Abstract
Purpose
To investigate the characteristics of complement activation products and angiogenic cytokines in the aqueous humor in eyes with pachychoroid neovasculopathy (PNV) and neovascular age-related macular degeneration (nAMD).
Methods
This was a prospective, comparative, observational study. All patients with choroidal neovascularization were classified as PNV without polyps, PNV with polyps (polypoidal choroidal vasculopathy [PCV]), or drusen-associated nAMD according to the presence or absence of pachychoroid features and soft drusen. This study included a total of 105 eyes. Aqueous humor samples were collected from 25 eyes with PNV without polyps, 23 eyes with PCV, and 24 eyes with drusen-associated nAMD before intravitreal anti-vascular endothelial growth factor (VEGF) injection and cataract surgery in 33 control eyes. Clinical samples were measured for complement component 3a (C3a), C4a, C5a, VEGF, and macrophage chemoattractant protein 1 (MCP-1) using a bead-based immunoassay.
Results
C3a and MCP-1 levels were significantly higher in PCV (P = 0.032 and P = 0.039, respectively) and drusen-associated nAMD (P = 0.01 for both comparisons) than in controls, and no difference was seen in C3a and MCP-1 levels between PNV and controls (P = 0.747 and P = 0.294, respectively). VEGF levels were significantly higher in PNV (P = 0.016), PCV (P = 0.009), and drusen-associated nAMD (P = 0.043) than in controls. In PNV, the VEGF levels elevated without elevated C3a and MCP-1.
Conclusions
PNV, PCV, and drusen-associated nAMD had significantly distinct profiles of complement activation products and cytokines in the aqueous humor.
Keywords: pachychoroid neovasculopathy, neovascular age-related macular degeneration, aqueous humor, complement, cytokine
Age-related macular degeneration (AMD) is a leading cause of irreversible visual impairment in older adults.1 AMD consists of two forms, dry and wet. Dry-form AMD shows atrophy of the retinal pigment epithelium (RPE). Choroidal neovascularization (CNV) is a common clinical feature in a type of wet-form AMD referred to as neovascular AMD (nAMD). CNV in nAMD is categorized as type 1 or type 2 based on the CNV location; CNV is located under the RPE in type 1 CNV and above the RPE in type 2 CNV.2 Typically, nAMD shows adjunctive clinical features, including multiple soft drusen and relatively thinner choroid, which can be observed clinically through optical coherence tomography (OCT).3
A new type 1 CNV with peculiar clinical features in older adults is referred to as polypoidal choroidal vasculopathy (PCV) or pachychoroid neovasculopathy (PNV). PCV was proposed by Yannuzzi et al.4 in 1990, and PNV was reported in 2015 by the same research group.5 Both PCV and PNV show common clinical features, except for polypoidal lesions. The common characteristics of PCV and PNV are thick choroid (so-called pachychoroid), few or no soft drusen, choroidal vascular hyperpermeability (CVH), dilated large choroidal vessel (pachyvessel), and predisposition of Asian populations.5,6 Differences in the pathophysiology of CNV among nAMD, PNV, and PCV have been noted in many published reports, but such differences are still controversial.
The cytokines play a pivotal role in CNV development. Previous studies have reported that the cytokine levels of IL-1β, IL-6, interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), and vascular endothelial growth factor (VEGF) may be involved in CNV formation. In addition, cytokine levels in the aqueous humor can be useful for predicting treatment effects.7,8 Among the cytokines mentioned above, VEGF7–16 and MCP-17,8,10,15,16 have been considered key cytokines in the development of CNV.17–19
The complement proteins are also important in CNV development. Deposition of complement proteins has been confirmed in drusen, and previous genetic studies have suggested involvement of the complement system with nAMD pathogenesis.20–24 It has been reported that complement proteins, such as Ba, complement component 3a (C3a), and C4a, which are complement-activation products of factor B, C3, and C4, respectively, were elevated in the aqueous humor of nAMD patients.25–27 Experimental research has suggested that local complement activation could upregulate angiogenic cytokines, resulting in CNV development.
Although it is assumed that complement proteins and cytokines play an important role in CNV formation, to our knowledge no reports have compared cytokines in the aqueous humor among PNV without polyps, PCV, and nAMD. Measurement of complement proteins and cytokines in the aqueous humor may reveal the pathophysiology of each CNV subtype. In the current study, we measured C3a, C4a, C5a, VEGF, and MCP-1 in the aqueous humor of eyes with PNV without polyps, PCV, or nAMD using cytometric bead arrays.
Methods
Patients
This prospective, comparative, observational study adhered to the tenets of the Declaration of Helsinki; the research ethics board approved the study before patient enrollment began at the Department of Ophthalmology of Fukushima Medical University Hospital, Fukushima City, Japan. All patients provided informed consent before enrollment.
