Abstract
Objective
To describe the proportions of asymptomatic, mild and severe diseases in infected pregnant women admitted for delivery. To compare maternal, fetal and neonatal outcomes of SARS-CoV-2 infected pregnant women with those of non-infected patients.
Study design
Through an universal PCR testing for SARS-COV-2 at admission (not symptoms-based), this prospective cohort study enrolled all pregnant women admitted for delivery between 16th of June and the 16th of August 2020 in the West French Guiana Hospital Center.
Results
507 pregnant women were included during the study period, of which 137 (27 %) were infected with SARS-COV-2. On admission, only 34/137 (24.8 %) of these patients presented with clinical symptoms. Among asymptomatic women, 16 /103 (15 %) became symptomatic after diagnosis. Throughout the delivery hospitalization and follow-up, 87/137 (63.5 %) remained always asymptomatic, 45/137 (32.8 %) developed a mild COVID-19 and 5/137 (3.6 %) developed a severe infection.
SARS-CoV-2 infected patients were more likely to have post-partum hemorrhage >500 mL (14.2 % vs 7.2 %, RR 2.0 [95 %CI 1.1–3.4]), to be transfused (5.5 % vs 1.1 %, RR 4.9 [1.5–16.6]), and to be hospitalized in ICU (3.6 % vs 0.8 %, RR 4.5 [95 %CI 1.1−18.6] than uninfected ones. Intra-uterine fetal demises were more common in infected mothers compared to controls (5.1 % vs 1.1 %, RR 4.7 [95 % CI 1.4–45.9).
Among 108 neonates from infected mothers tested at birth, none tested positive (0/108). When tested between 25 and 42 h after delivery, 4/29 (13.7 %) were positive for SARS-CoV-2 RT-PCR on nasopharyngeal swabs and remained asymptomatic.
Conclusion
Pregnant women admitted for delivery and diagnosed with a SARS-COV-2 infection through an universal screening were symptomatic in only a quarter of cases. Their risks of post-partum hemorrhage, transfusion and admission to ICU were higher than those of uninfected patients. They also presented a higher risk of intra-uterine fetal demise. There were no other differences in maternal, obstetrical or neonatal outcomes.
Keywords: Pregnancy, SARS-CoV-2 infection, Maternal outcomes, Stillbirth, Neonatal outcomes
Introduction
The recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a public health emergency declared by WHO as a global public health emergency [1]. Although there is a growing understanding of SARS-CoV-2, the outcomes associated with SARS-CoV2 infection during pregnancy remain limited and unclear. Based on previous viral exposition, pregnant women are particularly vulnerable to infections with potential for adverse pregnancy or perinatal outcomes [2,3]. Unfortunately, the lack of a comparative group makes COVID 19 studies not strong enough to found adequate power to assess these outcomes. Unfortunately, the infection is re-emerging in many countries; it is an urgent need to analyze the result of the first wave.
The lack of an universal screening at admission (most of patients tested being symptomatic) may lead to selection biases and to a misunderstanding of the potential consequences of SARS-COV-2 infection during pregnancy [[4], [5], [6]].
On the fetal side, many questions arise about the effect of symptomatic and asymptomatic SARS-CoV-2 infection during pregnancy. A few reports, based on biological and histological findings of electron microscopy and immunohistochemistry suggest vertical transmission of SARS-CoV-2 [7] but evidence is still uncertain.
In the last weeks, as many countries in Latin America, Covid-19 has rapidly spread to French Guiana territory, a French department located in South America, north of Brazil. Early in the beginning of the epidemic, we implemented universal testing with RT-PCR on nasopharyngeal swabs to identify SARS-COV-2 infections in pregnant women admitted to the maternity of the West French Guiana Hospital Center (Centre Hospitalier de l’Ouest Guyanais, CHOG).
The aims of this study were to describe the proportions of asymptomatic, mild and severe diseases in infected pregnant women admitted for delivery; and to compare maternal, fetal and neonatal outcomes of SARS-CoV-2 infected pregnant women with those of non-infected patients.
Materials and methods
This observational prospective cohort study carried out in the Department of Obstetrics and Gynecology of the CHOG (referral center of western French Guiana) during the first 8 weeks of the SARSCoV-2 outbreak in the West French Guiana territory, from the 16 of June to the 16 of August 2020.
Protocol description
The west French Guiana territory was spared from COVID 19 before the beginning of this report. At the beginning of the outbreak, our hospital implemented a systematic screening for all pregnant women admitted for delivery using SARS-CoV-2 real-time reverse-transcriptase polymerase-chain-reaction (RT-qPCR) assays (Xpert Xpress, Cepheid, Sunnyvale, USA) on nasopharyngeal swabs. At admission, both RT-qPCR and serological analysis were performed. Results were obtained after 51 min using a machine installed in the delivery room. Positive patients were isolated. Serologies were performed on Abbott ARCHITECT SARS-CoV-2 IgG assay (Abbott, Longford, Ireland). Testing for SARS-CoV-2 in placental tissue was performed using RT-qPCR in the National Reference Center of the Pasteur Institute, French Guiana.
