Abstract
Microwave irradiation (MWI), as a unique, effective, sustainable, more economic, and greener source of energy compared to conventional heating, is applied in different organic transformations to result in the rapid formation of desired compounds due to thermal/kinetic effects. In this review, we try to underscore the applications of microwave irradiation (MWI) in the synthesis of quinazoline and quinazolinone derivatives that have been achieved and reported on in the last two decades.
Keywords: quinazoline, quinazolinone, microwave irradiation, MWI, multicomponent reactions (MCRs), niementowski quinazoline synthesis
Introduction
In the past two decades, microwave irradiation (MWI) has become an effective, powerful, and green source of energy in the art of organic synthesis. As early as the 1950's, it was found that microwaves could heat water. The popular use of domestic microwave ovens happened during the1970 and 1980's as a result of actual and operative Japanese technology transfers and worldwide marketing. The use of MWI in chemical modification can, however, be traced back to the 1950's (Lidström et al., 2001; Bogdal et al., 2003; Kappe, 2004; de la Hoz et al., 2005). In 1986, Gedye et al. investigated four different types of organic reactions in a domestic microwave oven. They observed an increased rate of the formation of products compared to the rate of synthesis of the products under conventional reaction conditions (Gedye et al., 1986). Since then, the conduction of chemical reactions under MWI in the laboratory has started to gain wide recognition. However, MWI's role in organic synthesis has brought intense differences of opinion which resulted in diverse theories.
MW chemistry is the science of applying MWI to chemical reactions. When the rate of heat conduction is high between system areas, hot spots lacklong-term existence as the components reach thermal equilibrium rapidly. In a system where the heat transfer is slow, it would be possible to have the presence of a steady-state hot spot that may increase the rate of the chemical reaction within that hot spot. Based on this theory, specific molecules or certain functional groups within molecules should be excited when exposed to MWI. As a result, the time of distribution of thermal energy under MWI is much shorter than the period that thermal energy is distributed under ambient or conventional heating and laboratory conditions. The MW region of the electromagnetic spectrum lies between infra-red radiation and radio frequencies, relating to frequencies of 30 GHz to 300 MHz respectively. In order not to obstruct these uses, domestic MW ovens and industrial MW heaters are required to operate at either 2.45 GHz or 900 MHz, unless the apparatus is protected in such a way that no loss of radiation happens. Commonly, domestic MW ovens are designed and made to operate at 2.45 GHz (Mingos and Baghurst, 1991). Although synthetic chemists began to use domestic MW ovens in the mid-1980's, over the years, some problems have risen. The essential problem that one can have while using a commercial MW oven is caused by the nonhomogeneous distribution of energy. The other drawbacks involve the lack of temperature and pressure control, which does not allow for the use of flammable solvents and reproducibility (Bacher, 2016; Nain et al., 2019).
Consequently, special MW systems were designed for use in the laboratory, which demonstrates improved prospects for re-procreativity, fast synthesis, easy reaction optimization, and the potential exploration of new synthetic pathways. Dedicated microwave reactors allow fast heating of reaction mixtures to high pressures and temperatures–far above the boiling point of the used solvent. MWI can have certain advantages over conventional heating. Under MWI, reactions proceed to completion much faster, are performed under milder and greener reaction conditions, give higher chemical yields, require lower energy usage, and are selective or sometimes show different selectivity. Above all, its uses have become inevitable in cases when conventional approaches need constraining reaction conditions, when prolonged reaction times are required, or when certain selectivity is required. Thus, MW has been widely applied in the fields of organic and inorganic chemistry (Abramovitch, 1991; Loupy, 1993, 1994; Majetich and Hicks, 1994, 1995; Caddick, 1995; Strauss and Trainor, 1995).
Quinazoline and quinazolinone analogs are two important heterocyclic systems with diverse biological activities; thus, they have attracted much attention from synthetic organic chemists as desirable targets (Michael, 2004).
Quinazoline and quinazolinone derivatives constitute a noteworthy class of naturally occurring fused heterocycles (Wang et al., 1996; Sheu et al., 2000; Wu et al., 2001; Liu et al., 2018b), which were initially isolated from different natural sources such as plant families, microorganisms, and animals. Their structures were elucidated, showing diverse biological properties (Michael, 2003; Connolly et al., 2005; Mhaske and Argade, 2006; Khan et al., 2014, 2015; Kshirsagar, 2015). Several quinazoline and quinazolinone derivatives have passed clinical trials and are recognized as potential candidates for prescribed drugs (Inoue et al., 1976). Quinazoline and quinazolinone analogs possess a wide range of useful biological properties, including being antitumor (El-Azab et al., 2010; Sharma et al., 2013), anticancer (Wakeling et al., 2002; Vasdev et al., 2005; Chandregowda et al., 2009; Kabri et al., 2009; Pathania et al., 2014), anti-microbial (Gupta et al., 2008; Rohini et al., 2010; Rajasekaran et al., 2013), anti-bacterial (Mohammadi et al., 2014), anti-virus (Li et al., 1998), anti-inflammation, anti-tuberculosis (Nandy et al., 2006), and anti-obesity (Sasmal et al., 2012).
The first synthesis of the quinazoline nucleus was achieved and reported by Griess in 1869 through the reaction of anthranilic acid with cyanogen as a source of nitrogen (Griess, 1869). Von Niementowski optimized the reaction using amides instead of cyanogen (Von Niementowski, 1895). Owing to the diverse range of pharmacological activities, various synthetic routes for the synthesis of quinazolinone derivatives have been developed by employing 2-aminobenzoic acid, anthranilamide, anthranilonitrile, isatoic anhydride, 2-carbomethoxyphenyl isocyanate, N-arylnitrilium salts, and benzoxazines as starting materials (Michael, 2003; Connolly et al., 2005; Mhaske and Argade, 2006; Khan et al., 2014, 2015; Kshirsagar, 2015).
In 2007, a review on the synthesis of bioactive quinazolines and quinazolinones was published, focusing only on the synthesis of heterocyclic systems of specific biological activities or commercially important compounds (Besson and Chosson, 2007). In 2017, a review entitled “Green approaches toward [the] synthesis of substituted quinazolines” appeared in the chemical literature (Devi et al., 2017). Many researchers have used MWI as a green and sustainable source of energy in different organic transformations (Chatel and Varma, 2019). MWI has been frequently used in the synthesis of N-heterocyclic systems (Majumder et al., 2013). Applications of MWI as greener and more ecological trends emerge has also been extended to the synthesis of quinazoline and quinazolinone derivatives, particularly to those required for biological screening and in drug discovery studies, where rapid, high-yielding preparation of samples in highly pure forms are needed.
In the continuation of our interest in the chemistry of quinazolines and quinazilidinones (Heravi et al., 2008, 2009, 2010, 2011; Saeedi et al., 2011; Shiri et al., 2018) and our continuous attempts to conduct some of our reactions under MWI (Heravi et al., 1999, 2016; Valizadeh et al., 2010a,b, 2016; Fazeli et al., 2013; Heravi and Moghimi, 2013), in this review, we try to underscore all the successful strategies selected and progress made in the synthesis of quinazoline and quinazolinone derivatives, being conducted under MWI.
Synthesis of Quinazoline Derivatives
Intramolecular Heterocyclization
2-Benzimidazoylbenzamides have been employed as substrates for intramolecular heterocyclization to create quinazolines under MW conditions. For example, Pessoa-Mahana et al. reported the synthesis of 6-arylbenzimidazo[1,2-c]quinazolines 2 by intramolecular heterocyclization of 2-benzimidazoylbenzamides 1 under MWI in a solventless system (Pessoa-Mahana et al., 2004). Compounds 1 were efficiently and cleanly transformed into the corresponding tetracyclic compounds 2 in moderate yields under MWI in the presence of SiO2-MnO2 as a solid inorganic matrix in only 30–45 min (Scheme 1). The same reaction under conventional thermal conditions (anhydrous m-xylene, reflux) increased the reaction times. This novel and interesting strategy has shown the application of MWI in the heterocyclization for producing complex polycyclic systems.
Scheme 1.
MW–assisted synthesis of 2 through intramolecular heterocyclization of 1.
Intramolecular Friedel-Crafts-Type Cyclization
Intramolecular Friedel-Crafts-type cyclization strategy has been applied in the synthesis of quinazolines. An original and efficient method based on the MW–assisted intramolecular Friedel-Crafts-type cyclization of guanidines 3 to access 4-phenylquinazolines 4 in [OMIm]X ionic liquid in 10 min was reported by Debray et al. (2010). The reaction mechanism includes three steps as represented in Scheme 2. In the first step, the basic arylguanidine abstracted the [OMIm] H-2 with the generation of the carbene and the acylguanidinium species (I). The second step involved the addition of the corresponding N-heterocyclic carbene to the hydroxyimino tautomer (I), providing the highly electrophilic intermediate (II). In the next step, the intermediate (II) underwent Friedel-Crafts-type cyclization along with the elimination of imidazolium and water, completing the synthesis of the expected products 4. The use of traditional heating (110°C) instead of MWI required a longer time to achieve completion of this reaction (1 h instead of 10 min).
Scheme 2.
Mechanism of formation of 4 using ionic liquid as solvent under MWI.
Palladium-Catalyzed Annulation of N-Allylamidines
N-Allylamidines have been used as substrates for palladium-catalyzed annulation to produce the quinazolines. For example, Pd(OAc)2-catalyzed annulation strategy for the construction of multi-substituted quinazolines 6 from N-allylamidines 5 under MWI has been developed and reported (Xu et al., 2015). Quinazolines 6 were obtained as the major products through the isomerization of Pd-alkene complex intermediate of N-allylamidines 5 and C–H activation in the presence of a catalytic amount of Pd(OAc)2 under MWI at 170°C in a sealed tube (Scheme 3). It was found that heating the reaction mixture conventionally improved the yield of the reaction.
Scheme 3.

Pd(OAc)2-catalyzed annulation of 5 to create 6 under MWI.
Cyclocondensation Using Various Methods
MWI has been used to promote cyclocondensation of various nitrogen-containing precursors with ortho-esters, benzaldehydes, alcohols, amines, ammonium formate, nitriles, HMTA, and thiourea, as described below.
Ortho-esters
Ortho-esters have been condensed with aniline derivatives under MWI to produce quinazolinones. As an example in this regard, the synthesis of 6-substituted benzimidazo[1,2-c]-quinazolines 8 was accomplished through the cyclocondensation of 2-(2-aminophenyl)benzimidazole (7) with ortho-esters in N,N-dimethyl acetamide (DMAC) using MWI and in the absence of the catalyst (Scheme 4) (Khajavi et al., 1999a). Products 8 were obtained, which showed a relatively higher yield compared to under classical heating. A comparison of two reaction conditions showed the emphasis on MWI as the main factor in improving the product yields in 2–6 min, which involved a prolonged reaction time. The reaction also occurred in the absence of a solvent.
Scheme 4.
MW–assisted synthesis of 8 from 7 and ortho-esters.
Azizian et al. reported a strategy to rapidly prepare novel polyheterocyclic compounds 10, 6-substituted quinazolino[4,3-b]quinazolin-8-ones, through fusing 2-(o-aminophenyl)-4(3H)-quinazolinone (9) with a range of ortho-esters under solvent-free MW conditions in the absence of organic or inorganic reagents (Scheme 5) (Azizian et al., 2004b). The strategy offered several advantages comprising cleaner reaction profiles, short reaction times, simple work-up procedures, and high yield of products, making it a useful tool for the synthesis of target products.
Scheme 5.
MW–assisted synthesis of 10 form 9 and ortho-esters.
Benzaldehydes
Benzaldehydes have also been condensed and cyclized with various amines under MW conditions to synthesize quinazolinones. For example, Kumar et al. accomplished the synthesis of biologically important quinazoline derivatives using a domestic microwave oven (Kumar et al., 2004). The advantage of this process is better yields, shorter reaction times, lower energy inputs, and an easy work-up procedure, as well as it being less expensive and high-yielding. The aza-Wittig reaction of N-imidoyliminophosphorane (11) with aldehyde derivatives under domestic MWI at 300 W for a period of 3–4 min led to the formation of the desired quinazolines 12 in good yields (Scheme 6).
Scheme 6.
MW-promoted aza-Wittig synthesis of quinazolines 12.
Later, Kumar et al. synthesized quinazolines 12 via the MW–assisted direct condensation of N-arylamidine 13 with aldehydes, without their prior conversion to iminophosphoranes, in the absence of any Lewis acid catalyst (Kumar et al., 2005). This methodology could be employed to synthesize 2-secamino substituted quinazoline derivatives 14 and benzo[g]quinazolines 15 through the condensation of guanidines and N-naphthalen-1-yl-benzamidine with different aldehydes, respectively (Scheme 7).
Scheme 7.
MW–assisted synthesis of 12, 14, and 15.
A useful strategy to synthesize new quinazoline derivatives was developed by Maitraie et al. (2006). 2,6-Dicyanoaniline derivatives 16 on the reaction with Grignard reagents produced imine regioisomers 17 and 18, which, after separation and identification, reacted with various aldehydes under MWI with 450 W to provide novel quinazolines 19 and 20, respectively. Interestingly, when the same reaction was conducted under 300 W power of MWI, 2-dihydroquinazolines 21 and 22 were exclusively obtained (Scheme 8). Notably, quinazolines were obtained at a comparatively higher yield than the 1,2-dihydroquinazolines.
Scheme 8.
MW–assisted synthesis of quinazolines 19 and 20 from 17 and 18, respectively.
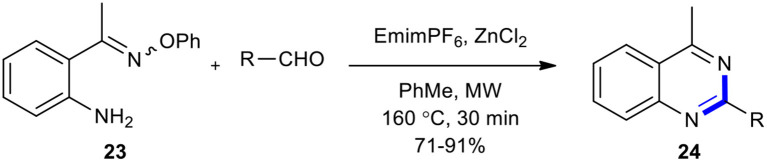
Portela-Cubillo et al. showed that MWI in the case of the reaction of 2-(aminoaryl)alkanone O-phenyl oxime (23) with aldehydes in the presence of emimPF6 as a catalyst and ZnCl2 as a co-catalyst in toluene is a suitable source of heating for the synthesis of quinazolines 24 in good to excellent yields (71–91%) (Scheme 9) (Portela-Cubillo et al., 2008). ZnCl2 in this reaction acted as a promoter which improved the yield of the products when less reactive carbonyl compounds were used. The notable features of this strategy involved mild reaction conditions, high yields, short reaction times, and no need for acids, bases, or metal catalysts. In addition, unlike other radical-mediated protocols, no initiators were required and no by-products contaminated the process.
Scheme 9.
MW–assisted synthesis of 24 from 23 and aldehydes using emimPF6 and ZnCl2.
Alcohols
Condensation of alcohols with imino-1,2,3-dithiazole 27 to 4-alkoxyquinazoline using MWI has been reported. Besson et al. employed MWI for the synthesis of 4-alkoxyquinazoline 28 through the reaction of the imino-1,2,3-dithiazole 27, prepared form aniline 25 and 4,5-dichloro-1,2,3-dithiazolium chloride (26), in the presence of sodium hydride in EtOH (Scheme 10) (Besson et al., 2000). Compound 28 was probably obtained via the addition of the alcohol to the cyano group, which was accompanied by cyclization and aromatization. Here, MWI was used as a powerful source of energy for accelerating the reactions and was cleaner compared to conventional heating (Besson and Rees, 1996; Besson et al., 2000).
Scheme 10.
MW–assisted synthesis of 28 from 27.
Aliphatic and Aromatic Amines
Aliphatic and aromatic amines have been employed as reactants to afford quinazolinones. Tsou et al. presented the synthesis of 6-nitro-4-(3-bromophenylamino)quinazoline (32) (Tsou et al., 2001). They reacted market purchasable 5-nitroanthranilonitrile (29) with N,N-dimethylformamide dimethyl acetal (DMF–DMA) as a reactant and solvent to give N-(2-cyano-4-nitrophenyl)-N,N-dimethylimidoformamide (30). The latter was then reacted with 3-bromoaniline (31) in AcOH under reflux to produce the desired compounds (32) (Scheme 11).
Scheme 11.
MW–assisted synthesis of 32 from 30 and 31.
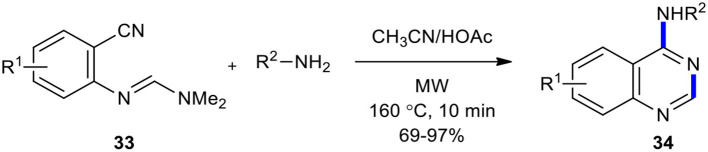
A simple, efficient, straightforward, and high-yielding strategy for the preparation of 4-aminoquinazoline derivatives 34 employing MW chemistry was reported (Yoon et al., 2004). The resulting products 34 were formed when the reaction between N-(2-cyanophenyl)-N,N-dimethylformamidine derivatives 33 and amines was conducted under the optimized reaction conditions (CH3CN/HOAc, MW, 160°C, 10 min) (Scheme 12).
Scheme 12.
MW–assisted synthesis of 4-aminoquinazolines 34 from 33 and amines.
Foucourt et al. (2010) re-investigated the MW conditions of Tsou's reaction (Tsou et al., 2001) with the aim to define a well-established protocol, enabling a high level of reproducibility as well as easy scaling-up to a multi-gram scale. They synthesized 4-anilino-6-nitroquinazolines 35 from 5-nitroanthranilonitrile (29) on a multi-gram scale through the MW–assisted condensation and Dimroth rearrangement. The best result in the synthesis of the intermediate 30 was carried out in only 2 min under MWI at atmospheric pressure at 70°C, which gave the products in almost quantitative yields. They also synthesized 4-arylaminoquinazolines 37 (Scheme 13). In this MW heating, acetic acid reacted with anilines to generate the corresponding acetamides and gave cleaner products. This methodology was also used to access Azixa, a microtubule destabilizing agent and apoptosis inducer, in two steps in 64% overall yields.
Scheme 13.
MW–assisted synthesis of 35 and 37 from 29 and 36, respectively.
Foucourt et al. could also develop their strategy for the efficient and simple production of a forty molecule library of novel 6,6,5-tricyclic thiazolo[5,4-f ]-quinazoline scaffold as interesting DYRK1A inhibitors (Foucourt et al., 2014). The synthesis of the target molecules was started with 6-aminobenzo[d]thiazole-2,7-dicarbonitrile (38) which, upon reaction with DMF–DMA under MWI at 70°C, yielded (E)-N'-(2,7-dicyanobenzo[d]thiazol-6-yl)-N,N-dimethylformimidamide (39) in good yield (86%). Next, formimidamide 39 was cyclized to thiazolo[5,4-f ]quinazoline-2-carbonitriles 40, the expected compounds, in 70–99% yields via MW–assisted thermal-sensitive Dimroth rearrangement using appropriate anilines in acetic acid at 118°C for 2–45 min (Scheme 14).