All patients were over 50 years old. Seventy-two eyes of patients with treatment-naïve PNV, PCV, and drusen-associated nAMD were included. Thirty-three eyes of 33 patients who had received cataract surgery were recruited as controls. All patients underwent comprehensive ophthalmic examinations, including slit-lamp biomicroscopy with a non-contact fundus lens. Color fundus photographs and fluorescein angiography (FA) images were obtained using the TRC-DX fundus camera (Topcon, Tokyo, Japan). Fundus autofluorescence and indocyanine green angiography (ICGA) images were obtained using the HRA2 (Heidelberg Engineering, Heidelberg, Germany). OCT (Spectralis; Heidelberg Engineering) and OCT angiography (PLEX Elite 9000; Carl Zeiss Meditec, Jena, Germany) were also performed. All controls underwent FA and ICGA.
Classification
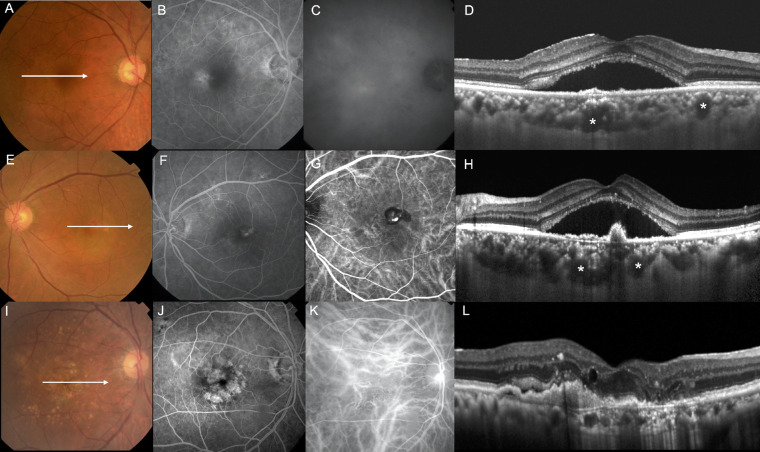
All eyes were classified into one of three subtypes: PNV, PCV, or drusen-associated nAMD (Table 1); Figure 1 shows the representative cases. Because the definition of PNV is still evolving, PNV was classified based on the latest reports.5,8,28 In our study, PNV characteristics included pachychoroid features, no soft drusen, and no polypoidal lesions (Figs. 1A–D). We defined pachychoroid features as satisfying all of the following criteria: (1) type-1 CNV detected in one or both eyes; (2) no or only non-extensive drusen (total area ≤ 125-µm circle) or hard drusen (≤63 µm) in both eyes; (3) dilated choroidal vessels below type 1 CNV detected by ICGA and OCT; (4) CVH detected on the late-phase ICGA images; and (5) the presence of central serous chorioretinopathy (CSC)- or pachychoroid pigment epitheliopathy-related RPE abnormalities independent of CNV lesions detected by FA or a history of CSC. PCV characteristics included pachychoroid features, polypoidal lesions, and no soft drusen (Figs. 1E–H). The eyes satisfying PNV criteria and having polypoidal lesions were defined as PCV. Polypoidal lesions were diagnosed in the presence of terminal aneurysmal dilatations with a branching vascular network in ICGA corresponding to steep RPE elevations on OCT images. When the eyes with definitive pachychoroid features showed pachydrusen (drusen-like deposits), the eyes were classified into PCV or PNV. We defined pachydrusen based on the following criteria reported in a recent review by Yanagi28: (1) size > 125 µm (large drusen), (2) irregular outer contours with better defined outer borders, (3) scattered distribution over the posterior pole, and (4) present in isolation or in groups of only a few yellow–white deposits. Subjects with soft drusen and pachydrusen or borderline cases were excluded. Drusen-associated nAMD had soft drusen in either eye and no pachychoroid features (Figs. 1I–L). Eyes with retinal angiomatous proliferation were excluded. This category would be the closest to Caucasian nAMD.
Table 1.
Disease Classification
| Pachychoroid | Soft | Polypoidal | |
|---|---|---|---|
| Disease | Features* | Drusen | Lesions |
| Pachychoroid spectrum disease | |||
| PNV | + | – | – |
| PCV | + | – | + |
| Drusen-associated nAMD | – | + | – |
Choroidal vascular hyperpermeability, dilated large choroidal vessel (pachyvessel), CSC- or PPE-related RPE abnormalities independent of CNV lesions, or a history of CSC.
Figure 1.
Right eye of a 53-year-old male with PNV: (A) Color fundus photography revealed no drusen. (B) FA in the early phase revealed granular leakage. (C) ICGA in the late phase revealed CVH. (D) OCT along the white lines demonstrated irregular RPE detachment, serous retinal detachment (SRD), and thick choroid with pachyvessels (asterisks). Left eye of a 62-year-old female with PCV: (E) Color fundus photography revealed SRD and polypoidal lesions. (F) FA in the early phase revealed granular leakage. (G) ICGA in the early phase revealed polypoidal lesions and branching vascular network. (H) OCT along the white lines demonstrated SRD, thick choroid with pachyvessels (asterisks), and acute RPE elevation (polyps). Right eye of an 80-year-old male with drusen-associated nAMD: (I) Color fundus photography revealed soft drusen and RPE abnormalities. (J) FA in the early phase revealed granular leakage and window defects. (K) ICGA in the early phase revealed CNV. (L) OCT along the white lines demonstrated SRD, macular edema, and relatively thin choroid.
