Abstract
The COVID-19 pandemic has affected 215 countries and territories around the world with 60,187,347 coronavirus cases and 17,125,719 currently infected patients confirmed as of the November 25, 2020. Currently, many countries are working on developing new vaccines and therapeutic drugs for this novel virus strain, and a few of them are in different phases of clinical trials. The advancement in high-throughput sequence technologies, along with the application of bioinformatics, offers invaluable knowledge on genomic characterization and molecular pathogenesis of coronaviruses. Recent multi-disciplinary studies using bioinformatics methods like sequence-similarity, phylogenomic, and computational structural biology have provided an in-depth understanding of the molecular and biochemical basis of infection, atomic-level recognition of the viral-host receptor interaction, functional annotation of important viral proteins, and evolutionary divergence across different strains. Additionally, various modern immunoinformatic approaches are also being used to target the most promiscuous antigenic epitopes from the SARS-CoV-2 proteome for accelerating the vaccine development process. In this review, we summarize various important computational tools and databases available for systematic sequence-structural study on coronaviruses. The features of these public resources have been comprehensively discussed, which may help experimental biologists with predictive insights useful for ongoing research efforts to find therapeutics against the infectious COVID-19 disease.
Keywords: Computational tools, Databases, Genomic, Coronavirus, SARS-CoV-2, COVID-19
Graphical abstract
Highlights
-
•
This review presents a comprehensive and up-to-date overview of newly developed computational resources focused on coronaviruses.
-
•
The resources are being categorized based on its utility and applications in coronaviruses study.
-
•
The features of each of the tools have been discussed along with their application in recent coronavirus research studies.
1. Introduction
Since the first discovery of coronavirus in domestic poultry in the 1930s, to date various novel coronaviruses strains have been often reported causing respiratory, gastrointestinal, liver, and neurologic diseases in animals, including humans [[1], [2], [3]]. The earlier human-infected coronavirus strains such as NL63, HKU1, 229E, and OC43 are known to be associated with mild to moderate upper-respiratory tract illnesses. While the three major human infected coronaviruses viz., the SARS-CoV-1 of 2002 popularly known as severe acute respiratory syndrome coronavirus [5], the 2012 middle east respiratory syndrome coronavirus (MERS-CoV) [6] and the new SARS-CoV-2, the cause of the current pandemic that was first identified in Wuhan City (China) in 2019 [7,8], are associated with a high rate of mortality and morbidity worldwide. Further, the World Health Organization (WHO) declared this pandemic as the Public Health Emergency of International Concern on January 30, 2020 [9]. All these three coronaviruses belong to the betacoronavirus genera of the coronaviridae family [10], with a genome size of approximately 30 Kb [11]. Comparative genomic analysis of SARS-CoV-2 sequences exhibited 79.5% and 96% nucleotide similarity with SARS-CoV-1 and SARSr-CoV-RaTG13 strain originated from bats, respectively. Thus, indicating its partial similarity with SARS-CoV-1 and bats as the most probable host organism [12]. Further, high sequence-structure conservation noted between SARS-CoV-2 and SARS-CoV-1 genomes, speculated that they have a common ACE2 receptor (angiotensin-converting enzyme 2) [13]. Moreover, in comparison to SARS-CoV-1 and MERS-CoV, SARS-CoV-2 has relatively higher contagiousness and morbidity (with a Ro value ranging from 2.2 to 3.77); but a lower mortality rate (3.4%) [14,15].
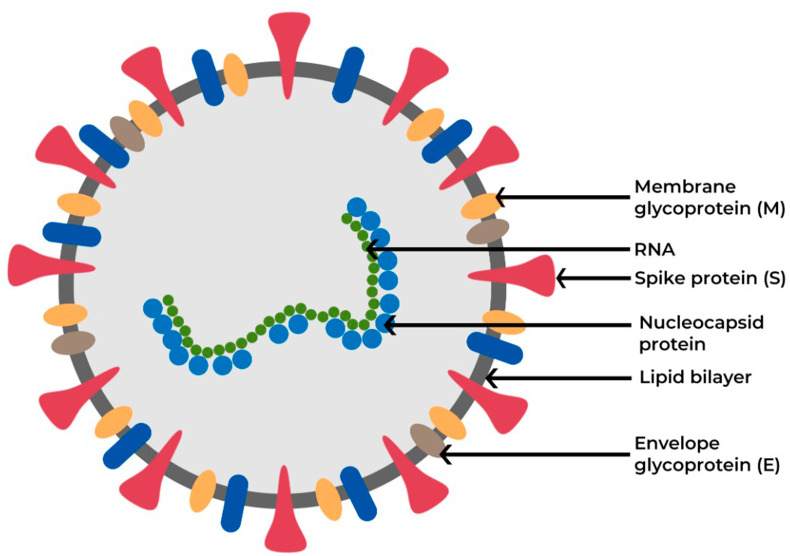
The four major structural glycoproteins responsible for coronavirus pathogenesis in the host are (i)Spike (S), (ii)Membrane (M), (iii) Nucleocapsid (N) and, (iv)Envelope (E) proteins (Fig. 1 ) [16]. Particularly, the receptor-binding domain (RBD) region of the S protein facilitates the viral entry into the host cells via binding to the receptor, ACE2 [17,18], triggering a cascade of multiple events initiating the fusion of both viral and host membranes [19]. Earlier studies on vaccine platforms for SARS-CoV-1 and MERS-CoV have identified that the RBD region of the S protein on the virus surface is the target for ideal vaccine development, as it reduces host immunopotentiation and generates neutralizing antibodies against coronavirus [[20], [21], [22]]. Many SARS-CoV-1 vaccines have been in the advanced stage of development and tested using various animal models [23]. Although these vaccines showed encouraging results in animal models, only a few of them have reached the initial phase I clinical trials before the next round of funding dried up. Nonetheless, virus eradication was possible through preventive measures and social distancing [23].Due to the absence of clinically approved vaccines against coronavirus, the COVID-19 disease has created a global menace to public health while the spread of the virus infection is continuing across the globe.
Fig. 1.
Schematic representation of the coronavirus surface glycoproteins crucial for viral entry and pathogenesis.
Access to multiple coronaviruses genome sequences now provides abundant opportunities for the application of bioinformatics approaches to unravel the evolutionary origin of new strains and the mechanism of viral entry and pathogenesis in the host. A study performed in this direction by Pachetti et al. has identified the genomic hotspots that are likely to mutate easily by carrying out genome alignment of 220 sequences from coronavirus isolates across several countries. With this prior knowledge, they hypothesized that the occurrence of such transformations might determine the variation in the mortality rate of SARS-CoV-2 infected people in different geographic regions [25]. Another recent study, using an integrated network approach by combining protein-protein interaction and SARS-CoV-2/Human Interactome, has shed light on the evolutionary origin of SARS-CoV-2 by finding out the mechanism of host interaction [26]. Besides, sequence information, the availability of crystal structures in RCSB PDB (Protein Data Bank) of SARS-CoV-1 (PDB Id: 2GHV) [27] and SAR-CoV-2 S proteins complexed with ACE2 receptors (PDB Id: 6M0J) [28] have identified the conserved residues responsible for the host receptor-binding in SARS-CoV-2 based on comparative sequence-structure similarity against SARS-CoV-1 [29,30]. Furthermore, using a computational approach the probable host range of SARS-CoV-2 has been predicted by analyzing the conserved amino acid properties located within the binding site interface [[30], [31], [32]]. Moreover, by employing molecular docking and dynamics simulation approach, the interaction of human SARS-CoV-2 E protein with various phytochemicals has been analyzed, which might be utilized as potential drugs for SARS-CoV-2 [33].While in silico approaches using immunoinformatic tools and databases are now indispensable in the search of potential immunogenic targets for drug and vaccine development and also have been demonstrated successfully in diseases like HIV-1 [34] and cancer [[35], [36], [37]], its application in the identification of potential vaccine candidates against coronaviruses is just beginning to emerge in the last few years [38].Recently, few studies using immunoinformatics and reverse vaccinology approach have identified promiscuous T-cell and B-cell epitopes as vaccine candidates against SARS-CoV-2 which may excite cellular and humoral immune response [[39], [40], [41], [42]]. Using similar bioinformatics methods, an epitope-based vaccine construct was suggested based on linear amino acid motifs which are found to be conserved in a wide range of coronaviruses including SARS-CoV-2 [43]. Similarly, using known immunogenic epitopes from the SARS-CoV-1 genome available on IEDB and ViPR immunology databases, homologous amino acids present at the equivalent position in SARS-CoV-2 have been proposed for vaccine design [44]. These studies conclusively highlight the potential utility of the next-generation bioinformatics approaches in providing greater insights into coronavirus genome structure and molecular mechanism of viral pathogenesis in the host organism.
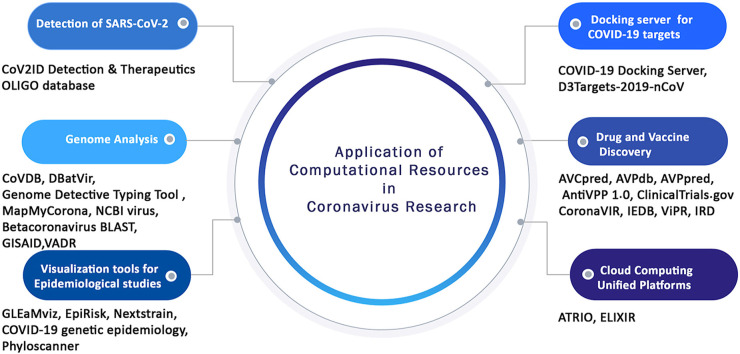
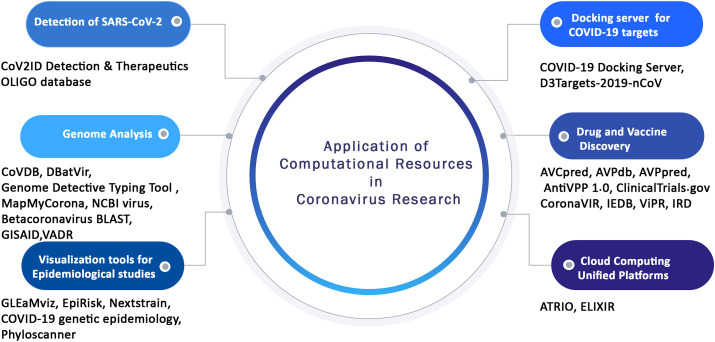
To further accelerate the ongoing experimental studies, various bioinformatics databases, tools, and web servers have been recently developed which systematically stores various genomic, epidemiology, and biological data pertinent to coronaviruses. These publicly available comprehensive resources can also be applied as platform tools for deriving useful information from complex and large datasets scattered across different databases. Therefore, in the present review for the first time, various computational resources have been discussed along with the integrated features they provide specifically for coronavirus research. We have categorized these popular tools and databases depending on the utility and application such as for the detection of coronavirus, comparative genomics analysis, vaccine and drug discovery, molecular docking, and other applications in coronaviruses study (Fig. 2 , Table 1 ). This review will certainly constitute an important resource for experimental biologists, immunologists, vaccinologists, and computational biologists across the globe to spur R&D efforts on designing strategies to combat COVID-19 disease.