Scheme 14.
MW–assisted synthesis of 40 from 39 and anilines.
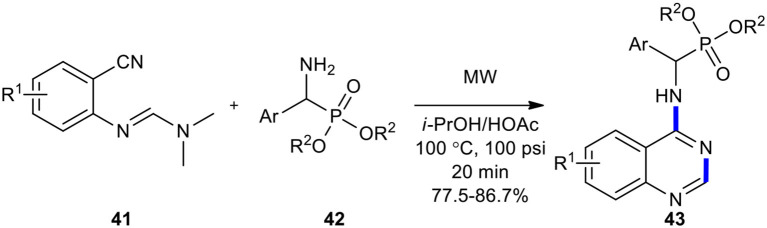
MW technology was employed as an efficient, mild, and environmentally friendly method to increase yields and shorten reaction times in the synthesis of (quinazolin-4-ylamino)methylphosphonates 43 (Luo et al., 2012). The desired products 43 were easily prepared in higher yields compared with a conventional mode of heating when N'-(substituted-2-cyanophenyl)-N,N-dimethylformamidines 41 was reacted with dialkyl amino (phenyl)methylphosphonates 42 under the optimized reaction conditions (MWI, i-PrOH/HOAc, 100°C, 20 min) (Scheme 15).
Scheme 15.
MW–assisted synthesis of 43 from 41 and 42.
Ammonium Formate
Ammonium formate (HCOONH4) have been employed as substrates for the synthesis of quinazolinones under MW conditions. For example, 2,4-disubstituted quinazolines 45 were obtained upon treatment of acylamides 44 with HCOONH4 as a source of NH3 under MW activation at 150°C for 4–20 min (Ferrini et al., 2007). Heating of the reaction probably led to the decomposition of HCOONH4 and generation of the imine which was cyclized in the acid environment owing to the presence of excess HCOONH4. A temperature of 150°C and the presence of the acid afforded the requisite quinazoline 45 (Scheme 16).
Scheme 16.

MW–assisted synthesis of 45 from 44 using ammonium formate.
Nitriles
Nitriles have been used as reactants in the synthesis of quinazolinone derivatives. A research group in the Universidad de Santiago de Compostela synthesized 4-aminoquinazolines 47 by the reaction of anthranilonitrile (46) with various aromatic nitriles in the presence of a catalytic amount of t-BuOK as a base in a domestic microwave oven as a heating device in the absence of solvent (Seijas et al., 2000). The products 47 were obtained in good to excellent yields and in a very short irradiation time (Scheme 17).
Scheme 17.

MW–assisted synthesis of 47 from 46 and nitriles.
A solvent-free, transition-metal-free, rapid, and efficient reaction to prepare 2,4-disubstituted quinazolines 49 via the Lewis-acid-catalyzed activation of nitriles and intramolecular cyclization in a one-pot reaction sequence was developed (Saikia et al., 2018). The reaction was performed in the presence of a catalytic amount of trimethylsilyltrifluoromethane sulfonate (TMSOTf) under MWI. Nitriles, employed as a nitrogen source and activated by TMSOTf, were reacted with 2-aminophenyl carbonyl compounds 48. The best result was achieved when 48 was treated with nitrile and TMSOTf under MWI at reflux 100°C for 10 min (Scheme 18). The significant features of this approach included clean and mild reaction conditions, very short reaction times, and a broad substrate scope.
Scheme 18.
MW–assisted synthesis of 49 from 48 and nitriles.
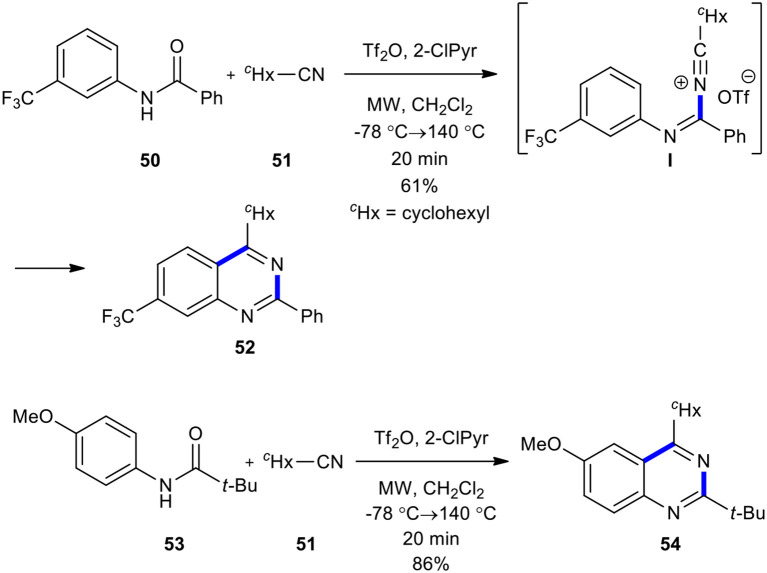
Movassaghi and Hill compared conventional heating and MWI in the condensation of amides with cyclohexanecarbonitrile as a nucleophile to construct an azaheterocycle scaffold (Hill and Movassaghi, 2008). The condensation of amide 50 with cyclohexanecarbonitrile (51) in the presence of trifluoromethanesulfonic anhydride (Tf2O) and 2-chloropyridine (2-ClPyr) in the sealed reaction vessel in CH2Cl2 under MWI heating at a lower temperature (−78°C) of 140°C for 20 min afforded the expected quinazoline 52 (Scheme 19). The above synthetic route could complete the conversion of the sterically hindered substrate, amide 53, into the desired quinazoline 54. This approach was found to be superior in comparison with heating in an oil bath. The MWI also shortened the reaction times and increased the yield of the products.
Scheme 19.
MW–assisted synthesis of 52 and 54 from 50 and 51, respectively.
The one-step synthesis of a new tricyclic product, 5-chloroimidazo[1,5-a]quinazolines 58, was reported by Li et al. via the reaction between N-acylanthranilic acids 55 and 2-amino acetamides 56 (or 2-amino-acetonitriles 57) in the presence of phosphorus oxychloride (POCl3) as a condensing reagent under MWI (Scheme 20) (Li et al., 2009). The introduction of EWG at the 5-position of 2-acetamidobenzoic acid improved the yield of the expected products.
Scheme 20.
MW–assisted synthesis of 58 from 55 and 56 (or 57) using POCl3 as condensing reagent.
HMTA
Hexamethylenetetramine (HMTA) has been condensed with ethyl phenylcarbamates under MW conditions to synthesize the quinazolinones. As an example, Chilin et al. established the benefits of using MWI in the one-pot synthesis of the quinazoline scaffold as a high-yielding and user-friendly protocol. This method used ethyl phenylcarbamates 59 as a simple and easily available starting material (Chilin et al., 2007). The reaction of compounds 59 with HMTA in trifluoroacetone (TFA) to carry out aminomethylation and intramolecular cyclization furnished the dihydropyrimidine ring, which was subjected to oxidative dehydrogenation upon exposure to K3Fe(CN)6 in aqueous ethanolic KOH to obtain the desired quinazolines 60 (Scheme 21).
Scheme 21.
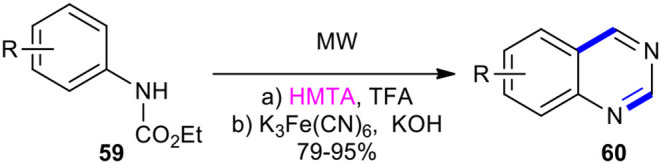
MW–assisted synthesis of 60 from 59 and HMTA.
Thiourea
Thiourea has been used as an efficient reagent for condensation with 2-aminobenzophenones to create the 4-aryl substituted quinazoline derivatives. A very simple and facile synthetic route for the direct conversion of substituted 2-aminobenzophenones 61 into 4-substituted quinazolines 62 under MWI in the presence of thiourea in dimethyl sulfoxide (DMSO) as a solvent and reagent was reported (Wang et al., 2016). The reaction was carried out through thermal decomposition of thiourea to generate carbodiimide and hydrogen sulfide, which were, respectively, reacted with 2-aminobenzophenone and DMSO to furnish 4-phenylquinazolin-2(1H)-imine intermediate and methanethiol (or other sulfur-containing molecules) as a complementary reducing agent. The reaction of 4-phenylquinazolin-2(1H)-imine intermediate with the sulfur-containing reducing agents formed 4-phenyl-1,2-dihydroquinazolin-2-amine which was subjected to the elimination of ammonia to complete the synthesis of substituted quinazoline derivatives 62 (Scheme 22).
Scheme 22.
MW–assisted synthesis of 62 from 61 using thiourea in dimethyl sulfoxide.
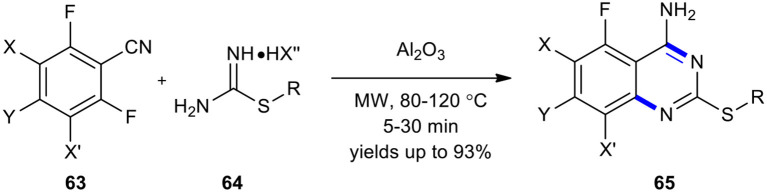
An inexpensive, environmentally benign, and efficient protocol was developed for the synthesis of the fluorinated 2-alkylthio-4-aminoquinazoline scaffold, with a broader sulfur-containing substrate scope, that combined the use of MWI and basic alumina, acting as the solid support agented base promoter (Liu et al., 2018a). The synthesis of 2-alkylthio-4-aminoquinazolines 65 was achieved by MW–assisted cyclization of o-fluorobenzonitriles 63 with S-alkyl isothiouronium salts 64 and basic alumina at 80 or 120°C for 5–30 min (Scheme 23). The use of MWI could considerably improve product yields.
Scheme 23.
MW–assisted cyclization of 63 with 64 using basic alumina to synthesize 65.
Three-Component Reaction
Multicomponent reaction (MCR) is an attractive synthetic strategy where three or more substrates combine to form a single product in a one-pot fashion. MCR is a useful and powerful tool that has been used for the rapid and efficient synthesis of various complex molecules and natural products (Armstrong et al., 1996; Toure and Hall, 2009). Moreover, it is characterized by its environmental friendliness, atom economy, high yields, time efficiency, and low waste production. As a type of MCR, one-pot condensation of aminobenzonitrile (or anthranilic acid and its derivatives), ortho-esters (or formic acid), and amines under MWI is one of the most straightforward procedures for the construction of quinazoline and quinazolinone derivatives.
Rad-Moghadam and Samavi used MW technology to develop a facile and rapid method for the synthesis of 4-aminoquinazolines (Rad-Moghadam and Samavi, 2006). The synthesis was accomplished in a few minutes through a one-pot multi-component reaction between 2-aminobenzonitrile (46), ortho-esters, and ammonium acetate (AcONH4) under solvent-free and MW conditions, leading to the desired products 66, 2-alkyl-4-aminoquinazolines, in good yields (Scheme 24). AcONH4 was employed as a separate synthon for the first time for N-3 in the synthesis of 4-aminoquinazolines. The results were compared with that of a multi-component reaction under solvent-free conditions. MW conditions with conventional heating (refluxing absolute ethanol) showed a preference for the first system.
Scheme 24.
MW–assisted MCR of 46, ortho-esters, and AcONH4 to produce 66.
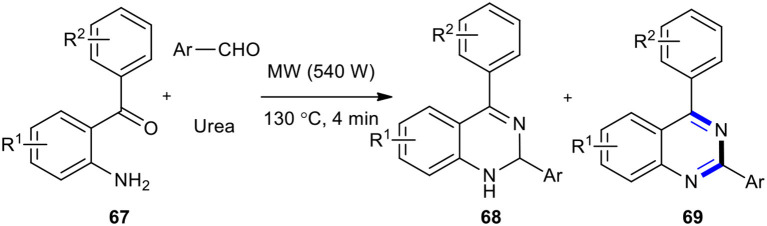
When 2-aminobenzophenones 67 were reacted with aldehydes and urea as an environmentally benign source of ammonia through one-pot three-component reaction under MW conditions (540 W and 130°C), a library of 2,4-disubstituted-1,2-dihydroquinazolines 68 as a major product with a small amount of quinazolines 69 as a minor product were obtained (Scheme 25) (Sarma and Prajapati, 2011). Their methodology also employed ammonium acetate as a good source of ammonia in place of urea. The salient features of this method were that it was rapid, simple, and clean, and had no need for a solvent or catalyst.
Scheme 25.
MW–assisted MCR of 67, aldehydes, and urea to afford 69.
Synthesis of Quinazolinone Derivatives
Radziszewski's Reaction Using UHP
The Radziszewski reaction is an organic reaction which involves the oxidation of nitriles using alkaline hydroperoxide (Radziszewski, 1885). Performing the reaction on o-amidobenzonitriles may result in the formation of quinazolin-4-(3H)-ones (Bogert and Hand, 1902). The Radziszewski reaction as a useful and efficient strategy has been employed in the synthesis of quinazolinones under MW conditions. For example, MWI was used in the cyclization of 2-chloro-N-(2-cyanophenyl)acetamide (70a) and 2-amidobenzamide derivatives 72 to quinazolinones (Kabri et al., 2009). It is noteworthy that compounds 70 and 72 were obtained as a solid since they could be directly converted into the target molecules. Compound 70a was subjected to Radziszewski's reaction in the presence of urea hydrogen peroxide (UHP) as a safe, mild, and non-hazardous oxidizing agent in the mixture of acetone/H2O (1:1, v/v) to afford 2-chloromethylquinazolin-4(3H)-one 71a. The reaction was performed under MW and classical conditions. In classical conditions, the product 71a was formed in a 55% yield after 30 h reaction time at 84°C, whereas, under MW conditions (500 W, 70?C, 1.5 h), the same product was obtained in a 78% yield. Cyclization of derivatives 72 to quinazolin-4(3H)-ones 71 was accomplished when K2CO3 in water was used under MWI at 80?C for 1 h at a power of 500 W, obtaining the expected compounds in good yields (Scheme 26).
Scheme 26.
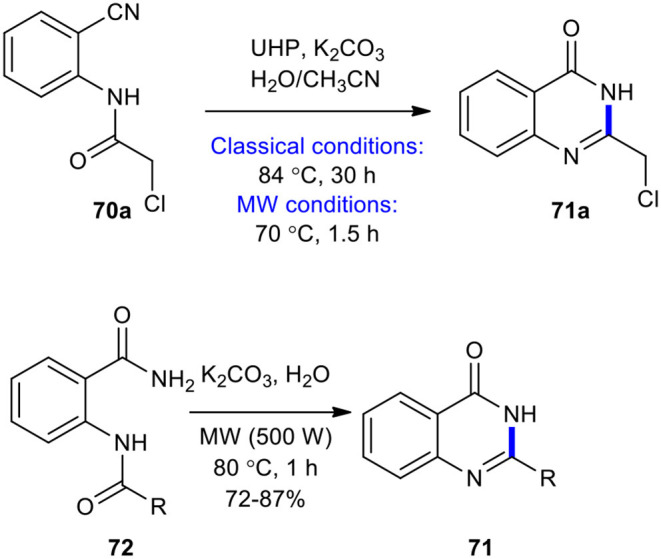
Radziszewski's reaction using UHP under conventional and MW conditions to afford 71a.
Double Cyclization
A double cyclization reaction was employed in the synthesis of natural products containing quinazolinone under MW conditions. For example, the direct double cyclization of tripeptides 73 using Sn(OTf)2 in DMF and MWI at 140°C to access the structurally more challenging members, quinazolinobenzodiazepines 74, including sclerotigenin, asperlicin C, and circumdatin F natural products and their analogous compounds, in 5–15 min was accomplished within a week to good yields (34–85%) (Scheme 27) (Tseng et al., 2009). Tin(II) triflate in this dehydrative cyclization reaction could serve as an effective Lewis acid.
Scheme 27.
MW–assisted direct double cyclization of 73 to afford 74.
Intramolecular Azido-Reductive Cyclization
Intramolecular azido-reductive cyclization strategy has previously been employed in the synthesis of natural products under MW conditions. For example, Kamal et al. developed a simple, mild, and efficient synthetic route to synthesize rutaecarpine, euxylophoricines A and C, and several analogs by using an intramolecular azido-reductive cyclization assisted by MWI of the corresponding substituted azido cyclic amides 75 as precursors (Kamal et al., 2011). The reduction of azido functionality in 2,3,4,9-tetrahydro-β-carbolin-1-one derivatives 75 with triphenylphosphine (PPh3) or nickel boride (Ni2B) as a reducing reagent in HCl–MeOH using a CEM Discovery MW reactor furnished the expected quinazolinones 76 in excellent yields (80–90%) (Scheme 28).
Scheme 28.
MW–assisted intramolecular azido-reductive cyclization of 75 to afford 76.
Niementowski Quinazoline Synthesis
The Niementowski quinazoline synthesis involves the reaction of anthranilic acids with amides to construct 4-oxo-3,4-dihydroquinazolines (3H-quinazolin-4-ones). It is the most commonly used synthetic method to form the 3H-quinazolin-4-one ring. This synthetic route generally involves lengthy and tedious conditions as well as high temperatures. MWI as a powerful technique is capable of improving and reducing reaction times and increasing the yield of the reaction more than the purely thermal heating source. The first highly accelerated Niementowski reaction of anthranilic acid (77) with formamide (or formanilide) using a house-hold unmodified microwave oven under solvent-free conditions was reported by Khajavi et al., leading to the high purity of quinazolinone derivatives 78 (Scheme 29) (Khajavi et al., 1998). The salient features of this method included operational simplicity and a simple work-up procedure.
Scheme 29.
MW–assisted Niementowski reaction to afford 78.
As a general, efficient, and useful method, the combination of supported reagents and MWI was used in solvent-free conditions to prepare the requested quinazolines 80 through Niementowski quinazoline synthesis (Balalaie et al., 2001). The reaction of anthranilic acids 79 with formamide under MW conditions for 4 min was achieved using acidic alumina, silica gel, and montmorillonite K-10 (MK-10). Among acidic solid-supported reagents, montmorillonite K-10 gave the best yield (Scheme 30). Solvent-free conditions, good yields, short reaction times, and a simple set-up and work-up procedure are advantages of this method.
Scheme 30.
MW–assisted Niementowski reaction of 79 and formamide using MK-10 to afford 80.