The exclusion criteria were eyes with a history of any other retinal diseases; uveitis; glaucoma, including ocular hypertension, with the use of antiglaucoma eye drops; any intraocular surgery, including cataract surgery; high myopia (spherical equivalent < –6 diopters or axial length > 26.5 mm); or eyes with a treatment history of intravitreal anti-VEGF injections. Patients were excluded who had any systemic diseases potentially involving complement system activation, such as diabetes, autoimmune diseases, cancer, cardiovascular disease, cerebrovascular disease, or systemic corticosteroid medications. In controls, eyes with soft drusen or pachychoroid features were excluded.
Aqueous Humor Collection
Aqueous humor samples of eyes with PNV, PCV, or drusen-associated nAMD were aspirated before intravitreal injection of aflibercept (Eylea; Regeneron Pharmaceuticals, Tarrytown, NY, USA) or ranibizumab (Lucentis; Genentech, South San Francisco, CA, USA) under topical anesthesia using a syringe with a 30-gauge needle (Nipro, Osaka, Japan). The procedure and timing of sample collection were standardized to minimize variance due to the sampling procedure. The aqueous humor of controls was aspirated before cataract surgery in the same manner. The aqueous humor samples were immediately mixed with 2 µL of protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) to prevent complement and anaphylatoxin activation. Aliquots of the obtained aqueous humor samples were stored at –80°C until analysis.
Measurement of Complement Activation Products and Cytokine Levels
Levels of anaphylatoxins (C3a, C4a, and C5a) in the aqueous humor were measured using a BD CBA Human Anaphylatoxin Kit (BD Biosciences, San Jose, CA, USA). The levels of cytokines (VEGF and MCP-1) in the aqueous humor were measured using a BD Human Soluble Protein Master Buffer Kit (BD Biosciences). The bead-based immunoassays were performed according to the manufacturer's instructions.
Image Analysis
Soft drusen and pigmentary abnormalities were evaluated using color fundus photographs. Soft drusen were graded based on the simplified severity scale for AMD from the Age-Related Eye Disease Study.29 The central retinal thickness (CRT), presence of subretinal and intraretinal fluid, and subfoveal choroidal thickness (SFCT) were evaluated on OCT images. CRT and SFCT were measured using the caliper function of the Spectralis instrument. CRT was defined as the distance between the RPE surface and the internal limiting membrane surface. SFCT was defined as the distance between the hyperreflective line corresponding to Bruch's membrane beneath the RPE and the chorioscleral border. Complete posterior vitreous detachment was defined as no vitreous attachment to the retina and disc surface on both vertical and horizontal spectral-domain OCT scans. The diagnosis of polypoidal lesions was confirmed by the presence of dilated polyps at the end of the branching vascular network on ICGA images.7 The presence of CVH was defined as multifocal hyperfluorescence seen on middle- and late-phase ICGA images, as judged by two masked retinal specialists (YK and YO). The kappa value of CVH judgment by the two graders was 0.72. When the retina specialists did not agree, a third grader (TS) made the final judgment regarding CVH. The greatest linear dimension was measured manually, using a tool in IMAGEnet software (Topcon), as the lesion dimension covering the area of CNV, including the areas of dye leakage, pigment epithelial detachment, and subretinal hemorrhage on fluorescein angiography. The CNV lesion size was calculated manually in each early ICGA image using the HRA2 Image Explorer “draw lesion” tool.
Statistical Analysis
The Mann–Whitney U test was used to compare the levels of anaphylatoxins and cytokines in the aqueous humor among each group. The Kruskal–Wallis test was used to compare the levels of anaphylatoxins and cytokines in the aqueous humor among the CNV subtypes. Post hoc analysis was performed using the Steel–Dwass procedure. Multidimensional scaling (MDS) multivariate analysis provides a means of visualizing the level of similarity of individual cases of a dataset.30 This method classifies relationships among objects by placing points in a low-dimensional space, as in the case of principal component analysis. The relationships are visualized so that similar ones are placed closer together and different ones are placed farther apart. Statistical analyses were performed using JMP 14 software (SAS Institute, Cary, NC, USA). P < 0.05 was considered statistically significant. The results of the power calculation are shown in Table 2.
Table 2.