Fig. 2.
Schematic representation of the different application of computational resources in coronavirus research.
Table 1.
An overview of computational databases, web servers, and tools useful for coronavirus research.
| Resources | URL | Release date | Key features | References |
|---|---|---|---|---|
| Detection of SARS-CoV-2 Coronavirus | ||||
| CoV2ID | http://covid.portugene.com/cgi-bin/COVid_home.cgi | 2020 | Repository of manually curated available oligonucleotides for SARS-CoV-2. CoV2ID has information on 52 oligonucleotides, 38 PCR primers, 14 probes, and 7 detection protocols to perform laboratory testing for SARS-CoV-2. | 45 |
| Resources for Host-Pathogen genome analysis | ||||
| CoVDB | http://covdb.microbiology.hku.hk | 2007 | Repository of known annotated coronavirus sequence with more than3000 records, belonging to various strains like coronavirus HKU1, SARS coronavirus belonging to bat, coronavirus HKU2 group 1 belonging to bat, coronaviruses 2c group coronaviruses group 2d, and 20 other coronaviruses strains. | 46 |
| DBatVir | http://www.mgc.ac.cn/DBatVir/ | 2014 | Repository of well-curated 4100 bat-associated viruses' genomes belonging to 23 viral families identified from 196 species of the bat across 69 countries. Presently, it contains 3873 sequences belonging to the coronaviridae family, of which 1393 are alphacoronavirus, 1181 betacoronavirus, and 1299 unclassified coronavirus. Further, DBatVir also provides details on the virus taxonomy lineage; host information such as sample type, collection date, and sampling country, and published data on the pathogenesis and epidemiology of several bat-associated emerging infectious diseases. | 47 |
| Genome Detective Typing Tool | www.genomedetective.com | 2020 | Web-based tool to identify and characterize the novel coronavirus genomes | 48 |
| MapMyCorona | http://shiny.mapmycorona.org/ | 2020 | MapMyCorona is an online tool using BLASTserver for identification of SARS CoV-2 sequences (protein or DNA) which finally displays the topmost hits in a well-organized spatial and temporal fashion. | |
| NCBI virus | https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/ | 2017 | A community web portal of sequences data of Virus with an integrative, value-added resource designed to support data retrieval, presentation, and investigation of various well-curated collection of sequences belonging to virus and datasets | 49 |
| Betacoronavirus BLAST | https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&BLAST_SPEC=Betacoronavirus&LINK_LOC=blasttab | 2020 | Recently developed BLAST database focused on the SARS-CoV-2, containing betacoronavirus nucleotide sequence data from GenBank and RefSeq. | 50 |
| GISAID | https://www.gisaid.org/ | 2017 | Repository of sequence, clinical and epidemiological data belonging to influenza viruses and recently included SARS-CoV-2 | 51 |
| VADR | https://github.com/nawrockie/vadr | 2019 | Web-server validates and annotates sequences submitted to the GenBank | 52 |
| Docking servers for COVID-19 targets-ligand interactions | ||||
| COVID-19 Docking Server | http://ncov.schanglab.org.cn | 2020 | A powerful web server that helps in predicting the binding interaction affinity of COVID-19 targets and the probable ligands like peptide, antibody, and small molecules. | 53 |
| D3Targets-2019-nCoV | https://www.d3pharma.com/D3Targets-2019-nCoV/index.php | 2020 | An online server developed to recognize the potential targets for antivirals against SARS-CoV-2 through protein structure-based virtual screening. | 54 |
| Resources for Drug Discovery | ||||
| AVCpred | http://crdd.osdd.net/servers/avcpred | 2017 | Web server to predict and design potential antiviral compounds against deadly viruses. | 55 |
| AVPdb | http://crdd.osdd.net/servers/avpdb | 2014 | Database of comprehensive experimentally validated Antiviral peptides (AVPs) targeting more than 60 therapeutically medically important SARS coronaviruses. AVPdb database also contains information of numerous peptide-like anti-coronavirus peptides with detailed mechanisms of their mode of action against SARS-CoV-1 | 56 |
| AVPpred and AntiVPP 1.0 | http://crdd.osdd.net/servers/avppred | |||
| https://github.com/bio-coding/AntiVPP | 2012 | |||
| 2019 | Comprehensive virus-specific web-resources to explore potential sequences of the peptide having antiviral properties against SARS coronaviruses of human. | 57, 58 | ||
| ClinicalTrials.gov COVID-19 related studies | https://clinicaltrials.gov/ct2/results/details?cond=COVID-19 | ClinicalTrials.gov is a government website with an easy access option to publicly and privately supported human clinical studies information with an extensive collection of diseases and conditions. On May 7, 2020, information on 1254 clinical studies on COVID-19 are available | ||
| Resources for vaccine discovery | ||||
| CoronaVIR | https://webs.iiitd.edu.in/raghava/coronavir/ | 2020 | CoronaVIR: a multi-omics repository of SARS-CoV-2 coronaviruses with detailed information on genomic, proteomic, diagnostic, and therapeutic knowledge to fight against the pandemic disease. | 59 |
| IEDB | www.iedb.org | 2019 | A free resource for searching and exploring experimentally validated T cell epitope and antibody -information about infectious disease, allergy, autoimmunity, and transplantation. Additionally, IEDB also provides several immunoinformatic tools for the prediction of putative epitopes from antigenic proteins, for further analysis of epitope data. | 60, 61 |
| ViPR | http://www.viprbrc.org | 2012 | ViPR is an NIAID-funded data repository and analysis tool for numerous human pathogenic viruses including the entire coronaviridae family. Recently, ViPR has developed the 2019 Novel Coronavirus (2019-nCoV) portal in ViPR to support research efforts on this emerging public health threat (https://www.viprbrc.org/brc/home.spg?decorator=corona_ncov). This open-access resource is updated daily and provides the necessary analytical tools and genomic data for SARS-CoV-2 and related betacoronaviruses. | 62 |
| IRD | Influenza Research Database like the ViPR Resource is also an NIAID-funded project with a specific focus on viral research pathogens causing (re)emerging infectious diseases. IRD along with ViPR maintains and updates the novel coronavirus (2019-nCoV) portal hosted in ViPR | 63 | ||
| Visualization tools to map the coronavirus spread | ||||
| GLEaMviz | http://www.gleamviz.org/ | 2011 | User-friendly graphical interface to explore the spread of human-to-human infectious diseases and emerging scenarios at the global scale | 64 |
| EpiRisk | https://epirisk.net/ | 2020 | Web-based interface for investigating the risk of the coronavirus | |
| Nextstrain COVID-19 genetic epidemiology | https://nextstrain.org | 2020 | It is a real-time genetic tracking tool to map how the virus spread and pathogen evolution to further accelerate research on COVID-19 disease; Nextstrain is incorporating country-wise genomes information of SARS-CoV-2 epidemiology of novel coronavirus analyses and situation report. | |
| Phyloscanner | https://github.com/BDI-pathogens/phyloscanner | 2018 | Analyses pathogen genetic diversity and relationships between and within hosts at once, in windows along the genome. | 65 |
| Cloud Computing Unified Platforms | ||||
| ATRIO: A Composable Cloud | https://atrio.io/ | ATRIO is a computing platform to eliminate the challenges between user infrastructure and cloud computing. Atrio is presently offering its users free high-performance GPU and CPU computing resources and containerized toolsets to facilitate COVID-19 research. | ||
| ELIXIR | https://elixir-europe.org/ | A group of Intergovernmental organizations working together to curate all resources of life science across Europe. Currently, ELIXIR's extends its services to support SARS-CoV-2 research by ensuring the exponential increasing genomic data, resources, and scientific publications on SARS-CoV-2 to be freely accessible to all across worldwide. | ||
2. Detection of SARS-Cov-2 coronavirus
2.1. CoV2ID detection & therapeutics OLIGO database
Rapid detection and monitoring of SARS-CoV-2 infection is the most pressing need for screening of carriers and preventing future epidemics. Although various techniques such as real-time reverse transcription-polymerase chain reaction (RT-qPCR) and sequencing methods are widely used for identification and characterization of coronaviruses [66], nevertheless the high mutation rates in the genomic sequences of SARS-CoV-2 is a major challenge for the efficiency of available assays [67]. In this context, CoV2ID (http://covid.portugene.com/cgi-bin/COVid_home.cgi) [45] is a promising web-based resource to facilitate molecular evaluation for detection of SARS-CoV-2 and treatment of COVID-19.CoV2ID provides a comprehensive, reviewed, and constantly updated catalog of oligonucleotides for SARS-CoV-2. The CoV2ID score indicates the level of conservation of the oligo according to the viral genetic diversity where the higher the score, the more conserved is the oligo. At present, CoV2ID contains information on 52 oligonucleotides, 38 PCR primers, 14 probes, and 7 detection protocols to perform laboratory testing for SARS-CoV-2. The database features various information about COVID-19 virus (including the isolate from Wuhan-Hu-1 for SARS-CoV-2) like the multiple sequence alignment results of SARS-CoV-2 strains, oligonucleotides manually curate from peer-reviewed publications along with information on its target type, techniques, target, sequence, genomic region, and detection protocol, diversity study at a genetic level across the genome of SARS-CoV-2 and a quick guide to perform laboratory testing for SARS-CoV-2.Users can retrieve an oligonucleotide with a specific CoV2ID score by browsing through the search tab or can manually design a novel oligonucleotide under the “Genome variation” of the database.
3. Resources for host-pathogen genomics analysis
The necessity of achieving an urgent solution for the ongoing pandemic has led to the sequencing of thousands of SARS-CoV-2 genomes. By leveraging the advancement of next-generation sequencing technology, more than 11500 complete SARS-CoV-2 genome sequences and over 6100partial genomes are available at NCBI to date (https://www.ncbi.nlm.nih.gov/). Among all known RNA viruses, coronaviruses possess the largest genomes (approximately 26,000 and 32,000 bases) with G + C contents varying from 32% to 43% [68]. Although wet lab techniques represent the most accurate method for viral genome annotation, however, these experiments are time-consuming and expensive [69]. In this context, computational tools and databases can become a reliable alternative, and cost economic approach for identifying, annotating, and understanding the genomic characteristics of the novel viruses [70].