The Niementowski reaction under MWI (60 W) was employed for the synthesis of 3H-quinazolin-4-one core 82 using an excess of formamide (5 equiv) as a fusion accelerator in a fixed temperature (150°C) (Scheme 31) (Alexandre et al., 2002). The expected products 82 were obtained in very good yields without any by-products.
Scheme 31.
MW–assisted Niementowski reaction using an excess of formamide to afford 82.
Alexandre et al. described an original synthetic route to the rare 8H-thiazolo[5,4-f ]quinazolin-9-one (85) and the novel 7H-thiazolo[4,5-h]quinazolin-6-one (86), starting with the formation of 3H-nitroquinazolin-4-ones 84 (Alexandre et al., 2003a). The MW–assisted Niementowski condensation using formamide under MW conditions (60 W, 150°C) for 40 min annulated rapidly the quinazolin-4-one rings 84 which, in several steps, were converted into products 85 and 86, with different functional group in different positions (Scheme 32).
Scheme 32.
The synthesis of 84 through MW–assisted Niementowski reaction toward 85 and 86.
Domon et al. prepared new triazabenzo[a]indeno[1,2-c]anthracen-5-ones and triazabenzo[a]indeno-[1,2-c]naphtacen-5-one 89 using MWI in dry media (Domon et al., 2001). Cyclization of anthranilic acid derivatives 87 using indoloquinazoline derivative 88 was performed with an excess of anthranilic acid, adsorbed on graphite, using MWI (120 W) at 140°C for 30 min (Scheme 33). Interestingly, they realized that the direct condensation of anthranilic acids with thioamides may be very difficult or unsuccessful; hence, they converted the mercapto group of thioquinazolines into a better leaving group. Their method gave products 89 in various yields without any by-products. Although MWI shortened the reaction time and provided a cleaner reaction compared to the same reaction in purely thermal procedures, it should have been performed at a higher temperature (140°C) due to graphite/MW interaction.
Scheme 33.
MW–assisted synthesis of 89 from 87 and 88.
To access 8H-quinazolino[4,3-b]quinazolin-8-ones 93, Alexandre et al. introduced two different leaving groups on the quinazoline ring (Scheme 34) (Alexandre et al., 2003b). In method A, 4-(thiomethyl)quinazolines 91 was condensed with anthranilic acids 90 on graphite under the optimized reaction conditions (MW, 60 W, 150°C, 30 min). In method B, the use of chlorine instead of thiomethyl group offered more straightforward access to target molecules 93 and afforded better yields than method A. It is noteworthy that method B employed acetic acid as a solvent while method A occurred in solvent-free conditions.
Scheme 34.
MW–assisted synthesis of 93 from 91 or 93.
The reaction of anthranilic acid with lactim ethers is one of the methods to synthesize the quinazoline scaffold. This reaction was relatively neglected in the past, due to its low yields and the possibility of epimerization of stereocenters adjacent to carbonyl groups (Rajappa and Advani, 1973, 1974; Caballero et al., 1998) when performed under thermal and solvent-free conditions. Cledera et al. improved the earlier protocol by utilizing MWI in the cyclocondensation of anthranilic acid with lactim ethers, preparing pyrazino[2,1-b]quinazoline-3,6-diones (Cledera et al., 2004). When the tetracyclic ardeemin fragment 94 was reacted with anthranilic acid (77) under MW conditions (600 W) for 3 min, a 6:1 mixture of the diastereoisomeric de-prenylardeemins 95 and 96 in 48% overall yield was obtained (Scheme 35). The results showed that the optimized reaction conditions not only improved yield and shortened the reaction time but also improved stereochemical integrity compared to the conventional conditions.
Scheme 35.
MW–assisted modified Niementowski condensation of 94 to afford 95 and 96.
Novel tetraaza-pentaphene-5,8-dione derivatives 98 could be synthesized from anthranilic acid (77) and the 2,3-condensed (3H)-quinazolin-4-ones 97 via a MW–assisted modified Niementowski condensation under pressure at 220°C (Scheme 36) (de Fatima Pereira et al., 2005).
Scheme 36.
MW–assisted modified Niementowski condensation of 97 to afford 98.
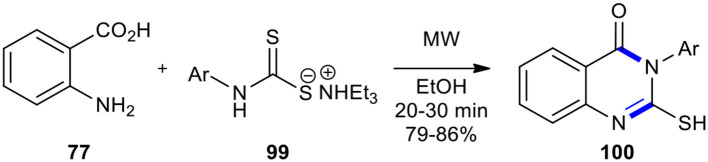
An efficient method for forming a series of 3-aryl 2-thioderivatives of quinazolinone 100 through the MW-promoted cyclocondensation of anthranilic acid (77) with thio carbamate salt of variously substituted anilines 99 in either solvent (ethanol) or solvent-free conditions was achieved (Scheme 37) (Patil et al., 2012). The result showed that during the operation of MWI, better yields were obtained in the presence of a solvent or solventless system although, under solvent-free conditions, the reaction time was shortened from 20–30 to 2–3 min. As expected, the MWI, compared to conventional heating, significantly reduced the reaction time and improved the reaction yield.
Scheme 37.
MW–assisted modified Niementowski condensation of 99 to afford 100.
Niementowski Modification of Friedlander Synthesis
The use of isatoic anhydride in place of anthranilic acid in the Niementowski reaction results in the Niementowski modification of Friedlander synthesis. The Niementowski modification of Friedlander synthesis has been employed in the synthesis quinazolinone scaffold. Yadav and Reddy reported the total synthesis of the cytotoxic alkaloid luotonin A (103a) for the first time in high yields based on the reaction of isatoic anhydride (101) with 3-oxo-1H-pyrrolo[3,4-b]quinoline (102) under MWI and solvent-free conditions (Yadav and Reddy, 2002). Using the present reaction conditions, methyl-substituted luotonin A (103b) was produced in good yield (87%) (Scheme 38). This process was new, efficient, simple, clean, rapid, and higher yielding than the reported strategies for the preparation of luotonin A. This approach used inexpensive reagents in this synthesis.
Scheme 38.
Niementowski modification of Friedlander synthesis of 102.
Azizian et al. previously prepared quinazolinone derivatives from isatin-3-imine and isatoic anhydride using MWI (Azizian et al., 2004a). The MW–assisted condensation of isatin-3-imines 104 with isatoic anhydride (101) using KF on alumina as a reusable catalyst in DMA in 4 min resulted in the formation of the new 6-arylimino-6H-indolo[2,1-b]quinazolin-12-ones 105 (Scheme 39). The reaction proceeded through ring-opening and cyclization.
Scheme 39.
MW–assisted Niementowski modification of the Friedlander synthesis of 104.
Later, the same group used an MW–assisted Niementowski modification of the Friedlander synthesis (Pater, 1971) to prepare 2-(o-aminophenyl)-4(3H)-quinazolinone (107) (Azizian et al., 2004b). When isatoic anhydride (107) was reacted with anthranilamide (106), the nucleophilic attack of the o-amino group of the anthranilamide opened the isatoic anhydride ring which upon reaction with Na2CO3 (eq.) gave 2-(o-aminophenyl)-4(3H)-quinazolinone (107) (Scheme 40).
Scheme 40.
MW–assisted Niementowski modification of the Friedlander synthesis of 106.
Metal-Catalyzed Cyclization of Benzoic Acids
Catalysis is the process of enhancing the rate of a chemical reaction by adding a substance known as a catalyst (McNaught and Wilkinson, 1997; Masel, 2001). Generally, metal catalysts are recognized as the most important factors in increasing catalyzed organic transformations. Particularly, transition metal-catalyzed reactions are fundamental for the convenient and effective synthesis of a wide range of organic compounds (Beller et al., 1998; Bates, 2012). The area of transition metal catalysis indisputably had an immense impact on basic research in academia and chemical industries, thus in turn having an effect on modern society and our daily life. That is well-recognized through several awarded Nobel Prizes during the twentieth century, stretching from Nobel Prize (2020) for the synthesis of ammonia to Richard Suzuki, Heck, and Negishi, who shared the 2010 the prize for their outstanding endeavors and achievements in transition metal-catalyzed cross-coupling reactions (Johansson Seechurn et al., 2012). Although the aforementioned reactions are considered as the most important transition-metal catalyses, a plethora of vital reactions can be effectively catalyzed by various transition metals including reductive elimination, oxidative addition, and transmetallation. Transition metal catalysis has also found extensive applications in the convenient and efficient synthesis of a wide variety of heterocyclic systems (Ma et al., 2017; Ramanathan and Liu, 2017; Tiwari and Bhanage, 2017; Chatterjee et al., 2018; Saikia et al., 2018; Chen et al., 2020; Debabrata et al., 2020; Ghosh et al., 2020; Janardhanan et al., 2020; Jiang et al., 2020; Kanwal et al., 2020; Kojima and Matsunaga, 2020; Li and Zhang, 2020; Nagata and Obora, 2020; Neto and Zeni, 2020a,b; Pal et al., 2020; Ratmanova et al., 2020; Sahiba and Agarwal, 2020; Sonawane et al., 2020; Xuan et al., 2020). However, the literature survey revealed only an example of zinc (Shi et al., 2004), a limited number of iron-catalyzed (Melvin et al., 1992; Valderrama et al., 1999; Kanth et al., 2006; Yin et al., 2012; Gopalaiah et al., 2017, 2019; Raut and Bhanage, 2017; Eidi et al., 2018), and relatively more copper-catalyzed reactions, leading to the synthesis of quinazoline and quinazolinone derivatives (Melvin et al., 1992; Chatterjee et al., 2018; Potuganti et al., 2018; Liang et al., 2019; Rodrigues et al., 2019; Donthiboina et al., 2020). The number of zinc, iron, and copper-catalyzed synthesis of quinazoline and quinazolinone derivatives were decreased when the literature survey was narrowed down to the choice of those reactions, performed specifically under MWI as an unconventional and environmentally benign source of energy, in accordance with the title of this review.
In this regard, the 2-nitro- and 2-azido substituted benzoic acid derivatives have been employed as substrates for the synthesis of quinazolinones. A simple, efficient, and mild method for the synthesis of (3H)-quinazolinone (109) under solvent-free conditions based on the MW-mediated reduction of nitro and azido arenes to N-arylformamides using Zn and ammonium formate (Zn–HCO2NH4) was developed by Kamal et al. (2004). To produce (3H)-quinazolinone 109, the 2-substituted azido- or nitrobenzoic acids 108 were transformed into their corresponding N-arylformamides 109 by employing Zn–HCO2NH4 under MWI at 300 W (Scheme 41). In this reaction, Zn acted as an efficient and inexpensive catalyst, while HCO2NH4 was decomposed to formamide, which in the following was condensed with the anthranilic acid. It is important to note that the reaction in the absence of MWI afforded amines instead of arylformamides.
Scheme 41.

One-pot MW–assisted Zn-catalyzed reduction and cyclocondensation of nitro and azido arenes 108 to produce 109.
As described above, apart from palladium and other transition metals, copper and iron species are catalysts of choice in the synthetic community. In general, they are advantageous, expedient, cost-effective, and relatively less toxic. Thus, iron (Correa et al., 2008; Li et al., 2013; Ghorai et al., 2017) and copper-catalyzed (Monnier and Taillefer, 2013) organic transformations have recently received enormous attention from synthetic organic research groups; they especially are the catalysts of choice for organic transformations requiring, C–N bond formation. In this regard, in recent years, applications of copper and iron-catalyzed transformations have overgrown and still are developing. They have been used in the synthesis of a wide variety of heterocyclic systems, especially N-heterocycles. In this regard, they have been used as effective catalysts in the synthesis of quinazoline and quinazolinone derivatives under different reaction conditions, involving conventional heating or green sources of energy such as ultrasound or MWI.
The reaction of 2-halobenzoic acids and ammonia as a source of nitrogen catalyzed either iron or copper in the presence of a base such as Cs2CO3 and NaOH leads to the construction of quinazolinones (Zhang et al., 2009; Ke et al., 2018; Radhakrishnan et al., 2018). However, in these reactions, stoichiometric amounts of bases were often necessary, and in some cases using ligands was also essential (Ley and Thomas, 2003; Corbet and Mignani, 2006). Since the scope of this review is limited to underlining the synthesis of quinazolinone and quinazolinone derivatives, successfully performed under MWI as a green source of energy (Zhou et al., 2008; Liu et al., 2009; Zhang et al., 2009; Xu and Fu, 2011, 2012; Xu et al., 2011; Sreenivas et al., 2012), in the following we try to describe the synthesis of the above-mentioned heterocycles being successfully conducted in the presence of either Fe or Cu species under MWI.
The 2-halobenzoic acids have been employed as substrates for transition metal-mediated cross-coupling and cyclization with urea derivatives to synthesize the 2-substituted quinazolinones in a single-pot operation. For example, Zhang et al. performed the reaction of substituted 2-halobenzoic acids 110 with amidines 111 in the presence of a Fe species, ligand, and base in a suitable solvent using MW heating to obtain quinazolinone derivatives 112 (Scheme 42) (Zhang et al., 2009). Worth noting is that the above reaction was conducted diversely with or without ligand in water or DMF, which afforded the respective products in moderate to high yields. As expected, 2-iodobenzoic acids were more reactive than 2-bromobenzoic acids and 2-chlorobenzoic acids. The features of this method included being green, rapid, highly efficient, versatile, inexpensive, and environmentally friendly, especially when water as a solvent is used.
Scheme 42.
MW–assisted iron-catalyzed cyclization of 110 with 111 to afford 112.
Copper continues to be one of the most employed and popular transition metal catalysts in synthetic organic chemistry. Furthermore, to its economic and environmental benefit over other transition metal catalysts, it is also abundant. Thus, nowadays, copper catalysis has attracted enormous attention from synthetic organic chemists. Several review articles collected and described the recent progress made in pleasing and exceptional copper catalysis in all areas of synthetic transformations (Monnier and Taillefer, 2013; Guo et al., 2015; Thapa et al., 2015; Tandon, 2019; Ghiazza and Tlili, 2020). Copper-based catalysts are also extensively employed in different chemical industries (Punniyamurthy and Rout, 2008). In this line, Ke et al. used copper as a catalyst instead of iron in the reaction of 2-halobenzoic acids with amidines (Ke et al., 2018). The synthesis of 37 examples of quinazolinones 115 was achieved in good to excellent yields (up to 94%) in 20 min when the substituted 2-halobenzoic acids 113 were coupled with amidines 114 in the presence of CuCl2, ligand, and NaOH in water under MWI at 120 W at room temperature (Scheme 43). The remarkable features of this strategy were using an inexpensive and commercially available and environmentally benign copper catalyst, activation at low temperatures, and there being no need for an inert atmosphere. Worth noting is that the relative reactivity of 2-halobenzoic acids was similar to that of Zhang's method, using iron species as catalyst (Zhang et al., 2009).
Scheme 43.
MW–assisted copper-catalyzed cyclization of 113 with 114 to afford 115.
In the following, Cu-catalyzed one-pot coupling and cyclization leading to the construction of N-heterocycles was studied (Radhakrishnan et al., 2018). The reaction of 2-halobenzoic acids 116 with guanidine hydrochloride (117) using MWI resulted in the formation of 2-aminoquinazoline analogs 118 (Scheme 44). The target products 118 were obtained in one-pot with high yield without the need of any additional ligand. Cu2O and Cs2CO3 proved to the best catalyst and base, respectively, for the MW–assisted Cu(I)-catalyzed reaction to form C-N bond. The advantages of C-N bond formation strategy are the same as described for Ke's method (Ke et al., 2018); there was also no need for any ligand.
Scheme 44.
MW–assisted copper-catalyzed cyclization of 116 with 117 to produce 118.
Cyclocondensation Using Different Methods
MWI has been employed to promote cyclocondensation of various nitrogen-containing precursors with ortho-esters, aldehydes, carboxylic acids, TEAF, benzyl alcohols, amines, thioamides, isothiocyanates, and potassium isopropyldithiocarbonate, as described below.
Ortho-esters
Ortho-esters have been condensed with 2-carbonyl substituted aniline derivatives under MW conditions to create variously substituted quinazolinones and their heterocycle-appended hybrids. Hazarkhani and Karimi synthesized a variety of new 3-(2-benzimidazolyl)-2-alkyl-4-(3H)-quinazolinones 120 in good to high yields in a facile and rapid manner through the reaction of 2-amino-N-(1-H-benzimidazol-2-yl)benzamide (119) with a set of ortho-esters under MWI (600 W) in the presence of p-toluenesulphonic acid (p-TsOH or PTSA) in DMAC (Scheme 45) (Hazarkhani and Karimi, 2003).
Scheme 45.
MW–assisted synthesis of 120 from 119 and ortho-esters using PTSA.
Numerous methods for the construction of the quinazolinone skeleton based on the cyclocondensation of 2-aminobenzamides with orto-esters using MWI have been reported in the literature. A variety of conditions in the absence of a solvent to the quinazolinone skeleton have been used. These make use of solid catalyst systems such as (SiO2/H2SO4) (Montazeri and Rad-Moghadam, 2004), montmorillonite K-10 (Dabiri et al., 2004b), AlCl3/ZnCl2-SiO2 (Dabiri et al., 2005), and HY-zeolite (Bakavoli et al., 2007; Montazeri et al., 2012a), as well as organocatalysts, including pentafluorophenylammonium triflate (PFPAT) (Montazeri et al., 2012b).
Aldehydes
Anthraniamide derivatives have also been condensed with various aldehydes under MW conditions to afford quinazolinones. The highly accelerated Niementowski synthesis of quinazolin-4(3H)-one using MW heating protocol as a key step was successfully applied for the construction of an important and key anticancer drug, Iressa (123) (Li et al., 2007). The 6,7-disubstituted quinazolin-4(3H)-one 122 was prepared in an 87% isolated yield from a 5-benzyloxy-4-methoxy-2-aminobenzamide (121) and formamide under the acidic catalytic amount of acetic acid by MWI at 300 W for 5 min, termed as the Niementowski synthesis. After several steps, the key intermediate 122 was converted into Iressa (123) as a pale yellow solid (Scheme 46). This method could improve the reaction conditions from 5 h of heating at 190°C to 5 min using MWI.
Scheme 46.
MW–assisted synthesis of 122 from 121 and formamide.
The condensation of anthranilamide (106) with different aromatic aldehydes in the presence of PTSA in polyethylene glycol (PEG-200/400) as a green solvent without the use of an oxidant under MW conditions (560 W in a domestic microwave oven) for 5–10 min afforded the corresponding 2-aryl- or 2-hetaryl-4(3H)-quinazolinones 124 in good to excellent yields with high purity (Scheme 47) (Deligeorgiev et al., 2010). This method provided several benefits, including novel environmentally friendly conditions, having a simple work-up procedure, carrying out the reactions in very short times, and isolating products only by filtration.