Levels of Complement Activation Products and Cytokines in the Aqueous Humor of Patients with CNV and in Controls
| Eyes with CNV | |||||||
|---|---|---|---|---|---|---|---|
| Analyte | PNV (n = 25) Median (IQR) | PCV (n = 23) Median (IQR) | Drusen-Associated nAMD (n = 24) Median (IQR) | P * [Statistical Power†] | Total (N = 72)Median (IQR) | Controls (n = 33) Median (IQR) | P ‡ [Statistical Power§] |
| C3a (ng/mL) | 2.34 (1.97–2.94) | 3.12 (2.21–3.48) | 2.95 (2.36–3.57) | 0.035 [0.65] | 2.7 (2.14–3.35) | 2.2 (1.52–2.82) | 0.004 [0.83] |
| C4a (ng/mL) | 1.47 (1.21–1.80) | 1.77 (1.48–2.08) | 1.97 (1.41–2.43) | 0.046 [0.58] | 1.69 (1.35–2.09) | 1.48 (1.03–1.92) | 0.11 [0.48] |
| C5a (ng/mL) | 0.018 (0.010–0.021) | 0.017 (0.009–0.031) | 0.027 (0.012–0.067) | 0.25 [0.50] | 0.018 (0.010–0.033) | 0.032 (0.015–0.056) | 0.049 [0.18] |
| VEGF (pg/mL) | 42.0 (26.3–53.2) | 40.4 (27.3–67.8) | 38.6 (28.5–49.1) | 0.79 [0.18] | 40.1 (27.3–52.6) | 25.3 (17.1–36.0) | 0.001 [0.80] |
| MCP-1 (pg/mL) | 273.9 (188.1–371.4) | 306.7 (246.7–427.4) | 311.9 (242.6–618.0) | 0.24 [0.47] | 305.8 (221.4–423.1) | 201.3 (122.4–305.5) | 0.001 [0.83] |
Kruskal–Wallis test among CNV subtypes.
Statistical power among the three categories.
Mann–Whitney U test between the eyes with CNV and controls.
Statistical power between total eyes with CNV and controls.
Results
Clinical Features
Table 3 shows the demographic and clinical characteristics of patients with PNV, PCV, or drusen-associated nAMD and of the controls. A total of 105 patients (72 patients with CNV, 33 controls) were enrolled. The mean age was 73.5 ± 8.9 years (mean ± SD) for eyes with CNV and 72.3 ± 6.3 years in the control group (P = 0.17). The mean age of the patients with drusen-associated nAMD was higher than that of the controls (P = 0.01) and the PNV group (P = 0.02) by pairwise analysis. The percentage of female patients with CNV (n = 21, 29.1%) was lower than that in the control group (n = 17, 51.5%; P = 0.28). In the 72 eyes with CNV, the number of eyes with PNV, PCV, or drusen-associated nAMD was 25 (35%), 23 (32%), and 24 (33%), respectively. There were no significant differences in age, sex, equivalent spherical power, incidence of posterior vitreous detachment, subretinal fluid and pigmentary abnormalities, greatest linear dimension, or CNV lesion size among the three subtypes. CRT for the PNV, PCV, and drusen-associated nAMD groups (median, 302, 414, and 392 µm, respectively; interquartile range [IQR], 221–441, 285–482, and 348–572, respectively; P = 0.029), incidence rates of intraretinal fluid (numbers of eyes, 1, 2, and 8, respectively; P = 0.011), soft drusen (numbers of eyes, 0, 0, and 11, respectively; P < 0.001), and SFCT (median, 340, 285, and 210 µm, respectively; IQR, 256–401, 226–353, and 146–258, respectively; P = 0.003) differed among the three subtypes. SFCT was greater in the PNV group (median, 340 µm; IQR, 257–401) than in the drusen-associated nAMD group (median, 210 µm; IQR, 146–258; P = 0.001), whereas there were no differences in SFCT between the PNV and PCV groups (median, 285 µm; IQR, 226–353; P = 0.301) (Fig. 2).
Table 3.