3.1. CoVDB
The comprehensive database for comparative analysis of coronavirus genes and genomes (CoVDB; http://covdb.microbiology.hku.hk) [46] is an user-friendly database of annotated coronavirus genomes that currently holds records on >3000 coronavirus sequences, belonging to coronavirus HKU1, bat SARS coronavirus, bat coronavirus HKU2group 1, coronaviruses group 2c, coronaviruses group 2d, and 20 other coronaviruses strains. This user-friendly platform enables fast and precise sequence retrieval in batch and comparative genome analysis across diverse phylogenetic groups. CoVDB provides information on genome organization of coronavirus, GC content, cleavage sites of the polyprotein, transcriptional regulatory sequences, acidic sequential tandem repeat, and RNA structures details. To further enhance its application, CoVDB also includes a BLAST tool to facilitate similarity search against coronavirus reference sequences within the database. This database includes both complete and incomplete genome and their respective genes sequences. This is beneficial because some of the genes viz., RNA-dependent RNA polymerase, S and N, are sequenced frequently as they are either most/least conserved, hence are crucial for primer design for RT PCR assays and evolutionary studies. These useful features will aid our understanding of the regulatory function and pathogenesis of the novel SARS-CoV-2 coronavirus.
Various reports on coronavirus research like characterization and isolation of novel betacoronavirus performed by Lau et al. [71], ecoepidemiology and comparative studies of different strains of SARS-related Rhinolophus Bat Coronavirus by Lau et al. [72], comparative genomics analysis of three avian coronaviruses by Woo et al. have demonstrated the utility of CoVDB as an important tool for batch sequence retrieval and relative investigation of coronavirus genes and genomes [73].
3.2. Database of bat-associated viruses (DBatVir)
It is reported that approximately 60% of the evolving communicable diseases agents affecting humans are of zoonotic origin [74] and bats are the most important reservoir for many viruses including the coronaviruses [[75], [76], [77], [78]].The Database of Bat-associated Viruses (DBatVir; http://www.mgc.ac.cn/DBatVir/) [47], is a well-curate repository for specific bat-associated animal viruses comprising of over 4100 bat-associated viruses genomes belonging to 23 identified viral families from 196 species of the bat. Additionally, this database currently contains 11,142 known viral sequences isolated from different bat samples and cataloged with the related metadata, like bat species, specimen type, location, and sampling time. Presently, it contains 3873 sequences belonging to the coronaviridae family, of which 1393 are alphacoronavirus, 1181 betacoronavirus, and 1299 unclassified coronavirus. Moreover, DBatVir provides details on the virus taxonomy lineage; host information such as sample type, collection date, and sampling country along with published data on the pathogenesis and epidemiology of several bat-associated emerging infectious diseases. In addition to the linear genomic map for each complete viral sequence, it also shows a global map that indicates the number of viruses detected in each country associated with the bat. With a highly intuitive web interface and in-built bioinformatics tools (BLAST search and Phylogeny analysis), DBatVir is a one-stop platform to study the bats’ virome diversity and genomic characterization of emerging bat originated coronaviruses diseases.
3.3. Genome detective Typing Tool
Genome Detective Typing Tool (www.genomedetective.com) [48] is an easy-to-use a tool that permits rapid characterization and identification of SARS-CoV-2 sequences isolated across the world. Users can submit up to one GB of next-generation sequencing datasets and 2000 sequences per submission in the FASTA format. At present, the tool contains a reference dataset of 435 whole-genome sequences (WGS) of which 47 belongs to SARS-CoV-2 genomes originated in Wuhan, and 386 WGS from the Virus Pathogen Resource (VIPR) database, which includes beta coronavirus, Human Coronavirus HKU1, Longquan Rl Rat coronavirus, MERS-CoV, Murine Hepatitis Virus, Rat Coronavirus, Rousettus Bat Coronavirus HKU9, SARS-CoV-1, Tylonycteris Bat Coronavirus HKU4, and Zaria_bat_coronavirus. The tool classifies the coronavirus sequences into its respective phylogenetic cluster with high specificity, sensitivity, and accuracy. Moreover, using a novel dynamic aligner, the web-based tool identifies mutations in genes and encoded proteins at the amino acid level, which may provide clues regarding the evolutionary divergence across novel strains and the resulting varied clinical manifestations.
Recently, a study employing the tool has shown that the novel SARS-CoV-2 shares 79.5% similarity with the genome of SARS-CoV-1 [79]. Also, another study demonstrated a variant analysis of SARS-CoV-2 genomes using this Typing Tool and found 483 variations within the 29,903 bp long genome of SARS-CoV-2, which comprises115 variations in the UTR region, 130 synonymous variations, 228 non-synonymous variations, 16 INDELs, and variations in two non-coding regions [80].
3.4. MapMyCorona
The MapMyCorona (http://shiny.mapmycorona.org/) developed under the de.NBI cloud resources offers a protein or nucleotide sequence similarity search of newly sequenced SARS-CoV-2 strains against the known genome sequences. The BLAST-server integrated into MapMyCorona for SAR-CoV-2 sequences shows the results spatially and temporally on a world map with various interesting display options to filter the results. Using this resource, a user can follow up and understand the trend of SARS-CoV-2 sequences across the geographical and temporal distribution and identify the pathway for the global spread of the emerging viral strains.
3.5. NCBI virus
NCBI virus [49] is a community portal especially created to retrieve, exhibit, and analyze a set of curated virus sequence data from RefSeq, GenBank, and other NCBI repositories. This resource is hosted by the National Center for Biotechnology Information (NCBI) and can be retrieved at https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/. NCBI virus integrates data processing pipelines that extract sequences from GenBank, presents gene and protein annotation and maps uniformly to their respective metadata terms. Moreover, the sequence data available at the NCBI virus can be searched either by virus or sequence and exported easily in various formats for further analysis. Currently, it includes data from four sequence groups: i) RefSeq: reference sequence records from one or more complete genome sequences for each viral species; ii) Complete nucleotide sequences of all NCBI viral nucleotide sequences (sequences that include the word ‘complete’ in the record's definition line); iii) Partial nucleotide sequences (sequences that are mentioned as ‘not complete’ according to the definition line); iv) Proviral sequences (sequences that contain "/proviral” source qualifier in the GenBank record). Besides influenza virus A, B or C, Norovirus, and Dengue virus, the NCBI virus portal house a focused resource for SARS-CoV-2 (https://submit.ncbi.nlm.nih.gov/sarscov2/)that allow to submit, search, retrieve, and analyze SARS-CoV-2 data. It currently includes 1206 nucleotide and 13,173 protein sequences along with specific information like GenBank accession ID, release date, species, sequence length, geographic location, host, isolation source, collection date, taxonomy, genotype, authors, publication, and bio-sample information. Besides, it has two web-based tools, ‘Align’ tool for multi-sequence alignment and ‘Build Phylogenetic tree’ for generating phylogenetic trees. Conclusively, the NCBI virus portal is an integrative resource to enhance the efficiency of archived data in various repositories like GenBank and other NCBI databases.
In a recent study for the identification of extra- and intra-cellular RNAs of SARS-CoV-2, Toptanet al., aligned 165 SARS-CoV-2 complete sequences using the NCBI Virus tool, and a consensus was created to further proceed with primer design and validation [81].Likewise, Dharavath et al., demonstrated the utility of the database by retrieving 93 SARS-CoV-2genome sequences, 6 human-specific SARS viruses, and 27,399 SARS/SARS-like viruses to understand the phylogenetic relationship across the various members of the coronaviridae family [82].
3.6. Betacoronavirus BLAST
To facilitate the study on zoonotic betacoronaviruses causing SARS-like disease in humans, NCBI-BLAST has developed a specific Betacoronavirus BLAST platform on Feb 03, 2020(https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&BLAST_SPEC=Betacoronavirus&LINK_LOC=blasttab) [50]. It allows BLAST search against Betacoronavirus databases containing the betacoronavirus nucleotide sequence data (taxid: 694002) from GenBank and RefSeq, and currently includes 9186 sequences (as of April 24, 2020). It supports three BLAST search options: i) BLASTN- a tool to search nucleotide sequences in the databases using a nucleotide query, ii) TBLASTN - a tool used to search translated nucleotide databases using a protein query and iii) TBLASTX-a tool used to find out distant relationships within nucleotide databases using a translated nucleotide query. Furthermore, depending on the input sequence, the user can choose a specific algorithm for retrieving the best similarity search results. The program selection options include i) MEGABLAST (default) for the comparative study of closely related sequences, ii) Dis-contiguous MEGABLAST allows mismatches for cross-species comparisons, and iii) BLASTN for short queries input and identifying short matches, it gives a better result for cross-species searches.
3.7. Global Initiative on Sharing All Influenza Data(GISAID)
The GISAID (Global Initiative on Sharing All Influenza Data (https://www.gisaid.org/))consortium assists rapid sharing of all influenza viruses data, including the SARS-CoV-2 coronavirus [51]. Further, the database also provides geographical sites along with species-specific data to help in understanding the evolution of viruses and their spread across geographical regions. Given the crucial importance of genetic data in improving our understanding of the progress of an epidemic infectious disease like COVID-19, GISAID promotes research collaboration among scientific communities based on open data sharing before publication. Various comparative genomic studies on the novel SARS-CoV-2 have used a GISAID database for retrieving both complete and partial nucleotide sequences [[83], [84], [85]].
3.8. Viral Annotation DefineR (VADR)
Viral Annotation DefineR (VADR, https://github.com/nawrockie/vadr) [52], a reference-based general software tool that validates and annotates sequences belonging to a virus from well-characterized species having genome less than 25 Kb in length for which a reference (e.g. RefSeq entry) exists. VADR evaluates the GenBank submitted sequence and compares it with matching RefSeq using numerous parameters such as early stop codon, low nucleotide similarity regions, to further decide on the automatic sequence entry into GenBank. VADR's output annotation for individual sequence includes a five-column feature table showing protein-coding regions, the “mature peptide” cleavage products of proteins, genes, non-coding RNAs, and structural RNA features. Re-built models are available for i) Norovirus and Dengue virus RefSeqs, along with other Flaviviridae and Caliciviridae RefSeqs, ii) metazoan Cytochrome c oxidase I (COX1) and iii) CoronaviridaeRefSeqs, including 2019-nCoV (NC_045512).
Recently, Edgar et al., developed a cloud computing architecture for sequence alignment called Serratus (https://github.com/ababaian/serratus), which integrates the VADR tool for reference-based genome annotation studies [86].