Scheme 47.
MW–assisted synthesis of 124 using PTSA in PEG as a solvent.
Recently, Kang et al. reported the MW–assisted synthesis of quinazolin-4(3H)-one derivatives 125 within several minutes under solvent-free and mild conditions (Kang et al., 2018). The products could be obtained in good to excellent yields when anthranilamide (106) was condensed with various aldehydes in the presence of a catalytic amount of commercially available antimony (III) trichloride (SbCl3) (Scheme 48).
Scheme 48.

MW–assisted synthesis of 125 using SbCl3.
Carboxylic Acids
Carboxylic acids have also been employed as reactants with 2-substituted anilines to afford quinazolino-4-one derivatives. MW-assisted cyclocondensation of 2-aminobenzamide (106) with carboxylic acids was achieved in 3-5 min under solvent-free conditions to afford a series of 2-substituted quinazolin-4(3H)-ones 126 as represented in Scheme 49 (Rahimizadeh et al., 2004). This transformation was 40-80 times faster than under conventional heating methods and obtained products in higher yields.
Scheme 49.
MW–assisted synthesis of 126 from 106 and carboxylic acids.
The acid-catalyzed cyclization with formic acid was used to synthesize the 3H-quinazolin-4-one (109) by the employment of MWI (Saari et al., 2011). The product 109 was obtained in a 64% yield from the reaction of 2-cyanoaniline (46) with formic acid and sulfuric acid under MWI at 100°C for 5 min (Scheme 50).
Scheme 50.

MW–assisted synthesis of 109 from 46 and formic acid.
TEAF
Triethylamine–formic acid mixture (TEAF) has been used as a substrate for transition metal-catalyzed transfer hydrogenation and condensation with o-nitrobenzamides to produce the quinazolinones in a single-pot operation. For example, a highly efficient one-pot method for the synthesis of quinazolin-4(3H)-one derivatives 128 was developed using the Pd-catalyzed transfer hydrogenation (CTH)/condensation cascade of o-nitrobenzamides 127 and azeotropic TEAF under MWI at 150°C for 8 min (Scheme 51) (Zhu et al., 2015). In this reaction, TEAF played a dual role as a good hydrogen source for the CTH reduction and as a source of mono-carbon for the subsequent cyclocondensation.
Scheme 51.

MW–assisted Pd-CTH/condensation cascade of 127 and TEAF to afford 128.
Benzyl Alcohols
Benzyl alcohols have also been condensed with o-aminobenzamide derivatives under MW conditions to afford quinazolinones. A new water-assisted strategy to quinazolinones 130 was developed through the reaction of o-aminobenzamides (106) with benzyl alcohols 129 using sodium chloride as a salting-out agent and tert-butyl hydroperoxide (TBHP) as an oxidant under metal-, ligand-, base-free and MW conditions (Scheme 52) (Dandia et al., 2018). The production of 130 was examined under MWI and conventional heating conditions. The superior salting-out effect of sodium chloride was observed when the reaction was performed under MW conditions, compared to the conventional method. Among varying MWI power (300, 400, and 500 W) and temperatures, the 400 W power at 80°C gave the best result to carry out the maximum conversion to the expected product.
Scheme 52.
MW–assisted synthesis of 130 from 106 and 129 using NaCl and TBHP.
Thioamides, Isothiocyanates, and Potassium Isopropyldithiocarbonate
Thioamides, isothiocyanates, and potassium isopropyldithiocarbonate have also been employed as reactants to effect cyclocondensation of anthranilamide derivatives to afford quinazolinones. Khajavi et al. previously employed the thioamides for the highly accelerated Niementowski reaction under MWI (Khajavi et al., 1998). They condensed anthranilic acid (106) with thioamides (thiobenzamide or thioacetamide) 131 in DMAC using a house-hold unmodified microwave oven to generate 2-substituted-4-(3H)-quinazolinones 132. The latter were obtained through a simple work-up procedure (Scheme 53). The conventionally thermalized approach for this reaction needed longer reaction times and resulted in the formation of 132 in lower yields.
Scheme 53.

MW–assisted highly accelerated Niementowski reaction of 106 and 131 to afford 132.
Tavallaii et al. previously reacted 2-aminobenzamide (106) with isothiocyanates (or isocyanates) 133 to access 2-(alkylamino) and 2-(arylamino)-4(3H) quinazolinones 134 (Tavalaie et al., 2007). The production of target molecules 134 was completed in high yields (78–98%) by applying MWI under solvent-free conditions, providing the best yields in a faster time in comparison to the conventional heating methods (Scheme 54).
Scheme 54.
MW–assisted Niementowski reaction of 106 and 133 to afford 134.
Kumar and Dubey in 2012 described a protocol which provided the safe, simple, rapid, inexpensive, and environmentally friendly process to 2-mercapto-quinazolinone (136) via the treatment of anthranilamide (106) as a bifunctional molecule with potassium isopropyldithiocarbonate (135) in a minimum quantity of DMF as solvent under MWI (Scheme 55) (Kumar and Dubey, 2012). While the reaction took 5 hours under reflux conditions, it lasted 5 min under MWI. Owing to high stability at higher temperatures and staying at room temperature for months, isopropyldithiocarbonate was used as a stable reagent in this method. These reaction conditions could also be applied to the transformation of various 1,2-bifunctional molecules to afford mercapto derivatives of benzimidazoles, benzothiazole and benzoxazole, and oxadiazoles.
Scheme 55.
MW–assisted Niementowski reaction of 106 and 135 to afford 136.
Aliphatic and Aromatic Amines
Benzoxazinones and their open-chain derivatives have also been employed as substrates for the synthesis of quinazolinones under MW conditions. For example, Khajavi et al. reported an efficient and practical synthesis of a number of variously substituted 4(3H)-quinazolinones 138 (Khajavi et al., 1999b). This transformation included the MWI in the combination of benzoxazinones 137 with amines in DMAC, enabling efficient access to this important class of heterocycles (Scheme 56). The MW–assisted chemistry provided increased product yields and shortened reaction times.
Scheme 56.
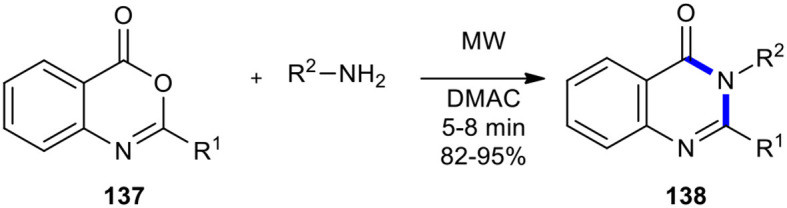
MW–assisted synthesis of 138 from 137 and amines in DMAC.
2,3-Disubstituted 3H-quinazolin-4-ones 140 with a broad chemistry scope were synthesized by Liu's research group by employing MWI on benzoxazinones 139 with amines (Liu et al., 2005b). Benzoxazinones 139 prepared by the condensation of anthranilic acids and either acyl chlorides or carboxylic acids using P(PhO)3 were, in turn, condensed with amines to provide the transient amidine salt species (I). This intermediate rapidly cyclized under the optimized MW conditions at 250°C in 3–10 min, yielding the expected 2,3-disubstituted 3H-quinazolin-4-ones 140 (Scheme 57).
Scheme 57.
MW–assisted synthesis of 140 from 139 and amines.
Laddha and Bhatnagar employed the MW-accelerated solution-phase method to 6,8-disubstituted 2-phenyl-3-(benzothiazol-2-yl)-4-[3H]-quinazolinones 143, bearing various substituents on the benzothiazole ring (Laddha and Bhatnagar, 2008). The synthesis involved the cyclocondensation of 2-phenyl-4H-benzo[d][1,3]oxazine-4-ones 141 with 2-aminobenzothiazoles 142 in dry pyridine under MWI at 210 W and reflux temperature (Scheme 58). This procedure proceeded very cleanly and formed products in a fast and easy work-up procedure without any traces of side products, presenting the advantage of a high rate and better yields than the conventional procedure.
Scheme 58.
MW-accelerated solution-phase synthesis of 143 from 141 and 142.
Various 2-styryl benzoxazinone derivatives 144 were utilized with 2-aminothiazoles 145 using pyridine-DMF as a co-solvent under MWI in the synthesis of newer 3-thiazole substituted 2-styryl-4(3H)-quinazolinones 146 (Jagani et al., 2012). The products 146 were obtained in good yields within an appropriate time of MWI at 350 W (Scheme 59). It is interesting to know that the reaction either in pyridine as a solvent proceeded sluggishly or in DMF reduced yields.
Scheme 59.
MW–assisted synthesis of 146 from 144 and 145.
Priya et al. prepared compound 147 by the reaction of anthranilic acid (77) with Vilsmeier reagent (DMF/POCl3) (Heravi et al., 2018). They then reacted 147 with differently substituted anilines under MW for 2–4 min to synthesize 4-(3H)-quinazolinone derivatives 148 in good yields (Scheme 60) (Priya et al., 2011). The use of MWI in this reaction shows its value in providing increased yields in short reaction times.
Scheme 60.
MW–assisted synthesis of 148 from 147 and anilines.
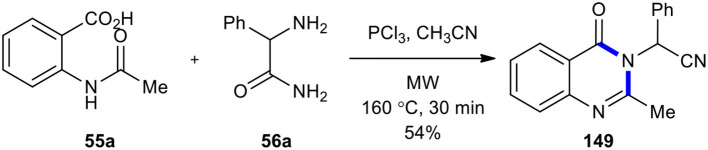
In 1946, Grimmel's research group reported the synthesis of quinazolinones through the treatment of N-acetylanthranilic acids with aromatic amines using the condensing agents including POCl3, phosphorus trichloride (PCl3), or thionyl chloride(SOCl2) in toluene or xylene (Grimmel et al., 1946). This method has been frequently used to synthesize a wide range of quinazolinone derivatives (Wolfe et al., 1990; van Zyl, 2001; Bhatti and Seshadri, 2004; Storelli et al., 2005; Giri et al., 2009). In 2009, MWI was used to synthesize 2-(2-methyl-4-oxoquinazolin-3(4H)-yl)-2-phenylacetonitrile (149) (Li et al., 2009). When N-acetylanthranilic acid (55a) was reacted with the amino group of 2-amino-2-phenylacetamide (56a) using PCl3 as a condensing reagent in acetonitrile under MWI at 160°C for 30 min, quinazolinone was formed with concomitant carboxamide dehydration to give 2-(2-methyl-4-oxoquinazolin-3(4H)-yl)-2-phenylacetonitrile (149) in a 54% yield (Scheme 61).
Scheme 61.
MW–assisted synthesis of 149 from 55a and 56a using PCl3.
Jagani et al. (2011) optimized Grimmel's method (Grimmel et al., 1946) using MWI to construct N-(4-(N-(4-oxo-2-methyl/aryl-substituted quinazolin-3(4H)-yl)sulfamoyl)phenyl)acetamides 152, allowing the improvement of rate and yields. The quinazolinone products 152 were synthesized by the reaction between N-acetylanthranilic acids 150 and 4-acetamidobenzenesulfonyl hydrazide (151) in the presence of an amount of PCl3 in THF as a solvent under MW conditions (350 W) (Scheme 62).
Scheme 62.
MW–assisted synthesis of 152 from 150 and 151 using PCl3.
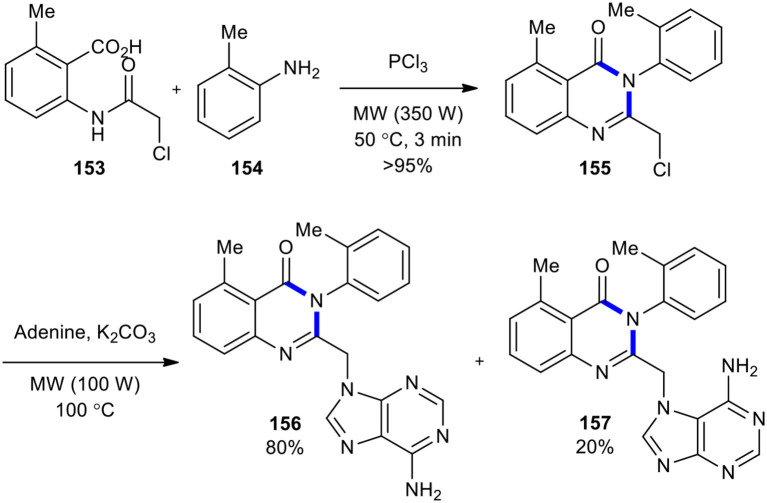
A protocol that involved an environmentally friendly MW–assisted cyclization reaction to construct 2-(chloromethyl)-5-methyl-3-(o-tolyl)quinazolin-4(3H)-one (155) toward the medicinally important purine quinazolinone scaffold was devised and presented (Sawant et al., 2012). The reaction of compound 153 with 2-methyl aniline (154) and PCl3 as a cyclizing agent under MW conditions (350 W) at 50°C for 3 min yielded 2-(chloromethyl)-5-methyl-3-o-tolylquinazolin-4(3H)-one (155) in excellent yield (>95%). Compound 155 without purification was subjected to a coupling reaction with adenine to give the expected molecules 156 and 157 (Scheme 63).
Scheme 63.
MW–assisted synthesis of 155 and further transformation into 156 and 157.
Three-Component Reaction
MCR, as one of the most straightforward strategies, has been extensively employed under MWI for the synthesis of quinazolinone derivatives. For example, the preparation of substituted quinazolin-4(3H)-one derivatives 158 by the one-pot three-component cyclocondensation of anthranilic acid (77) with an amine and formic acid (or ortho-esters) under MWI was reported by Rad-Moghadam and Khajavi (Scheme 64) (Rad-Moghadam and Khajavi, 1998). Formic acid and amines were used in place of amides or amidines utilized in the Niementowski reaction, providing 3-substituted-4(3H)quinazolinones 158 instead of the resulting 2-substituted derivative. The reaction was completed in a few minutes in the absence of solvent or any dehydrating agents. As a result, the reaction with ortho-esters required a catalytic amount of PTSA. The reaction occurred within a much reduced time under MWI with respect to the conventional heating procedure (Leiby, 1985).
Scheme 64.
MW–assisted MCR of 77, amine, and ortho-esters using PTSA to produce 158.
A modification of the above method, which is environmentally friendly, involved the synthesis of fluorine-containing 4(3H)-quinazolinones 160 using fluorinated anilines 159 in the absence of a solvent and catalysts (Scheme 65) (Wenli et al., 2011).
Scheme 65.
MW–assisted MCR of 77, 159, and triethoxymethane to create 160.
The demand for increasingly clean and efficient chemical synthesis of 4-(3H)-quinazolinone from anthranilic acid, amines, and ortho-esters or formic acid under MW conditions paved the way for the use of Yb(OTf)3 (Wang et al., 2003), NaHSO4-SiO2 (Das and Banerjee, 2004), silica gel/FeCl3 (Chari et al., 2006), La(NO3)3, Bi(TFA)3-[nbp]FeCl4 (Khosropour et al., 2006), Nafion-H (perfluorinated resin supported sulfonic acid) (Lingaiah et al., 2006), H-Y-zeolites (Bakavoli et al., 2007), Keggin-type heteropolyacids (HPAs) (Ighilahriz et al., 2008), and silica sulfuric acid (SSA) (Koroji et al., 2018) as solid supported catalysts and PTSA (Narasimhulu et al., 2006) and SnCl4 (Oskooie et al., 2007) as catalysts. It is noteworthy that the use of ammonium acetate as a source of amine in the condensation with anthranilic acid (77) and ortho-esters under MWI in the absence of a solvent yielded the corresponding 2-substituted quinazolin-4(3H)-ones 161 in a few minutes (Scheme 66) (Rad-Moghadam and Mohseni, 2003).
Scheme 66.
MW–assisted MCR of 77, ammonium acetate, and ortho-esters to synthesize 161.
One of the methods for the synthesis of substituted (3H)-quinazolin-4-one derivatives involved a one-pot three-component condensation of isatoic anhydride (101) with amines and ortho-esters. In 2014, Dabiri et al. developed this method by employing MWI in the presence of PTSA as a catalyst, resulting in the formation of 2,3-disubstituted 4(3H)-quinazolinones 162 in good to excellent yields (Scheme 67) (Dabiri et al., 2004a). They also compared the resulting products synthesized under the two conditions of MW and classical heating, which indicated the superiority of MW conditions. The method offered several advantages including a cleaner reaction, short reaction times, a high yield of products, and an easy experimental work-up procedure.
Scheme 67.
MW–assisted MCR of 101, amines, and ortho-esters using PTSA to afford 162.
Later, the application of the AlCl3/ZnCl2-SiO2 Lewis acid system instead of PTSA under both MWI and conventional heating methods was studied by the same group. Disubstituted (3H)-quinazolin-4-ones 163 were synthesized in good yields under conventional conditions, although slightly higher yields were obtained under MWI (Scheme 68) (Dabiri et al., 2005). It should be noted that the work-up of products under both conditions was very easy since they were washed with hot ethanol and then filtrated and recrystallized.
Scheme 68.
MW–assisted MCR of 101, amines, and ortho-esters using AlCl3/ZnCl2-SiO2.
KAl(SO4)2∙12H2O (Alum) is an inexpensive and nontoxic reagent which is very soluble in water and is also recyclable. Its ability as an effective catalyst was demonstrated in the MW–assisted synthesis of 2-alkyl and 2-aryl-4(3H)-quinazolinones. Twenty 2,3-disubstituted-4(3H)-quinazolinones 165 were synthesized through a three-component reaction between isatoic anhydrides 164, ortho-esters, and amines (Scheme 69) (Mohammadi and Sadat Hossini, 2011). The high yield of products and simple experimental work-up procedures are the salient features of this method.
Scheme 69.
MW–assisted MCR of 164, amines, and ortho-esters using Alum to afford 165.
The synthesis of fluorinated 2,3-disubstituted quinazolin-4(3H)-ones 167 was reported by Dandia et al. by developing a “green chemistry procedure” using a neat one-pot three-component cyclocondensation under MW conditions (Dandia et al., 2004). Treatment of anthranilic acid (77) with phenyl acetyl chloride (166) and substituted anilines in the microwave oven at 640 W for 4–5 min without using any solvent, dehydrating agent, or support produced the target molecules 167 (Scheme 70). The MW chemistry in this reaction allowed significant rate enhancement and good to excellent yields, compared to the conventional procedure.
Scheme 70.
MW–assisted MCR of 77, 166, and anilines to afford 167.
