Abstract
Chronic massive pericardial effusion without cardiac tamponade is relatively rare. Nearly half of all patients with chronic large pericardial effusion are asymptomatic. We report a case of a 77-year-old man who presented with an asymptomatic chronic massive pericardial effusion, with no evidence of cardiac tamponade or pericardial constriction during a 10-year follow-up. The patient had a complex history of lymph node tuberculosis, hypertension, hypothyroidism, and polycythemia vera, as well as high-dose 31P radiation exposure 45 years ago. There was no evidence of tuberculosis infection, hypothyroidism, malignant tumor, severe heart failure, uremia, trauma, severe bacterial or fungal infection, chronic myeloid leukemia, or bone marrow fibrosis after admission. The patient underwent pericardiocentesis twice. The pericardial effusion comprised exudate fluid with a high proportion of monocytes. The patient refused indwelling catheter drainage or pericardiectomy. The likely final diagnosis was recurrent chronic large idiopathic pericardial effusion.
Keywords: Pericardial effusion, elderly, case report, literature review, asymptomatic, chronic disease, idiopathic
Introduction
The clinical spectrum and presentation of pericardial effusion vary from asymptomatic effusion to cardiac tamponade. Although relatively rare and with diverse etiologies, the most commonly reported form is pericardial effusion secondary to hypothyroidism, tuberculosis, and infection.1 Compared with other etiologies, recurrent chronic moderate to large idiopathic pericardial effusion is relatively rare. Clinically, there is no unified criterion or guidelines for “benign” pericardial effusion (without cardiac tamponade). A variety of conditions can lead to pericardial effusion, and the characteristics of the effusion, such as exudates or transudates and levels of adenosine deaminase (ADA) and interferon gamma (IFN-γ),2 are significant in making a diagnosis. The primary pathogenesis should be treated prior to the pericardial effusion itself, unless critical conditions such as cardiac tamponade or pericardial constriction are also present. The management of pericardial effusion is partially determined by the patient’s hemodynamic status, the volume of the effusion, and the severity of inflammation. Some effusions may decrease or even vanish after treatment of the primary condition, but pericardiocentesis should be the first priority in the case of cardiac tamponade. Pericardial constriction should also be treated actively to improve the patient’s prognosis, and the use of drugs or invasive therapies should be considered in the context of the hemodynamic disorders and cardiac tamponade.
Case report
A 77-year-old man complained of a 1-month history of dyspnea. He had received amiodarone (cumulative dose about 18 g) for atrial fibrillation 10 years ago. No pericardial effusion was detected by echocardiography prior to amiodarone treatment; however, 2 months after amiodarone treatment he developed pitting edema of the extremities, together with dyspnea and chest constriction. Echocardiography revealed a moderate to large pericardial effusion, and B-mode ultrasound showed mild pleural effusion and ascites. A thyroid function test showed hypothyroidism and thyroid puncture biopsy followed by pathologic investigation confirmed Hashimoto’s thyroiditis. His symptoms of edema and dyspnea became more severe after levothyroxine replacement therapy, despite normal thyroid function. Although his pleural effusion and ascites disappeared, a moderate to large pericardial effusion persisted for 10 years. His activity tolerance remained stable without cardiac tamponade. However, the patient was admitted because of worsening dyspnea.
The patient had a history of cervical lymph node tuberculosis for >40 years, hypertension, polycythemia vera with splenomegaly, hepatomegaly, hyperuricemia, and duodenal ulcer for >30 years, chronic renal insufficiency for >20 years, and atrial fibrillation for >10 years. The patient was a nuclear physicist and had received a high dose of 31P radiation exposure without protection 45 years ago. One colleague had died of hepatic malignant tumor; however, no other colleagues had any records of pericardial effusion, polycythemia vera, other hematologic diseases, or malignant tumors. The patient had no family history of inherent diseases.