3.9. Docking servers for COVID-19 targets-ligand interactions
Various virtual screening analysis has exploited the major proteins targets, including 3C-like proteinase (Mpro) [87,88], ACE2 [89], papain-like proteinase (PLpro) [90] and furin [91] to discover effective drugs against the deadly coronavirus. However, none of the clinical studies provides effective therapies to combat COVID-19 disease. Thus, identification of potential drug targets by employing virtual screening and docking analysis is highly essential for a detailed investigation of protein structural and functional mechanisms against the SARS-CoV-2. Recently many tools and web-servers have been developed to assist in coronavirus-specific docking studies which have been discussed in this section.
3.10. COVID-19 docking server
COVID-19 Docking Server (http://ncov.schanglab.org.cn) [53] is a web-based server that helps in predicting the binding interaction between the targets (COVID-19) and the ligands such as small molecules, peptide, and antibody. The COVID-19 Docking Server employs AutoDock Vina tools for docking of small-molecule, CoDockPP tool for peptide and antibody interaction, and JSMol for molecular visualization of the developed structures. Although COVID-19 Docking Server has been developed very recently, it is being used widely for in silico screening of lead compounds for drug discovery against COVID-19 disease [[92], [93], [94]]. For instance, a recent study [92] using the COVID-19 Docking Server proposed the two compounds anisotine and vasicoline extracted from the Justicia adhatoda leaves as promising inhibitors for the treatment of COVID-19. In another study, the binding affinity of selected bioactive compounds against Mpro and RNA-dependent RNA polymerase (RdRp) is performed with the online COVID-19 Docking Server. Results indicated that cyanidin, daidzein, genistein, phycocyanobilin, and riboflavin, are effective inhibitor bioactive compounds to Mpro and RdRp compared to known antiviral drugs [93]. Likewise, COVID-19 Docking server was used to study the binding affinities of FDA approved drugs and to recognize the key amino acid residues as key protein targets against SARS-CoV-2 [94].
3.11. D3Targets-2019-nCoV
D3Targets-2019-nCoV (https://www.d3pharma.com/D3Targets-2019-nCoV/index.php) [54] is a web server used to identify the targets for antiviral potential against SARS-CoV-2 through virtual screening of protein structure. This docking server comprehends 42 proteins (20 SARS-CoV-2 encoded proteins; 22 proteins belonging to humans involved in virus infection, replication and release) including 69 different structural conformations and 557 potential ligand-binding pockets. The D3Targets-2019-nCoV utilizes the D3Pockets server [95], (https://www.d3pharma.com/D3Pocket/index.php), to scientifically predict the possible binding pockets for every protein. Further, it employs velocity-scaling optimized replica exchange molecular dynamics (vsREMD) simulations [96] to explore variable druggable conformations for each potential drug target. The docking server supports query molecule files in numerous formats, viz., SDF, mol2, mol, smiles, and PDB formats; and uses Open Babel (version 2.4.0) [97] to convert it to mol2 format and MMFF94 force field by RDKit (open-source cheminformatics software, version 2019.09.3, GitHub, Inc.) to generate 3D structures from 2D coordinates of the query molecule. For each query molecule, D3Targets-2019-nCoV generates docking scores for each target protein downloadable with their coordinate files (ligand-protein interaction) and have an average running time of about an hour. The tool has been successfully validated for six potential antiviral agents including the active form of remdesivir, favipiravir, ribavirin, penciclovir, N3 compound, and teriflunomide against important SARS-CoV-2 proteins.
4. Resources for drug discovery
To date, there is no definitive FDA-approved antiviral-drug for the COVID-19 treatment. Besides, most of the treatment strategies concentrate only on symptomatic management and supportive therapy [98].Various research organizations are tirelessly working hard to evaluate multiple compounds that can inhibit the spread of SARS-CoV-2 in humans. Nevertheless, these efforts are tedious and involve a meticulously extensive process. Thus, in addition to experimental work, specialized computational resources focusing on antiviral compounds/peptides can be utilized to evaluate the identified antivirals and repurpose them against single agents or in combinations to effectively control the spread of the virus [98,99].
4.1. Antiviral Compound Prediction (AVCpred)
Antiviral Compound Prediction (AVCpred; http://crdd.osdd.net/servers/avcpred) [55] is a freely accessible web server using a quantitative structure-activity relationship (QSAR) based approach to predict and design potential antiviral compounds (AVCs) against deadly viruses like SARS coronavirus, influenza virus, human immunodeficiency virus, human herpes virus (HHV). AVCpred can regressively calculate the inhibition percentage value of a chemical compound against the virus as well as provides various other properties like charge, hydrogen, and Lipinski bond donors/acceptors, logP value, molecular weight, structure, rigid and rotatable bonds to identify the molecular structures of the probable drug target, for each query molecule. Besides drug designing, virtual screening, and collecting experimentally validated AVCs, AVCpred also offers two built-in user-friendly modules, i) ‘Design analogs’ - used to identify potential analogs of an existing AVC, it allows analogs design based on particular building blocks and predicts their inhibition properties against the viruses; ii) ‘Draw tool’ - it predicts the two-dimensional structure of an antiviral molecule using Marvin editor. As identifying novel and potential viral inhibitors is a major concern in the development of effective treatment against various harmful human viruses, AVCpred can be a promising tool for drug candidate's identification against viral proteins strains of novel coronavirus SARS-CoV-2.
Balmeh et al., in their study of predicting therapeutic targets for COVID-19, has illustrated the use of AVCpred for the determination of antiviral characteristics of the beneficial phytochemical compounds [100]. To speed up the novel drug discovery and development procedure and to recognize novel antiviral drug candidate, another study focusing on SARS-CoV-2 performed by Jomhor et al., have utilized AVCpred to assess the antiviral properties of 111 natural and synthetic compounds, to further study the interactions against the binding site SB domain- ACE2 [101].
4.2. Database of Antiviral Peptides (AVPdb)
Database of Antiviral Peptides (AVPdb; http://crdd.osdd.net/servers/avpdb) [56] is an exclusive resource for experimentally proved Antiviral peptides (AVPs) targeting over 60 therapeutically significant viruses, including SARS coronaviruses. AVPdb database also contains information on different anti-coronavirus peptides along with the mode of mechanisms to combat SARS-CoV-1. It can also be employed for MERS-CoV and other human coronaviruses for the high level of sequence and structure conservancy exhibited by these viruses. Presently, AVPdb contains 2059 experimentally verified natural peptides and 624 modified peptides including 76 from SARS-CoV-1Sprotein. For each record in the database, it harbors detailed information about peptide sequences, nomenclature, target virus (with taxonomy), peptide source, UniProt accession number, Cell line, peptide inhibitory activity, Targeted protein or molecular, and PubMed IDs with links. Besides, the user-friendly browsing and searching functionalities, the database provides details about the physicochemical properties and 3D structure for individual AVPs. AVPdb database has many inbuilt tools like AVPdb MAP helps in searching the perfectly matching peptide available; AVPdb BLAST for performing sequence alignment of query peptide against all the peptide sequences in the repository; and Physicochemical Properties Calculator tool to calculate the composition of amino acid, hydrophobicity, β-sheets preference, α-helix frequency, charge of amino acid, and polarity for query peptide sequence. Studies identified that peptides are extremely powerful signal transduction agents for viral diseases and therefore have a promising role for a successful therapeutic option for emerging pathogens of SARS and MERS coronavirus. Hence, AVPdb could be an important resource for the identification of potential antiviral peptides as potential therapeutic options against coronaviruses. AVPdb database was used recently to screen potential AVP against the SARSCoV-2 spike protein [102].
4.3. AVPpred &AntiVPP 1.0
To expedite the antiviral drug discovery processes, AVPpred (http://crdd.osdd.net/servers/avppred) [57] and AntiVPP 1.0 (https://github.com/bio-coding/AntiVPP) [58] are two virus-specific resources to explore potential peptides as therapeutic agents for various infectious diseases caused by viral pathogens. AVPpred and AntiVPP 1.0 use a support vector machine and Random Forest algorithm respectively, for predicting antiviral peptides. Using AVPpred a comparative genomic study revealed that a peptide KWPWYIWLGFIAGLI shows high binding affinity with spike protein (0.98 prediction score) and also found that no AVPs were associated with N protein and ORF7a, while an AVP VNCLDDRCILHCANF is associated with both NSP7 and NSP10 [103].
4.4. ClinicalTrials.gov COVID-19 related studies
ClinicalTrials.gov (https://clinicaltrials.gov/ct2/results/details?cond=COVID-19) is a government website updated daily and provides information on human clinical studies carried out both publicly and privately on an extensive range of diseases conditions. The website is hosted by the National Library of Medicine (NLM) at the National Institutes of Health (NIH).Each record provides detailed information about the study protocol and other information like disease condition, description and study design, requirements for participation, study locations and contact details of the concerned person, and linking health information on the web sites. With the implementation of new guidelines and strict regulations, the rate of clinical study registration has increased with more sponsors and investigators have also voluntarily registered their studies.
The clinicalTrials.gov provides detailed information about the various drugs under clinical study using human volunteers to add knowledge to medical science. The site includes information on both interventional and observational studies. On May 7, 2020, information on 1254 clinical studies on COVID-19 are available, 430 studies on SARS-CoV-2, 36 studies on 2019-nCoV, 20 studies on 2019 novel coronavirus, and one on Wuhan coronavirus.
5. Resources for vaccine discovery
Various research organizations are working relentlessly investigating many probable vaccine candidates and technologies for SARS-CoV-2 such as the subunit vaccines, nucleic acid vaccines, and whole virus vaccines [104]. As of August 2020, 234 vaccine candidates were under development; however, none of the candidates has completed successful clinical trials to prove its efficacy and safety [105].Keeping in view the current COVID-19 situation, it is very much essential to expeditiously combine computational and experimental vaccine designing techniques to reduce the cost and time in the identification of target vaccine candidates [106], followed by further evaluation for safety and efficacy.
5.1. CoronaVIR
CoronaVIR (https://webs.iiitd.edu.in/raghava/coronavir/) [59] a multi-omics website that contains detailed information about genomic, proteomic, therapeutic, and diagnostic knowledge of novel SARS-CoV-2coronaviruses, that have been manually curated from literature, existing databases and also contain predicted useful information obtained using various computational tools. To provide a holistic platform, four major modules, viz., “Genomics”, “Diagnosis”, “Immunotherapy” and “Drug Designing” have been integrated with CoronaVIR. The Genomics module contains genome data of different coronaviruses strains to facilitate genomic level alterations studies. The Diagnosis module provides updated information on widely used diagnostics tests for this virus as well as five novel universal primers set predicted using in silico approach. The Immunotherapy module contains many computationally predicted antigenic peptide sequences (B-cell and T-cell epitopes) which might elicit antibody-mediated immunity and cellular immune responses against the coronavirus infection. Lastly, the drug module provides tertiary structure information of important FDA approved drug molecules, drug targets, repurposing drugs, and monoclonal antibodies.
