of HMGB1. The association of TLR4 and RAGE (receptor for advanced glycation end products) with PEDV pathogenesis through the infection in Vero cells was confirmed [225].
Recent studies also showed the benefit of glycyrrhizin in treating upper respiratory tract infections; and Rhizoma Phragmitis improved the function of upper respiratory mucosal immune system [226], [227]. Glycyrrhizin also inhibited viral adsorption and penetration [228], [229]. Despite lots of research, its effects on SARS-CoV2 is still unclear.
5.3. Berberine
Berberine, is an alkaloid derived from Hydrastis canadensis L., the Chinese herb Huanglian, and many other plants. It is commonly used in the Chinese traditional medicine as an antimicrobial agent for treatment of infectious diarrhea and dysentery. Berberine has also shown some impacts on the cardiovascular system including negative chronotropic, positive inotropic, antiarrhythmic, and vasodilative effects [230]. Besides, it is regarded as an anti-diabetic agent. Berberine alters the glucose metabolism via glycolysis stimulation, which is mediated through boosting glucokinase activity and insulin secretion, as well as inhibiting hepatic gluconeogenesis and adipogenesis. Its ability in activation of 5′AMPK appears to be its main function [231]. Berberine also exerts some immunomodulatory effects. It stimulated IL-12 secretion and conversely inhibited IL-6 production, thereby enhancing the production of IFN-γ and decreasing the IL-4 level in the antigen-primed CD4+ T cells [85], [86]. Berberine exhibited a stimulatory effect on Th1 cytokine production in CD4+ T cells as well as Th2 inhibition [232]. Another study indicated that berberine suppressed the basal- and TPA-mediated PGE2 level and COX-2 expression by prevention of AP-1 binding [233]. Berberine presented activity against different viruses such as SARS-CoV-1 (IC50 2 µg/mL) [234], HSV, Chikungunya virus (CHIKV) [235], [236], HCV [237], and influenza virus [238]. Therefore, further investigations on its possible impacts on COVID-19 are warranted.
6. Conclusion and future direction
There are no approved therapeutic agent available for the treatment of COVID-19 yet, and the current treatments mostly focus on the supportive care or drug therapy based on the formerly approved antiviral drugs administered in other viral infections and experimental studies [239]. Another issue regarding COVID-19 global pandemic is the lack of available vaccines to control the prevalence of new cases. Although robust evidence regarding the nature of long-term protection against human coronaviruses has not been found, the data gathered from previous studies on different types of human coronaviruses suggest that virus-specific antibody titers gradually drops over time and may only establish partial and temporary protection against COVID-19 [240], [241], which raises questions regarding the efficacy of prophylactic vaccines for long-term protection against COVID-19. On the other hand, the cost of development and clinical trials considering the time required to approve a vaccine is significant. Therefore, a variety of approved antiviral agents with IFN-modifying potential reviewed here, could be noticed as drug candidates targeted for repurposing in the treatment of COVID-19. Despite the fact that the adverse effects of some of these drugs may overweight their benefits, studying their mechanism and structure may give a clue for designing novel drugs in future. Furthermore, the antiviral effect and IFN-modifying mechanisms possessed by some of these drugs might lead to a synergistic effect against SARS-CoV-2. Further investigations are needed to evaluate such possibilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107245.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.De Haan C.A., et al. Mapping of the coronavirus membrane protein domains involved in interaction with the spike protein. J. Virol. 1999;73(9):7441–7452. doi: 10.1128/jvi.73.9.7441-7452.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.-F.-W., et al. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21(10):544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Lee A.J., Ashkar A.A. The dual nature of type I and type II interferons. Front. Immunol. 2018;9:2061. doi: 10.3389/fimmu.2018.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crouse J., Kalinke U., Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 2015;15(4):231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 6.Hadjadj J., et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. MedRxiv. 2020 doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco-Melo D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020 doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owji H., Negahdaripour M., Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020 doi: 10.1016/j.intimp.2020.106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park A., Iwasaki A. Type I and Type III interferons-induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N., et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe. 2020;28(3) doi: 10.1016/j.chom.2020.07.005. 455–464.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monk P.D., et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Resp. Med. 2020 doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung S.-Y., et al. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020;9(1):558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozafari N., et al. Inflammation: a bridge between diabetes and COVID-19, and possible management with sitagliptin. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokugamage K.G., et al. SARS-CoV-2 is sensitive to type I interferon pretreatment. BioRxiv. 2020 doi: 10.1101/2020.03.07.982264. [DOI] [Google Scholar]
- 16.Molony R.D., Malawista A., Montgomery R.R. Reduced dynamic range of antiviral innate immune responses in aging. Exp. Gerontol. 2018;107:130–135. doi: 10.1016/j.exger.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A. Maiti, African-American Population Is More Vulnerable to Sars-CoV2 Infection and IFN-Beta Supplement Can Be An Effective Treatment. Available at SSRN 3566710, 2020. Hhtps://doi.org/10.1007/s00251-020-01174-6.