The reason of this policy was to better manage hospitalization, avoid intra hospital contamination and to protect others parturient and medical staff. Antenatal care was provided by obstetricians and midwifes in accordance with the clinical practice of the French College of Gynecologists and Obstetricians. All patients were clinically evaluated. Fetal wellbeing was confirmed by cardiotocography. If necessary, ultrasonographic evaluation was performed. All women presented with a fever were also screened for Dengue virus.
CHOG policy authorize mothers to practice skin-to-skin care and breastfeed in the delivery room. She had to wear a surgical mask and to practice proper hand hygiene before skin-to-skin contact, breastfeeding, and routine care. All patients were monitored 14 days after hospital discharge either by telephone call or by home visit according to their clinical forms.
Outcomes and variables definitions
Inclusion criteria were pregnant women admitted for delivery beyond 15 weeks of gestation and tested for SARS-CoV-2 infection on an RT-PCR assay performed on a nasopharyngeal swab. This population was divided into two groups: an exposed group, defined as confirmed SARS-CoV2 infected pregnant women (positive RT-PCR); and a control group, defined by a negative SARS-CoV2 RT-PCR with negative serology when available.
Primary outcome
We evaluated the proportions of asymptomatic, mild and severe diseases among SARS-COV-2 infected pregnant women, according to the World Health Organization criteria [8].
Adverse maternal outcomes included maternal death, intensive care unit (ICU) admission and oxygen support (non-invasive ventilation, endotracheal intubation). Adverse obstetrical outcomes included preterm delivery (spontaneous or induced delivery <34wg), operative vaginal delivery or emergency cesarean section, acute fetal distress, postpartum hemorrhage (>500 mL) and transfusion.
Adverse fetal outcomes included late miscarriages (>14 wg) and intra-uterine fetal demises (spontaneous antepartum fetal death >20 wg).
Adverse neonatal outcomes included per-partum or neonatal death, NICU admission, respiratory distress, seizures, Apgar score ≤7 at 1 min, umbilical venous lactate ≥ 5 mmol/l at birth and a low birthweight (<3rd percentile, according to Intergrowth21st charts).
Secondary outcomes
All maternal, obstetrical and neonatal complications directly related or not related to COVID-19 infection were reported. Neonatal testing for SARS-COV-2 infection at birth and 24–48 h after birth, using RT-PCR assays on naso-pharyngeal swabs, was also reported.
Data collection
Data were collected prospectively and anonymously using an excel spreadsheet. The follow-up was completed on August 31, 2020. In compliance with the Regulation (EU) 2016/679 of the European Parliament on the protection of personal data a privacy impact assessment of the study was carried out according to the methodology described by the French data protection (CNIL). The risk mapping report was approved by the establishment's ethics committee (decision CHOG 2020-08-17).
Statistical analysis
Data were analyzed using STATA® v13.0. Student’s t-test was used to compare continuous variables, whereas the comparison of proportions was performed using the chi-square test or Fisher’s exact test, as appropriate. The unpaired Student’s t-test and the Mann–Whitney U test were used to compare groups of continuous normally and non-normally distributed variables, respectively. Presented P-values were two-sided and the significance level was set to 5 % (p = 0.05) for all statistical tests. Relative risks were estimated using a generalized linear model, adjusted for unbalanced maternal characteristics that could represent confounding factors and stratified for potential interactions with primary outcomes. Neonatal outcomes analysis included a robust variance option for twin pregnancies in the model.