5.2. Immune epitope database and Analysis Resource (IEDB)
The Immune Epitope Database and Analysis Resource (IEDB, www.iedb.org) [60,61] a freely available repository, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID).This database provides directories of experimentally validated antibody and T-cell epitope related information in human and primates in infectious diseases such as allergy, autoimmunity, and transplantation. Also, IEDB provides several immunoinformatic tools for the prediction of putative epitopes from antigenic proteins and analysis of epitope data. Particularly, IEDB covers tool for T-cell epitope prediction and binding affinity to MHC class I & II binding, as well as specific tools each for linear and discontinuous B-cell epitope predictions. Besides the epitope prediction tool, IEDB also houses a variety of immunoinformatics tools for the detailed analysis of epitopes.
Recently, promiscuous epitopes were evaluated as candidate targets for immune responses against SARS-CoV-2 has been mapped using the known epitopes at the equivalent position in SARS-CoV-1 and MERS-CoV present at the IEDB server [42]. Similar in silico studies with the help of prediction and analysis tools of IEDB have identified potential vaccine candidates belonging to B-cell and T-cell epitopes from SARS-CoV-2 antigenic proteins, using epitopes conservancy, potential immunogenicity, and conservancy analysis [[39], [40], [41], [42]]. Therefore, IEDB is a useful resource in the development of vaccines against SARS-CoV-2 coronaviruses usingepitope-based subunit.
5.3. Virus Pathogen Resource (ViPR)
The Virus Pathogen Resource (ViPR; http://www.viprbrc.org) [62], supported by, NIAID, is a data repository of pathogenic viruses and hosts a plethora of tools for the analysis of multiple human pathogenic viruses belonging to the broad families, including the entire coronaviridae family. Currently, ViPR contains major pathogenic viruses with single-stranded positive-sense RNA belonging to the family (Togaviridae, Caliciviridae, Coronaviridae, Hepeviridae, Flaviviridae, and Picornaviridae); single-stranded negative-sense RNA (Paramyxoviridae, Bunyavirales, Filoviridae, Pneumoviridae, and Rhabdoviridae); double-stranded RNA (Reoviridae); double-stranded DNA (Herpesviridae and Poxviridae). Users can select Virus Taxonomy Browser and select the family from the drop-down menu. Multiple user-friendly search options are provided to view data contents based on strains and sequences, 3D protein structures, immune epitopes, antiviral drugs, host factor data, and plasmid data. Additionally, several analytical and visualization tools for performing sequence alignment, phylogenetic tree, sequence variation (SNP), BLAST search, genome annotator, PCR primer design, and metadata-driven comparative analysis. The ‘Workbench’ feature of ViPR allows users to store and share results for future use.
Recently, ViPR has developed the 2019 Novel Coronavirus (2019-nCoV) portal in ViPR to support research efforts on this emerging public health threat(https://www.viprbrc.org/brc/home.spg?decorator=corona_ncov).This open-access resource is updated daily and provides genomic data and analytical tools for SARS-CoV-2 and related betacoronaviruses. This page also provides useful information regarding the virus such as a brief description of the viral structure, phylogeny, links to GenBank, epidemiology reports, and links to recent publications on SARS-CoV-2.
5.4. Influenza Research Database (IRD)
Influenza Research Database (https://www.fludb.org/brc/home.spg?decorator=influenza, IRD) [69] like the ViPR, is an NIAID funded resources that support the research of viral pathogens causing (re)emerging infectious diseases. IRD and ViPR integrate data from external sources (like GenBank, UniProt, IEDB, Protein Data Bank, etc.), direct submissions, and internal curation and analysis pipelines, and provide a suite of bioinformatics analysis and visualization tools to expedite virology research. The IRD database along with the ViPR maintains and updates the novel coronavirus portal hosted in ViPR (https://www.viprbrc.org/brc/home.spg?decorator=corona_ncov).
5.5. Visualization tools to map the coronavirus spread
For effective and preventive control measures against the ongoing pandemic, immediate surveillance and monitoring of the spread of disease are highly essential. Notably, re-emerging infectious diseases are major health problems occurring in a particular geographic location or population. Nevertheless, they can rapidly disperse globally/locally with specific modes of transmission and pandemic capability. The emergence of various infectious diseases is particularly related to human factors viz., travel, population density, the interaction between humans and wildlife, trade, and environmental factors [107]. However, advancement in the field of computational technologies has aided efforts of disease surveillance and also guided the inefficient construction of mathematical models to gain significant insights into the disease dynamics and epidemic prediction [107]. As revealed during the 2003 SARS outbreak, H1N1 influenza pandemic in 2009 and 2012 MERS-CoV, various computational techniques for epidemic models help in real-time analysis of the public health crisis [107,108]. These computational models have the unique ability to recognize disease hotspots of different emerging infectious diseases, including identification of which emerging pathogen is more likely to occur in which particular hotspot.
5.6. The global epidemic and mobility model (GLEAMviz) and EpiRisk
The global epidemic and mobility model (GLEAMviz, http://www.gleamviz.org/) [64] have a user-friendly graphical interface to explore the emerging spread of human-to-human infectious diseases scenarios at the global scale. The simulation engine of the GLEAMviz visualizes a theoretical metapopulation taking into account the data-driven schemes for the short-range and long-range locomotion of peoples across inter-population levels. These combinations with coarse-grained techniques help in the illustration of the infection dynamics within each subpopulation. In addition to various customized visualization features, it also provides an interactive visualization of the spatial and temporal evolution of the epidemic predicted using a set of graphs.
Concerning the current coronavirus breakthrough, a specific platform called EpiRisk (https://epirisk.net/) is developed by the GLEAMviz team. EpiRisk is based on the computational platform intended to provide a quick and reliable estimate of the probability of infected individuals moving from sites of disease outbreak to other new areas in the world through air transport and daily commuting patterns. It is widely utilized for introductory scrutiny of the outbreak ofCOVID-19 from Wuhan province. Considering the present scenario of the global COVID-19 outbreak, a recent study has illustrated the utility of GLEAMviz epidemiological models to simulate the spread of infectious diseases originating from distant locations [109].
5.7. Nextstrain
Nextstrain (https://nextstrain.org) is a real-time genetic tracking tool to map how the virus spread and pathogen evolution. Nextstrain provides an updated profile of publicly existing data with powerful analytic and visualization tools for use by the global scientific community. To further accelerate research on COVID-19 disease, Nextstrain is incorporating SARS-CoV-2 genomes and started sharing country-wise genomic epidemiology of novel coronavirus analyses and situation reports. A genomic epidemiology map of different SARS-CoV-2 isolates has been demonstrated using NextStrain tools [110].
5.8. Phyloscanner
Understanding the transmission route of pathogens is important for identifying epidemiological risk factors to avoid the spreading of emerging infectious diseases in densely human population regions. A new software tool called Phyloscanner (https://github.com/BDI-pathogens/phyloscanner.) aims to analyze pathogen genetic diversity and relationships between and within hosts along the genome [65]. The tool helps in the identification of the mode of disease transmission, multiple infections, recombination, and contamination across pathogen genomic profiles by performing programmed phylogenetic analysis using the data generated from NGS deep sequencing data, or multiple genotypes per host generated by other analysis techniques.
6. Cloud computing unified platforms
Due to the ongoing pandemic, most of the health care centers, clinicians, and medical researchers are analyzing huge amounts of data in an effort for the identification of fully effective coronavirus therapeutics to combat COVID-19. In this context, cloud computing platforms can play a major role in unifying all the biological resources and providing flexibility to access these data and resources effectively. To accelerate the ongoing efforts to battle against SARS-CoV-2, few important cloud computing platforms have been discussed in this section which will assist in COVID-19 related research.
6.1. ATRIO
ATRIO (https://atrio.io/) is a computing platform to eliminate the challenges between user infrastructure and cloud computing. It provides an interface that unifies any common infrastructure and cloud services to a single computing network. Atrio is presently offering its users free high-performance GPU and CPU computing resources and containerized toolsets to facilitate COVID-19 research. Besides protein structural analysis and pathogen behavior simulations, several other applications like case reports analysis, trial results, demographic, and genome processing can be addressed through the BIG Data/AI application services in ATRIO. With these features and resources, ATRIO aims to expedite the drugs and vaccine development process to combat human coronavirus infection and also other infectious diseases affecting global public health.
6.2. ELIXIR
ELIXIR (https://elixir-europe.org/) is an organization formed by various intergovernmental bodies to bring together resources of life science including databases, software tools, training materials, cloud storage, and supercomputers in one common platform to enable scientists to freely access services that are vital for their research. Currently, ELIXIR's extends its services to support SARS-CoV-2 research by ensuring the exponential increasing genomic data, resources, and scientific publications on SARS-CoV-2 to be freely accessible to all across worldwide. The web resource enables users to retrieve multiple information relevant to COVID-19 by providing access to important resources like BridgeDB dataset (https://zenodo.org/record/3735705#.Xor6zW57kkg) which comprise of gene/protein mapping of human and SARS-related coronavirus derived from Wikidata; ViralZone (https://viralzone.expasy.org/8996), that provides access to proteome data as well as cross-links to complementary resources of SARS-CoV-2; Cellosaurus (https://web.expasy.org/cellosaurus/sars-cov-2.html) that furnishes updated information on the SARS-CoV-2 cell lines; Guide to Pharmacology (http://www.guidetopharmacology.org/coronavirus.jsp) containing curated data on SARS-CoV-2 targets and captures the pharmacological approaches being studied to mitigate the impact of COVID-19; Human Protein Atlas (https://www.proteinatlas.org/humanproteome/sars-cov-2) which offers data on human proteins and their interaction with SARS-CoV-2; and the FAIRDOM Hub (https://fairdomhub.org/projects/190) that provide the outputs of the Disease Map Consortium resource, a molecular repository of all known host-pathogen interactions specific to SARS-CoV-2.