- 18.Mosaddeghi P., Shahabinezhad F., Dorvash M., Goodarzi M., Negahdaripour M. Harnessing the non-specific immunogenic effects of available vaccines to combat COVID-19. Hum. Vaccin. Immunother. 2020;13:1–12. doi: 10.1080/21645515.2020.1833577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siu K.-L., et al. Suppression of innate antiviral response by severe acute respiratory syndrome coronavirus M protein is mediated through the first transmembrane domain. Cell. Mol. Immunol. 2014;11(2):141–149. doi: 10.1038/cmi.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kindler E., Thiel V., Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv. Virus Res. 2016;96:219–243. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoops K., et al. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6(9) doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvet M., et al. In vitro reconstitution of SARS-coronavirus mRNA cap methylation. PLoS Pathog. 2010;6(4) doi: 10.1371/journal.ppat.1000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., et al. Functional screen reveals SARS coronavirus nonstructural protein nsp14 as a novel cap N7 methyltransferase. Proc. Natl. Acad. Sci. 2009;106(9):3484–3489. doi: 10.1073/pnas.0808790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y., et al. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell. 2013;4(12):951–961. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X., et al. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopecky-Bromberg S.A., et al. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81(2):548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornbrough J.M., et al. Middle East respiratory syndrome coronavirus NS4b protein inhibits host RNase L activation. MBio. 2016;7(2) doi: 10.1128/mBio.00258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wathelet M.G., et al. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 2007;81(21):11620–11633. doi: 10.1128/JVI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastard P., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin F.-C., Young H.A. Interferons: success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014;25(4):369–376. doi: 10.1016/j.cytogfr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Channappanavar R., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pushpakom S., et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 34.Andersen P.I., et al. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negahdaripour M. A world of changes: the inheritance of COVID-19. Iran. J. Med. Sci. 2020;45(3):155–156. doi: 10.30476/ijms.2020.46530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negahdaripour M. The rise and fall in therapeutic candidates for COVID-19. Iran. J. Med. Sci. 2020;45(4):231. doi: 10.30476/ijms.2020.46689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S., et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wishart D.S., et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C., et al. Azithromycin protects against zika virus infection by upregulating virus-induced Type I and III interferon responses. Antimicrob. Agents Chemother. 2019;63(12) doi: 10.1128/AAC.00394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 41.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Gautret P., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gopinath S., et al. Topical application of aminoglycoside antibiotics enhances host resistance to viral infections in a microbiota-independent manner. Nat. Microbiol. 2018;3(5):611–621. doi: 10.1038/s41564-018-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H., et al. Aminoglycoside antibiotics bind to the influenza A virus RNA promoter. Mol. BioSyst. 2012;8(11):2857–2859. doi: 10.1039/c2mb25333j. [DOI] [PubMed] [Google Scholar]
- 45.https://www.drugs.com/.
- 46.https://clinicaltrials.gov/ct2/show/NCT04341493.
- 47.Hargest V., et al. Astrovirus replication is inhibited by nitazoxanide in vitro and in vivo. J. Virol. 2020;94(5) doi: 10.1128/JVI.01706-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elazar M., et al. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2α via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009;137(5):1827–1835. doi: 10.1053/j.gastro.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 49.M. Kelleni, Nitazoxanide/Azithromycin combination for COVID-19: a suggested new protocol for COVID-19 early management, 2020. [DOI] [PMC free article] [PubMed]
- 50.Xu J., et al. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect. Dis. 2020;6(5):909–915. doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shie J.-J., et al. Discovery of potent anilide inhibitors against the severe acute respiratory syndrome 3CL protease. J. Med. Chem. 2005;48(13):4469–4473. doi: 10.1021/jm050184y. [DOI] [PubMed] [Google Scholar]
- 52.https://clinicaltrials.gov/.
- 53.Pindiprolu S., Pindiprolu S.H. Plausible mechanisms of Niclosamide as an antiviral agent against COVID-19. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schloer S., et al. The clinically licensed antifungal drug itraconazole inhibits influenza virus in vitro and in vivo. Emerg. Microbes Infect. 2019;8(1):80–93. doi: 10.1080/22221751.2018.1559709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevenson N.J., et al. Ribavirin enhances IFN-α signalling and MxA expression: a novel immune modulation mechanism during treatment of HCV. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W.-L., et al. Ribavirin enhances the action of interferon-α against hepatitis C virus by promoting the p53 activity through the ERK1/2 pathway. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0043824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel D.A., et al. High-throughput screening normalized to biological response: application to antiviral drug discovery. J. Biomol. Screen. 