Results
Study population
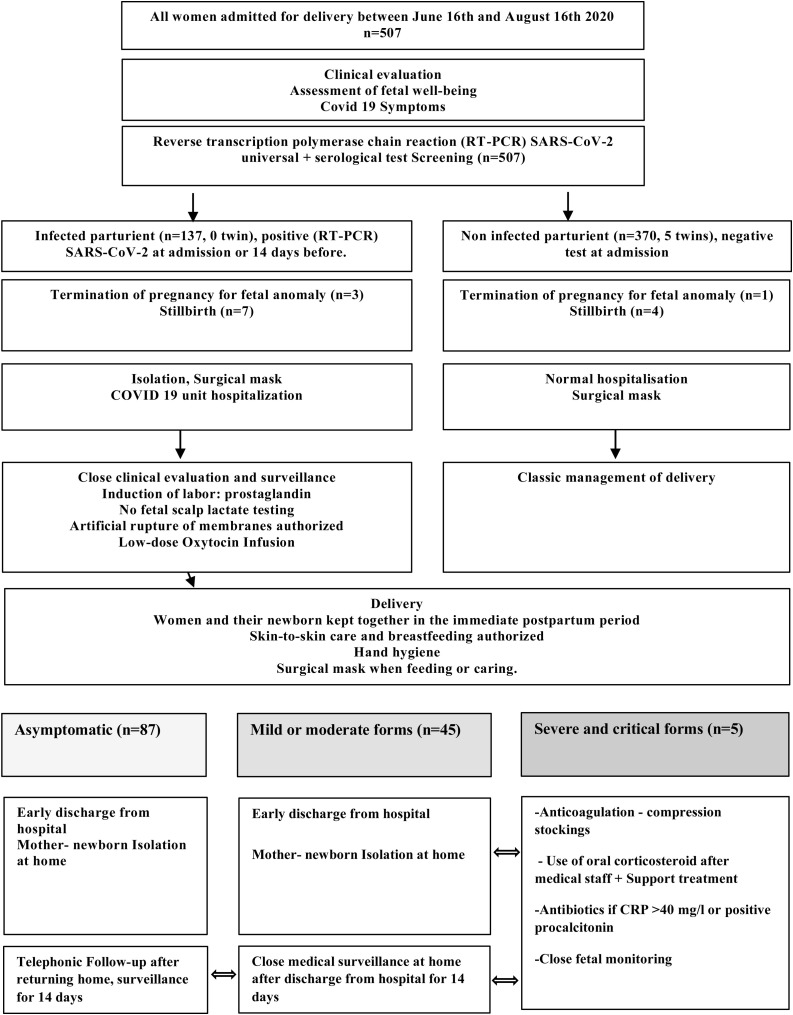
During the study period, 507 pregnant women were admitted for delivery at the CHOG. Among them 137/507 (27 %) had a confirmed SARS-CoV-2 infection with positive RT-PCR on nasopharyngeal swabs (Fig. 1 ). In the control group, all patient had negative RT-PCR SARS-COV-2. In SARS-COV-2 RT-PCR positive mothers, results of serology were obtained in 76 patients (55.4 %). It was negative in 36.8 % (28/ 76), positive in 60.5 % (46/76) and doubtful in 2 cases (2.6 %). Among those tested negative on RT-PCR (i.e. control group), serological testing was performed in 21.6 % (80/370) and was negative for all cases.
Fig. 1.
Flow diagram of CHOG maternity protocol during the SARS-CoV-2 epidemic.
There were 127 alive neonates from positive mothers and 364 neonates ≥25 WG from negative mothers including five sets of twins. We have documented 11 stillbirths, 4 termination of pregnancy for fetal anomalies and 6 extremely preterm infants 22−25WG (live births followed by a neonatal death).
Baseline characteristics
Maternal characteristics and comorbidities are presented in Table 1 .
Table 1.
Comparison of Maternal baseline characteristics and pregnancy co-morbidities between the groups of SARS-CoV-2 infected and non-infected pregnant women.
| SARS-CoV-2 Infected parturient (n = 137) | SARS-CoV-2 Non infected parturient (n = 370) | P-Value | |
|---|---|---|---|
| Maternal age, median (IQR) | 25 (21,31) | 25 (21,33) | 0.29 |
| Age over 35 years | 18 (13.1 %) | 72 (19 %) | 0.12 |
| BMI, median (IQR), kg/m2 | 26.1 (22.3, 30.8) | 26.6 (22.9, 31.0) | 0.57 |
| BMI over 30 Kg/m2 | 48 (35.0 %) | 122 (32.9 %) | 0.74 |
| Gravida, median (IQR) | 3 (2,6) | 3 (2,6) | 0.84 |
| Parity, median (IQR) | 2 (1,5) | 2 (0,4) | 0.4 |
| Nulliparous | 30 (21.9 %) | 94 (25.4 %) | 0.48 |
| Scarred uterus | 22 (16.1 %) | 67 (18.1 %) | 0.68 |
| Term ≥41 weeks of gestation | 10 (7.2 %) | 27 (7.2 %) | 1 |
| Medical condition before pregnancy | |||
| Chronic hypertension | 2 (1.4 %) | 6 (1.6 %) | 1* |
| Diabetes | 0 (0.0 %) | 5 (1.35 %) | 0.33* |
| Asthma | 0 (0.0 %) | 7 (1.8 %) | 0.19* |
| Cardiovascular disease | 1 (0.72 %) | 2 (0.5 %) | 1* |
| Homozygote drepanocytosis | 2 (1.4 %) | 2 (0.5 %) | 0.29* |
| Anemia | 5 (3.6 %) | 7 (1.8 %) | 0.32* |
| Co-morbidities during pregnancy | |||
| Dichorionic twins | 0 (0.0 %) | 5 (1.4 %) | 0.3 |
| Preeclampsia /hypertension during pregnancy | 15 (10.9 %) | 31 (8.3 %) | 0.47 |
| Anemia | 11 (8.0 %) | 39 (10.5 %) | 0.5 |
| Small-for-Gestational-Age fetus/fetal growth restriction | 3 (2.1 %) | 6 (1.6 %) | 0.7* |
| Premature labor | 7 (5.1 %) | 16 (4.3 %) | 0.89 |
| Gestational diabetes | 13 (9.4 %) | 30 (8.1 %) | 0.75 |
| Co infection | |||
| Dengue infection | 1 (0.7 %) | 2 (0.5 %) | 1* |
| Hepatitis B chronic | 6 (4.3 %) | 5 (1.3 %) | 0.07* |
| Syphilis | 3 (2.1 %) | 2 (0.5 %) | 0.12* |
| HIV | 0 (0.0 %) | 5 (1.3 %) | 0.3* |
Anemia was defined as hemoglobin under 10 g/dl.