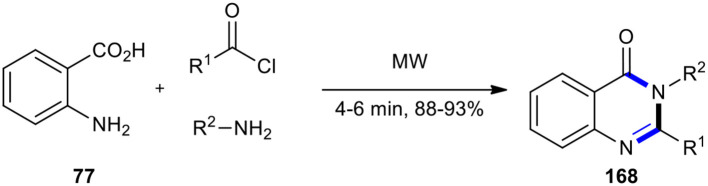
An approach that utilized anthranilic acid and formamide to the synthesis of quinazolin-4(3H)-one core was improved by using the neat MW–assisted three-component reaction of anthranilic acid (77), acyl chlorides, and amines (Dandia et al., 2005). The reaction was completed in a shorter time with a facile work-up procedure, making it a general protocol to produce the expected molecules 168 with a range of acid chlorides and amines. Using MWI at 640 watts, the reaction between anthranilic acid, acid chlorides, and amines furnished 2-benzyl-3-aryl-quinazolin-4-[3H]-ones 168 in high yields and reduced the time (Scheme 71).
Scheme 71.
MW–assisted MCR of 77, acyl chlorides, and amines to afford 168.
After a decade, the same procedure in the presence of two long-chain double SO3H-functionalized acidic ionic liquids, SBAILs-1 and 2, as reusable catalysts, was reported (Scheme 72) (Li et al., 2015). Although the authors have expanded the production of 2,3-disubstituted 4(3H)-quinazolinone derivatives 169, one product formed (R1 = Ph, R2 = 4-Cl-C6H5), which was produced from the previous method (Dandia et al., 2005), with a lesser yield (Li et al., 2015).
Scheme 72.
MW–assisted MCR of 77, acyl chlorides, and amines using SBAILs-1 and 2.
Two new Brønsted acidic ionic liquids, including 1-(4-sulfonic)-benzyl-3-methylimidazolium hydrogen sulfate (170a) and N-(4-sulfonic)-benzyl-pyridium hydrogen sulfate (170b), were utilized as catalyst and reaction medium, respectively, to lead to the synthesis of 2-substituted-4(3H)-quinazolinones 171 in satisfactory yields (Li et al., 2010). The reaction proceeded through a one-pot three-component reaction under MW conditions for 6 min, starting from anthranilic acid (77), acyl chlorides, and ammonium acetate (Scheme 73).
Scheme 73.
MW–assisted MCR of 77, acyl chlorides, and NH4OAc using BAIL to afford 171.
Liu et al. developed a practical and efficient protocol that employed an MW–assisted one-pot three-component reaction for the synthesis of various 2,3-disubstituted 3H-quinazolin-4-ones 173, starting from anthranilic acids 172, carboxylic acids, and amines (Scheme 74) (Liu et al., 2005b). The use of MW–assisted chemistry increased the product yields and shortened the reaction times.
Scheme 74.
MW–assisted MCR of 172, carboxylic acids, and amines to afford 173.
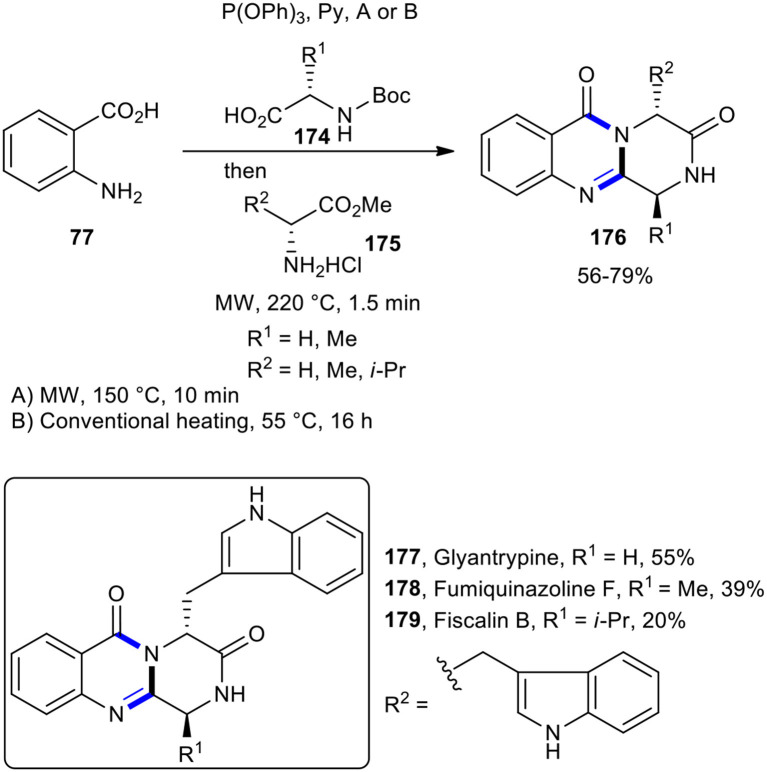
This MW–assisted one-pot methodology could be adapted for the synthesis of 4-quinazoline-3,6-diones 176 and also for the total synthesis of a number of natural products containing core, accommodating an expanded array of carboxylic acids and amines (Liu et al., 2005c). Using selected Boc-amino acids and amino acid esters under MWI, total synthesis of the alkaloids glyantrypine (177), fumiquinazoline F (178), and fiscalin B (179) was accomplished in overall yields of 55, 39, and 20%, respectively (Scheme 75).
Scheme 75.
The synthesis of 176 and total synthesis of 177, 178, and 179 under MWI.
Liu's research group synthesized circumdatin E analogs 183 and 184 by using MW–assisted one-pot three-component sequential reactions (Liu et al., 2005a). The subjection of anthranilic acids 180a and 180b to MWI three-component reaction with N-Boc-proline (181) using P(OPh)3 reagent in pyridine at 150°C in 10 min, followed by the addition of methyl anthranilate (182) and further conditions (MW, 230°C, 15 min), resulted in the formation of the expected compounds 183 and 184 as analogs of circumdatin E in 34 and 29% yields, respectively (Scheme 76).
Scheme 76.
MW–assisted synthesis of circumdatin E analogs 183 and 184.
Wan et al. reported Pd-catalyzed aminocarbonylations of aryl halides by the employment of MW-accelerated decomposition of formamide to ammonia (NH3) and carbon monoxide (CO) (Wan et al., 2003). After a couple of years, Nouira et al. achieved this reaction by using NH3 as the sole synthon for introducing a nitrogen atom in a heterocyclic ring via MW–assisted three-component reactions with anthranilic acid (77) and carboxylic anhydride, leading to the fast and safe synthesis of some quinazolin-4-one derivatives 185 (Scheme 77) (Nouira et al., 2008). Their work confirmed that the use of MWI may lead to different behaviors of reactants, depending on parameters such as power input, reached temperature, and pressure in the vials. Using modern MW technology, full control and fine-tuning of these parameters can be achieved.
Scheme 77.
MW–assisted MCR of 77, carboxylic anhydride, and formamide to afford 185.
Lin et al. discovered a rapid, direct, and practical method for the construction of a quinazolinone scaffold by using iron pentacarbonyl (Fe(CO)5) as a reducing agent and CO source under MW and base-free conditions (Lin et al., 2017). The process involved the Pd/Fe(CO)5-catalyzed reduction of o-nitrobenzamides to o-aminobenzamides, followed by reductive carbonylation of aryl iodides into amide intermediates and subsequent intramolecular ring closure. To access 2-substituted quinazolinones 187, various o-nitrobenzamides 186 and aryl iodides using various palladium catalysts, ligands, and solvents were employed. The best reaction conditions were found to use Pd(Cl)2 as a catalyst, xantphos as a ligand, and ethanol as a solvent at 110°C in 30 min under MWI in good yields (Scheme 78).
Scheme 78.
MW–assisted iron pentacarbonyl mediated carbonylation of aryl iodides.
Conclusions
In this review, we highlighted the application of MWI in the synthesis of quinazolines and quinazolinones. Routes and strategies used here have opened a gateway to the wider applications of MW chemistry in both academia and industry. Several quinazoline and quinazolinone derivatives were provided with a broad substrate scope in a variety of MW systems, e.g. the domestic microwave oven. Many reactions were examined under both MW and conventional heating conditions in which the superiority of MW conditions was demonstrated. MWI acted as a powerful technique that could reduce the reaction times (more often a few minutes), enhance the yield of the products, increase the purity of the resulting product, and decrease the usage of organic solvents and by-products more than the conventional heating procedure. The salient features of MW reactions in these syntheses include environmental friendliness, more economic value, good atom economy, and simple experimental work-up procedure. Due to previous features and the lack of a need for solvents, reagents, and ligands in many cases, MW technology was able to promote the principles of green chemistry. It should be noted that the reactions may occur under the conditions of the MW heating or at room temperature, indicating the significant effect of temperature on the reaction. In many reactions, it is shown that focused MWI with proper control of power and temperature is more important and efficient than multimode MWI or conventional heating sources. Most of the strategies developed for the synthesis of quinazoline and quinazolinone derivatives under MWI were successfully achieved under metal-free conditions. The merits of metal-free reactions involve the absence of a costly metal catalyst and supporting ligands, comparatively milder reaction conditions, insensitivity to moisture, and easy workup procedures. Few examples of synthesis of quinazoline and quinazolinone derivatives using transition metals such as zinc, iron, and copper were described.
Author Contributions
MH has been invited by the editor, developed the idea, and drafted the presented manuscript and worked on it. LM presented the idea and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to the Alzahra University Research Council for partial financial assistance. MH also appreciates the financial support from the Iran National Science Foundation (9400159), granted via an individually granted research chair.
References
- Abramovitch R. A. (1991). Applications of microwave energy in organic chemistry. A review. Org. Prep. Proced. Int. 23, 683–711. 10.1080/00304949109458244 [DOI] [Google Scholar]
- Alexandre F.-R., Berecibar A., Besson T. (2002). Microwave-assisted Niementowski reaction. Back to the roots. Tetrahedron Lett. 43, 3911–3913. 10.1016/S0040-4039(02)00619-6 [DOI] [Google Scholar]
- Alexandre F.-R., Berecibar A., Wrigglesworth R., Besson T. (2003a). Efficient synthesis of thiazoloquinazolinone derivatives. Tetrahedron Lett. 44, 4455–4458. 10.1016/S0040-4039(03)01026-8 [DOI] [Google Scholar]
- Alexandre F.-R., Berecibar A., Wrigglesworth R., Besson T. (2003b). Novel series of 8H-quinazolino[4,3-b]quinazolin-8-ones via two Niementowski condensations. Tetrahedron 59, 1413–1419. 10.1016/S0040-4020(03)00053-X [DOI] [Google Scholar]
- Armstrong R. W., Combs A. P., Tempest P. A., Brown S. D., Keating T. A. (1996). Multiple-component condensation strategies for combinatorial library synthesis. Acc. Chem. Res. 29, 123–131. 10.1021/ar9502083 [DOI] [Google Scholar]
- Azizian J., Mohammadi A. A., Ardakani F. (2004a). A simple and efficient synthesis of new 6-arylimino-6H-indolo[2,1-b]quinazolin-12-ones under microwave irradiation. Heterocycles 63, 791–795. 10.3987/COM-03-9937 [DOI] [Google Scholar]
- Azizian J., Mohammadi A. A., Karimi A. R., Mohammadizadeh M. R. (2004b). Synthesis of some new 6-substituted quinazolino[4,3-b]quinazolin-8-ones under solvent-free conditions. J. Chem. Res. 2004, 435–437. 10.3184/0308234041423736 [DOI] [Google Scholar]
- Bacher A. (2016). Microwave Chemistry in Organic Synthesis. Los Angeles, CA: UCLA. [Google Scholar]
- Bakavoli M., Sabzevari O., Rahimizadeh M. (2007). HY-zeolites induced heterocyclization: highly efficient synthesis of substituted-quinazolin-4(3H)ones under microwave irradiation. Chin. Chem. Lett. 18, 533–535. 10.1016/j.cclet.2007.03.029 [DOI] [Google Scholar]
- Balalaie S., Sharifi A., Ahangarian B., Kowsari E. (2001). Microwave enhanced synthesis of quinazolines in solvent-free condition. Heterocycl. Commun. 7, 337–340. 10.1515/HC.2001.7.4.337 [DOI] [Google Scholar]
- Bates R. (2012). Organic Synthesis Using Transition Metals. New York, NY: John Wiley & Sons. 10.1002/9781119942863 [DOI] [Google Scholar]
- Beller M., Riermeier T. H., Stark G. (1998). Transition Metals for Organic Synthesis. Weinheim: VCH; 10.1002/9783527619399 [DOI] [Google Scholar]
- Besson T., Chosson E. (2007). Microwave-assisted synthesis of bioactive quinazolines and quinazolinones. Comb. Chem. High Throughput Screen. 10, 903–917. 10.2174/138620707783220356 [DOI] [PubMed] [Google Scholar]
- Besson T., Guillard J., Rees C. W. (2000). Multistep synthesis of thiazoloquinazolines under microwave irradiation in solution. Tetrahedron Lett. 41, 1027–1030. 10.1016/S0040-4039(99)02221-2 [DOI] [Google Scholar]
- Besson T., Rees C. W. (1996). New route to 4-alkoxyquinazoline-2-carbonitriles. J. Chem. Soc. Perkin Trans. 1, 2857–2860. 10.1039/p19960002857 [DOI] [Google Scholar]
- Bhatti H. S., Seshadri S. (2004). Synthesis and fastness properties of styryl and azo disperse dyes derived from 6-nitro substituted 3-aryl-2-methyl-4(3H)-quinazolinone. Color. Technol. 120, 151–155. 10.1111/j.1478-4408.2004.tb00221.x [DOI] [Google Scholar]
- Bogdal D., Penczek P., Pielichowski J., Prociak A. (2003). Microwave assisted synthesis, crosslinking, and processing of polymeric materials. Adv. Polym. Sci. 163, 194–263. 10.1007/b11051 [DOI] [Google Scholar]
- Bogert M. T., Hand W. F. (1902). The synthesis of alkylketodihydroquinazolines from anthranilic nitrile. J Am. Chem. Soc. 24, 1031–1050. 10.1021/ja02025a001 [DOI] [Google Scholar]
- Caballero E., Avendaño C., Menéndez J. C. (1998). On the fate of the tryptophan stereocenter during the synthesis of hexacyclic analogues of N-acetylardeemin. Tetrahedron Asymmetry 9, 3025–3038. 10.1016/S0957-4166(98)00307-3 [DOI] [Google Scholar]
- Caddick S. (1995). Microwave assisted organic reactions. Tetrahedron 51, 10403–10432. 10.1016/0040-4020(95)00662-R [DOI] [Google Scholar]
- Chandregowda V., Kush A. K., Reddy G. C. (2009). Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur. J. Med. Chem. 44, 3046–3055. 10.1016/j.ejmech.2008.07.023 [DOI] [PubMed] [Google Scholar]
- Chari M. A., Shobha D., Mukkanti K. (2006). Silica gel/FeCl3: an efficient and recyclable heterogenous catalyst for one step synthesis of 4(3H)-quinazolinones under solvent free conditions. Catal. Commun. 7, 787–790. 10.1016/j.catcom.2006.02.026 [DOI] [Google Scholar]
- Chatel G., Varma R. S. (2019). Ultrasound and microwave irradiation: contributions of alternative physicochemical activation methods to green chemistry. Green Chem. 21, 6043–6050. 10.1039/C9GC02534K [DOI] [Google Scholar]
- Chatterjee T., Kim D. I., Cho E. J. (2018). Base-promoted synthesis of 2-aryl quinazolines from 2-aminobenzylamines in water. J. Org. Chem. 83, 7423–7430. 10.1021/acs.joc.8b00327 [DOI] [PubMed] [Google Scholar]
- Chen Z., Wang L.-C., Wu X.-F. (2020). Carbonylative synthesis of heterocycles involving diverse CO surrogates. Chem. Commun. 56, 6016–30. 10.1039/D0CC01504K [DOI] [PubMed] [Google Scholar]
- Chilin A., Marzaro G., Zanatta S., Guiotto A. (2007). A microwave improvement in the synthesis of the quinazoline scaffold. Tetrahedron Lett. 48, 3229–3231. 10.1016/j.tetlet.2007.03.027 [DOI] [Google Scholar]
- Cledera P., Sanchez J. D., Caballero E., Avendano C., Ramos M. T., Menendez J. C. (2004). Solvent-free cyclocondensation of lactim ethers with anthranilic acid under microwave irradiation. Synlett 2004, 0803–0806. 10.1055/s-2004-817784 [DOI] [Google Scholar]
- Connolly D. J., Cusack D., O'Sullivan T. P., Guiry P. J. (2005). Synthesis of quinazolinones and quinazolines. Tetrahedron 61, 10153–10202. 10.1016/j.tet.2005.07.010 [DOI] [Google Scholar]
- Corbet J.-P., Mignani G. (2006). Selected patented cross-coupling reaction technologies. Chem. Rev. 106, 2651–2710. 10.1021/cr0505268 [DOI] [PubMed] [Google Scholar]
- Correa A., Mancheño O. G., Bolm C. (2008). Iron-catalysed carbon–heteroatom and heteroatom–heteroatom bond forming processes. Chem. Soc. Rev. 37, 1108–1117. 10.1039/b801794h [DOI] [PubMed] [Google Scholar]
- Dabiri M., Salehi P., Khajavi M. S. (2004a). Microwave-assisted one-pot three component synthesis of some new 4(3H)-quinazolinone derivatives. Heterocycles 63, 1417–1421. 10.3987/COM-04-10042 [DOI] [Google Scholar]
- Dabiri M., Salehi P., Mohammadi A. A., Baghbanzadeh M. (2005). One-pot synthesis of mono-and disubstituted (3H)-quinazolin-4-ones in dry media under microwave irradiation. Synth. Commun. 35, 279–287. 10.1081/SCC-200048462 [DOI] [Google Scholar]
- Dabiri M., Salehi P., Mohammadi A. A., Baghbanzadeh M., Kozehgiry G. (2004b). Montmorillonite K-10 catalysed solvent-free synthesis of 2, 3-disubstituted-4(3H)quinazolinones under microwave irradiation. J. Chem. Res. 2004, 570–572. 10.3184/0308234042563866 [DOI] [Google Scholar]
- Dandia A., Sharma R., Indora A., Parewa V. (2018). Kosmotropes perturbation and ambiphilic dual activation: responsible features for the construction of C-N bond towards the synthesis of quinazolin 4(3H)-ones in water. ChemistrySelect 3, 8285–8290. 10.1002/slct.201801224 [DOI] [Google Scholar]