The patient’s vital signs showed blood pressure of 140/85 mmHg, pulse 80 beats/minute, respiration 20 breaths/minute, and temperature 36.5°C. Physical examination revealed signs of jugular venous engorgement, peripheral edema, and dry skin. His thyroid was not palpable. There was bilateral basal rale in both lungs and distant heart sound without rub or murmur. His heart rate was 90 beats/minute and irregular, with pulse deficit. His abdomen was distended and his navel was protruding. The lower boundary of the liver and spleen were located at subcostal four and six fingers, respectively, and were hard on palpation. He had pitting edema, pigmentation, and low temperature in both lower extremities. The median cubital vein pressure was 14 to 16 cmH2O. Neurological examination results were normal.
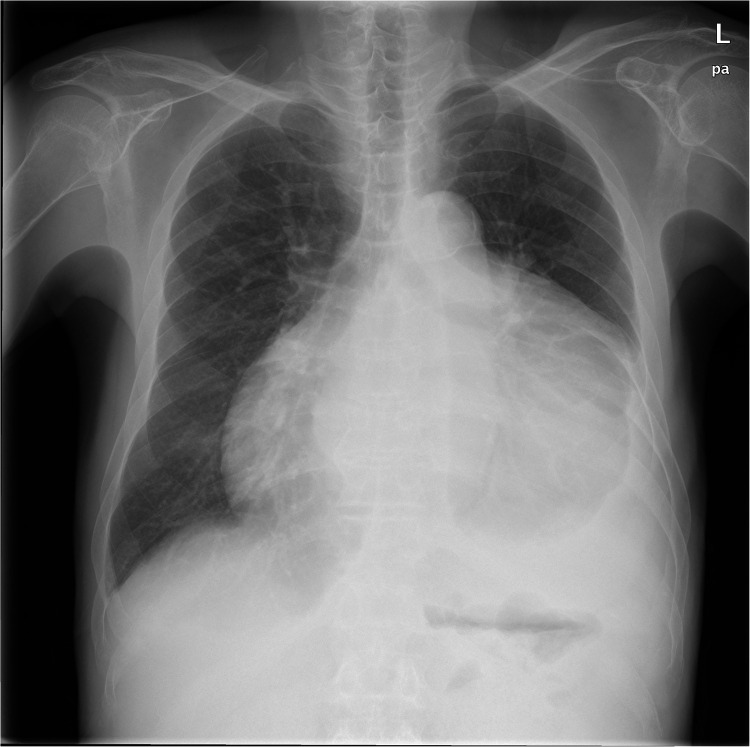
An electrocardiogram indicated atrial fibrillation, with low amplitude QRS complex. A chest X-ray showed extraordinary cardiac enlargement (Figure 1). The echocardiogram revealed a massive pericardial effusion with no signs of pericardial constriction (Figure 2). A computed tomography (CT) scan of the chest revealed hepatosplenomegaly, a widened portal vein, and massive pericardial effusion, with no evidence of neoplastic pericardial involvement. The maximum thickness of the pericardium (including pericardial effusion) measured by CT was 3.2 cm, with no thickening of the pericardial wall (Figure 3).
Figure 1.
Chest X-ray at admission. Chest radiography suggested pericardial effusion indicated by enlarged cardiac silhouette with globular appearance. No sign of pericardial calcification.
Figure 2.
Typical echocardiographic images. (a, b) Two-dimensional echocardiography images showed an echo-free space between the parietal pericardium and the epicardial surface of the myocardium.
Figure 3.
Typical computed tomography (CT) images. (a, b) Contrast CT scans of chest and abdomen showed a massive pericardial effusion, hepatosplenomegaly, and widened portal vein without malignant signs.
Arterial blood gas analysis revealed no hypoxia or hypercapnia. Biochemistry laboratory tests showed the following results: total cholesterol 115.8 mg/dL, triglycerides 70.8 mg/dL, creatine 1.02 mg/dL, creatine phosphokinase 85 U/L, lactate dehydrogenase (LDH) 166 U/L, and N-terminal pro brain natriuretic peptide 6873 pg/mL. Complete blood count showed normal white blood cell count and normal C-reactive protein, but his hemoglobin was 167 g/L and platelet count was 62 × 109/L.