IQR, InterQuartile Range; BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fisher's exact test.
There were no significant differences between the two groups of infected and non-infected pregnant women for age, BMI, BMI > 30 kg/m2, comorbidities, gravida, parity, scarred uterus, and pathologies during pregnancy before testing. Twin pregnancies were only present in the control group (5/370, 1.4 %).
Clinical and biological course of patients diagnosed with SARS-COV-2 infection at admission
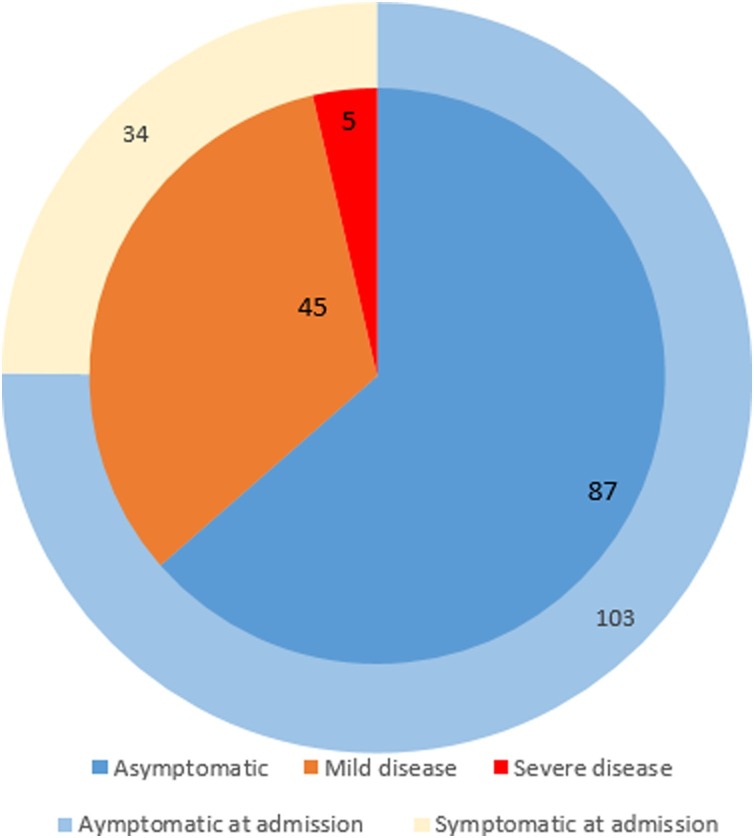
On admission, only 34/137 (24.8 %) of positive patients presented clinical symptoms of COVID 19. Among 103 asymptomatic women (103/137, 75.2 %), 16/103 (15 %) became symptomatic after diagnosis, often occurring after returning home (including two severe cases).
The most reported symptoms were fever (21/50, 42 %), headache (17/50, 34 %), loss of taste and smell (12/50, 24 %), tiredness (9/50, 18 %), cough (8/50, 16 %), diarrhea or abdominal pain (3/50, 6 %) and shortness of breath (2/50, 4 %).
Throughout the delivery hospitalization and the follow-up after returning home, 87/137 (63.5 %) were completely asymptomatic, 45/137 (32.8 %) had mild COVID-19 and 5/137 (3.6 %) had severe infection according to World Health Organization criteria (Fig. 2 ). Four patients (2.9 %) were re-admitted for complication related to SARS-CoV-2 infection.
Fig. 2.
Clinical course of 137 pregnant women diagnosed with SARS-CoV-2 infection at delivery.
The commonest laboratory abnormalities were raised inflammatory markers of C-reactive protein or procalcitonin (35 %), thrombopenia (10.1 %), lymphopenia (2.1 %) and elevated transaminases (3.6 %).
Obstetrical outcomes
There were no differences in mode of delivery (operative vaginal delivery, cesarean section before labor, or cesarean section in labor) and abnormal fetal heart rate (Table 2 ).
Table 2.