6.3. Current research strategies and future perspectives
In the absence of any vaccine against coronavirus, various alternatives encompassing monoclonal antibodies, interferon, oligonucleotide, and peptides based therapies are being currently studied to fight against the disease [111]. In addition, several promising drug molecules are observed to have properties effective against SARS-CoV-2 coronavirus in cell lines studies [112]. Many researchers have studied the effect of hydroxychloroquine, a conventional drug used for the treatment of malaria, on SARS-CoV-2 in vitro, and the results have been found promising [113]. In an early report, Nafamostat, an inhibitor, used as an anti-pancreatitis and anticoagulant to treat cystic fibrosis, was also shown to possess have mucolytic action that can inhibit lung function deterioration caused by coronaviruses [114]. Remdesivir, anadenosine triphosphate prodrug, is shown to play an important role against SARS-CoV-2 by impeding the polymerase activity of RNAand has been suggested as the most promising candidate to treat COVID-19 according to WHO [115]. Based on the results of phase III trials performed using remdesivir as shown by ACTT study (directed by NIAID) and SIMPLE study (managed by Gilead), the US Food and Drug Administration (USFDA) has recently approved the usage of remdesivir to cure COVID-19 infections, through the Special Emergency Use Authorization [116,117].The combination of ritonavir and lopinavir protease inhibitors (treatment for HIV), in the presence or absence of IFNβ, has also been suggested to be a potential candidate against SARS-CoV-2, by preventing the 3-chymotrypsin-like protease of the virus [118]. Moreover, an earlier study based on the influenza virus showed that the addition of umifenovir (inhibitor of viral fusion using human cell membranes) to the combination of ritonavir and lopinavir resulted in quick elimination of nasopharyngeal virus and regression of lung imaging, compared to the patients receiving both ritonavir and lopinavir monotherapy [119].
WHO conducted a study named SOLIDARITY, trial to help in rapid identification of the most effective antiviral candidate against SARS-CoV-2 using ritonavir, lopinavir, and remdesivir + chloroquine combination [120]. The main objective of the trial is to track mortality rate, duration of hospitalization, identification of patients requiring intensive medical support, and administration of these drug candidates. A similar study “Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy)” is also initiated by the National Institutes of Health and Medical Research (France) to check the efficacy and safety of the aforementioned antiviral drugs. It was found that the invasion of SARS-CoV-2 into the host cell results in the evoke of an immune response, resulting in secretion of cytokine (including IL-6), and causes systemic and tissue inflammation, pulmonary fibrosis, and fever [121]. According to ClinicalTrials.gov, a novel, double-blind, placebo-controlled phase III study ‘COVACTA’, investigated the overall safety and efficacy of intravenous tocilizumab (basically used in rheumatology), a monoclonal antibody with the property to inhibit interleukin-6 (IL-6) receptor, in adult patients hospitalized with COVID-19 infection [122].
A recent report shows that SARS-CoV-2 needs Transmembrane Serine Protease 2 (TMPRSS2) for penetration into the host and further revealed that Camostat Mesylate, a serine protease inhibitor prevents the entry of the SARS-CoV-2 into the lung cells [123]. Later, it was shown that nafamostat blocked SARS-CoV-2 infection in human lung cells with 15-fold-higher efficiency in comparison to Camostat Mesylate, [124].At present, the efficacy of Camostat Mesylate (CamoCO-19 study) and Nafamostat (RACONA study) on COVID-19 infection in the clinical trials is underway. A clinical trial has revealed that the combination of hydroxychloroquine and azithromycin treatment is efficient in the reduction of viral load in SARS-CoV-2 infected patients [125].
A docking study showed that amodiaquine can act as an alternative inhibitor to SARS-CoV-2 in comparison to the approved medicines, such as hydroxychloroquine, and remdesivir [126]. Moreover, studies using novel AI-based systems approach identified Vitamin E, ruxolitinib, and glutamine to have a high binding affinity with ACE 2 [127].Recently Hemmat et al., suggested that serpins and arginase inhibitors can be effective against SARS-CoV-1 infection, and can also be effective against SARS-CoV-2 as it shares high similarity with SARS-CoV-1 [128]. Using integrated molecular modeling approaches, various reports indicated that phytochemicals such as carvacrol, oleanolic acid, and ursolic acid, might act as probable inhibitors in modulating the Mpro protein function and regulating replication of virus [129]. A computational study has hypothesized that plant-origin compounds viz., bisdemethoxycurcumin, demethoxycurcumin, scutellarin, myricetin, and quercetin could act as possible drug candidates against Mpro and NSP15 proteins of SARS-CoV-2 [130].Further, repurposed existing drugs viz., disulfiram, carmofur, ebselen, shikonin, tideglusib, PX-12, and TDZD-8 might be promising inhibitors targeting Mpro [131]. Similarly, Isavuconazonium (triazole), α-KI (ketoamide), and Pentagastrin (peptide) are suggested as possible drug candidates to treat infected patients with COVID-19 [132].Currently, several worldwide research organizations and pharmaceutical companies are steadily involved in developing an effective vaccine against the virulent SARS-CoV-2 virus [133]. According to the WHO (as of August 2020), a total of146 vaccines were identified as probable candidates which are under the pre-clinical stage, 36 vaccine candidates were in clinical research (24 in Phase I–II trials, and 12 in Phase II–III trials; https://www.who.int/). At present, to develop a potential rapid vaccine on high priority, various computational based reports [106,[134], [135], [136]] have applied immunoinformatics approach to design promiscuous multi-epitope vaccine candidates using a combination of B-cell and T-cell epitopes which would provoke both cellular and humoral immune responses to successfully combat COVID-19 disease.
7. Conclusion
Several recent studies by integrating in silico sequence-structural analysis have advanced our understanding of the molecular and evolutionary origin of SARS-CoV-2, detailed mechanism of viral-host binding interaction as well as identification of potential antiviral peptides and epitopic vaccine candidates as potential therapeutic options against coronaviruses. These advances are complemented by the development of novel computational databases and tools specific for coronavirus study which not only have accelerated research efforts to prevent COVID-19 but also strive to assemble the huge amount of genomic data and important research findings on freely accessible unified platforms to disseminate to the wider scientific community across the globe for further application. This review presents a comprehensive and up-to-date overview of newly developed computational resources including tools for coronavirus detection, resources for host-pathogen genome analysis, web servers for vaccine/drug discovery, docking tools for COVID-19 targets-ligand interactions, and visualization tools to real-time map the coronavirus spread and evolution, which will not only assist in handling this pandemic but also will make us better prepared for the re-emerging coronaviruses outbreak.
Funding statement
The author received no funding from an external source.
Author contribution
Rajiv kangabam: Writing- Original draft preparation; Susrita Sahoo: Writing- Original draft preparation, Arpan Ghosh- Writing- Original draft preparation; Riya Roy: Writing- Original draft preparation; Yumnam Silla: Writing- Original draft preparation; Namrata Misra: Conceptualization, Writing- Reviewing and Editing; MrutyunjaySuar: Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the help rendered by Mr. Saikat Gupta, Associate - Design & Campaign at KIIT-TBI, Bhubaneswar in designing figures of the manuscript
References
- 1.Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- 2.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mubarak A., Alturaiki W., Hemida M.G. Middle east respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019;2019:6491738. doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GorbalenyaAEBakerSC, Baric R.S., Groot J.R., Drosten C., Gulyaeva A.A. Severe acute respiratory syndrome-related coronavirus–The species and its viruses, a statement of the Coronavirus Study Group. BioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . 2020. Novel Coronavirus (2019-nCoV) Situation Reports. [Google Scholar]
- 10.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;580:E7. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Hu Y. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan. China. bioRxiv. 2020 [Google Scholar]
- 12.C hen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al, Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Yu T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M., Kleine-Weber H., Krüger N., Mueller M.A., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020 doi: 10.1101/2020.01.31.929042. [DOI] [Google Scholar]
- 14.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Trav. Med. 2020;27(2) doi: 10.1093/jtm/taaa021. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie M., Chen Q. Insight into 2019 novel coronavirus—an updated intrim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panda P.K., Arul M.N., Patel P., Verma S.K., Luo W., Rubahn H.G., Mishra Y.K., Suar M., Ahuja R. Structure-based drug designing and immunoinformatics approach for SARS-CoV-2. Science advances. 2020 Jul 1;6(28) doi: 10.1126/sciadv.abb8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehury B., Raina V., Misra N., Suar M. Effect of mutation on structure, function and dynamics of receptor binding domain of human SARS-CoV-2 with host cell receptor ACE2: a molecular dynamics simulations study. J. Biomol. Struct. Dyn. 2020 Aug 6:1–5. doi: 10.1080/07391102.2020.1802348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S., He Y., Liu S. SARS vaccine development. Emerg. Infect. Dis. 2005;11(7):1016–1020. doi: 10.3201/eid1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schindewolf C., Menachery V.D. Middle east respiratory syndrome vaccine candidates: cautious optimism. Viruses. 2019;11(1):E74. doi: 10.3390/v11010074. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tse L.V., Meganck R.M., Graham R.L., Baric R.S. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front. Microbiol. 2020;11:658. doi: 10.3389/fmicb.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pachetti M., Marini B., Benedetti F., Giudici F., Mauro E., Storici P. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020 Apr;18(1):179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzzi P.H., Mercatelli D., Ceraolo C., Giorgi F.M. Master regulator analysis of the SARS-CoV-2/human interactome. J. Clin. Med. 2020;9(4):E982. doi: 10.3390/jcm9040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 28.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 Mar 30:1–9. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 29.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020 Mar 17;94(7) doi: 10.1128/JVI.00127-20. : e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.OthmanH BouslamaZ., Brandenburg J.T., Rocha J., Hamdi Y., Ghedira K., Abid N.S., Hazelhurst S. In silico study of the spike protein from SARS-CoV-2 interaction with ACE2: similarity with SARS-CoV, hot-spot analysis and effect of the receptor polymorphism 2020. BioRxiv. 2020 doi: 10.1101/2020.03.04.976027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020 Mar 27;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta M.K., Vemula S., Donde R., Gouda G., Behera L., Vadde R. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1751300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z., Xiao Y., Chen Y.H. Epitope-vaccine strategy against HIV-1: today and tomorrow. Immunobiology. 2003;208(4):423–428. doi: 10.1078/0171-2985-00286. [DOI] [PubMed] [Google Scholar]
- 35.Mocellin S., Pilati P., Nitti D. Peptide-based anticancer vaccines: recent advances and future perspectives. Curr. Med. Chem. 2009;16:4779–4796. doi: 10.2174/092986709789909648. [DOI] [PubMed] [Google Scholar]