The patient’s thyroid function was normal under levothyroxine replacement therapy: thyroid-stimulating hormone 3.4 μIU/mL, free T4 1.5 ng/dL, free T3 4.4 pg/mL, antithyroglobulin 32 U/mL, antithyroid peroxidase antibodies 27 U/mL, and thyroid stimulation-blocking antibody was negative.
The patient underwent pericardiocentesis with a wide-bore needle. The pericardial effusion showed yellow, epinephelos liquid with positive Rivalta test. The specific gravity was >1.025 and nucleated cells were 8920 × 106/L, with a monocyte proportion >85%. Total protein in the pericardial effusion was 46 g/L (serum level 64 g/L), albumin 28 g/L (serum level 40 g/L), LDH 117 U/L (serum level 166 U/L), glucose 4.6 mmol/L (serum level 5.1 mmol/L), and ADA 5.8 U/L (serum level 7.3 U/L). There were no signs of bacterial or fungal infection or tuberculosis based on smear examination and negative polymerase chain reaction (PCR) amplification of Mycobacterium tuberculosis. Carbohydrate antigen 125 (CA125) levels in the pericardial effusion ranged from 398 to 826 U/mL, with normal carbohydrate antigen 199 (CA199) and carcinoembryonic antigen (CEA). No tumor cells were detected in repeated smears. The patient also underwent thoracentesis, and routine tests of the pleural effusion showed similar characteristics to the pericardial effusion. Based on these findings, we did not perform paracentesis.
Primary treatment included thyroid hormone replacement therapy, intermittent diuretics, anti-platelet treatment (long-term, low-dose aspirin) to prevent thromboembolism, leukocyte-stimulating interferon for polycythemia vera, anti-hypertensive treatment, and an angiotensin converting enzyme inhibitor to improve ventricular remodeling.
Partial pericardial resection was recommended by the consulting cardiologist, but was refused by the patient. The patient showed no symptoms or signs of advanced heart failure or cardiac tamponade and no decrease in activity endurance during follow-up (Table 1). There were no signs of pericardial constriction detected at the last echocardiographic study.
Table 1.
Results of consequential echocardiographic imaging during follow-up.
|
Date |
|||||
|---|---|---|---|---|---|
| 2010-7-6 | 2010-7-16 | 2010-8-6 | 2011-7-29 | 2011-8-20 | |
| After puncture | After puncture | ||||
| Left atrial diameter (mm) | 54 | 55 | 59 | 65 | 61 |
| Left ventricular end-diastolic diameter (mm) | 55 | 50 | 53 | 57 | 56 |
| Left ventricular end-systolic diameter (mm) | 34 | 31 | 36 | 40 | 37 |
| Right atrial diameter(mm) | 57 × 47 | 57 × 48 | 62 × 45 | 68 × 48 | 68 × 52 |
| Interventricular septal thickness (mm) | 10 | 11 | 11 | 11 | 12 |
| Left ventricular wall thickness (mm) | 10 | 11 | 12 | 11 | 12 |
| Pulmonary artery systolic pressure (mmHg) | 41.4 | 38 | 59.6 | 51.3 | 41 |
| Left ventricular ejection fraction (%) | 67% | 68% | 59% | 57% | 62% |
| Pericardial effusion (cm) | |||||
| Left ventricular posterior wall | 5.9 | 2.1 | 5.0 | 1.9 | 0.5 |
| left ventricular lateral wall | 5.2 | 3.2 | 1.1 | 2.4 | 0.4 |
| Apical | 1.3 | 1.1 | 0.6 | ||
| Right ventricular free wall | 2.8 | 0.4 | 1.1 | 0.6 | 0.3 |
| top of the right atrium | 2.8 | 2.4 | 1.6 | 2.0 | 1.1 |
| Right ventricular anterior wall | 0.8 | 1.0 | 0.4 | ||
This case report was authorized by the Bioethics Committee of Beijing Friendship Hospital (document no: 2019-P2-208-01) and the need for informed consent was waived.