- Dandia A., Singh R., Sarawgi P. (2004). Green chemical multi-component one-pot synthesis of fluorinated 2,3-disubstituted quinazolin-4(3H)-ones under solvent-free conditions and their anti-fungal activity. J. Fluor. Chem. 125, 1835–1840. 10.1016/j.jfluchem.2004.06.009 [DOI] [Google Scholar]
- Dandia A., Singh R., Sarawgi P. (2005). Reinvestigation of the synthesis of 2-benzyl-3-aryl-quinazolin-4-[3H]-ones. An improved multi-component procedure using microwaves. Org. Prep. Proced. Int. 37, 397–402. 10.1080/00304940509354972 [DOI] [Google Scholar]
- Das B., Banerjee J. (2004). Silica-supported sodium hydrogen sulfate and amberlyst-15: two efficient heterogeneous catalysts for single-step synthesis of 4(3H)-quinazolinones from anthranilic acid, ortho esters, and amines under solvent free conditions. Chem. Lett. 33, 960–961. 10.1246/cl.2004.960 [DOI] [Google Scholar]
- de Fatima Pereira M., Picot L., Guillon J., Léger J.-M., Jarry C., Thiéry V., et al. (2005). Efficient synthesis of novel pentacyclic 6,7-dihydro-5a,7a,13,14-tetraaza-pentaphene-5,8-diones. Tetrahedron Lett. 46, 3445–3447. 10.1016/j.tetlet.2005.03.133 [DOI] [Google Scholar]
- de la Hoz A., Diaz-Ortiz A., Moreno A. (2005). Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 34, 164–178. 10.1039/B411438H [DOI] [PubMed] [Google Scholar]
- Debabrata M., Alessio B., Giuseppe F., Giuseppe Z. (2020). Transition metal promoted cascade heterocycles synthesis via C-H functionalization. Chemistry. 10.1002/chem.202001832 [DOI] [PubMed] [Google Scholar]
- Debray J., Lévêque J.-M., Philouze C., Draye M., Demeunynck M. (2010). Swift and efficient synthesis of 4-phenylquinazolines: involvement of N-heterocyclic carbene in the key cyclization step. J. Org. Chem. 75, 2092–2095. 10.1021/jo902726k [DOI] [PubMed] [Google Scholar]
- Deligeorgiev T. G., Kaloyanova S., Vasilev A., Vaquero J.-J., Alvarez-Builla J., Cuadro A. M. (2010). Novel environmentally benign procedure for the synthesis of 2-aryl-and 2-hetaryl-4 (3H)-quinazolinones. Color. Technol. 126, 24–30. 10.1111/j.1478-4408.2010.00223.x [DOI] [Google Scholar]
- Devi P., Srivastava A., Srivastava K., Bishnoi A. (2017). Green approaches towards the synthesis of substituted quinazolines. Curr. Green Chem. 4, 25–37. 10.2174/2213346104666170704153434 [DOI] [Google Scholar]
- Domon L., Le Coeur C., Grelard A., Thiéry V., Besson T. (2001). Efficient modified von Niementowski synthesis of novel derivatives of 5a,14b,15-triazabenzo[a]indeno[1,2-c]anthracen-5-one from indolo[1,2-c]quinazoline. Tetrahedron Lett. 42, 6671–6674. 10.1016/S0040-4039(01)01364-8 [DOI] [Google Scholar]
- Donthiboina K., Mani G. S., Shankaraiah N., Kamal A. (2020). Iodine-mediated oxidative annulation by C–C cleavage: a Domino synthetic approach to quinazolinones and benzo[4,5] imidazo[1,2-c]quinazolines. ChemistrySelect 5, 3923–3928. 10.1002/slct.202000682 [DOI] [Google Scholar]
- Eidi E., Kassaee M. Z., Nasresfahani Z., Cummings P. T. (2018). Synthesis of quinazolines over recyclable Fe3O4@ SiO2-PrNH2-Fe3+ nanoparticles: a green, efficient, and solvent-free protocol. Appl. Organomet. Chem. 32, e4573 10.1002/aoc.4573 [DOI] [Google Scholar]
- El-Azab A. S., Al-Omar M. A., Alaa A.-M., Abdel-Aziz N. I., Magda A.-A., Aleisa A. M., et al. (2010). Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: molecular docking study. Eur. J. Med. Chem. 45, 4188–4198. 10.1016/j.ejmech.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Fazeli A., Oskooie H. A., Beheshtiha Y. S., Heravi M. M., Moghaddam F. M., Foroushani B. K. (2013). Synthesis of 1,4-disubstituted 1,2,3-triazoles from aromatic a-bromoketones, sodium azide and terminal acetylenes via Cu/Cu(OTf)2-catalyzed click reaction under microwave irradiation. Z. Naturforsch B. 68, 391–396. 10.5560/znb.2013-3043 [DOI] [Google Scholar]
- Ferrini S., Ponticelli F., Taddei M. (2007). Convenient synthetic approach to 2,4-disubstituted quinazolines. Org. Lett. 9, 69–72. 10.1021/ol062540s [DOI] [PubMed] [Google Scholar]
- Foucourt A., Dubouilh-Benard C., Chosson E., Corbière C., Buquet C., Iannelli M., et al. (2010). Microwave-accelerated Dimroth rearrangement for the synthesis of 4-anilino-6-nitroquinazolines. Application to an efficient synthesis of a microtubule destabilizing agent. Tetrahedron 66, 4495–4502. 10.1016/j.tet.2010.04.066 [DOI] [Google Scholar]
- Foucourt A., Hédou D., Dubouilh-Benard C., Désir,é L., Casagrande A.-S., Leblond B., et al. (2014). Design and synthesis of thiazolo[5,4-f]quinazolines as DYRK1A inhibitors, part I. Molecules 19, 15546–15571. 10.3390/molecules191015546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedye R., Smith F., Westaway K., Ali H., Baldisera L. (1986). The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 27, 279–282. 10.1016/S0040-4039(00)83996-9 [DOI] [Google Scholar]
- Ghiazza C., Tlili A. (2020). Copper-promoted/copper-catalyzed trifluoromethylselenolation reactions. Beilstein J. Org. Chem. 16, 305–316. 10.3762/bjoc.16.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorai S. K., Gopalsamuthiram V. G., Jawalekar A. M., Patre R. E., Pal S. (2017). Iron catalyzed C-N bond formation. Tetrahedron 73, 1769–1794. 10.1016/j.tet.2017.02.033 [DOI] [Google Scholar]
- Ghosh P., Ganguly B., Das S. (2020). C–H functionalization of quinazolinones by transition metal catalysis. Org. Biomol. Chem. 18, 4497–518. 10.1039/D0OB00742K [DOI] [PubMed] [Google Scholar]
- Giri R. S., Thaker H. M., Giordano T., Williams J., Rogers D., Sudersanam V., et al. (2009). Design, synthesis and characterization of novel 2-(2, 4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-κB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur. J. Med. Chem. 44, 2184–2189. 10.1016/j.ejmech.2008.10.031 [DOI] [PubMed] [Google Scholar]
- Gopalaiah K., Saini A., Devi A. (2017). Iron-catalyzed cascade reaction of 2-aminobenzyl alcohols with benzylamines: synthesis of quinazolines by trapping of ammonia. Org. Biomol. Chem. 15, 5781–5789. 10.1039/C7OB01159H [DOI] [PubMed] [Google Scholar]
- Gopalaiah K., Tiwari A., Choudhary R., Mahiya K. (2019). Straightforward access to 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides and quinazolines via iron-catalyzed aerobic oxidative condensation of amines. ChemistrySelect 4, 5200–5205. 10.1002/slct.201900850 [DOI] [Google Scholar]
- Griess P. (1869). Ueber die einwirkung des cyans auf anthranilsäure. Ber. Dtsch. Chem. Ges. 2, 415–418. 10.1002/cber.186900201180 [DOI] [Google Scholar]
- Grimmel H. W., Guenther A., Morgan J. F. (1946). A new synthesis of 4-quinazolones. J. Am. Chem. Soc. 68, 542–543. 10.1021/ja01208a002 [DOI] [PubMed] [Google Scholar]
- Guo X.-X., Gu D.-W., Wu Z., Zhang W. (2015). Copper-catalyzed C–H functionalization reactions: efficient synthesis of heterocycles. Chem. Rev. 115, 1622–1651. 10.1021/cr500410y [DOI] [PubMed] [Google Scholar]
- Gupta V., Kashaw S. K., Jatav V., Mishra P. (2008). Synthesis and antimicrobial activity of some new 3–[5-(4-substituted) phenyl-1,3,4-oxadiazole-2yl]-2-styrylquinazoline-4(3H)-ones. Med. Chem. Res. 17, 205–211. 10.1007/s00044-007-9054-3 [DOI] [Google Scholar]
- Hazarkhani H., Karimi B. (2003). A facile synthesis of new 3-(2-benzimidazolyl)-2-alkyl-4-(3H)-quinazolinones under microwave irradiation. Tetrahedron 59, 4757–4760. 10.1016/S0040-4020(03)00696-3 [DOI] [Google Scholar]
- Heravi M., Ghavidel M., Heidari B. (2016). Microwave-assisted Biginelli reaction: an old reaction, a new perspective. Curr. Org. Synth. 13, 569–600. 10.2174/1570179413666151218202307 [DOI] [Google Scholar]
- Heravi M., Moghimi S. (2013). Solid-supported reagents in organic synthesis using microwave irradiation. Curr. Org. Chem. 17, 504–527. 10.2174/1385272811317050007 [DOI] [Google Scholar]
- Heravi M. M., Ajami D., Mojtahedi M. M., Ghassemzadeh M. (1999). A convenient oxidative deprotection of tetrahydropyranyl ethers with iron(III) nitrate and clay under microwave irradiation in solvent free conditions. Tetrahedron Lett. 40, 561–562. 10.1016/S0040-4039(98)02355-7 [DOI] [Google Scholar]
- Heravi M. M., Derikvand F., Ranjbar L. (2010). Sulfamic acid–catalyzed, three-component, one-pot synthesis of [1,2,4]triazolo/benzimidazolo quinazolinone derivatives. Synth. Commun. 40, 677–685. 10.1080/00397910903009489 [DOI] [Google Scholar]
- Heravi M. M., Ghavidel M., Mohammadkhani L. (2018). Beyond a solvent: triple roles of dimethylformamide in organic chemistry. RSC Adv. 8, 27832–27862. 10.1039/C8RA04985H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heravi M. M., Ranjbar L., Derikvand F., Alimadadi B., Oskooie H. A., Bamoharram F. F. (2008). A three component one-pot procedure for the synthesis of [1,2,4]triazolo/benzimidazolo-quinazolinone derivatives in the presence of H6P2W18O62·18H2O as a green and reusable catalyst. Mol. Divers. 12, 181–185. 10.1007/s11030-008-9086-8 [DOI] [PubMed] [Google Scholar]
- Heravi M. M., Sadjadi S., Sadjadi S., Oskooie H. A., Bamoharram F. F. (2009). Rapid and efficient synthesis of 4(3H)-quinazolinones under ultra sonic irradiation using silica-supported Preyssler nano particles. Ultrason. Sonochem. 16, 708–710. 10.1016/j.ultsonch.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Heravi M. M., Tavakoli-Hoseini N., Bamoharram F. F. (2011). Brønsted acidic ionic liquids: new, efficient, and green promoter system for the synthesis of 4(3H)-quinazolinones. Synth. Commun. 41, 707–714. 10.1080/00397911003640108 [DOI] [Google Scholar]
- Hill M. D., Movassaghi M. (2008). Observations on the use of microwave irradiation in azaheterocycle synthesis. Tetrahedron Lett. 49, 4286–4288. 10.1016/j.tetlet.2008.04.143 [DOI] [Google Scholar]
- Ighilahriz K., Boutemeur B., Chami F., Rabia C., Hamdi M., Hamdi S. M. (2008). A microwave-assisted and heteropolyacids-catalysed cyclocondensation reaction for the synthesis of 4(3H)-quinazolinones. Molecules 13, 779–789. 10.3390/molecules13040779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I., Oine T., Yamada Y., Tani J., Ishida R., Ochiai T. (1976). 2-Fluoromethyl-3-o-tolyl-6-amino-4(3H)-quinazolinone. United States patent US 3966731. [Google Scholar]
- Jagani C. L., Sojitra N. A., Vanparia S. F., Patel T. S., Dixit R. B., Dixit B. C. (2011). A convergent microwave assisted synthesis of 4-amino-N-(4-oxo-2-substituted-4H-quinazolin-3-yl) benzenesulfonamide derivatives. Arkivoc 9, 221–237. 10.3998/ark.5550190.0012.916 [DOI] [Google Scholar]
- Jagani C. L., Sojitra N. A., Vanparia S. F., Patel T. S., Dixit R. B., Dixit B. C. (2012). Microwave promoted synthesis and antimicrobial activity of 3-thiazole substituted 2-styryl-4(3H)-quinazolinone derivatives. J. Saudi Chem. Soc. 16, 363–369. 10.1016/j.jscs.2011.02.001 [DOI] [Google Scholar]
- Janardhanan J.C., Bhaskaran R.P., Praveen V.K., Narayanapillai M., P Babu B. (2020). Recent advances in the transition metal catalyzed synthesis of indazoles. Asian J. Org. Chem. 14, 6638–6650. 10.1039/C6OB00944A [DOI] [Google Scholar]
- Jiang X., Wei X., Lin F., Zhang Z., Yao G., Yang S., et al. (2020). Substrate-controlled [5+1] annulation of 5-amino-1H-phenylpyrazoles with alkenes: divergent synthesis of multisubstituted 4,5-dihydropyrazolo[1,5-a]quinazolines. Eur. J. Org. Chem. 2020, 3997–4003. 10.1002/ejoc.202000536 [DOI] [Google Scholar]
- Johansson Seechurn C. C. C., Kitching M. O., Colacot T. J., Snieckus V. (2012). Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 51, 5062–5085. 10.1002/anie.201107017 [DOI] [PubMed] [Google Scholar]
- Kabri Y., Gellis A., Vanelle P. (2009). Microwave-assisted synthesis in aqueous medium of new quinazoline derivatives as anticancer agent precursors. Green Chem. 11, 201–208. 10.1039/B816723K [DOI] [Google Scholar]
- Kamal A., Reddy K. S., Prasad B. R., Babu A. H., Ramana A. V. (2004). Microwave enhanced reduction of nitro and azido arenes to N-arylformamides employing Zn–HCOONH4: synthesis of 4 (3H)-quinazolinones and pyrrolo[2,1-c][1,4]benzodiazepines. Tetrahedron Lett. 45, 6517–6521. 10.1016/j.tetlet.2004.06.112 [DOI] [Google Scholar]
- Kamal A., Reddy M. K., Reddy T. S., Santos L. S., Shankaraiah N. (2011). Total synthesis of rutaecarpine and analogues by tandem azido reductive cyclization assisted by microwave irradiation. Synlett 2011, 61–64. 10.1055/s-0030-1259095 [DOI] [Google Scholar]
- Kang H., Wang W., Sun Q., Yang S., Jin J., Zhang X., et al. (2018). Microwave-assisted synthesis of quinazolin-4(3H)-ones catalyzed by SbCl3. Heterocycl Commun. 24, 293–296. 10.1515/hc-2018-0115 [DOI] [Google Scholar]
- Kanth S. R., Reddy G. V., Maitraie D., Narsaiah B., Rao P. S., Kumar K. R., et al. (2006). Iron(III)chloride catalysed three-component Grieco condensation: synthesis of tetrahydropyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidines/quinazolines. J. Fluor. Chem. 127, 1211–1221. 10.1016/j.jfluchem.2006.06.017 [DOI] [Google Scholar]
- Kanwal I., Mujahid A., Rasool N., Rizwan K., Malik A., Ahmad G., et al. (2020). Palladium and copper catalyzed sonogashira cross coupling an excellent methodology for C-C bond formation over 17 years: a review. Catalysts 10:443 10.3390/catal10040443 [DOI] [Google Scholar]
- Kappe C. O. (2004). Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 43, 6250–6284. 10.1002/anie.200400655 [DOI] [PubMed] [Google Scholar]
- Ke F., Liu C., Zhang P., Xu J., Chen X. (2018). Efficient and selective microwave-assisted copper-catalyzed synthesis of quinazolinone derivatives in aqueous. Synth. Commun. 48, 3089–3098. 10.1080/00397911.2018.1533974 [DOI] [Google Scholar]
- Khajavi M. S., Afshani P., Rad Moghadam K. (1998). Microwave irradiation promoted the Niementowski reaction preparation of substituted quinazolinones and quinolines. Iran. J. Chem. Chem. Eng. 17, 29–32. [Google Scholar]
- Khajavi M. S., Rad-Moghadam K., Hazarkhani H. (1999a). A facile synthesis of 6-substituted benzimidazo[1,2-c]-quinazolines under microwave irradiation. Synth. Commun. 29, 2617–2624. 10.1080/00397919908086422 [DOI] [Google Scholar]
- Khajavi M. S., Sadat Hosseini S. S., Montazari N. (1999b). Microwave irradiation promoted reactions of benzoxazin-4-ones with primary amines. preparation of 4(2= 3H)-quinazolinones. Iran. J. Chem. Chem. Eng. 18, 30–32. [Google Scholar]
- Khan I., Ibrar A., Abbas N., Saeed A. (2014). Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur. J. Med. Chem. 76, 193–244. 10.1016/j.ejmech.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Khan I., Ibrar A., Ahmed W., Saeed A. (2015). Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: the advances continue. Eur. J. Med. Chem. 90, 124–169. 10.1016/j.ejmech.2014.10.084 [DOI] [PubMed] [Google Scholar]
- Khosropour A. R., Mohammadpoor-Baltork I., Ghorbankhani H. (2006). Bi(TFA)3-[nbp]FeCl4: a new, efficient and reusable promoter system for the synthesis of 4(3H)-quinazolinone derivatives. Tetrahedron Lett. 47, 3561–3564. 10.1016/j.tetlet.2006.03.079 [DOI] [Google Scholar]
- Kojima M., Matsunaga S. (2020). The merger of photoredox and cobalt catalysis. Trends Chem. 2, 410–426. 10.1016/j.trechm.2020.01.004 [DOI] [Google Scholar]