2014;19(1):119–130. doi: 10.1177/1087057113496848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel D.A., et al. High throughput screening for small molecule enhancers of the interferon signaling pathway to drive next-generation antiviral drug discovery. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0036594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marinello J., Delcuratolo M., Capranico G. Anthracyclines as topoisomerase II poisons: from early studies to new perspectives. Int. J. Mol. Sci. 2018;19(11):3480. doi: 10.3390/ijms19113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chandra A., et al. Identification of potential inhibitors of SARS-COV-2 endoribonuclease (EndoU) from FDA approved drugs: a drug repurposing approach to find therapeutics for COID19. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1775127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montoya M.C., Krysan D.J. Repurposing estrogen receptor antagonists for the treatment of infectious disease. MBio. 2018;9(6):e02272–e2318. doi: 10.1128/mBio.02272-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duran A., et al. Gefitinib and pyrrolidine dithiocarbamate decrease viral replication and cytokine production in dengue virus infected human monocyte cultures. Life Sci. 2017;191:180–185. doi: 10.1016/j.lfs.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 63.Lulli D., Carbone M.L., Pastore S. The MEK inhibitors trametinib and cobimetinib induce a type I interferon response in human keratinocytes. Int. J. Mol. Sci. 2017;18(10):2227. doi: 10.3390/ijms18102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pillaiyar T., Manickam M., Jung S. Middle East respiratory syndrome-coronavirus (MERS-CoV): an updated overview and pharmacotherapeutics. Med chem. 2015;5(8):361–372. [Google Scholar]
- 65.Ke Y.Y., et al. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomed. J. 2020 doi: 10.1016/j.bj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denisova O.V., et al. Obatoclax, saliphenylhalamide, and gemcitabine inhibit influenza a virus infection. J. Biol. Chem. 2012;287(42):35324–35332. doi: 10.1074/jbc.M112.392142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alfano G., et al. Antiviral activity of sirolimus in an HIV-positive kidney transplant recipient. Int. J. STD AIDS. 2019;30(9):919–922. doi: 10.1177/0956462419839520. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Y., et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6(1):1–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan Q., et al. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55(6):1673–1683. doi: 10.1002/hep.25562. [DOI] [PubMed] [Google Scholar]
- 70.Azeez S.A., et al. State-of-the-art tools to identify druggable protein ligand of SARS-CoV-2. Arch. Med. Sci. 2020;16(3):497–507. doi: 10.5114/aoms.2020.94046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trujillo-Murillo K., et al. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology. 2008;47(5):1462–1472. doi: 10.1002/hep.22215. [DOI] [PubMed] [Google Scholar]
- 72.Andreone P., et al. In vitro effect of indomethacin and interferon-α on Th1 and Th2 cytokine synthesis in patients with chronic hepatitis C. Cytokine. 2004;26(3):95–101. doi: 10.1016/j.cyto.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 73.Marinella M.A. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int. J. Clin. Pract. 2020 doi: 10.1111/ijcp.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsai W.-L., et al. Metformin activates type I interferon signaling against HCV via activation of adenosine monophosphate-activated protein kinase. Oncotarget. 2017;8(54):91928. doi: 10.18632/oncotarget.20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burke J., Platanias L.C., Fish E. Beta interferon regulation of glucose metabolism is PI3K/Akt dependent and important for antiviral activity against coxsackievirus B3. J. Virol. 2014;88(6):3485–3495. doi: 10.1128/JVI.02649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pal R., Bhadada S.K. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes Res. Clin. Pract. 2020;163 doi: 10.1016/j.diabres.2020.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young K.-C., et al. Fluoxetine a novel anti-hepatitis C virus agent via ROS-, JNK-, and PPARβ/γ-dependent pathways. Antiviral Res. 2014;110:158–167. doi: 10.1016/j.antiviral.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Gehring G., et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J. Antimicrob. Chemother. 2014;69(8):2123–2131. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castiglione V., et al. Statin therapy in COVID-19 infection. Eur. Heart J.—Cardiovasc. Pharmacother. 2020;6(4):258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumaki Y., Morrey J.D., Barnard D.L. Effect of statin treatments on highly pathogenic avian influenza H5N1, seasonal and H1N1pdm09 virus infections in BALB/c mice. Future Virol. 2012;7(8):801–818. doi: 10.2217/fvl.12.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng J., et al. Protective effect of fluvastatin on influenza virus infection. Mol. Med. Rep. 2014;9(6):2221–2226. doi: 10.3892/mmr.2014.2076. [DOI] [PubMed] [Google Scholar]
- 82.Batista M.N., et al. Caffeine inhibits hepatitis C virus replication in vitro. Arch. Virol. 2015;160(2):399–407. doi: 10.1007/s00705-014-2302-1. [DOI] [PubMed] [Google Scholar]
- 83.Michaelis M., et al. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo P., Liu D., Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahmoud M.A., et al. In vivo interrelationship between insulin resistance and interferon gamma production: protective and therapeutic effect of berberine. Evidence-Based Complem. Alternative Med. 2016 doi: 10.1155/2016/2039897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aziz M., et al. Immunomodulatory effect of Berberis vulgaris extracts on murine splenocytes and enrichment of dendritic cells in vitro. Biotechnol. Biotechnol. Equip. 2015;29(6):1149–1155. [Google Scholar]