Discussion
Pericardial effusion is a relatively common clinical syndrome. The diagnosis of pericardial effusion is generally comfirmed by echocardiography, but determining its etiology may be difficult. Although many cases are idiopathic, a careful review of the patient’s medical history, physical examination, and laboratory tests can reveal the etiology in most patients. Broad and untargeted tests aimed at identifying the accurate diagnosis should be avoided.
Previous studies found that the most common causes of pericardial effusion were infections (viruses, bacteria, especially Mycobacterium tuberculosis), malignant tumors, connective tissue diseases, pericardial injury syndrome (e.g. post-acute myocardial infarction, post-traumatic pericarditis), metabolic diseases (e.g. hypothyroidism), myocardial pericardial disease (pericarditis, myocarditis, and heart failure), uremia, and idiopathic pericardial effusion. Tuberculosis is considered to be a major cause of pericardial effusion in developing countries. Laboratory analysis of the pericaridal effusion can faciliate the diagnosis of infectious and neoplastic pericardial effusions. Elevated tumor markers, such as CEA and carbohydrate antigens, may be detected in cases of suspected malignant diseases. ADA, LDH, and IFN-γ, as well as PCR analysis of tuberculosis, should be analyzed in cases with suspected tuberculosis pericardial effusion. The white cell count is usually elevated in patients with pericardial effusion induced by inflammatory and infectious diseases, but reduced in pericardial effusion related to myxedema and hypothyroidism.
The current patient presented with a >10-year history of chronic massive pericardial effusion, with no evidence of pericardial tamponade. The effusion was exudative according to routine and biochemical tests. Laboratory and imaging tests found no evidence of infection, neoplasm, trauma, uremia, post-acute myocardial infarction effusion, or aortic dessection. However, the patient had a history of hypothyroidism, high-dose 31P radiation, and polycythemia vera.
A review of the common etiologies below may aid an accurate diagnosis.
Regarding tuberculosis-related cases, previous studies found that about 10% of patients with tuberculous pericardial effusion developed pericardial tamponade.3,4 Over 80% of tuberculous pericardial effusions show typical exudative characteristics with a high protein content and nucleated cells, predominantly lymphocytes and monocytes. About 10% to 55% of patients with tuberculous pericarditis have positive acid-fast staining or positive Mycobacterium tuberculosis culture. Pericardial effusion caused by tuberculosis can be diagnosed by PCR amplification of tubercle bacillus DNA fragments.5
Measurement of ADA in the effusion is a fast and accurate method for diagnosing tuberculous pericarditis, with a reported diagnostic efficiency of 83% to 100%, sensitivity of 89% to 100%, and specificity of 74% to 100%.6 Mycobacterium tuberculosis-specific T cells release IFN-γ, which is a sign of tuberculosis infection. Although this test has some diagnostic value, it cannot differentiate between active and past infections,7 with a sensitivity of 73.0% to 100% (median 87.6%) and specificity of 85.0% to 99.6% (median 96.6%).2 Although the current patient had a history of lymph node tuberculosis, he showed no signs of fever, night sweats, weight loss, or other symptoms of tuberculosis, with negative serum tuberculosis antibody, negative pure protein derivative, normal erythrocyte sedimentation rate, negative in vitro IFN-γ and lymphocyte culture, normal ADA in the pericardial effusion, no sign of pericardial calcification, negative pericardial and pleural effusion tuberculous smears, and normal mycobacterium PCR amplification. There was thus no evidence of tuberculosis. However, several studies2,4 reported that patients in whom tuberculous pericardial effusion could not be confirmed were nevertheless administered experimental anti-tuberculosis treatment to reduce the pericardial effusion and prevent pericardial calcification or cardiac tamponade.