Demographics of Patients with CNV and Controls
| Eyes with CNV | |||||||
|---|---|---|---|---|---|---|---|
| PNV (n = 25) | PCV (n = 23) | Drusen-Associated nAMD (n = 24) | P * | Total CNV (N = 72) | Controls (n = 33) | P † | |
| Age (y), mean ± SD | 70.8 ± 9.4 | 72.6 ± 7.7 | 76.7 ± 8.6 | 0.054 | 73.5 ± 8.9 | 72.3 ± 6.3 | 0.17 |
| Sex, female, n (%) | 4 (16.0) | 6 (26.1) | 11 (45.8) | 0.066 | 21 (29.1) | 17 (51.5) | 0.028 |
| Complete PVD, n (%) | 12 (48.0) | 13 (56.5) | 11 (45.8) | 0.95 | 39 (54.2) | 20 (60.6) | 0.54 |
| Spherical equivalent (D), median (IQR) | 0.38 (–1.31 to 1.44) | 0 (–1.38 to 0.75) | –0.25 (–1.66 to 1.50) | 0.79 | 0.06 (–1.37 to 1.47) | –0.25 (–1.87 to 0.25) | 0.20 |
| CRT (µm), median (IQR) | 302 (221–441) | 414 (285–482) | 392 (348–572) | 0.029 | 379 (284–480) | — | — |
| SFCT (µm), median (IQR) | 340 (256–401) | 285 (226–353) | 210 (146–258) | 0.003 | 274 (204–351) | — | — |
| GLD (µm), median (IQR) | 3.5 (2.7–4.7) | 3.9 (3.5–5.0) | 4.9 (3.2–6.4) | 0.13 | 4.0 (2.9–5.2) | — | — |
| CNV size (mm2), median (IQR) | 1.4 (0.6–4.2) | 1.1 (0.7–2.5) | 2.2 (1.2–4.7) | 0.11 | 1.4 (0.8–3.5) | — | — |
| SRF, n (%) | 20 (80.0) | 21 (91.3) | 17 (70.8) | 0.18 | 58 (80.5) | — | — |
| IRF, n (%) | 1 (4.0) | 2 (8.7) | 8 (33.3) | 0.011 | 11 (15.3) | — | — |
| Soft drusen, n (%) | 0 (0) | 0 (0) | 11 (45.8) | <0.001 | 11 (10.6) | — | — |
| Pigmentary abnormalities, n (%) | 21 (84.0) | 20 (86.9) | 21 (87.5) | 0.93 | 62 (59.1) | — | — |
GLD, greatest linear dimension; SRF, subretinal fluid; IRF, intraretinal fluid.
Kruskal–Wallis test among CNV subtypes.
Mann–Whitney U test between the eyes with CNV and controls.
Figure 2.
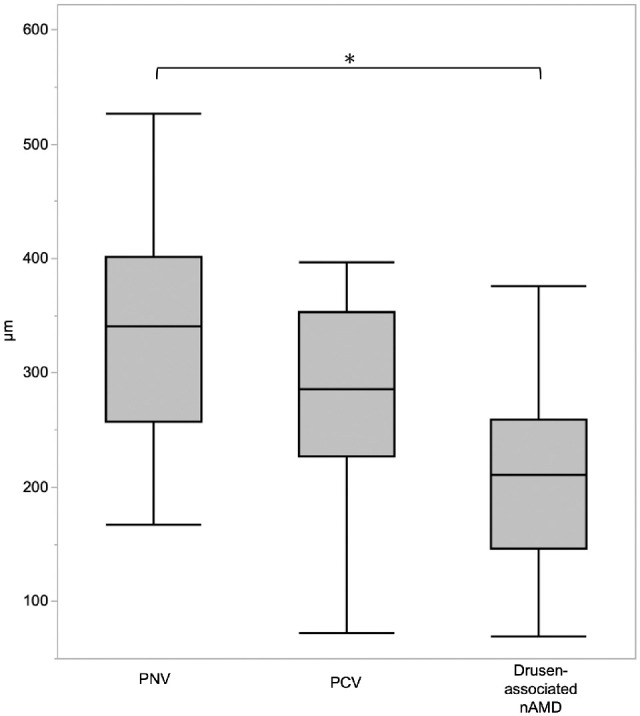
Box plot showing SFCT of CNV patients. SFCT of PNV was greater than that for drusen-associated nAMD (P = 0.001). *P < 0.05, Steel–Dwass test.
Concentrations of Complement Activation Products and Cytokines
The median levels of C3a, C4a, C5a, VEGF, and MCP-1 in the aqueous humor were 2.70 ng/mL (IQR, 2.14–3.35), 1.69 ng/mL (IQR, 1.35–2.09), 0.018 ng/mL (IQR, 0.010–0.033), 40.1 pg/mL (IQR, 27.3–52.6), and 305.8 pg/mL (IQR, 221.4–423.1), respectively, in the eyes with CNV. In the controls, the respective levels were 2.20 ng/mL (IQR, 1.52–2.82), 1.48 ng/mL (IQR, 1.03–1.92), 0.032 ng/mL (IQR, 0.015–0.056), 25.3 pg/mL (IQR, 17.1–36.0), and 201.3 pg/mL (IQR, 122.4–305.5) (Table 3). The concentrations of C3a (P = 0.004), VEGF (P = 0.001), and MCP-1 (P = 0.001) were significantly higher in the eyes with CNV compared to controls. Because the C5a level was lower than the limit of detection in seven eyes, they were excluded. Although the C5a level in the eyes with CNV was lower than in the control group (P = 0.049), the statistical power was low (0.18).