- 87.Menzel M., et al. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugamata R., et al. Leucomycin A 3, a 16-membered macrolide antibiotic, inhibits influenza A virus infection and disease progression. J. Antibiotics. 2014;67(3):213–222. doi: 10.1038/ja.2013.132. [DOI] [PubMed] [Google Scholar]
- 89.Zeng S., et al. Spiramycin and azithromycin, safe for administration to children, exert antiviral activity against enterovirus A71 in vitro and in vivo. Int. J. Antimicrob. Agents. 2019;53(4):362–369. doi: 10.1016/j.ijantimicag.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Ishaqui A.A., et al. Assessment of efficacy of Oseltamivir-Azithromycin combination therapy in prevention of Influenza-A (H1N1) pdm09 infection complications and rapidity of symptoms relief. Expert Rev. Respiratory Med. 2020:1–9. doi: 10.1080/17476348.2020.1730180. [DOI] [PubMed] [Google Scholar]
- 91.Schögler A., et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur. Respir. J. 2015;45(2):428–439. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- 92.Menzel M., et al. Azithromycin augments rhinovirus-induced IFNβ via cytosolic MDA5 in experimental models of asthma exacerbation. Oncotarget. 2017;8(19):31601. doi: 10.18632/oncotarget.16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ulrich H., Pillat M.M. CD147 as a Target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Reports. 2020:1–7. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin S.-J., et al. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int. Immunopharmacol. 2016;40:318–326. doi: 10.1016/j.intimp.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 95.Kuo C.-H., et al. Azithromycin suppresses Th1-and Th2-related chemokines IP-10/MDC in human monocytic cell line. J. Microbiol. Immunol. Infect. 2019;52(6):872–879. doi: 10.1016/j.jmii.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Mosquera R.A., et al. Role of prophylactic azithromycin to reduce airway inflammation and mortality in a RSV mouse infection model. Pediatr. Pulmonol. 2018;53(5):567–574. doi: 10.1002/ppul.23956. [DOI] [PubMed] [Google Scholar]
- 97.Damle B., et al. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andreani J., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Molina J.M., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;10 doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamada Y., et al. Inhibition of influenza A virus replication by a kanamycin derivative. Antiviral Res. 1991;15(3):171–182. doi: 10.1016/0166-3542(91)90064-x. [DOI] [PubMed] [Google Scholar]
- 101.Colombani T., et al. Lipidic aminoglycoside derivatives: a new class of immunomodulators inducing a potent innate immune stimulation. Adv. Sci. (Weinh) 2019;6(16):1900288. doi: 10.1002/advs.201900288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.White A.C., Jr Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Rev. Anti-infective Therapy. 2004;2(1):43–49. doi: 10.1586/14787210.2.1.43. [DOI] [PubMed] [Google Scholar]
- 103.Lam K.K., et al. Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis. PLoS Pathog. 2012;8(5):1002691. doi: 10.1371/journal.ppat.1002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hong S.K., et al. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int. Immunopharmacol. 2012;13(1):23–27. doi: 10.1016/j.intimp.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 105.Jasenosky L.D., et al. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus. iScience. 2019;19:1279–1290. doi: 10.1016/j.isci.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health. 2016;9(3):227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rossignol J.-F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haffizulla J., et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet. Infect. Dis. 2014;14(7):609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andrews P., Thyssen J., Lorke D. The biology and toxicology of molluscicides, Bayluscide. Pharmacol. Therap. 1982;19(2):245–295. doi: 10.1016/0163-7258(82)90064-x. [DOI] [PubMed] [Google Scholar]
- 110.Pearson R.D., Hewlett E.L. Niclosamide therapy for tapeworm infections. Ann. Internal Med. 1985;102(4):550–551. doi: 10.7326/0003-4819-102-4-550. [DOI] [PubMed] [Google Scholar]
- 111.Selection, W.E.C.o.t., U.o.E. Medicines, and W.H. Organization, The Selection and Use of Essential Medicines, vol. 950, 2008, World Health Organization. ISSN 05123054.
- 112.Weinbach E.C., Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969;221(5185):1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- 113.Chen W., et al. Niclosamide: beyond an antihelminthic drug. Cell. Signal. 2018;41:89–96. doi: 10.1016/j.cellsig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li M., et al. Suppression of lysosome function induces autophagy via a feedback down-regulation of MTOR complex 1 (MTORC1) activity. J. Biol. Chem. 2013;288(50):35769–35780. doi: 10.1074/jbc.M113.511212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang L., et al. Inhibitory effects of niclosamide on inflammation and migration of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflamm. Res. 2015;64(3–4):225–233. doi: 10.1007/s00011-015-0801-5. [DOI] [PubMed] [Google Scholar]
- 116.Ren X., et al. Identification of niclosamide as a new small-molecule inhibitor of the STAT3 signaling pathway. ACS Med. Chem. Lett. 2010;1(9):454–459. doi: 10.1021/ml100146z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu C.-J., et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004;48(7):2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wen C.-C., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50(17):4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 119.Anand K., et al. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 120.Chou K.-C., Wei D.-Q., Zhong W.-Z. Binding mechanism of coronavirus main proteinase with ligands and its implication to drug design against SARS. Biochem. Biophys. Res. Commun. 2003;308(1):148–151. doi: 10.1016/S0006-291X(03)01342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gassen N.C., et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 2019;10(1):1–16. doi: 10.1038/s41467-019-13659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Testoni B., et al. Ribavirin restores IFNalpha responsiveness in HCV-infected livers by epigenetic remodelling at interferon stimulated genes. Gut. 2016;65(4):672–682. doi: 10.1136/gutjnl-2014-309011. [DOI] [PubMed] [Google Scholar]