- 36.Lazoura E., Lodding J., Farrugia W., Ramsland P.A., Stevens J., Wilson I.A. Enhanced major histocompatibility complex class I binding and immune responses through anchor modification of the non‐canonical tumour‐associated mucin 1‐8 peptide. Immunology. 2006;119(3):306–316. doi: 10.1111/j.1365-2567.2006.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietersz G.A., Pouniotis D.S., Apostolopoulos V. Design of peptide-based vaccines for cancer. Curr. Med. Chem. 2006;13(14):1591–1607. doi: 10.2174/092986706777441922. [DOI] [PubMed] [Google Scholar]
- 38.Oli A.N., Obialor W.O., Ifeanyichukwu M.O., Odimegwu D.C., Okoyeh J.N., Emechebe G.O. ImmunoTargets Ther. 2020;9:13–30. doi: 10.2147/ITT.S241064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C. Development of epitope‐based peptide vaccine against novel coronavirus 2019 (SARS‐COV‐2): immunoinformatics approach. Immunoinformatics approach. J. Med. Virol. 2020;92(6):618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S. Drug and vaccine design against novel coronavirus (2019-nCoV) spike protein through computational approach. Preprints. 2020:2020020071. doi: 10.20944/preprints202002.0071.v1. [DOI] [Google Scholar]
- 41.Feng Y., Qiu M., Zou S., Li Y., Luo K., Chen R. Multi-epitope vaccine design using an immunoinformatics approach for 2019 novel coronavirus in China (SARS-CoV-2) bioRxiv. 2020 doi: 10.1101/2020.03.03.962332. [DOI] [Google Scholar]
- 42.Enayatkhani M., Hasaniazad M., Faezi S., Guklani H., Davoodian P., Ahmadi N. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: an in silico study. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1756411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robson B. Computers and viral diseases. Preliminary bioinformatics studies on the design of a synthetic vaccine and a preventative peptidomimetic antagonist against the SARS-CoV-2 (2019-nCoV, COVID-19) coronavirus. Comput. Biol. Med. 2020;119:103670. doi: 10.1016/j.compbiomed.2020.103670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020 Apr 8;27(4):671–680. doi: 10.1016/j.chom.2020.03.002. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carneiro J., Pereira F. 2020. CoV2ID: Detection and Therapeutics Oligo Database for SARS-CoV-2. bioRxiv. [DOI] [Google Scholar]
- 46.Huang Y., Lau S.K., Woo P.C., Yuen K.Y. CoVDB: a comprehensive database for comparative analysis of coronavirus genes and genomes. Nucleic Acids Res. 2008;36:D504–D511. doi: 10.1093/nar/gkm754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Liu B., Yang J., Jin Q. vol. 2014. Database; Oxford): 2014. p. bau021. (DBatVir: the Database of Bat-Associated Viruses). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cleemput S., Dumon W., Fonseca V., Abdool Karim W., Giovanetti M., Alcantara L.C. Genome Detective Coronavirus Typing Tool for rapid identification and characterization of novel coronavirus genomes. Bioinformatics. 2020 doi: 10.1093/bioinformatics/btaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatcher E.L., Zhdanov S.A., Bao Y., Blinkova O., Nawrocki E.P., Ostapchuck Y. Virus Variation Resource–improved response to emergent viral outbreaks. Nucleic Acids Res. 2017 Jan 4;45(D1):D482–D490. doi: 10.1093/nar/gkw1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 51.Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data–from vision to reality. Euro Surveill. 2017;22(13) doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schäffer A.A., Hatcher E., Yankie L., Shonkwiler L., Brister J.R., Karsch-Mizrachi I. VADR: validation and annotation of virus sequence submissions to GenBank. BioRxiv. 2019 doi: 10.1101/852657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong R, Yang G, Xue R, Liu M, Wang F, Hu J, et al. COVID-19 Docking Server: an Interactive Server for Docking Small Molecules, Peptides and Antibodies against Potential Targets of COVID-19. arXiv preprint arXiv:2003.00163. 2020. Doi: arXiv:2003.00163vol. 1. [DOI] [PMC free article] [PubMed]
- 54.Shi Y., Zhang X., Mu K., Peng C., Zhu Z., Wang X. D3Targets-2019-nCoV: a webserver for predicting drug targets and for multi-target and multi-site based virtual screening against COVID-19. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qureshi A., Kaur G., Kumar M. AVC pred: an integrated web server for prediction and design of antiviral compounds. Chem. Biol. Drug Des. 2017;89:74–83. doi: 10.1111/cbdd.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qureshi A., Thakur N., Tandon H., Kumar M. AVPdb: a database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 2014;42:D1147–D1153. doi: 10.1093/nar/gkt1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thakur N., Qureshi A., Kumar M. AVPpred: collection and prediction of highly effective antiviral peptides. Nucleic Acids Res. 2012;40:W199–W204. doi: 10.1093/nar/gks450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lissabet J.F., Belén L.H., Farias J.G. AntiVPP 1.0: a portable tool for prediction of antiviral peptides. Comput. Biol. Med. 2019;107:127–130. doi: 10.1016/j.compbiomed.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patiyal S., Kaur D., Kaur H., Sharma N., Dhall A., Sahai S. A web-based platform on COVID-19 to maintain Predicted Diagnostic, Drug and Vaccine candidates. OSF Preprints. 2020 doi: 10.31219/osf.io/xegzu. [DOI] [PubMed] [Google Scholar]
- 60.Dhanda S.K., Mahajan S., Paul S., Yan Z., Kim H., Jespersen M.C. IEDB-AR: immune epitope database—analysis resource in 2019. Nucleic Acids Res. 2019;47:W502–W506. doi: 10.1093/nar/gkz452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vita R., Mahajan S., Overton J.A., Dhanda S.K., Martini S., Cantrell J.R. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. 2019;47:D339–D343. doi: 10.1093/nar/gky1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pickett B.E., Greer D.S., Zhang Y., Stewart L., Zhou L., Sun G. Virus pathogen database and analysis resource (ViPR): a comprehensive bioinformatics database and analysis resource for the coronavirus research community. Viruses. 2012;4:3209–3226. doi: 10.3390/v4113209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Aevermann B.D., Anderson T.K., Burke D.F., Dauphin G., Gu Z. Influenza Research Database: an integrated bioinformatics resource for influenza virus research. Nucleic Acids Res. 2017;45:D466–D474. doi: 10.1093/nar/gkw857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van den Broeck W., Gioannini C., Gonçalves B., Quaggiotto M., Colizza V., Vespignani A. The GLEaMviz computational tool, a publicly available software to explore realistic epidemic spreading scenarios at the global scale. BMC Infect. Dis. 2011;11:37. doi: 10.1186/1471-2334-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wymant C., Hall M., Ratmann O., Bonsall D., Golubchik T., de Cesare M. PHYLOSCANNER: inferring transmission from within-and between-host pathogen genetic diversity. Mol. Biol. Evol. 2018 Mar 1;35:719–733. doi: 10.1093/molbev/msx304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb 22;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pachetti M., Marini B., Benedetti F., Giudici F., Mauro E., Storici P., Masciovecchio C., Angeletti S., Ciccozzi M., Gallo R.C., Zella D. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020 Dec;18:1–9. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020 Mar doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armstrong G.L., MacCannell D.R., Taylor J., Carleton H.A., Neuhaus E.B., Bradbury R.S., Posey J.E., Gwinn M. Pathogen genomics in public health. N. Engl. J. Med. 2019 Dec 26;381(26):2569–2580. doi: 10.1056/NEJMsr1813907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma D., Priyadarshini P., Vrati S. Unraveling the web of viroinformatics: computational tools and databases in virus research. J. Virol. 2015 Feb 1;89(3):1489–1501. doi: 10.1128/JVI.02027-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lau S.K., Woo P.C., Yip C.C., Fan R.Y., Huang Y., Wang M., Guo R., Lam C.S., Tsang A.K., Lai K.K., Chan K.H. Isolation and characterization of a novel Betacoronavirus subgroup A coronavirus, rabbit coronavirus HKU14, from domestic rabbits. J. Virol. 2012 May 15;86(10):5481–5496. doi: 10.1128/JVI.06927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lau S.K., Li K.S., Huang Y., Shek C.T., Tse H., Wang M., Choi G.K., Xu H., Lam C.S., Guo R., Chan K.H. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J. Virol. 2010 Mar 15;84(6):2808–2819. doi: 10.1128/JVI.02219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woo P.C., Lau S.K., Lam C.S., Lai K.K., Huang Y., Lee P., Luk G.S., Dyrting K.C., Chan K.H., Yuen K.Y. Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. J. Virol. 2009 Jan 15;83(2):908–917. doi: 10.1128/JVI.01977-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008 Feb;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Afelt A., Frutos R., Devaux C. Bats, coronaviruses, and deforestation: toward the emergence of novel infectious diseases? Front. Microbiol. 2018 Apr 11;9:702. doi: 10.3389/fmicb.2018.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006 Jul 1;19(3):531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong S., Lau S., Woo P., Yuen K.Y. Bats as a continuing source of emerging infections in humans. Rev. Med. Virol. 2007 Mar;17(2):67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng B.J., Guan Y., Wong K.H., Zhou J., Wong K.L., Young B.W., Lu L.W., Lee S.S. SARS-related virus predating SARS outbreak, Hong Kong. Emerg. Infect. Dis. 2004 Feb;10(2):176. doi: 10.3201/eid1002.030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sardar R., Satish D., Birla S., Gupta D. Integrative analyses of SARS-CoV-2 genomes from different geographical locations reveal unique features potentially consequential to host-virus interaction, pathogenesis and clues for novel therapies. Heliyon. 2020 Aug 20 doi: 10.1016/j.heliyon.2020.e04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lokman S.M., Rasheduzzaman M., Salauddin A., Barua R., Tanzina A.Y., Rumi M.H., Hossain M.I., Siddiki A.Z., Mannan A., Hasan M.M. Exploring the genomic and proteomic variations of SARS-CoV-2 spike glycoprotein: a computational biology approach. Infect. Genet. Evol. 2020 Jun 2:104389. doi: 10.1016/j.meegid.2020.104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toptan T., Hoehl S., Westhaus S., Bojkova D., Berger A., Rotter B., Hoffmeier K., Cinatl J., Ciesek S., Widera M. Optimized qRT-PCR approach for the detection of intra-and extra-cellular SARS-CoV-2 RNAs. Int. J. Mol. Sci. 2020 Jan;21(12):4396. doi: 10.3390/ijms21124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dharavath B., Yadav N., Desai S., Sunder R., Mishra R., Ketkar M., Bhanshe P., Gupta A., Redhu A.K., Patkar N., Dutt S. A one-step, one-tube real-time RT-PCR based assay with an automated analysis for detection of SARS-CoV-2. Heliyon. 2020 Jul 1;6(7) doi: 10.1016/j.heliyon.2020.e04405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ceraolo C., Giorgi F.M. Genomic variance of the 2019‐nCoV coronavirus. J. Med. Virol. 2020 May;92(5):522–528. doi: 10.1002/jmv.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen Z., Xiao Y., Kang L., Ma W., Shi L., Zhang L., Zhou Z., Yang J., Zhong J., Yang D. Genomic diversity of severe acute respiratory syndrome–coronavirus 2 in patients with coronavirus disease. Clin. Infect. Dis. 2020;4:536. doi: 10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020 Aug 20;182(4):812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edgar R.C., Taylor J., Altman T., Barbera P., Meleshko D., Lin V., Lohr D., Novakovsky G., Al-Shayeb B., Banfield J.F., Korobeynikov A. Petabase-scale sequence alignment catalyses viral discovery. bioRxiv. 2020 Jan 1 doi: 10.1101/2020.08.07.241729. [DOI] [PubMed] [Google Scholar]
- 87.Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. Journal of Genetics and Genomics. 2020 Feb 20;47(2):119. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y., Zhang J., Wang N., Li H., Shi Y., Guo G., Liu K., Zeng H., Zou Q. Therapeutic drugs targeting 2019-nCoV main protease by high-throughput screening. BioRxiv. 2020 Jan 1 doi: 10.1101/2020.01.28.922922. [DOI] [Google Scholar]
- 89.Cui Q, Huang C, Ji X, Zhang W, Zhang F, Wang L. Possible inhibitors of ACE2, the receptor of 2019-nCoV. Preprints 2020, 2020020047 (doi: 10.20944/preprints202002.0047.v1).