The post hoc analysis among the subtypes showed that C3a levels were significantly higher in the PCV group (P = 0.032) and drusen-associated nAMD group (P = 0.01) than in the control group (Fig. 3). There were no significant differences in C4a levels between the eyes with CNV and the controls (P = 0.11). There were also no differences in C5a levels among the PNV, PCV, and drusen-associated nAMD groups and the controls. VEGF levels in the PNV (P = 0.016), PCV (P = 0.009), and drusen-associated nAMD (P = 0.043) groups were higher than in the control group. There were no differences in VEGF levels among the PNV, PCV, and drusen-associated nAMD groups. MCP-1 levels in the PCV (P = 0.039) and drusen-associated nAMD (P = 0.011) groups were significantly higher than in the controls, but they did not differ significantly between the PNV group and the controls (P = 0.294).
Figure 3.
Box plot showing median levels of complement activation products and cytokines in aqueous humor of CNV patients and controls. *P < 0.05, Steel–Dwass test. (A) Median levels of C3a were higher in PCV and drusen-associated nAMD than in controls (PCV vs. controls, P = 0.032; drusen-associated nAMD vs. controls, P = 0.010). (B) There were no significant differences in the levels of C4a for each CNV subtype. (C) The levels of C5a were higher in controls than in PNV (P = 0.039). (D) VEGF levels were higher in all CNV subtypes compared to controls (PNV vs. controls, P = 0.016; PCV vs. controls, P = 0.009; drusen-associated nAMD vs. controls, P = 0.043). (E) MCP-1 levels were higher in PCV and drusen-associated nAMD than in controls (PCV vs. controls, P = 0.039; drusen-associated nAMD vs. controls, P = 0.011).
In the controls, significant correlations were seen among C3a, C4a, C5a, VEGF, and MCP-1, except for C3a versus VEGF (P = 0.069) and C5a versus VEGF (P = 0.145) (3Table 4). In the PNV, no significant differences were seen among the proteins measured except for C3a versus C4a (rs = 0.578, P = 0.003). In the PCV group, positive correlations were seen between C3a and VEGF (rs = 0.601, P = 0.002), C3a and MCP-1 (rs = 0.608, P = 0.002), C4a and MCP-1 (rs = 0.452, P = 0.031), and VEGF and MCP-1 (rs = 0.767, P < 0.001). In the drusen-associated nAMD group, positive correlations were seen between C3a and C4a (rs = 0.664, P < 0.001), C3a and C5a (rs = 0.501, P = 0.014), C3a and MCP-1 (rs = 0.619, P = 0.001), C4a and MCP-1 (rs = 0.452, P = 0.027), C5a and MCP-1 (rs = 0.444, P = 0.034), and VEGF and MCP-1 (rs = 0.629, P = 0.001). VEGF levels were not correlated with C3a, C4a, and C5a levels, but MCP-1 levels were correlated with all of them in the drusen-associated nAMD group.
Table 4.
Spearman's Rank Correlation Coefficients for Complement Activation Products and Cytokines in the Aqueous Humor of Patients with CNV and in Controls
| C3a | C4a | C5a | VEGF | MCP-1 | |
|---|---|---|---|---|---|
| Controls | |||||
| C3a | 1 | — | — | — | — |
| C4a | 0.767* | 1 | — | — | — |
| C5a | 0.619* | 0.625* | 1 | — | — |
| VEGF | 0.321 | 0.405* | 0.263 | 1 | — |
| MCP-1 | 0.511* | 0.578* | 0.351* | 0.589* | 1 |
| PNV | |||||
| C3a | 1 | — | — | — | — |
| C4a | 0.578* | 1 | — | — | — |
| C5a | 0.045 | 0.133 | 1 | — | — |
| VEGF | –0.049 | –0.029 | 0.164 | 1 | — |
| MCP-1 | 0.187 | 0.142 | –0.089 | 0.372 | 1 |
| PCV | |||||
| C3a | 1 | — | — | — | — |
| C4a | 0.402 | 1 | — | — | — |
| C5a | 0.235 | –0.142 | 1 | — | — |
| VEGF | 0.601* | 0.295 | 0.218 | 1 | — |
| MCP-1 | 0.608* | 0.452* | –0.101 | 0.767* | 1 |
| Drusen-associated nAMD | |||||
| C3a | 1 | — | — | — | — |
| C4a | 0.664* | 1 | — | — | — |
| C5a | 0.501* | –0.012 | 1 | — | — |
| VEGF | 0.331 | 0.325 | 0.281 | 1 | — |
| MCP-1 | 0.619* | 0.452* | 0.444* | 0.629* | 1 |
P < 0.05 was considered significant.
Similarity
MDS was determined by plotting the mean value of each concentration on a map to visualize the similarities of the complement activation products and cytokines in each group (Fig. 4). The PNV, PCV, and drusen-associated nAMD mean values were plotted at almost the same distances from the controls. The PCV mean value was plotted between drusen-associated nAMD and PNV. Among the complement activation products and cytokines measured in the current study, all three subtypes had low similarity to the controls; the profiles of PNV and drusen-associated nAMD differed the most.
Figure 4.