- Koroji S., Mohammadi A. A., Mehrshad M. (2018). An efficient one-pot three-component synthesis of some new 3-(benzo[d]thiazol-2-yl)-2-alkyl-4(3H)-quinazolinones using silica sulfuric acid as a heterogeneous catalyst. J. Heterocycl. Chem. 55, 2647–2651. 10.1002/jhet.3312 [DOI] [Google Scholar]
- Kshirsagar U. A. (2015). Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 13, 9336–9352. 10.1039/C5OB01379H [DOI] [PubMed] [Google Scholar]
- Kumar N. D., Dubey P. K. (2012). An expeditious microwave-assisted synthesis of mercapto benzazoles, quinazolinone and oxadiazoles. Indian J. Chem. 51B, 1619–1622. Available online at: http://nopr.niscair.res.in/handle/123456789/14985 [Google Scholar]
- Kumar V., Mohan C., Gupta M., Mahajan M. P. (2005). A catalyst-and solvent-free selective approach to biologically important quinazolines and benzo[g]quinazoline. Tetrahedron 61, 3533–3538. 10.1016/j.tet.2005.01.118 [DOI] [Google Scholar]
- Kumar V., Sharma A., Mahajan M. P. (2004). Microwave assisted Aza-Wittig reactions of N-imidoyliminophosphorane with aldehydes: a convenient and selective route to quinazolines. Synth. Commun. 34, 49–53. 10.1081/SCC-120027237 [DOI] [Google Scholar]
- Laddha S. S., Bhatnagar S. P. (2008). Rapid microwave-assisted solution phase synthesis of 6,8-disubstituted 2-phenyl-3-(substituted-benzothiazol-2-yl)-4-[3H]-quinazolinones as novel anticonvulsants. Phosphorus Sulfur Relat. Elem. 183, 2262–2273. 10.1080/10426500801957766 [DOI] [Google Scholar]
- Leiby R. W. (1985). Synthesis of 3-amino-4(3H)-quinazolinones from N-(2-carbomethoxyphenyl)imidate esters. J. Org. Chem. 50, 2926–2929. 10.1021/jo00216a023 [DOI] [Google Scholar]
- Ley S. V., Thomas A. W. (2003). Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. 42, 5400–5449. 10.1002/anie.200300594 [DOI] [PubMed] [Google Scholar]
- Li F., Feng Y., Meng Q., Li W., Li Z., Wang Q., et al. (2007). An efficient construction of quinazolin-4(3H)-ones under microwave irradiation. Arkivoc 1, 40–50. 10.3998/ark.5550190.0008.105 [DOI] [Google Scholar]
- Li G., Kakarla R., Gerritz S. W., Pendri A., Ma B. (2009). A facile one-step synthesis of 5-chloro-imidazo[1,5-a]quinazoline by microwave irradiation. Tetrahedron Lett. 50, 6048–6052. 10.1016/j.tetlet.2009.08.054 [DOI] [Google Scholar]
- Li H. Y., Huang R. Q., Qiu D. W., Yang Z., Liu X., Ma J. A., et al. (1998). Synthesis and bioactivity of 4-quinazoline oxime ethers. Prog. Nat. Sci. 8, 359–365. [Google Scholar]
- Li W., Zhang J. (2020). Synthesis of heterocycles through denitrogenative cyclization of triazoles and benzotriazoles. Chem. Eur. J. 26, 11931–11945. 10.1002/chem.202000674 [DOI] [PubMed] [Google Scholar]
- Li W., Zheng X., Li Z. (2013). Iron-catalyzed C-C bond cleavage and C-N bond formation. Adv. Synth. Catal. 355, 181–190. 10.1002/adsc.201200324 [DOI] [Google Scholar]
- Li X., Lin Q., Eli W. (2010). Microwave-assisted synthesis of 2-substituted-4(3H)-quinazolinones in bronsted acidic ionic liquids. Chinese J. Org. Chem. 30, 452–455. Available online at: http://sioc-journal.cn/Jwk_yjhx/EN/Y2010/V30/I03/452 [Google Scholar]
- Li X., Lin Q., Wang L. (2015). One-pot solvent-free synthesis of 2,3-disubstituted 4(3H)-quinazolinones catalyzed by long-chain double SO3H-functionalized Brønsted acidic ionic liquids under microwave irradiation. J. Iran. Chem. Soc. 12, 897–901. 10.1007/s13738-014-0553-0 [DOI] [Google Scholar]
- Liang E., Wu Y., Chen J., Xiong W., Zhao J., Yao X., et al. (2019). Copper-catalyzed aerobic oxidative cyclization protocol for the synthesis of quinazolines via amination of C(sp3)-H bonds of methylazaarenes. Tetrahedron 75:130783 10.1016/j.tet.2019.130783 [DOI] [Google Scholar]
- Lidström P., Tierney J., Watheyb B., Westmana J. (2001). Microwave assisted organic synthesisd-a review. Tetrahedron 57, 9225–9283. 10.1016/S0040-4020(01)00906-1 [DOI] [Google Scholar]
- Lin W.-H., Wu W.-C., Selvaraju M., Sun C.-M. (2017). One-pot synthesis of benzazoles and quinazolinones via iron pentacarbonyl mediated carbonylation of aryl iodides under microwave irradiation. Org. Chem. Front. 4, 392–397. 10.1039/C6QO00733C [DOI] [Google Scholar]
- Lingaiah B. V., Ezikiel G., Yakaiah T., Reddy G. V., Rao P. S. (2006). Nafion-H: An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of 2,3-disubstituted 4-(3H)-quinazolinones under solvent-free microwave irradiation conditions. Synlett 2006, 2507–2509. 10.1055/s-2006-950428 [DOI] [Google Scholar]
- Liu J., Wang Y.-L., Zhang J.-H., Yang J.-S., Mou H.-C., Lin J., et al. (2018a). Phosphatase CDC25B inhibitors produced by basic alumina-supported one-pot gram-scale synthesis of fluorinated 2-alkylthio-4-aminoquinazolines using microwave irradiation. ACS omega 3, 4534–4544. 10.1021/acsomega.8b00640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-F., Kaselj M., Isome Y., Chapnick J., Zhang B., Bi G., et al. (2005a). Microwave-assisted concise total syntheses of quinazolinobenzodiazepine alkaloids. J. Org. Chem. 70, 10488–10493. 10.1021/jo051876x [DOI] [PubMed] [Google Scholar]
- Liu J.-F., Lee J., Dalton A. M., Bi G., Yu L., Baldino C. M., et al. (2005b). Microwave-assisted one-pot synthesis of 2, 3-disubstituted 3H-quinazolin-4-ones. Tetrahedron Lett. 46, 1241–1244. 10.1016/j.tetlet.2005.01.008 [DOI] [Google Scholar]
- Liu J.-F., Ye P., Zhang B., Bi G., Sargent K., Yu L., et al. (2005c). Three-component one-pot total syntheses of glyantrypine, fumiquinazoline F, and fiscalin B promoted by microwave irradiation. J. Org. Chem. 70, 6339–6345. 10.1021/jo0508043 [DOI] [PubMed] [Google Scholar]
- Liu R., Li H., Yang J., An Z. (2018b). Quinazolinones isolated from Aspergillus sp., an endophytic fungus of Astragalus membranaceus. Chem. Nat. Compd. 54, 808–810. 10.1007/s10600-018-2484-y [DOI] [Google Scholar]
- Liu X., Fu H., Jiang Y., Zhao Y. (2009). A simple and efficient approach to quinazolinones under mild copper-catalyzed conditions. Angew. Chem. 121, 354–357. 10.1002/ange.200804675 [DOI] [PubMed] [Google Scholar]
- Loupy A. (1993). Les micro-ondes en synthèse organique: une méthodologie propre et performante: Préparation d'échantillons. Spectra Analyse 22, 33–38. [Google Scholar]
- Loupy A. (1994). Les micro-ondes: leurs applications potentielles dans la chimie des corps gras. Oléagineux Corps gras, Lipides. 1, 62–68. [Google Scholar]
- Luo H., Hu D., Wu J., He M., Jin L., Yang S., et al. (2012). Rapid synthesis and antiviral activity of (quinazolin-4-ylamino)methyl-phosphonates through microwave irradiation. Int. J. Mol. Sci. 13, 6730–6746. 10.3390/ijms13066730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Wan Y., Hong C., Li M., Hu X., Mo W., et al. (2017). ABNO-catalyzed aerobic oxidative synthesis of 2-substituted 4H-3,1-benzoxazines and quinazolines. Eur. J. Org. Chem. 2017, 3335–3342. 10.1002/ejoc.201700384 [DOI] [PubMed] [Google Scholar]
- Maitraie D., Yakaiah T., Srinivas K., Reddy G. V., Ravikanth S., Narsaiah B., et al. (2006). Regioselective addition of Grignard reagents to 2,6-dicyanoanilines and cyclization to new quinazoline derivatives under thermal/microwave irradiation conditions. J. Fluor. Chem. 127, 351–359. 10.1016/j.jfluchem.2006.01.003 [DOI] [Google Scholar]
- Majetich G., Hicks R. (1994). Applications of microwave accelerated organic chemistry. Res. Chem. Intermed. 20:61 10.1163/156856794X00072 [DOI] [Google Scholar]
- Majetich G., Hicks R. (1995). The use of microwave heating to promote organic reactions. J. Microw. Power Electromagn. Energ. 30, 27–45. 10.1080/08327823.1995.11688258 [DOI] [Google Scholar]
- Majumder A., Gupta R., Jain A. (2013). Microwave-assisted synthesis of nitrogen-containing heterocycles. Green Chem. Lett. Rev. 6, 151–182. 10.1080/17518253.2012.733032 [DOI] [Google Scholar]
- Masel R. I. (2001). Chemical Kinetics and Catalysis. New York, NY: Wiley. [Google Scholar]
- McNaught A. D., Wilkinson A. (1997). Compendium of Chemical Terminology. Oxford: Blackwell Science Oxford. [Google Scholar]
- Melvin J. Y., McCowan J. R., Towner R. D., Ho P. P. K., Phebus L. A., Ruterbories K. J., et al. (1992). Structurally novel antiarrhythmic/antioxidant quinazolines. Bioorg. Med. Chem. Lett. 2, 1121–1126. 10.1016/S0960-894X(00)80631-9 [DOI] [Google Scholar]
- Mhaske S. B., Argade N. P. (2006). The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 62, 9787–9826. 10.1016/j.tet.2006.07.098 [DOI] [Google Scholar]
- Michael J. P. (2003). Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 20, 476–493. 10.1039/b208140g [DOI] [PubMed] [Google Scholar]
- Michael J. P. (2004). Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 21, 650–668. 10.1039/b310691h [DOI] [PubMed] [Google Scholar]
- Mingos D. M. P., Baghurst D. R. (1991). Microwave-assisted reactions and processes. Chem. Soc. Rev 20, 1–47. 10.1039/cs9912000001 [DOI] [Google Scholar]
- Mohammadi A. A., Askari S., Rohi H., Soorki A. A. (2014). Design, synthesis, and antibacterial evaluation of some novel 3′-(phenylamino)-1′ H-spiro [indoline-3,2′-quinazoline]-2,4′(3′ H)-dione derivatives. Synth. Commun. 44, 457–467. 10.1080/00397911.2013.796992 [DOI] [Google Scholar]
- Mohammadi A. A., Sadat Hossini S. (2011). KAl(SO4)2·12H2O (Alum) catalyzed one-pot three-component synthesis of 2-alkyl and 2-aryl-4(3H)-quinazolinone under microwave irradiation and solvent free conditions. Chin. Chem. Lett. 29, 1982–1984. 10.1002/cjoc.201180344 [DOI] [Google Scholar]
- Monnier F., Taillefer M. (2013). Copper-catalyzed C(aryl)–N bond formation,” in Amination and Formation of sp2 C-N Bonds, eds Taillefer M., Ma D. (Berlin, Heidelberg: Springer; ), 173–204. 10.1007/3418_2013_69 [DOI] [Google Scholar]
- Montazeri N., Pourshamsian K., Fomani A., Kalantarian S. J. (2012a). Synthesis of 2,3-disubstituted-4(3H)-quinazolinones using HY-zeolite as reusable catalyst under microwave irradiation. Asian J. Chem. 24:2805 Available online at: http://www.asianjournalofchemistry.co.in/user/journal/viewarticle.aspx?ArticleID=24_6_98 [Google Scholar]
- Montazeri N., Pourshamsian K., Rahgol M., Bayazi M. (2012b). Pentafluorophenylammonium triflate: an efficient, practical and environmental friendly catalyst for synthesis of quinazolin-4(3H)-ones. Asian J. Chem. 24, 5361–5364. Available online at: http://www.asianjournalofchemistry.co.in/user/journal/viewarticle.aspx?ArticleID=24_11_129
- Montazeri N., Rad-Moghadam K. (2004). A convenient synthesis of substituted quinazolin-4(3H)-ones under microwave and solvent-free conditions. Phosphorus, Sulfur Relat. Elem. 179, 2533–2536. 10.1080/10426500490485606 [DOI] [Google Scholar]
- Nagata T., Obora Y. (2020). Transition-metal-mediated/catalyzed synthesis of pyridines, pyrimidines, and triazines by [2+ 2+ 2] cycloaddition reactions. Asian J. Org. Chem. 9, 1532–1547. 10.1002/ajoc.202000240 [DOI] [Google Scholar]
- Nain S., Singh R., Ravichandran S. (2019). Importance of microwave heating in organic synthesis. Adv. J. Chem. A. 2, 94–104. 10.29088/SAMI/AJCA.2019.2.94104 [DOI] [Google Scholar]
- Nandy P., Vishalakshi M. T., Bhat A. R. (2006). Synthesis and antitubercular activity of Mannich bases of 2-methyl-3H-quinazolin-4-ones. Indian J. Heterocycl. Chem. 15, 293–294. Available online at: https://www.scopus.com/inward/record.uri?eid=2-s2.0-33747797083&partnerID=40&md5=e71ece6ed4e8ebce925122620b81eb22 [Google Scholar]
- Narasimhulu M., Mahesh K. C., Reddy T. S., Rajesh K., Venkateswarlu Y. (2006). Lanthanum (III) nitrate hexahydrate or p-toluenesulfonic acid catalyzed one-pot synthesis of 4(3H)-quinazolinones under solvent-free conditions. Tetrahedron Lett. 47, 4381–4383. 10.1016/j.tetlet.2006.04.096 [DOI] [Google Scholar]
- Neto J., Zeni G. (2020a). Alkynes and nitrogen compounds: useful substrates for the synthesis of pyrazoles. Chem. Eur. J. 26:8175–8189. 10.1002/chem.202083761 [DOI] [PubMed] [Google Scholar]
- Neto J. S. S., Zeni G. (2020b). A decade of advances in the reaction of nitrogen sources and alkynes for the synthesis of triazoles. Coord. Chem. Rev. 409:213217 10.1016/j.ccr.2020.213217 [DOI] [Google Scholar]
- Nobel Prize (2020). The Nobel Prize in Chemistry 1918. Available online at: http://nobelprize.org/nobel_prizes/chemistry/laureates/1918/index.html
- Nouira I., Kostakis I. K., Dubouilh C., Chosson E., Iannelli M., Besson T. (2008). Decomposition of formamide assisted by microwaves, a tool for synthesis of nitrogen-containing heterocycles. Tetrahedron Lett. 49, 7033–7036. 10.1016/j.tetlet.2008.09.135 [DOI] [Google Scholar]
- Oskooie H. A., Baghernezhad B., Heravi M. M. (2007). SnCl4 center dot 4H(2)O as an efficient catalyst for the synthesis of 4(3H)-quinazolinone derivatives. Indian J. Heterocy. Chem. 17, 95–96. Available online at: https://link.springer.com/articles/cas-redirect/1%3ACAS%3A528%3ADC%252BD2sXht1Wktr7E [Google Scholar]
- Pal T., Lahiri G. K., Maiti D. (2020). Copper in efficient synthesis of aromatic heterocycles with single heteroatom. Eur. J. Org. Chem. 10.1002/ejoc.202000688. [Epub ahead of print]. [DOI] [Google Scholar]
- Pater R. (1971). 2-Aryl-4(3H)quinazolinones. J. Heterocycl. Chem. 8, 699–702. 10.1002/jhet.5570080501 [DOI] [Google Scholar]
- Pathania D., Sechi M., Palomba M., Sanna V., Berrettini F., Sias A., et al. (2014). Design and discovery of novel quinazolinedione-based redox modulators as therapies for pancreatic cancer. Biochim. Biophys. Acta 1840, 332–343. 10.1016/j.bbagen.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Patil S., Jadhav S. D., Shejwal R. V., Deshmukh M. B. (2012). Microwave-assisted cyclocondensation for the synthesis of 3-aryl-2-thioquinazolin-4(3H)-ones. Asian J. Chem. 24, 1858–1860. Available online at: http://www.asianjournalofchemistry.co.in/user/journal/viewarticle.aspx?ArticleID=24_4_99 [Google Scholar]
- Pessoa-Mahana H., Pessoa-Mahana C. D., Salazar R., Valderrama J. A., Saez E., Araya-Maturana R. (2004). Solvent-free synthesis of 6-arylbenzimidazo[1,2-c]quinazolines under microwave irradiation. Synthesis 2004, 436–440. 10.1055/s-2004-815924 [DOI] [Google Scholar]
- Portela-Cubillo F., Scott J. S., Walton J. C. (2008). 2-(Aminoaryl) alkanone O-phenyl oximes: versatile reagents for syntheses of quinazolines. Catal. Commun. 25, 2935–2937. 10.1039/b803630f [DOI] [PubMed] [Google Scholar]
- Potuganti G. R., Indukuri D. R., Alla M. (2018). An efficient one-pot multicomponent synthesis of tetracyclic quinazolino[4,3-b]quinazolines by sequential C–N bond formation and copper-mediated aerobic oxidative cyclization. Synlett 29, 1717–1722. 10.1055/s-0036-1591578 [DOI] [Google Scholar]
- Priya M. G. R., Zulykama Y., Girija K., Murugesh S., Perumal P. T. (2011). Synthesis of 4-(3H)-quinazolinones by microwave assisted tandem reaction and evaluation of their antibacterial and antifungal activities. Indian J. Chem. 50B, 98–102. 10.1002/chin.201118143 [DOI] [Google Scholar]
- Punniyamurthy T., Rout L. (2008). Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 252, 134–154. 10.1016/j.ccr.2007.04.003 [DOI] [Google Scholar]
- Radhakrishnan K., Das S., Kundu L. M. (2018). Synthesis of size-expanded nucleobase analogues for artificial base-pairing using a ligand-free, microwave-assisted copper(I)-catalyzed reaction. ChemistrySelect 3, 13098–13102. 10.1002/slct.201802455 [DOI] [Google Scholar]