Comparison of obstetrical, maternal and fetal outcomes between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infected and non-infected pregnant women admitted for delivery.
| SARS-CoV-2 Infected Pregnant women |
Non infected Pregnant women | Relative Risks [95 %CI] | P value | |
|---|---|---|---|---|
| Outcomes of pregnancy | n = 137 | n = 370 (5 twin pregnancies) | ||
| Alive neonates > 25wg | 127 (92.7 %) | 364 (98.4 %) | 0.9 [0.9–1.0] | 0.0012 |
| Extremely preterm infants (22−23 + 6WG) (live births followed by a neonatal death) |
0 (0.0 %) | 6 (1.6 %) | 0.17 | |
| Intrauterine fetal death (19–35 W G) | 7 (5.1 %) | 4 (1.1 %) | 4.7 [1.4–15.9] | 0.0057 |
| Termination of pregnancy for fetal abnormalities | 3 (2.2 %) | 1 (0.3 %) | 0.06 | |
| Obstetric Outcomes, pregnancy ≥25WG | n = 127 | n = 359 (5 twin pregnancies) | ||
| Meconium-stained amniotic Fluid | 8 (6.3 %) | 30 (8.4 %) | 0.58 | |
| Fever during labor | 7 (5.5 %) | 9 (2.5 %) | 0.17 | |
| Uterine hyperkinesia / Hypertonia | 4 (3.1 %) | 15 (4.1 %) | 0.79 | |
| Abnormal fetal heart rate patterns | 23 (18.1 %) | 53 (14.8 %) | 0.45 | |
| Spontaneous preterm delivery <37 + 0 weeks | 11 (8.7 %) | 36 (10.0 %) | 0.78 | |
| Spontaneous preterm delivery <34 + 0 weeks | 1 (0.8 %) | 9 (2.6 %) | 0.4 | |
| Medically indicated preterm birth < 34 + 0 weeks | 0 (0.0 %) | 3 (0.8 %) | 0.57 | |
| Normal vaginal delivery | 101 (79.5 %) | 271 (75.4 %) | 0.42 | |
| Operative vaginal delivery | 7 (5.5 %) | 25 (7.0 %) | 0.71 | |
| Cesarean delivery before labor | 6 (4.7 %) | 18 (5.0 %) | 1 | |
| Cesarean delivery during labor | 13 (10.2 %) | 44 (12.3 %) | 0.65 | |
| Postpartum hemorrhage > 500 cc | 18 (14.2 %) | 26 (7.2 %) | 2.0 [1.1–3.4] | 0.0193 |
| Transfusion after hemorrhage | 7 (5.5 %) | 4 (1.1 %) | 4.9 [1.5–16.6] | 0.004 |
| Readmission | 4 (3.1 %) | 0 (0.0 %) | 0.004 | |
| Maternal complications (n = 137) | n = 137 | n = 370 (5 twin pregnancies) | ||
| Intensive care unit admission | 5 (3.6 %) | 3 (0.8 %) | 4.5 [1.1−18.6] | 0.0227 |
| Intensive care unit admission directly related to COVID 19 | 3 (2.2 %) | – | – | – |
| Oxygen support (nasal or non-invasive ventilation) related to COVID 19 | 3 (2.2 %) | – | – | – |
| Re hospitalization related to COVID 19 | 4 (2.9 %) | – | – | – |
| Endotracheal intubation for complication of COVID 19 | 0 (0.0 %) | – | – | – |
| Maternal death | 0 (0.0 %) | 0 (0.0 %) | – | – |
Risk Differences with 95 % confidence intervals are presented. Twin pregnancies were tested as effect-modifier and did not change the crude analysis reported.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CI, confidence intervals; WG, weeks of gestation.
There were no significant differences in spontaneous births before 37 weeks’ gestation (8.6 % vs 10 %, p = 0.78) and before 34 weeks’ gestation (0.7 % vs 2.5 %, p = 0.46). The risk of iatrogenic preterm birth and caesarean delivery was not significatively increased (0% vs 0.8 %, p = 0.57).
None of the cesarean sections were performed for worsening maternal respiratory status. In two patients with severe COVID-19, C-section extraction was contemplated for women under oxygen therapy at 35 + 1 WG and 34 + 6 WG. Finally, the delivery was postponed after stabilization of respiratory condition. Evolution was good under curative anticoagulation, high doses of oral Prednisone and antibiotics. They gave normal vaginal birth after spontaneous labor.
In post-partum period, SARS-CoV-2 infected patients were more likely to have post-partum hemorrhage >500 mL (18/127, 14.2 % vs 26/359, 7.2 %) and to be transfused (7/127, 5.5 % vs 4/359, 1.1 %) when compared to non-infected patients: RR 2.0 [95 %CI 1.1–3.4] and RR 4.9 [95 %CI 1.5–16.6], respectively. Twin pregnancies were tested as an effect-modifier on these outcomes and did not modify the crude analysis since neither PPH nor transfusion occurred in twin pregnancies. Biological analysis showed hemostatic disorder before delivery only in 2/18 of these patients with post-partum hemorrhage (prothrombin time at 60 %, prolonged activated partial thromboplastin time). We do not observe any difference in the clinical forms, maternal or fetal outcomes when considering sub analysis including positive and negative serology.