- 90.Arya R., Das A., Prashar V., Kumar M. Potential inhibitors against papain-like protease of novel coronavirus (SARS-CoV-2) from FDA approved drugs. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11860011.v2. [DOI] [Google Scholar]
- 91.Wu Canrong, Yang Y., Yang L.I., Zhang P., Wang Y., Wang Q. Furin, a potential therapeutic target for COVID-19. China. 2020 doi: 10.12074/202002.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bag A, Bag A. reportJusticia Adhatoda Leaves Extract Is a Strong Remedy for COVID-19–Case Report Analysis and Docking Based Study.
- 93.Pendyala B, Patras A. In silico Screening of Food Bioactive Compounds to Predict Potential Inhibitors of COVID-19 Main protease (Mpro) and RNA-dependent RNA polymerase (RdRp).ChemRxiv. Preprint. 10.26434/chemrxiv.12051927.v2. [DOI]
- 94.Barik A, Rai G, Modi G. Molecular Docking and Binding Mode Analysis of Selected FDA Approved Drugs against COVID-19 Selected Key Protein Targets: an Effort towards Drug Repurposing to Identify the Combination Therapy to Combat COVID-19. arXiv preprint arXiv:2004.06447. 2020. doi: arXiv:2004.06447vol. 1.
- 95.Chen Z., Zhang X., Peng C., Wang J., Xu Z., Chen K. D3Pockets: a method and web server for systematic analysis of protein pocket dynamics. J. Chem. Inf. Model. 2019;59:3353–3358. doi: 10.1021/acs.jcim.9b00332. [DOI] [PubMed] [Google Scholar]
- 96.Wang J., Peng C., Yu Y., Chen Z., Xu Z., Cai T. Exploring conformational change of adenylate kinase by replica exchange molecular dynamic simulation. Biophys. J. 2020;118:1009–1018. doi: 10.1016/j.bpj.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: an open chemical toolbox. J. Cheminf. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saxena A. Drug targets for COVID-19 therapeutics: ongoing global efforts. J. Biosci. 2020 Dec;45(1):1–24. doi: 10.1007/s12038-020-00067-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alexander S.P., Armstrong J., Davenport A.P., Davies J., Faccenda E., Harding S.D., Levi‐Schaffer F., Maguire J.J., Pawson A.J., Southan C., Spedding M.J. A rational roadmap for SARS‐CoV‐2/COVID‐19 pharmacotherapeutic research and development. IUPHAR review “XXX”. Br. J. Pharmacol. 2020 May 1 doi: 10.1111/bph.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balmeh N., Mahmoudi S., Mohammadi N., Karabedianhajiabadi A. Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors. Informatics in Medicine Unlocked. 2020 Aug 7 doi: 10.1016/j.imu.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jomhori M, Mosaddeghi H. Investigation of interaction between close and open types of SARS-Cov-2 spike glycoprotein with synthesized and natural Compounds as inhibitor; Molecular Docking Study. Doi: 10.21203/rs.3.rs-38089/v1. [DOI]
- 102.Barh D, Tiwari S, Andrade BS, Giovanetti M, Kumavath R, Ghosh P, Góes-Neto A, Alcantara LC, Azevedo V. Potential chimeric peptides to block the SARS-CoV-2 Spike RBD. Preprints 2020, 2020040347 (doi: 10.20944/preprints202004.0347.v1).
- 103.Sardar R., Satish D., Birla S., Gupta D. Integrative analyses of SARS-CoV-2 genomes from different geographical locations reveal unique features potentially consequential to host-virus interaction, pathogenesis and clues for novel therapies. Heliyon. 2020 Aug 20 doi: 10.1016/j.heliyon.2020.e04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen W.H., Strych U., Hotez P.J. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020:1–4. doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karch C.P., Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016;120:1–14. doi: 10.1016/j.bcp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahapatra S., Sahoo S., Dehury B., Raina V., Patro S., Misra N., Suar M. Designing an efficient multi-epitope vaccine displaying interactions with diverse HLA molecules for an efficient humoral and cellular immune response to prevent COVID-19 infection. Expet Rev. Vaccine. 2020 Sep 1 doi: 10.1080/14760584.2020.1811091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christaki E. New technologies in predicting, preventing and controlling emerging infectious diseases. Virulence. 2015 Aug 18;6(6):558–565. doi: 10.1080/21505594.2015.1040975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balcan D., Hu H., Goncalves B., Bajardi P., Poletto C., Ramasco J.J., Paolotti D., Perra N., Tizzoni M., Van den Broeck W., Colizza V. Seasonal transmission potential and activity peaks of the new influenza A (H1N1): a Monte Carlo likelihood analysis based on human mobility. BMC Med. 2009 Dec 1;7(1):45. doi: 10.1186/1741-7015-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nikolaou P., Dimitriou L. Identification of critical airports for controlling global infectious disease outbreaks: stress-tests focusing in Europe. J. Air Transport. Manag. 2020 Apr 10 doi: 10.1016/j.jairtraman.2020.101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Del Pozo C.H., Prosper F., Romero J.P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 Apr 24 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Akhter S., Akhtar S. Emerging coronavirus diseases and future perspectives. VirusDisease. 2020 Jun 23:1–8. doi: 10.1007/s13337-020-00590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J., Tsatsakis A. Zinc and respiratory tract infections: perspectives for COVID-19. Int. J. Mol. Med. 2020 Jul 1;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J.I., Matsuda Z. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob. Agents Chemother. 2016 Nov 1;60(11):6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Islam M.T., Nasiruddin M., Khan I.N., Mishra S.K., Kudrat-E-Zahan M., Riaz T.A., Ali E.S., Rahman M.S., Mubarak M.S., Martorell M., Cho W.C. A perspective on emerging therapeutic interventions for COVID-19. Frontiers in public health. 2020;8 doi: 10.3389/fpubh.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.NIH NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19. National Institute of Allergy and Infectious Diseases (NIH); 2020. [Google Scholar]
- 117.Gilead Gilead Announces Results from Phase 3 Trial of Investigational Antiviral Remdesivir in Patients with Severe COVID-vol. 19. (2020).
- 118.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004 Mar 1;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. Unit. States Am. 2017 Jan 10;114(2):206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oslo University Hospital the Efficacy of Different Anti-viral Drugs in COVID 19 Infected Patients. 2020. NCT04321616. [Google Scholar]
- 121.Vanden Eynde J.J. COVID-19: an update about the discovery clinical trial. Pharmaceuticals. 2020 May;13(5):98. doi: 10.3390/ph13050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoffmann-La Roche . 2020. A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients with Severe COVID-19 Pneumonia (COVACTA). NCT04320615.https://clinicaltrials.gov/ Available online at: [Google Scholar]
- 123.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Mar 5 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. 2020 Apr 20. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrobial Agents and Chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gautret P., Lagier J.C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Hagar M., Ahmed H.A., Aljohani G., Alhaddad O.A. Investigation of some antiviral N-heterocycles as COVID 19 drug: molecular docking and DFT calculations. Int. J. Mol. Sci. 2020 Jan;21(11):3922. doi: 10.3390/ijms21113922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J., Zhang J., Cha Y., Kolitz S., Funt J., Chong R.E., Barrett S., Kusko R., Zeskind B., Kaufman H. Advanced bioinformatics rapidly identifies existing therapeutics for patients with coronavirus disease-2019 (COVID-19) J. Transl. Med. 2020 Dec;18(1):1–9. doi: 10.1186/s12967-020-02430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hemmat N., Derakhshani A., BannazadehBaghi H., Silvestris N., Baradaran B., De Summa S. Neutrophils, crucial, or harmful immune cells involved in coronavirus infection: a bioinformatics study. Front. Genet. 2020 Jun 9;11:641. doi: 10.3389/fgene.2020.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar A., Choudhir G., Shukla S.K., Sharma M., Tyagi P., Bhushan A., Rathore M. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020 May 20:1–21. doi: 10.1080/07391102.2020.1772112. (just-accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma A., Goyal S., Yadav A.K., Kumar P., Gupta L. In-silico screening of plant-derived antivirals against main protease, 3CLpro and endoribonuclease, NSP15 proteins of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020 Aug 18:1–5. doi: 10.1080/07391102.2020.1808077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020 Apr 9:1–5. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 132.Achilonu I., Iwuchukwu E.A., Achilonu O.J., Fernandes M.A., Sayed Y. Targeting the SARS-CoV-2 main protease using FDA-approved Isavuconazonium, a P2-P3 α-ketoamide derivative and Pentagastrin: an in-silico drug discovery approach. J. Mol. Graph. Model. 2020 Sep 2 doi: 10.1016/j.jmgm.2020.107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Badgujar K.C., Badgujar V.C., Badgujar S.B. Vaccine development against coronavirus (2003 to present): an overview, recent advances, current scenario, opportunities and challenges. Diabetes & Metabolic Syndrome: Clin. Res. Rev. 2020 Sep 1;14(5):1361–1376. doi: 10.1016/j.dsx.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tamalika K., Utkarsh N., Srijita B., Debashrito D., Filippo C., Mueller D.M., Srivastava A.P. A candidate multi-epitope vaccine against SARS-CoV-2. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-67749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kiyotani K., Toyoshima Y., Nemoto K., Nakamura Y. Bioinformatic prediction of potential T cell epitopes for SARS-Cov-2. J. Hum. Genet. 2020 Jul;65(7):569–575. doi: 10.1038/s10038-020-0771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Lee S.S., Chakraborty C. Development of epitope‐based peptide vaccine against novel coronavirus 2019 (SARS‐COV‐2): immunoinformatics approach. J. Med. Virol. 2020 Jun;92(6):618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]