- 123.Liu W.L., et al. Ribavirin enhances the action of interferon-alpha against hepatitis C virus by promoting the p53 activity through the ERK1/2 pathway. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0043824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barnard D.L., et al. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res. 2006;71(1):53–63. doi: 10.1016/j.antiviral.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khalili J.S., et al. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morgenstern B., et al. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem. Biophys. Res. Commun. 2005;326(4):905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martschick A., et al. The pathogenetic mechanism of anthracycline-induced palmar-plantar erythrodysesthesia. Anticancer Res. 2009;29(6):2307–2313. [PubMed] [Google Scholar]
- 129.Cohen J.I. New activities for old antibiotics. Nat. Microbiol. 2018;3(5):531–532. doi: 10.1038/s41564-018-0152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jordan V.C. The science of selective estrogen receptor modulators: concept to clinical practice. Clin. Cancer Res. 2006;12(17):5010–5013. doi: 10.1158/1078-0432.CCR-06-1136. [DOI] [PubMed] [Google Scholar]
- 131.Grainger D.J., Metcalfe J.C. Tamoxifen: teaching an old drug new tricks? Nat. Med. 1996;2(4):381–385. doi: 10.1038/nm0496-381. [DOI] [PubMed] [Google Scholar]
- 132.Cham L.B., et al. Tamoxifen protects from vesicular stomatitis virus infection. Pharmaceuticals (Basel) 2019;12(4) doi: 10.3390/ph12040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Post A.E.M., et al. Interferon-stimulated genes are involved in cross-resistance to radiotherapy in tamoxifen-resistant breast cancer. Clin. Cancer Res. 2018;24(14):3397–3408. doi: 10.1158/1078-0432.CCR-17-2551. [DOI] [PubMed] [Google Scholar]
- 134.Swaisland H.C., et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin. Pharmacokinet. 2005;44(10):1067–1081. doi: 10.2165/00003088-200544100-00005. [DOI] [PubMed] [Google Scholar]
- 135.Chen Y., et al. Pharmacokinetic and pharmacodynamic study of Gefitinib in a mouse model of non-small-cell lung carcinoma with brain metastasis. Lung Cancer. 2013;82(2):313–318. doi: 10.1016/j.lungcan.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 136.Yan M., et al. Knockdown of PLAT enhances the anticancer effect of gefitinib in non-small cell lung cancer. J. Thorac Dis. 2020;12(3):712–723. doi: 10.21037/jtd.2019.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.AlAsmari A.F., et al. Liraglutide attenuates gefitinib-induced cardiotoxicity and promotes cardioprotection through the regulation of MAPK/NF-kappaB signaling pathways. Saudi Pharm. J. 2020;28(4):509–518. doi: 10.1016/j.jsps.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thota R., Johnson D.B., Sosman J.A. Trametinib in the treatment of melanoma. Expert. Opin. Biol. Ther. 2015;15(5):735–747. doi: 10.1517/14712598.2015.1026323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scarfo L., et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reynolds J.K., Levien T.L. Quality-of-life assessment in phase III clinical trials of gemcitabine in non-small-cell lung cancer. Drugs Aging. 2008;25(11):893–911. doi: 10.2165/0002512-200825110-00001. [DOI] [PubMed] [Google Scholar]
- 141.de Sousa Cavalcante L., Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol. 2014;741:8–16. doi: 10.1016/j.ejphar.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 142.Honeywell R.J., et al. Inhibition of thymidylate synthase by 2′, 2′-difluoro-2′-deoxycytidine (Gemcitabine) and its metabolite 2′, 2′-difluoro-2′-deoxyuridine. Int. J. Biochem. Cell Biol. 2015;60:73–81. doi: 10.1016/j.biocel.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 143.Mini E., et al. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006;17(suppl_5):v7–v12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 144.Lee K., et al. Gemcitabine, a broad-spectrum antiviral drug, suppresses enterovirus infections through innate immunity induced by the inhibition of pyrimidine biosynthesis and nucleotide depletion. Oncotarget. 2017;8(70) doi: 10.18632/oncotarget.23258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leaman D.W., et al. Roles of JAKs in activation of STATs and stimulation of c-fos gene expression by epidermal growth factor. Mol. Cell. Biol. 1996;16(1):369–375. doi: 10.1128/mcb.16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shresta S., et al. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J. Immunol. 2005;175(6):3946–3954. doi: 10.4049/jimmunol.175.6.3946. [DOI] [PubMed] [Google Scholar]