Pericardial effusion may also be caused by hypothyroidism. The incidence of pericardial effusion in patients with early-stage hypothyroidism is about 3%,8 reaching as high as 80% once myxedema appears.9 Hypothyroidism may lead to increased capillary permeability, lymphatic reflux disorder, and protein leaking into the interstitial space, subsequently resulting in pericardial effusion. Although hypothyroidism accounts for only about 1.5% of cases of pericardial effusion, it is a main cause of massive pericardial effusion. Pericardial effusions caused by hypothyroidism can be completely absorbed after thyroid hormone replacement therapy. The present patient had normal thyroid function 10 years previously, but pericardial effusion appeared after the development of hypothyroidism and then grew rapidly, together with pleural effusion and ascites. However, his pleural effusion and ascites disappeared soon after thyroid hormone replacement therapy, while a medium to large pericardial effusion persisted for 10 years. The patient’s thyroid function was normal after hormone replacement therapy, suggesting that Hashimoto’s thyroiditis and hypothyroidism were probably not the cause of his persistent pericardial effusion.
Iatrogenic radiation is commonly applied in mediastinal radiotherapy for malignant tumors, and the pericardium is commonly involved.10 Pericardial diseases may develop and remain symptomless for months to years after radiation,11 or restrictive pericarditis may occur soon after radiation, without a chronic course.12 Pericardial resection is the most effective therapy for restrictive pericarditis. The current patient had a history of high-dose 31P radiation exposure over 30 years ago; however, the radiation was systemic rather than focused on the chest. Moreover, colleagues who received similar radiation doses showed no pericardial diseases. Based on the above facts, we concluded that the pericardial effusion in this patient was unlikely to have been caused by radiation exposure.
The patient had polycythemia vera, possibly caused by 31P exposure, as well as hepatosplenomegaly and portal hypertension associated with the polycythemia vera. Reports of polycythemia vera complicated with pericardial effusion are limited, and pericardial effusion usually only occurred when the polycythemia vera developed to myelofibrosis or chronic myeloid leukemia.13 The present patient received bone marrow biopsy twice, and bone marrow pathology showed no signs of myelofibrosis or chronic myeloid leukemia, suggesting that his pericardial effusion was not induced by polycythemia vera.
Regarding nonspecific pericardial effusion and chronic idiopathic recurrent pericarditis, a previous study14 prospectively evaluated 1108 patients with pericarditis from 1977 to 1992. Among 461 patients with large pericardial effusion, 28 had idiopathic massive chronic effusion. The researchers accordingly concluded that idiopathic massive chronic pericardial effusion could be well tolerated for a long period in most patients.
A systematic review15 of all published studies of recurrent pericarditis from 1966 to 2006 analyzed 230 patients with idiopathic recurrent pericarditis from eight studies. The incidence of pericardial tamponade was only 3.5% during an average follow-up of >5 years, and there were no cases of constrictive pericarditis or left ventricular dysfunction.
Previous studies also demonstrated that the etiology of massive pericardial effusion remained ‘idiopathic’ in 7% to 48% of patients, including Han Chinese people.16–18
An observational study, representing the largest prospective cohort study reporting the outcome of idiopathic massive chronic pericardial effusion, showed that the outcome of ‘idiopathic’ massive chronic pericardial effusion was usually benign, and the risk of cardiac tamponade was only 2.2% per year.19 Idiopathic recurrent pericarditis thus has a relatively good prognosis, with rare complications and no report of restrictive pericarditis.
Based on the above studies and observations, we concluded that the most likely diagnosis in the current patient was recurrent chronic large idiopathic pericardial effusion. After 1 year of follow-up, the patient had normal thyroid funcion, and unchanged complete blood count and liver and kidney functions. The pericardial effusion volume remained massive without tamponade or calcification. Repeated pericardiocentesis revealed exudate with a high proportion of monocytes. All imaging features, including echocardiography, and chest and abdominal CT scans, were largely unchanged.