Maternal outcomes
SARS-CoV-2 infected patients were more often admitted to intensive care unit when compared to non-infected patients: 3.7 % (5/137) vs 0.8 % (3/370), 4.5 [95 %CI 1.1−18.6]. Four patients were retested when readmitted for complication related to COVID 19 and they remained positive. Intensive care unit admission was required in 2.1 % (3/137) for complication related to COVID 19, with no need of invasive ventilation. No maternal deaths occurred among these patients.
Fetal and neonatal outcomes
There were statistically significant differences between the two groups concerning fetal adverse outcomes (Table 2). SARS-CoV-2 infected pregnant women were less likely to give birth to alive neonates. The incidence of intra-uterine fetal demise was significantly higher in SARS-CoV-2 infected pregnant women compared to uninfected ones: 5.1 % (7/137) vs 1.1 % (4/370), RR 4.7 [1.4–15.9]. All stillbirth occurred in asymptomatic (n = 6) and mild COVID 19 (n = 1) patients, between 19 and 35 weeks of gestation. No medication related to COVID 19 was administered to these patients before fetal demise. We tested placenta for RT-PCR of SARS-CoV-2 in 5 cases of intra uterine fetal demise. Placental fragments were negative for SARS-CoV-2. Unfortunately, in only one case family consented for fetal autopsy. Exam did not detect any significant abnormality in the fetus but showed vascular alteration in the placenta. For the others 6 cases, only histopathological examinations of the placenta were done. It showed acute chorioamnionitis in five cases, and retroplacental hematoma in one case.
In all cases (7 patients), induction of labor was performed using association of mifepristone and Misoprostol and they delivered normally.
There were no differences in the main clinical (birth weight, Apgar score, respiratory distress, oxygen administration, NICU admission, neonatal seizure, or neonatal death) or biological (umbilical venous lactate) neonatal outcomes between the two groups (Table 3 ). All neonates continued to be clinically well and are growing appropriately.
Table 3.
Comparison of neonatal outcomes between neonates from Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infected mothers and those from non-infected mothers.
| Neonates from infected women (n = 127) | Neonates from non-infected women (n = 364) | P-value | |
|---|---|---|---|
| Birth weight (mean ± SD g) | 3090 (2755, 3376) | 3112 (2779, 3396) | 0.6 |
| Apgar score ≤7 at 1 min | 14 (11 %) | 31 (8.5 %) | 0.5 |
| Apgar score ≤7 at 5 min | 4 (3.1 %) | 12 (3.2 %) | 1 |
| Umbilical venous lactate ≥5 mmol/l | 13 (10.7 %) | 53 (14.5 %) | 0.28 |
| Respiratory distress | 4 (3.1 %) | 16 (4.3 %) | 0.79 |
| Neonatal ICU admission | 3 (2.3 %) | 12 (3.2 %) | 0.76 |
| Neonatology admission | 11 (8.6 %) | 36 (9.8 %) | 0.82 |
| Seizure | 0 (0.0 %) | 2 (0.5 %) | NS |
| Neonatal death | 0 (0.0 %) | 0 (0.0 %) | NS |
| SARS-CoV-2 RT PCR testing | |||
| At birth (n = 108) | 0 (0.0 %) | – | – |
| 24−48 h after birth (n = 29) | 4 (13.8 %) | – | – |
| Complication related to Covid 19 | 0 (0.0 %) | – | – |
| Re hospitalization related to COVID19 | 0 (0.0 %) | – | – |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ICU, intensive care unit.
Twins (n = 5, non-infected group) were considered as two separate neonates, the model included a robust variance option for twin pregnancies.
No separation of mother –newborn, skin-to-skin and breakfasting allowed. Mothers were delivered protective measures (hand washing, breast cleaning and wearing a mask when breakfasting).
Among 108 neonates from infected mothers tested at birth, none tested positive (0/108). When tested between 25 and 42 h after delivery, 4/29 of neonates (13.7 %) were positive for SARS-CoV-2 RT-PCR on nasopharyngeal swabs. Two neonates were retested positive 3 and 6 days after. Newborns were breastfed, never separated from their mothers, and received medical surveillance at home for one month. All positive neonates remained asymptomatic.
Discussion
Principal findings of the study
Only a quarter of pregnant women who presented SARS-COV-2 infection in the peri-partum period had symptoms on admission. SARS-COV-2 infected patients had increased risks of maternal (ICU admission, post-partum hemorrhage and transfusion) and fetal (intrauterine fetal demise) adverse outcomes. The risk of intrauterine fetal demise did not depend on the clinical form at admission; thus, the population of asymptomatic pregnant women is not spared of such adverse outcomes.