- 147.Shin H.J., Kim C., Cho S. Gemcitabine and nucleos (t) ide synthesis inhibitors are broad-spectrum antiviral drugs that activate innate immunity. Viruses. 2018;10(4):211. doi: 10.3390/v10040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stamelos V.A., Redman C.W., Richardson A. Understanding sensitivity to BH3 mimetics: ABT-737 as a case study to foresee the complexities of personalized medicine. J. Mol. Signal. 2012;7(1):12. doi: 10.1186/1750-2187-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Konopleva M., et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68(9):3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smoot R.L., et al. A Bax-mediated mechanism for obatoclax-induced apoptosis of cholangiocarcinoma cells. Cancer Res. 2010;70(5):1960–1969. doi: 10.1158/0008-5472.CAN-09-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McCoy F., et al. Obatoclax induces Atg7-dependent autophagy independent of beclin-1 and BAX/BAK. Cell Death Dis. 2010;1(12) doi: 10.1038/cddis.2010.86. e108–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kuivanen S., et al. Obatoclax, saliphenylhalamide and gemcitabine inhibit Zika virus infection in vitro and differentially affect cellular signaling, transcription and metabolism. Antiviral Res. 2017;139:117–128. doi: 10.1016/j.antiviral.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 153.Andersen P.I., et al. Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses. 2019;11(10):964. doi: 10.3390/v11100964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim Y.-J., et al. The ReFRAME library as a comprehensive drug repurposing library to identify mammarenavirus inhibitors. Antiviral Res. 2019;169 doi: 10.1016/j.antiviral.2019.104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Varghese F.S., et al. Obatoclax inhibits alphavirus membrane fusion by neutralizing the acidic environment of endocytic compartments. Antimicrob. Agents Chemother. 2017;61(3):e02227–e2316. doi: 10.1128/AAC.02227-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Halloran P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 157.Hackl A., Ehren R., Weber L.T. Effect of mycophenolic acid in experimental, nontransplant glomerular diseases: new mechanisms beyond immune cells. Pediatric Nephrol. 2017;32(8):1315–1322. doi: 10.1007/s00467-016-3437-y. [DOI] [PubMed] [Google Scholar]
- 158.Abadja F., et al. Impact of mycophenolic acid and tacrolimus on Th17-related immune response. Transplantation. 2011;92(4):396–403. doi: 10.1097/TP.0b013e3182247b5f. [DOI] [PubMed] [Google Scholar]
- 159.Russell B., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lin M.-H., et al. Disulfiram can inhibit mers and sars coronavirus papain-like proteases via different modes. Antiviral Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheng K.-W., et al. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chan J.F., et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013;67(6):606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shen L., et al. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 2019;93(12):e00023–e119. doi: 10.1128/JVI.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hart B.J., et al. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. General Virol. 2014;95(Pt 3):571. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chan J.F.-W., et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mo Y., Fisher D. A review of treatment modalities for Middle East respiratory syndrome. J. Antimicrob. Chemother. 2016;71(12):3340–3350. doi: 10.1093/jac/dkw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Al Ghamdi M., et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect. Dis. 2016;16(1):174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim M.Y., et al. Synergistic inhibition of tumor necrosis factor-alpha-stimulated pro-inflammatory cytokine expression in HaCaT cells by a combination of rapamycin and mycophenolic acid. Ann. Dermatol. 2015;27(1):32–39. doi: 10.5021/ad.2015.27.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao W., et al. Toll-like receptor–mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI (3) K-mTOR-p70S6K pathway. Nat. Immunol. 2008;9(10):1157. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou X., et al. Rapamycin and everolimus facilitate hepatitis E virus replication: revealing a basal defense mechanism of PI3K-PKB-mTOR pathway. J. Hepatol. 2014;61(4):746–754. doi: 10.1016/j.jhep.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 171.Huang C.-T., et al. Rapamycin adjuvant and exacerbation of severe influenza in an experimental mouse model. Sci. Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-04365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jung U., et al. Ex vivo rapamycin generates Th1/Tc1 or Th2/Tc2 Effector T cells with enhanced in vivo function and differential sensitivity to post-transplant rapamycin therapy. Biol. Blood Marrow Transplant. 2006;12(9):905–918. doi: 10.1016/j.bbmt.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 173.Chen X.L., et al. Rapamycin enhances IFN-gamma and IL-4 production in co-culture of gdelta T and dendritic cells from mice with lipopolysaccharide-induced acute lung injury. Genet. Mol. Res. 2016;15(2) doi: 10.4238/gmr.15027511. [DOI] [PubMed] [Google Scholar]
- 174.Kindrachuk J., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sargiacomo C., Sotgia F., Lisanti M.P. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY) 2020;12(8):6511. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mannick J.B., et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6(268) doi: 10.1126/scitranslmed.3009892. 268ra179-268ra179. [DOI] [PubMed] [Google Scholar]
- 177.Lehrer S. Inhaled biguanides and mTOR inhibition for influenza and coronavirus. World Acad. Sci. J. 2020;2(3) doi: 10.3892/wasj.2020.42. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Glatthaar-Saalmüller B., Mair K.H., Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract—an in vitro study. Influenza Other Respir. Viruses. 2017;11(1):85–92. doi: 10.1111/irv.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yousefi S., et al. Effect of acetyl salicylic acid on production and action of leukocyte-derived interferons. Antimicrob. Agents Chemother. 1987;31(1):114–116. doi: 10.1128/aac.31.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cesario T., Yousefi S., Carandang G. The regulation of interferon production by aspirin, other inhibitors of the cyclooxygenase pathway and agents influencing calcium channel flux. Bull. N. Y. Acad. Med. 1989;65(1):26. [PMC free article] [PubMed] [Google Scholar]
- 181.Li T., et al. Aspirin enhances IFN-α-induced growth inhibition and apoptosis of hepatocellular carcinoma via JAK1/STAT1 pathway. Cancer Gene Ther. 2013;20(6):366–374. doi: 10.1038/cgt.2013.29. [DOI] [PubMed] [Google Scholar]