In terms of its management and prognosis, therapy for pericardial effusion should be aimed at its etiology if its cause is evident; however, various treatment options can be considered if the diagnosis is unclear or idiopathic.
Drug therapies are available for pericardial effusion, of which non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, and glucocorticoids are the most frequently used.
NSAIDs can relieve chest pain and alleviate inflammation. In an observational study,20 ibuprofen (300–800 mg every 6–8 hours or 600–2400 once daily), aspirin (650 mg every 6 hours or 2–4 g once daily), or diclofenac (150–200 mg once a day) were effective in 85% to 90% of patients with symptomatic pericardial effusion. Long-term treatment with NSAIDs needs to be administered in combination with mucosal protective agents to prevent gastrointestinal ulcers. NSAIDs should be continued until the patient’s symptoms improve and inflammatory markers normalize.21
The COlchicine for acute PEricarditis (COPE) trial22 was the first large randomized prospective study investigating standard treatment combined with colchicine for acute pericarditis. A total of 120 patients were enrolled and the mean follow-up duration was 18 months. The recurrence rate of effusion was reduced from 32.3% to 10.7% by the combination of standard therapy and colchicine. The design of the COlchicine for REcurrent pericarditis (CORE) trial23 was similar to the COPE trial, and colchicine significantly reduced the recurrence rate of pericardial effusion after a mean follow-up of 20 months.
Use of glucocorticoids was an independent risk factor for the recurrence of pericardial effusion in the COPE and CORE studies, probably because glucocorticoids can interfere with the efficacy of colchicine. Glucocorticoids should thus only be recommended in patients with pericarditis caused by immune or connective tissue diseases. A previous systematic review of randomized controlled trials investigating the effectiveness of adjuvant corticosteroids in tuberculous pericarditis showed that steroids could have positive effects on mortality and morbidity in patients with tuberculous pericarditis, but the included trials were too small to draw any conclusions and further large placebo-controlled trials are required.24 Treatment with colchicine has been reported to be highly effective in preventing recurrent pericarditis, while pretreatment with corticosteroids exacerbates and extends the course of recurrent pericarditis.25 The results of the IMPI trial, as the largest randomized controlled trial of glucocorticoid therapy in patients with tuberculous pericarditis, found no significant difference in primary outcomes between patients who received prednisolone and placebo.26
Pericardiocentesis is a life-saving intervention for acute cardiac tamponade, and should be performed immediately once severe pericardial effusion has been confirmed (with unstable hemodynamics). According to the Mayo Clinic Experience, echocardiography-guided pericardiocentesis is a simple, safe, and effective procedure for significant postoperative pericardial effusions.27
Regarding indwelling catheter drainage, it is recommended that an indwelling pigtail drainage catheter can be introduced over a guidewire during pericardiocentesis in patients with recurrent pericardial effusion caused by metastatic cancer. Pericardiocentesis with catheter drainage was found to be a relatively safe and effective treatment for pericardial effusion caused by malignant diseases.28
Surgical therapy is another option for pericardial effusion. Fenestration of the pericardium is the most common surgical treatment of chronic pericardial effusion, with the additional benefit of providing pericardial tissue for pathological diagnosis.
In the current case, the patient received long-term low-dose aspirin therapy but no glucocorticoids, because of the lack of evidence of pericardial adhesion or immune etiology. He refused both indwelling catheter drainage and surgical therapy.
The prognosis of pericardial effusion is essentially related to its etiology and the volume of the effusion. Idiopathic pericarditis has a very low risk of constrictive pericarditis. Based on a large sample-size, idiopathic massive chronic pericardial effusion may be well-tolerated for long periods in most patients, although severe tamponade can develop unexpectedly at any time.14 A recent study of a large cohort of patients with idiopathic large chronic pericardial effusion reported that its evolution was usually benign, with reduction in the size of the effusion in most cases and regression in about 40% of cases.19 In a meta-analysis of outcomes of pericardial effusion including 17,022 patients, the average mortality was 14.5%, but the correlation between idiopathic pericardial effusion and a poor prognosis was unclear.29 The frequency of follow-up of patients with pericardial effusion is mainly based on its etiology. Patients with large-volume effusions should be monitored by echocardiography, while urgent invasive procedures should be performed if cardiac tamponade or constrictive pericarditis occurs.