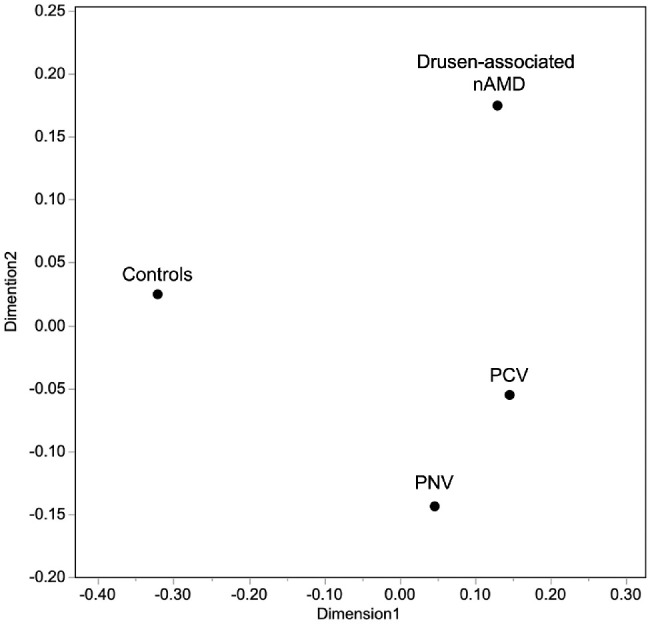
Multidimensional scaling plot for the means of C3a, C4a, C5a, VEGF, and MCP-1.
Discussion
C3a, VEGF, and MCP-1 levels in the aqueous humor of the eyes with drusen-associated nAMD and PCV were higher than those of controls. By contrast, C3a and MCP-1 levels in the aqueous humor of the eyes with PNV did not differ from those in the control group, but the VEGF level was significantly higher in the PNV than in the control group. The measurement items showing the correlation were different for each disease type.
Previous genetic studies have suggested an association between the complement system and AMD.22,31 Also, elevation of complement activation products (complement factors Ba, H, I, C3a,25,26 and C4a27) in the aqueous humor have been reported. Many cytokines have also been reported as causative factors associated with nAMD, VEGF, MCP-1, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, IL-6, IL-8, IP-10, C-reactive protein, angiopoietin-2, human growth factor, tissue inhibitor of metalloproteinases 1, C-X-C motif chemokines 12 and 13, tumor necrosis factor-α, IL-31, leukemia inhibitory factor, and stromal cell-derived factor-1-α.7–16,32–36 A recent study that summarized these cytokine networks using cluster analysis suggested that VEGF and MCP-1 were key factors in the development of nAMD.15 Although complement factors and angiogenic cytokines were associated with nAMD development, their relationships in the aqueous humor have not been fully determined.
The present study showed that C3a, VEGF, and MCP-1 levels were elevated in drusen-associated nAMD. Previous studies have suggested that C3a and C5a can be involved in angiogenesis, especially in eyes with drusen.17,19 MCP-1 can reportedly initiate angiogenesis by stimulating macrophages and the RPE. In the current study, although VEGF levels were elevated in drusen-associated nAMD, there was no correlation with C3a, C4a, or C5a; however, the VEGF elevation was correlated with an elevation in MCP-1. A positive regulatory feedback loop between VEGF and MCP-1 expression by vascular endothelial cells has been suggested.37 The profile of the aqueous humor suggests that an increase in VEGF production could be mediated by MCP-1. MCP-1 exhibits a chemotactic activity for monocytes and basophils. Histopathologic studies suggest that monocytes accumulate in the choroidal neovascular membrane and choroid in nAMD.38,39 Macrophages can reportedly be involved in angiogenesis40 and may play a pivotal role in CNV development in eyes with drusen.
Aqueous humors of the PNV group showed low C3a and high VEGF levels, whereas aqueous humors of the drusen-associated nAMD group showed high C3a and high VEGF levels. One could speculate that PNV might be early-stage nAMD in which drusen is inconspicuous; however, it has been reported that C3a was elevated even in early-stage nAMD.26 The pathophysiology may be fundamentally different between PNV and drusen-associated nAMD. It seems unlikely that neovascularization in PNV is driven by the complement system. Previous studies have reported a large choroidal vein and Sattler's layer attenuation on OCT images in PNV. Pang and Freund5 and Cheung et al.6 postulated that focal circulatory disturbances in areas of Sattler's layer attenuation in the pachychoroid may upregulate VEGF, resulting in CNV development. Hypoxia of the choriocapillaris can induce VEGF without complement activation and promote neovascularization (hypoxia-driven angiogenesis). This hypothesis may explain our results.
Although pachychoroid disease is characterized by a thick choroid, the pathogenesis of the choroidal thickening has not been elucidated. SFCT was significantly greater in the PNV group than in the drusen-associated nAMD group in the current study. The high VEGF and low inflammation indicated by C3a and MCP-1 can be associated with choroidal thickening.