- 182.FitzGerald G.A. Misguided drug advice for COVID-19. Science. 2020;367(6485):1434. doi: 10.1126/science.abb8034. [DOI] [PubMed] [Google Scholar]
- 183.Amici C., et al. Indomethacin has a potent antiviral activity against SARS coronavirus. Antiviral Therapy. 2006;11(8):1021. [PubMed] [Google Scholar]
- 184.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rena G., Pearson E.R., Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56(9):1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marcucci F., et al. Context-dependent pharmacological effects of metformin on the immune system. Trends Pharmacol. Sci. 2020;41(3):162–171. doi: 10.1016/j.tips.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 187.Chen Y.C., et al. Suppressive effects of metformin on T-helper 1-related chemokines expression in the human monocytic leukemia cell line THP-1. Endocr. Res. 2018;43(4):228–234. doi: 10.1080/07435800.2018.1460605. [DOI] [PubMed] [Google Scholar]
- 188.Duggal N.A. Reversing the immune ageing clock: lifestyle modifications and pharmacological interventions. Biogerontology. 2018;19(6):481–496. doi: 10.1007/s10522-018-9771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.El-Arabey A.A., Abdalla M. Metformin and COVID-19: a novel deal of an Old Drug. J. Med. Virol. 2020 doi: 10.1002/jmv.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cava C., Bertoli G., Castiglioni I. In silico discovery of candidate drugs against covid-19. Viruses. 2020;12(4):404. doi: 10.3390/v12040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lin Y.-M., et al. Fluoxetine regulates cell growth inhibition of interferon-α. Int. J. Oncol. 2016;49(4):1746–1754. doi: 10.3892/ijo.2016.3650. [DOI] [PubMed] [Google Scholar]
- 192.Kubera M., et al. Anti-inflammatory effects of antidepressants through suppression of the interferon-γ/interleukin-10 production ratio. J. Clin. Psychopharmacol. 2001;21(2):199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 193.Maes M. The immunoregulatory effects of antidepressants. Human Psychopharmacol.: Clin. Exp. 2001;16(1):95–103. doi: 10.1002/hup.191. [DOI] [PubMed] [Google Scholar]
- 194.Khakzad M.R., et al. Effect of verapamil on bronchial goblet cells of asthma: an experimental study on sensitized animals. Pulm. Pharmacol. Ther. 2012;25(2):163–168. doi: 10.1016/j.pupt.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 195.Matsumori A., Nishio R., Nose Y. Calcium channel blockers differentially modulate cytokine production by peripheral blood mononuclear cells. Circ. J. 2010;74(3):567–571. doi: 10.1253/circj.cj-09-0467. [DOI] [PubMed] [Google Scholar]
- 196.Hashioka S., Klegeris A., McGeer P.L. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology. 2012;63(4):685–691. doi: 10.1016/j.neuropharm.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 197.Ali N., et al. Fluvastatin interferes with hepatitis C virus replication via microtubule bundling and a doublecortin-like kinase-mediated mechanism. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rao G.A., Pandya P.K. Statin therapy improves sustained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology. 2011;140(1):144–152. doi: 10.1053/j.gastro.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 199.Glück B., et al. Simvastatin treatment showed no prophylactic effect in influenza virus-infected mice. J. Med. Virol. 2013;85(11):1978–1982. doi: 10.1002/jmv.23682. [DOI] [PubMed] [Google Scholar]
- 200.Wickert L.E., et al. Simvastatin attenuates rhinovirus-induced interferon and CXCL10 secretion from monocytic cells in vitro. J. Leukoc. Biol. 2014;95(6):951–959. doi: 10.1189/jlb.0713413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu Z., et al. Evaluation of the efficacy and safety of a statin/caffeine combination against H5N1, H3N2 and H1N1 virus infection in BALB/c mice. Eur. J. Pharm. Sci. 2009;38(3):215–223. doi: 10.1016/j.ejps.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 202.Rahal E.A., et al. The impact of prophylactic antiviral agents and statin administration on graft longevity in kidney allograft recipients. Immunopharmacol. Immunotoxicol. 2012;34(5):763–767. doi: 10.3109/08923973.2011.653648. [DOI] [PubMed] [Google Scholar]
- 203.Gluck B., et al. Simvastatin treatment showed no prophylactic effect in influenza virus-infected mice. J. Med. Virol. 2013;85(11):1978–1982. doi: 10.1002/jmv.23682. [DOI] [PubMed] [Google Scholar]
- 204.Schultz N.E.Ø., et al. Statin treatment before stroke reduces pro-inflammatory cytokine levels after stroke. Neurol. Res. 2019;41(4):289–297. doi: 10.1080/01616412.2018.1558000. [DOI] [PubMed] [Google Scholar]
- 205.Cheng S.M., et al. Modulation of human T cells signaling transduction by lovastatin. Int. J. Cardiol. 2010;140(1):24–33. doi: 10.1016/j.ijcard.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 206.Krysiak R., Okopień B. Lymphocyte-suppressing action of simvastatin in patients with isolated hypertriglyceridemia. Pharmacol. Rep. 2013;65(3):756–760. doi: 10.1016/s1734-1140(13)71056-9. [DOI] [PubMed] [Google Scholar]
- 207.Lee C.S., et al. Simvastatin acts as an inhibitor of interferon gamma-induced cycloxygenase-2 expression in human THP-1 cells, but not in murine RAW264. 7 cells. Biocell. 2009 [PubMed] [Google Scholar]
- 208.Dashti-Khavidaki S., Khalili H. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy: J. Human Pharmacol. Drug Therapy. 2020;40(5):484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gu W., et al. Simvastatin alleviates airway inflammation and remodelling through up-regulation of autophagy in mouse models of asthma. Respirology. 2017;22(3):533–541. doi: 10.1111/resp.12926. [DOI] [PubMed] [Google Scholar]