This study focused on the consequences of SARS-COV-2 infection diagnosed in the peri-partum period. For this reason, we considered only the beginning of the epidemic. Control group was composed of patients who tested negative for SARS-COV-2 on RT-PCR (systematic screening) and serology when available.
It was carried out in a maternity hospital in French Guiana, a French department located in South America north of Brazil. The French Guiana population is very heterogeneous with 70 % of patients of African origin with many other Amerindian, Asian, and European communities. In our population, we noticed a high rate of positive pregnant women (27 %) in comparison to other reported series (<1 % in Southern California [6] and 13.7 % New York City [19]). Lifestyle, low income and limited education among population in west French Guiana territory could probably explain such results.
The findings of this study suggest that SARS-CoV-2 does not affect pregnancy only through complications of hypoxemia and circulatory insufficiency. The most important question is whether the adverse outcomes in pregnant women infected by SARS CoV2 resulted from direct effects of SARS-CoV-2 in the maternofetal compartment and therefore a possible vertical transmission. The exact mechanisms remain unclear. Is it hemodynamic, vascular, immunological, or hormonal?
The damage of the virus in the vascular system has been reported and could influence the feto- placental unit, notably with coagulopathy associated with COVID-19 [9,10]. The SARS-CoV-2 virus binds to angiotensin-converting enzyme 2 (ACE2) [11] found in many different human organs. ACE2 is a key part of the renin angiotensin system (RAS) and a counterbalance to angiotensin-converting enzyme 1 (ACE1) and angiotensin II. Angiotensin II is proinflammatory, vasoconstrictive [12]. The mechanism is similar to those described in preeclampsia and HELLP syndrome. Federici et al. reported hypertension and biological disorders suggesting pre-eclampsia and HELLP syndrome in a pregnant woman at 23.5 weeks of gestation with Covid-19 complications (acute respiratory distress syndrome treated with invasive mechanical ventilation) [13].
Regarding mode of delivery, early experiences reported an important rate of cesarean section (50–91%) [14,15] among severe cases of Covid 19.
Fortunately, epidemic studies in pregnant women reported that as much as 90 % of SARS-CoV-2–positive cases were asymptomatic [[16], [17], [18]]. In our experience, we did not find an increased risk of cesarean section in either the symptomatic or asymptomatic group compared to the control group. It might be due to the protective effect of ethnicity [19], genetic diversity of the virus [20] or the relatively small sample of severe SARS-CoV-2 infection. Severe forms of COVID 19 or administration of oxygen therapy should not be an indication for premature extraction if stabilization of respiratory condition is possible. This was the case for two of our patients and another one reported by Federici et al. [14].
Many authors [[21], [22], [23]] alerted about collateral damage, deleterious effects caused by the restrictions taken to limit the spread of the pandemic. Pregnant women who have multiple interactions with the health care system can been greatly affected by these actions. These effects are difficult to measure in clinical practice. The report of Khalil et al. demonstrates an increase in the stillbirth rate during the pandemic at St George’s University Hospital, London and attributed this to indirect factors without being able to exclude a direct consequence of SARS-CoV-2 infection [22]. Changes in obstetric services and health system disruption may have played a role in observed adverse outcomes in our cohort but it would also have affected the control group.
Although first findings from a small group of cases suggested there is no vertical transmission in women who develop COVID-19 [24,25], rare observations reported vertical transmission of SARS-CoV-2 with biological (RT-PCR in amniotic fluid) and histological findings based on electron microscopy and immunohistochemistry [26,27].
Main strengths and limitations of the study
To the best of our knowledge, this is the first comparative study to report such increasing risks in mothers positive for SARS-COV-2 infection. We have ensured that the groups were highly comparable considering homogeneous population and a single protocol of management in order to minimize the effect of any confounding factors that can influence outcomes. Given the re-emergence of the virus in many countries and the lack of vaccine, our findings could help inform patients and healthcare providers, as well as develop appropriate management protocols for pregnant women with COVID-19. SARS-COV-2 testing in all patients admitted for delivery, regardless of their symptoms at admission and without lost to follow-up, ensures a risk of selection bias as low as possible.
The findings of this study must be seen in light of some limitations. Firstly, this single-center cohort presents a relatively small number of patients infected with SARS-COV-2. We chose to limit the study at the first period of the epidemic to avoid including patients who tested positive for SARS-COV-2 but without active infection (pre-partum infection with negative PCR at admission). Our group of infected patients permitted to highlight higher risks of adverse maternal and fetal outcomes, but we cannot exclude that a larger group could have permitted to describe more subtle consequences of COVID-19 during pregnancy. At the opposite, the small number in control group might affect comparisons of some adverse outcomes as post-partum haemorrhage.