In summary, we report on an elderly male patient with a complex medical history and comorbidities, including hypothyroidism, high-dose 31P radiation exposure, polycythemia vera, and previous tuberculosis infection. His massive pericardial effusion lasted for over 10 years, without pericardial tamponade or constrictive pericarditis. Active tuberculosis infection, cancer, severe heart failure, uremia, cardiac trauma, severe bacterial or fungal infection, chronic myelogenous leukemia, and myelofibrosis were all excluded. The final diagnosis was recurrent chronic large idiopathic pericardial effusion, and we recommended regular echocardiography monitoring during follow-up. Moreover, although indwelling catheter drainage or surgical therapy may be considered as effective therapies, these were refused by the patient.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Research Foundation of Beijing Friendship Hospital, Capital Medical University [grant numbers yyqdkt2015-26 and yyqdkt2018-jx6].
ORCID iDs: Ying-shuo Huang https://orcid.org/0000-0002-5682-4661
References
- 1.Karam N, Patel P, DeFilippi C. Diagnosis and management of chronic pericardial effusions. Am J Med Sci 2001; 322: 79–87. [DOI] [PubMed] [Google Scholar]
- 2.Syed FF, Mayosi BM. A modern approach to tuberculous pericarditis. Prog Cardiovasc Dis 2007; 50: 218–236. DOI 10.1016/j.pcad.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Little WC, Freeman GL. Pericardial disease. Circulation 2006; 113: 1622–1632. DOI 10.1161/CIRCULATIONAHA.105.561514. [DOI] [PubMed] [Google Scholar]
- 4.Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation 2005; 112: 3608–3616. DOI 10.1161/CIRCULATIONAHA.105.543066. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Lee CW, Lee SG, et al. Comparison of polymerase chain reaction with adenosine deaminase activity in pericardial fluid for the diagnosis of tuberculous pericarditis. Am J Med 2002; 113: 519–521. [DOI] [PubMed] [Google Scholar]
- 6.Reuter H, Burgess LJ, Carstens ME, et al. Adenosine deaminase activity–more than a diagnostic tool in tuberculous pericarditis. Cardiovasc J S Afr 2005; 16: 143–147. [PubMed] [Google Scholar]
- 7.Higuchi K, Harada N, Fukazawa K, et al. Relationship between whole-blood interferon-gamma responses and the risk of active tuberculosis. Tuberculosis 2008; 88: 244–248. DOI 10.1016/j.tube.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Hardisty CA, Naik DR, Munro DS. Pericardial effusion in hypothyroidism. Clin Endocrinol (Oxf) 1980; 13: 349–354. [DOI] [PubMed] [Google Scholar]
- 9.Kabadi UM, Kumar SP. Pericardial effusion in primary hypothyroidism. Am Heart J 1990; 120: 1393–1395. [DOI] [PubMed] [Google Scholar]
- 10.Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015; 36: 2921–2964. DOI 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2013; 26: 1013–1032. DOI 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Szpakowski N, Desai MY. Radiation-associated pericardial disease. Curr Cardiol Rep 2019; 21: 97. DOI 10.1007/s11886-019-1192-y. [DOI] [PubMed] [Google Scholar]
- 13.Mesa RA, Barosi G, Cervantes F, et al. Myelofibrosis with myeloid metaplasia: disease overview and non-transplant treatment options. Best Pract Res Clin Haematol 2006; 19: 495–517. DOI 10.1016/j.beha.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Sagrista-Sauleda J, Angel J, Permanyer-Miralda G, et al. Long-term follow-up of idiopathic chronic pericardial effusion. N Engl J Med 1999; 341: 2054–2059. DOI 10.1056/NEJM199912303412704. [DOI] [PubMed] [Google Scholar]