- Rad-Moghadam K., Khajavi M. S. (1998). One-pot synthesis of substituted quinazolin-4(3H)-ones under microwave irradiation. J. Chem. Res. 11, 702–703. 10.1039/a802853b [DOI] [Google Scholar]
- Rad-Moghadam K., Mohseni M. (2003). An expeditious and solvent-free route to the synthesis of 2-substituted quinazolin-4(3H)-ones under microwave conditions. J. Chem. Res. 2003, 487–488. 10.3184/030823403103174632 [DOI] [Google Scholar]
- Rad-Moghadam K., Samavi L. (2006). One-pot three-component synthesis of 2-substituted 4-aminoquinazolines. J. Heterocycl. Chem. 43, 913–916. 10.1002/jhet.5570430415 [DOI] [Google Scholar]
- Radziszewski B. (1885). Ueber die oxydation mittelst wasserstoffsuperoxyd. ?Ber. Dtsch. Chem. Ges. 18, 355–356. 10.1002/cber.18850180171 [DOI] [Google Scholar]
- Rahimizadeh M., Tavallai Z., Bakavoli M. (2004). Microwave irradiation in solvent-free conditions: preparation of 2-substituted 4(3H)-quinazolinones by heterocyclisation of 2-aminobenzamide with carboxylic acids. Indian J. Chem. 43B, 679–681. 10.1002/chin.200428167 [DOI] [Google Scholar]
- Rajappa S., Advani B. G. (1973). Iminoethers—III: Tetracyclic quinazolones derived from cyclodipeptide mono iminoethers. Tetrahedron 29, 1299–1302. 10.1016/S0040-4020(01)83147-1 [DOI] [Google Scholar]
- Rajappa S., Advani B. G. (1974). Conformational studies on tricyclic quinazolones derived from cyclodipeptides incorporating sarcosine. J. Chem. Soc. Perkin Trans. 1, 2122–2124. 10.1039/p19740002122 [DOI] [PubMed] [Google Scholar]
- Rajasekaran A., Rajamanickam V., Darlinquine S. (2013). Synthesis of some new thioxoquinazolinone derivatives and a study on their anticonvulsant and antimicrobial activities. Eur. Rev. Med. Pharmacol. Sci. 17, 95–104. Available online at: https://www.europeanreview.org/article/1108 [PubMed] [Google Scholar]
- Ramanathan M., Liu S.-T. (2017). Preparation of quinazolines via a 2+2+2 annulation from aryldiazonium salts and nitriles. J. Org. Chem. 82, 8290–8295. 10.1021/acs.joc.7b01325 [DOI] [PubMed] [Google Scholar]
- Ratmanova N. K., Andreev I. A., Leontiev A. V., Momotova D., Novoselov A. M., Ivanova O. A., et al. (2020). Strategic approaches to the synthesis of pyrrolizidine and indolizidine alkaloids. Tetrahedron 76:131031 10.1016/j.tet.2020.131031 [DOI] [Google Scholar]
- Raut A. B., Bhanage B. M. (2017). Cuprous oxide nanoparticle supported on iron oxide (Cu2O-Fe3O4): magnetically separable and reusable nanocatalyst for the synthesis of quinazolines. ChemistrySelect 2, 10055–10060. 10.1002/slct.201701251 [DOI] [Google Scholar]
- Rodrigues R., Tran L. Q., Darses B., Dauban P., Neuville L. (2019). Copper-promoted tandem three-component access to quinazolin-4(H)-imines and benzimidazo[1,2-c]quinazolines. Adv. Synth. Catal. 361, 4454–4460. 10.1002/adsc.201900658 [DOI] [Google Scholar]
- Rohini R., Reddy P. M., Shanker K., Hu A., Ravinder V. (2010). Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c]quinazolines. Eur. J. Med. Chem. 45, 1200–1205. 10.1016/j.ejmech.2009.11.038 [DOI] [PubMed] [Google Scholar]
- Saari R., Törm,ä J.-C., Nevalainen T. (2011). Microwave-assisted synthesis of quinoline, isoquinoline, quinoxaline and quinazoline derivatives as CB2 receptor agonists. Bioorg. Med. Chem. 19, 939–950. 10.1016/j.bmc.2010.11.059 [DOI] [PubMed] [Google Scholar]
- Saeedi M., Beheshtiha Y. S., Heravi M. M. (2011). Synthesis of novel tetrahydro[4,5]imidazo[2,1-b]-chromeno[4,3,2-de]quinazoline and benzothiazol-2-ylaminoxanthenone derivatives. Heterocycles 83, 1831–1841. 10.3987/COM-11-12201 [DOI] [Google Scholar]
- Sahiba N., Agarwal S. (2020). Recent advances in the synthesis of perimidines and their applications. Top. Curr. Chem. 378, 1–47. 10.1007/s41061-020-00307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia U. P., Borah G., Pahari P. (2018). Lewis-acid-catalysed activation of nitriles: a microwave-assisted solvent-free synthesis of 2,4-disubstituted quinazolines and 1,3-diazaspiro[5.5]undec-1-enes. Eur. J. Org. Chem. 2018, 1211–1217. 10.1002/ejoc.201701585 [DOI] [Google Scholar]
- Sarma R., Prajapati D. (2011). Microwave-promoted efficient synthesis of dihydroquinazolines. Green Chem. 13, 718–722. 10.1039/c0gc00838a [DOI] [Google Scholar]
- Sasmal S., Balaji G., Reddy H. R. K., Balasubrahmanyam D., Srinivas G., Kyasa S., et al. (2012). Design and optimization of quinazoline derivatives as melanin concentrating hormone receptor 1 (MCHR1) antagonists. Bioorg. Med. Chem. Lett. 22, 3157–3162. 10.1016/j.bmcl.2012.03.050 [DOI] [PubMed] [Google Scholar]
- Sawant S. D., Srinivas M., Reddy G. L., Rao V. V., Singh P. P., Vishwakarma R. A. (2012). One-pot multicomponent synthesis of medicinally important purine quinazolinone derivatives. Tetrahedron Lett. 53, 6195–6198. 10.1016/j.tetlet.2012.08.137 [DOI] [Google Scholar]
- Seijas J. A., Vázquez-Tato M. P., Martinez M. M. (2000). Microwave enhanced synthesis of 4-aminoquinazolines. Tetrahedron Lett. 41, 2215–2217. 10.1016/S0040-4039(00)00090-3 [DOI] [Google Scholar]
- Sharma A., Luxami V., Paul K. (2013). Synthesis, single crystal and antitumor activities of benzimidazole–quinazoline hybrids. Bioorg. Med. Chem. Lett. 23, 3288–3294. 10.1016/j.bmcl.2013.03.107 [DOI] [PubMed] [Google Scholar]
- Sheu J. R., Hung W. C., Wu C. H., Lee Y. M., Yen M. H. (2000). Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br. J. Haematol. 110, 110–115. 10.1046/j.1365-2141.2000.01953.x [DOI] [PubMed] [Google Scholar]
- Shi D., Wang J., Shi C., Rong L., Zhuang Q., Hu H. (2004). Synthesis of quinazolines and imidazo[1,2-c]quinazolines with the aid of a low-valent titanium reagent. Synlett 2004, 1098–1100. 10.1055/s-2004-822893 [DOI] [Google Scholar]
- Shiri M., Heravi M. M., Faghihi Z., Zadsirjan V., Mohammadnejad M., Ranjbar M. (2018). Tandem and transition metal-free synthesis of novel benzoimidazo-quinazoline as highly selective Hg2+ sensors. Res. Chem. Intermed. 44, 2439–2449. 10.1007/s11164-017-3239-2 [DOI] [Google Scholar]
- Sonawane A. D., Sonawane R. A., Ninomiya M., Koketsu M. (2020). Synthesis of seleno-heterocycles via electrophilic/radical cyclization of alkyne containing heteroatoms. Adv Synthesis Catalysis. 362, 3485–3515. 10.1002/adsc.202000490 [DOI] [Google Scholar]
- Sreenivas D. K., Ramkumar N., Nagarajan R. (2012). Copper-mediated domino synthesis of pyrimido[4,5-b]carbazolones via Ullmann N-arylation and aerobic oxidative C–H amidation. Org. Biomol. Chem. 10, 3417–3423. 10.1039/c2ob07179g [DOI] [PubMed] [Google Scholar]
- Storelli S., Verdijk P., Verzijl D., Timmerman H., van de Stolpe A. C., Tensen C. P., et al. (2005). Synthesis and structure–activity relationship of 3-phenyl-3H-quinazolin-4-one derivatives as CXCR3 chemokine receptor antagonists. Bioorg. Med. Chem. 15, 2910–2913. 10.1016/j.bmcl.2005.03.070 [DOI] [PubMed] [Google Scholar]
- Strauss C. R., Trainor R. W. (1995). Developments in microwave-assisted organic chemistry. Aust. J. Chem. 48, 1665–1692. 10.1071/CH9951665 [DOI] [Google Scholar]
- Tandon P. K. (2019). Catalytic applications of copper species in organic transformations: a review. JoCC 1, 21–34. Available online at: http://engineeringjournals.stmjournals.in/index.php/JoCC/article/view/2336 [Google Scholar]
- Tavalaie Z., Sabzevari O., Bakavoli M., Rahimizadeh M. (2007). A novel synthesis of 2-(alkylamino) and 2-(arylamino)-4 (3H) quinazolinones by heterotrocyclization of 2-aminobenzamide with isothiocyanates (or isocyanates) under microwave irradiation. Sci. Iran. 14, 320–322. Available online at: https://www.sid.ir/en/journal/ViewPaper.aspx?ID=92576 [Google Scholar]
- Thapa S., Shrestha B., Gurung S. K., Giri R. (2015). Copper-catalysed cross-coupling: an untapped potential. Org. Biomol. Chem. 13, 4816–4827. 10.1039/C5OB00200A [DOI] [PubMed] [Google Scholar]
- Tiwari A. R., Bhanage B. M. (2017). Chemoselective cleavage of C(CO)–C bond: molecular iodinecatalyzed synthesis of quinazolines through sp3 C–H bond functionalization of aryl methyl ketones. Asian J. Org. Chem. 6, 831–836. 10.1002/ajoc.201700217 [DOI] [Google Scholar]
- Toure B. B., Hall D. G. (2009). Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 109, 4439–4486. 10.1021/cr800296p [DOI] [PubMed] [Google Scholar]
- Tseng M.-C., Lai C.-Y., Chu Y.-W., Chu Y.-H. (2009). Tin triflate-mediated total synthesis of circumdatin F, sclerotigenin, asperlicin C, and other quinazolino[3,2-a][1,4]benzodiazepines. Catal. Commun. 4, 445–447. 10.1039/B813880J [DOI] [PubMed] [Google Scholar]
- Tsou H.-R., Mamuya N., Johnson B. D., Reich M. F., Gruber B. C., Ye F., et al. (2001). 6-Substituted-4-(3-bromophenylamino) quinazolines as putative irreversible inhibitors of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER-2) tyrosine kinases with enhanced antitumor activity. J. Med. Chem. 44, 2719–2734. 10.1021/jm0005555 [DOI] [PubMed] [Google Scholar]
- Valderrama J. A., Pessoa Mahana H., Sarras G., Tapia R. (1999). Access to quinazolines from 2-nitrobenzaldehyde and arylamines. Chemistry. 51, 2193–2201. 10.3987/COM-99-8606 [DOI] [Google Scholar]
- Valizadeh H., Dinparast L., Noorshargh S., Heravi M. M. (2016). Microwave assisted synthesis of hydroxychromenes using imidazole-functionalized silica nanoparticles as a catalyst under solvent-free conditions. C R Chim. 19, 395–402. 10.1016/j.crci.2015.11.010 [DOI] [Google Scholar]
- Valizadeh H., Heravi M. M., Amiri M. (2010a). Conventional and microwave-assisted facile one-pot synthesis of N-substituted pyrrole-2,3,4,5-tetracarboxylates under neat conditions. Synth. Commun. 40, 3699–3706. 10.1080/00397910903531680 [DOI] [Google Scholar]
- Valizadeh H., Heravi M. M., Amiri M. (2010b). Unexpected synthesis of N-methylbenzo[d]isoxazolium hydroxides under microwave irradiation conditions. Mol. Divers. 14, 575–579. 10.1007/s11030-009-9189-x [DOI] [PubMed] [Google Scholar]
- van Zyl E. F. (2001). A survey of reported synthesis of methaqualone and some positional and structural isomers. Forensic Sci. Int. 122, 142–149. 10.1016/S0379-0738(01)00484-4 [DOI] [PubMed] [Google Scholar]
- Vasdev N., Dorff P. N., Gibbs A. R., Nandanan E., Reid L. M., O'Neil J. P., et al. (2005). Synthesis of 6-acrylamido-4-(2-[18F] fluoroanilino) quinazoline: a prospective irreversible EGFR binding probe. J. Lablelled Compd. Rad. 48, 109–115. 10.1002/jlcr.903 [DOI] [Google Scholar]
- Von Niementowski S. (1895). Synthesen von chinazolinverbindungen. J. Prakt. Chem. 51, 564–572. 10.1002/prac.18950510150 [DOI] [Google Scholar]
- Wakeling A. E., Guy S. P., Woodburn J. R., Ashton S. E., Curry B. J., Barker A. J., et al. (2002). ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 62, 5749–5754. Available online at: https://cancerres.aacrjournals.org/content/62/20/5749 [PubMed] [Google Scholar]
- Wan Y., Alterman M., Larhed M., Hallberg A. (2003). Formamide as a combined ammonia synthon and carbon monoxide source in fast palladium-catalyzed aminocarbonylations of aryl halides. J. Comb. Chem. 5, 82–84. 10.1021/cc0200843 [DOI] [PubMed] [Google Scholar]
- Wang D. Z., Yan L., Ma L. (2016). Facile preparation of 4-substituted quinazoline derivatives. J. Vis. Exp. 108:e53662. 10.3791/53662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-J., Shan J., Pang P., Yang M., Chou C.-J., Chen C.-F. (1996). The vasorelaxing action of rutaecarpine: direct paradoxical effects on intracellular calcium concentration of vascular smooth muscle and endothelial cells. J. Pharmacol. Exp. Ther. 276, 1016–1021. [PubMed] [Google Scholar]
- Wang L., Xia J., Qin F., Qian C., Sun J. (2003). Yb(OTf)3-catalyzed one-pot synthesis of quinazolin-4(3H)-ones from anthranilic acid, amines and ortho esters (or formic acid) in solvent-free conditions. Synthesis 2003, 1241–1247. 10.1055/s-2003-39397 [DOI] [Google Scholar]
- Wenli S., Jianmin Z., Jianan J., Xiaolei C. (2011). One-pot preparation of fluorinated 4(3H)-quinazolinones under microwave irradiation and solvent-free conditions. Chinese J. Org. Chem. 31, 1272–1276. Available online at: http://sioc-journal.cn/Jwk_yjhx/EN/Y2011/V31/I08/1272 [Google Scholar]
- Wolfe J. F., Rathman T. L., Sleevi M. C., Campbell J. A., Greenwood T. D. (1990). Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones. J. Med. Chem. 33, 161–166. 10.1021/jm00163a027 [DOI] [PubMed] [Google Scholar]
- Wu S.-N., Lo Y.-K., Chen H., Li H.-F., Chiang H.-T. (2001). Rutaecarpine-induced block of delayed rectifier K+ current in NG108-15 neuronal cells. Neuropharmacology 41, 834–843. 10.1016/S0028-3908(01)00114-9 [DOI] [PubMed] [Google Scholar]
- Xu H., Fu H. (2012). Copper-catalyzed one-pot synthesis of imidazo/benzoimidazoquinazolinones by sequential Ullmann-Type coupling and intramolecular C-H amidation. Chem. Eur. J. 18, 1180–1186. 10.1002/chem.201102794 [DOI] [PubMed] [Google Scholar]
- Xu L., Li H., Liao Z., Lou K., Xie H., Li H., et al. (2015). Divergent synthesis of imidazoles and quinazolines via Pd(OAc)2-catalyzed annulation of N-allylamidines. Org. Lett. 17, 3434–3437. 10.1021/acs.orglett.5b01435 [DOI] [PubMed] [Google Scholar]
- Xu W., Fu H. (2011). Amino acids as the nitrogen-containing motifs in copper-catalyzed domino synthesis of N-heterocycles. J. Org. Chem. 76, 3846–3852. 10.1021/jo2002227 [DOI] [PubMed] [Google Scholar]
- Xu W., Jin Y., Liu H., Jiang Y., Hua F. (2011). Copper-catalyzed domino synthesis of quinazolinones via Ullmann-type coupling and aerobic oxidative C–H amidation. Org. Lett. 13, 1274–1277. 10.1021/ol1030266 [DOI] [PubMed] [Google Scholar]
- Xuan J., He X.-K., Xiao W.-J. (2020). Visible light-promoted ring-opening functionalization of three-membered carbo-and heterocycles. Chem. Soc. Rev. 49, 2546–2556. 10.1039/C9CS00523D [DOI] [PubMed] [Google Scholar]
- Yadav J. S., Reddy B. V. S. (2002). Microwave-assisted rapid synthesis of the cytotoxic alkaloid luotonin A. Tetrahedron Lett. 43, 1905–1907. 10.1016/S0040-4039(02)00135-1 [DOI] [Google Scholar]
- Yin P., Liu N., Deng Y.-X., Chen Y., Deng Y., He L. (2012). Synthesis of 2,4-diaminoquinazolines and tricyclic quinazolines by cascade reductive cyclization of methyl N-cyano-2-nitrobenzimidates. J. Org. Chem. 77, 2649–2658. 10.1021/jo2023697 [DOI] [PubMed] [Google Scholar]
- Yoon D. S., Han Y., Stark T. M., Haber J. C., Gregg B. T., Stankovich S. B. (2004). Efficient synthesis of 4-aminoquinazoline and thieno[3,2-d]pyrimidin-4-ylamine derivatives by microwave irradiation. Org. Lett. 6, 4775–4778. 10.1021/ol047919y [DOI] [PubMed] [Google Scholar]
- Zhang X., Ye D., Sun H., Guo D., Wang J., Huang H., et al. (2009). Microwave-assisted synthesis of quinazolinone derivatives by efficient and rapid iron-catalyzed cyclization in water. Green Chem. 11, 1881–1888. 10.1039/b916124b [DOI] [Google Scholar]
- Zhou J., Fu L., Lv M., Liu J., Pei D., Ding K. (2008). Copper(I)iodide catalyzed domino process to quinazolin-4(3H)-ones. Synthesis 2008, 3974–3980. 10.1055/s-0028-1083245 [DOI] [Google Scholar]
- Zhu K., Hao J.-H., Zhang C.-P., Zhang J., Feng Y., Qin H.-L. (2015). Diversified facile synthesis of benzimidazoles, quinazolin-4(3H)-ones and 1,4-benzodiazepine-2,5-diones via palladium-catalyzed transfer hydrogenation/condensation cascade of nitro arenes under microwave irradiation. RSC Adv. 5, 11132–11135. 10.1039/C4RA15765F [DOI] [Google Scholar]