Secondly, ethnicity and socioeconomic status were not reported in this study. Social precariousness and co-morbidities during pregnancy are particularly high in western French Guiana and could increase the risk of infection as well as the risk of adverse outcomes, and then be considered as a confounding factors. The rates of co-morbidities were relatively balanced across the groups. The socio-economic status of patients enrolled was not reported, and it is paramount to acknowledge that it might contribute to the increase of adverse outcomes observed in SARS-COV-2 infected patients if it was lower in this group.
Thirdly, at the beginning of the outbreak, we did not collect placenta and fetal samples for RT-PCR testing in the first cases of stillbirth. This could underestimate the risks of vertical transmission and subsequent adverse fetal outcomes.
Finally, French Guiana territory is epidemic of other virus such as Dengue Virus. Active circulation of Dengue virus during the same period can influence the outcomes of pregnancy but not the comparison between the two groups. The small number of positive cases during the study period minimizes the influence of this factor on the results.
Conclusion
In conclusion, SARS-CoV-2 infection in parturient admitted for delivery seems to be symptomatic in only a quarter of cases. SARS-CoV-2 infected mothers presented higher risks of maternal and fetal adverse outcomes: ICU admission, post-partum hemorrhage, transfusion and fetal loss. Fetal outcomes did not depend on the clinical form of Covid 19. For mothers, special awareness should be taken about significant risks of post-partum hemorrhage. Until more is known about this disease, it is paramount to perform close monitoring for infected pregnant women especially in immediate post-partum period.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. published online Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockman L.J., Lowther S.A., Coy K., Saw J., Parashar U.D. SARS during pregnancy, United States. Emerg Infect Dis. 2004;10(9):1689–1690. doi: 10.3201/eid1009.040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell K.H., Tornatore J.M., Lawrence K.E., Illuzzi J.L., Sussman L.S., Lipkind H.S. Prevalence of SARS-CoV-2 among patients admitted for childbirth in Southern Connecticut. JAMA. 2020;323(24):2520–2522. doi: 10.1001/jama.2020.8904. [published online ahead of print, 2020 May 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil A., Hill R., Ladhani S., Pattisson K., O’Brien P. Severe acute respiratory syndrome coronavirus 2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.005. Published online May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sisman J., Jaleel M.A., Moreno W., Rajaram V., Collins R.R.J., Savani R.C. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr Infect Dis J. 2020;39(9):e265–e267. doi: 10.1097/INF.0000000000002815. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 2020. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) Available at: https://www.who.int/publications-detail/report-of-the-whochina-joint-mission-on-coronavirus-disease-2019-(covid-19). Retrieved July, 2020. [Google Scholar]
- 9.Miesbach W., Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620938149. 1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess D.C., Eldahshan W., Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11(3):322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federici L., Picone O., Dreyfuss D., Sibiude J. Successful continuation of pregnancy in a patient with COVID-19-related ARDS. BMJ Case Rep. 2020;13(8):e237511. doi: 10.1136/bcr-2020-237511. Published 2020 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalil A., Kalafat E., Benlioglu C., O'Brien P., Morris E., Draycott T. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. E Clin Med. 2020;25 doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell K.H., Tornatore J.M., Lawrence K.E., Illuzzi J.L., Sussman L.S., Lipkind H.S. Prevalence of SARS-CoV-2 among patients admitted for childbirth in Southern Connecticut. JAMA. 2020;323(24):2520–2522. doi: 10.1001/jama.2020.8904. [published online ahead of print, 2020 May 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil A., Hill R., Ladhani S., Pattisson K., O’Brien P. Severe acute respiratory syndrome coronavirus 2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.005. Published online May 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel P., Hiam L., Sowemimo A., Devakumar D., McKee M. Ethnicity and covid-19. BMJ. 2020;369:m2282. doi: 10.1136/bmj.m2282. Published 2020 Jun 11. [DOI] [PubMed] [Google Scholar]
- 20.Young B.E., Fong S.W., Chan Y.H., Mak T.M., Ang L.W., Anderson D.E. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31757-8. [published online ahead of print, 2020 Aug 18] S0140-6736(20)31757-31758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil A., von Dadelszen P., Draycott T., Ugwumadu A., O’Brien P., Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324(7):705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberton T., Carter E.D., Chou V.B., Stegmuller A.R., Jackson B.D., Tam Y. Early estimates of the indirect effects of the COVID‐19 pandemic on maternal and child mortality in low‐income and middle‐income countries: a modelling study. Lancet Glob Health. 2020;8:e901–908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casadio P., Youssef A., Arena A., Gamal N., Pilu G., Seracchioli R. Increased rate of ruptured ectopic pregnancy in COVID-19 pandemic: analysis from the North of Italy. Ultrasound Obstet Gynecol. 2020;56(2):289. doi: 10.1002/uog.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records [published correction appears in Lancet. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richtmann R., Torloni M.R., Oyamada Otani A.R., Levi J.E., Crema Tobara M., de Almeida Silva C. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;27 doi: 10.1016/j.crwh.2020.e00243. Published 2020 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]