- 15.Imazio M, Brucato A, Adler Y, et al. Prognosis of idiopathic recurrent pericarditis as determined from previously published reports. Am J Cardiol 2007; 100: 1026–1028. DOI 10.1016/j.amjcard.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Sagrista-Sauleda J, Merce J, Permanyer-Miralda G, et al. Clinical clues to the causes of large pericardial effusions. Am J Med 2000; 109: 95–101. DOI 10.1016/s0002-9343(00)00459-9. [DOI] [PubMed] [Google Scholar]
- 17.Levy PY, Corey R, Berger P, et al. Etiologic diagnosis of 204 pericardial effusions. Medicine (Baltimore) 2003; 82: 385–391. DOI 10.1097/01.md.0000101574.54295.73. [DOI] [PubMed] [Google Scholar]
- 18.Ma W, Liu J, Zeng Y, et al. Causes of moderate to large pericardial effusion requiring pericardiocentesis in 140 Han Chinese patients. Herz 2012; 37: 183–187. DOI 10.1007/s00059-011-3428-5. [DOI] [PubMed] [Google Scholar]
- 19.Imazio M, Lazaros G, Valenti A, et al. Outcomes of idiopathic chronic large pericardial effusion. Heart 2019; 105: 477–481. DOI 10.1136/heartjnl-2018-313532. [DOI] [PubMed] [Google Scholar]
- 20.Brucato A, Brambilla G, Adler Y, et al. Therapy for recurrent acute pericarditis: a rheumatological solution? Clin Exp Rheumatol 2006; 24: 45–50. [PubMed] [Google Scholar]
- 21.McNamara N, Ibrahim A, Satti Z, et al. Acute pericarditis: a review of current diagnostic and management guidelines. Future Cardiol 2019; 15: 119–126. DOI 10.2217/fca-2017-0102. [DOI] [PubMed] [Google Scholar]
- 22.Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005; 112: 2012–2016. DOI 10.1161/CIRCULATIONAHA.105.542738. [DOI] [PubMed] [Google Scholar]
- 23.Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med 2005; 165: 1987–1991. DOI 10.1001/archinte.165.17.1987. [DOI] [PubMed] [Google Scholar]
- 24.Ntsekhe M, Wiysonge C, Volmink JA, et al. Adjuvant corticosteroids for tuberculous pericarditis: promising, but not proven. QJM 2003; 96: 593–599. DOI 10.1093/qjmed/hcg100. [DOI] [PubMed] [Google Scholar]
- 25.Artom G, Koren-Morag N, Spodick DH, et al. Pretreatment with corticosteroids attenuates the efficacy of colchicine in preventing recurrent pericarditis: a multi-centre all-case analysis. Eur Heart J 2005; 26: 723–727. DOI 10.1093/eurheartj/ehi197. [DOI] [PubMed] [Google Scholar]
- 26.Mayosi BM, Ntsekhe M, Bosch J, et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med 2014; 371: 1121–1130. DOI 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang TS, Barnes ME, Hayes SN, et al. Clinical and echocardiographic characteristics of significant pericardial effusions following cardiothoracic surgery and outcomes of echo-guided pericardiocentesis for management: Mayo Clinic experience, 1979-1998. Chest 1999; 116: 322–331. [DOI] [PubMed] [Google Scholar]
- 28.Tsang TS, Seward JB, Barnes ME, et al. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc 2000; 75: 248–253. DOI 10.4065/75.3.248. [DOI] [PubMed] [Google Scholar]
- 29.De Filippo O, Gatti P, Rettegno S, et al. Is pericardial effusion a negative prognostic marker? Meta-analysis of outcomes of pericardial effusion. J Cardiovasc Med (Hagerstown) 2019; 20: 39–45. DOI 10.2459/Jcm.0000000000000720. [DOI] [PubMed] [Google Scholar]