- 210.Horrigan L.A., Kelly J.P., Connor T.J. Immunomodulatory effects of caffeine: friend or foe? Pharmacol. Ther. 2006;111(3):877–892. doi: 10.1016/j.pharmthera.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 211.Tej G.N.V.C., et al. Caffeine-enhanced anti-tumor immune response through decreased expression of PD1 on infiltrated cytotoxic T lymphocytes. Eur. J. Pharmacol. 2019;859 doi: 10.1016/j.ejphar.2019.172538. [DOI] [PubMed] [Google Scholar]
- 212.Sheth C.C., et al. Modulation of salivary cytokines in response to alcohol, tobacco and caffeine consumption: a pilot study. Sci. Rep. 2018;8(1):1–7. doi: 10.1038/s41598-018-35094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gantait A., et al. Quantification of glycyrrhizin in Glycyrrhiza glabra extract by validated HPTLC densitometry. J. AOAC Int. 2010;93(2):492–495. [PubMed] [Google Scholar]
- 214.Seki H., et al. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell. 2011;23(11):4112–4123. doi: 10.1105/tpc.110.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Seki H., et al. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc. Natl. Acad. Sci. 2008;105(37):14204–14209. doi: 10.1073/pnas.0803876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ito M., et al. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)] Antiviral Res. 1987;7(3):127–137. doi: 10.1016/0166-3542(87)90001-5. [DOI] [PubMed] [Google Scholar]
- 217.Ito M., et al. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV) Antiviral Res. 1988;10(6):289–298. doi: 10.1016/0166-3542(88)90047-2. [DOI] [PubMed] [Google Scholar]
- 218.Nasrollahi V., et al. The effect of drought stress on the expression of key genes involved in the biosynthesis of triterpenoid saponins in liquorice (Glycyrrhiza glabra) Phytochemistry. 2014;103:32–37. doi: 10.1016/j.phytochem.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tong T., et al. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16(13):1906206. doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wolk K., et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 2006;36(5):1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 221.Brito-Luna M., et al. Correlation of IL-12, IL-22, and IL-23 in patients with psoriasis and metabolic syndrome. Preliminary report. Cytokine. 2016;85:130–136. doi: 10.1016/j.cyto.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 222.Tu C.-T., et al. Glycyrrhizin regulates CD4+ T cell response during liver fibrogenesis via JNK, ERK and PI3K/AKT pathway. Int. Immunopharmacol. 2012;14(4):410–421. doi: 10.1016/j.intimp.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 223.Xiao X., Tang M., Li B. Treatment of psoriasis by compound glycyrrhizin injection and its effects on peripheral blood Th17 cell proportion and IL-22 concentration. Int. J. Clin. Exp. Med. 2018;11(2):1291–1297. [Google Scholar]
- 224.Gao R., et al. Glycyrrhizin inhibits PEDV infection and proinflammatory cytokine secretion via the HMGB1/TLR4-MAPK p38 pathway. Int. J. Mol. Sci. 2020;21(8):2961. doi: 10.3390/ijms21082961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Huan C.-C., et al. Glycyrrhizin inhibits porcine epidemic diarrhea virus infection and attenuates the proinflammatory responses by inhibition of high mobility group box-1 protein. Arch. Virol. 2017;162(6):1467–1476. doi: 10.1007/s00705-017-3259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu L.-S., et al. The effects and mechanism of yinqiao powder on upper respiratory tract infection. Int. J. Biotechnol. Wellness Indust. 2015;4(2):57–60. [Google Scholar]
- 227.Fu Y.-J., et al. Effects of different principles of traditional Chinese medicine treatment on TLR7/NF-κB signaling pathway in influenza virus infected mice. Chinese Med. 2018;13(1):42. doi: 10.1186/s13020-018-0199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cinatl J., et al. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pilcher H. Liquorice may tackle SARS. Nature. 2003 doi: 10.1038/news030609-16. [DOI] [Google Scholar]
- 230.Lau C.W., et al. Cardiovascular actions of berberine. Cardiovasc. Drug Rev. 2001;19(3):234–244. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 231.Cicero A.F., Tartagni E. Antidiabetic properties of berberine: from cellular pharmacology to clinical effects. Hospital Practice. 2012;40(2):56–63. doi: 10.3810/hp.2012.04.970. [DOI] [PubMed] [Google Scholar]
- 232.Kim T.S., et al. Induction of interleukin-12 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid, deviates CD4+ T cells from a Th2 to a Th1 response. Immunology. 2003;109(3):407–414. doi: 10.1046/j.1365-2567.2003.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kuo C.-L., Chi C.-W., Liu T.-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203(2):127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 234.Kim H.-Y., et al. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J. Clin. Virol. 2008;41(2):122–128. doi: 10.1016/j.jcv.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chin L.W., et al. Anti-herpes simplex virus effects of berberine from Coptidis rhizoma, a major component of a Chinese herbal medicine, Ching-Wei-San. Arch. Virol. 2010;155(12):1933–1941. doi: 10.1007/s00705-010-0779-9. [DOI] [PubMed] [Google Scholar]
- 236.Varghese F.S., et al. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antiviral Res. 2016;126:117–124. doi: 10.1016/j.antiviral.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 237.Hung T.-C., et al. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine. 2019;53:62–69. doi: 10.1016/j.phymed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 238.Ryu Y.B., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Negahdaripour M. The battle against COVID-19: Where do we stand now? Iran. J. Med. Sci. 2020;45(2):81. doi: 10.30476/ijms.2020.46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Traggiai E., et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Adams E.R., et al. Antibody testing for COVID-19: a report from the National COVID Scientific Advisory Panel. Wellcome Open Res. 2020;5(139):139. doi: 10.12688/wellcomeopenres.15927.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.