C3a, VEGF, and MCP-1 levels were elevated in the PCV group, similar to what was observed for the drusen-associated nAMD group, whereas the clinical features of PCV mostly overlapped with those of PNV. The obvious difference between PNV and PCV was the polypoidal lesions. Recent experimental reports have suggested that C3a can stimulate depositions of the collagenous layer and result in loosened adhesion of the RPE.41 If hypoxia-driven angiogenesis occurs and neovascular vessels extend to the areas with focally loosened RPE adhesion, then tangled vessels could form under the RPE. In turn, circulatory abnormalities caused by tangled vessels could induce complement activation.
Individual difference of immunity may be associated with C3a and MCP-1 levels. Systemically, C3a levels in plasma increase,42 but not those of MCP-1, in PCV patients.43 The chemokine receptors between PCV and nAMD were reportedly different.44 The difference of systemic immunity may change the phenotypes of CNV. Further studies are needed to elucidate the pathophysiology of PCV.
The current study had several limitations. First, we did not measure other angiogenic cytokines, such as IL-6, IL-8, and IP-10, or complement factors B, D, H, and I. To determine interactions between the complement system and angiogenic cytokines, we must measure these proteins extensively. Second, we could not measure one of the complement activation products, C5a, in all cases, because of the measurement range of the cytometric bead array. C5a has various important effects on the inflammatory process, including increased vascular permeability, release of proinflammatory molecules, and monocyte migration. Measurement of C5a levels in the aqueous humor by enzyme-linked immunosorbent assays should be required in the future. Third, all proteins associated with CNV under the retina may not reflect the aqueous humor profiles, although we have previously confirmed correlation of anaphylatoxin levels between the vitreous and aqueous humor.45 We should also consider protein production from other parts of the eye when we interpret cytokine concentrations in the aqueous humor. Fourth, we had to recruit control eyes with cataract, which could be associated with intraocular inflammation; however, it is ethically difficult to collect aqueous humor from completely healthy eyes. The severity of cataract should be evaluated in future studies. Fifth, there were significant differences in age and gender among the PNV, PCV, and drusen-associated nAMD groups. These differences could have influenced the results and should be taken into consideration when interpreting differences among the three categories.
In conclusion, PNV, PCV, and drusen-associated nAMD showed significantly distinct profiles of complement activation products and cytokines in the aqueous humor. Our results suggest that differences in the actions of complement systems and cytokines led to differences in the respective pathophysiologies of PNV, PCV, and drusen-associated nAMD. The profiling of complement activation products and cytokines in the aqueous humor can be useful to evaluating the biologic characteristics of CNV.
Acknowledgments
The authors thank Yumi Ishida for providing detailed advice regarding protein measurement.
Supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI JP17K11427).
Disclosure: Y. Kato, None; Y. Oguchi, None; T. Omori, None; H. Shintake, None; R. Tomita, None; A. Kasai, None; M. Ogasawara, None; Y. Sugano, None; K. Itagaki, None; A. Ojima, None; T. Machida, None; H. Sekine, None; T. Sekiryu, Alcon Japan (F), Novartis Pharmaceuticals (F), Bayer Yakuhin (F), Santen Pharmaceutical (F)
References
- 1. Congdon N, O'Colmain B, Klaver CC, et al.. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004; 122(4): 477–485. [DOI] [PubMed] [Google Scholar]
- 2. Gass JDM. Stereoscopic Atlas of Macular Diseases. St. Louis, MO: Mosby; 1997: 26–30. [Google Scholar]
- 3. Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016; 50: 1–24. [DOI] [PubMed] [Google Scholar]
- 4. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10(1): 1–8. [PubMed] [Google Scholar]
- 5. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015; 35(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 6. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye (Lond). 2019; 33(1): 14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakamoto S, Takahashi H, Tan X, et al.. Changes in multiple cytokine concentrations in the aqueous humour of neovascular age-related macular degeneration after 2 months of ranibizumab therapy. Br J Ophthalmol. 2018; 102(4): 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terao N, Koizumi H, Kojima K, et al.. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci Rep. 2018; 8(1): 10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Funk M, Karl D, Georgopoulos M, et al.. Neovascular age-related macular degeneration: intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology. 2009; 116(12): 2393–2399. [DOI] [PubMed] [Google Scholar]
- 10. Jonas JB, Tao Y, Neumaier M, Findeisen P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch Ophthalmol. 2010; 128(10): 1281–1286. [DOI] [PubMed] [Google Scholar]
- 11. Miao H, Tao Y, Li XX. Inflammatory cytokines in aqueous humor of patients with choroidal neovascularization. Mol Vis. 2012; 18: 574–580. [PMC free article] [PubMed] [Google Scholar]
- 12. Muether PS, Neuhann I, Buhl C, Hermann MM, Kirchhof B, Fauser S. Intraocular growth factors and cytokines in patients with dry and neovascular age-related macular degeneration. Retina. 2013; 33(9): 1809–1814. [DOI] [PubMed] [Google Scholar]
- 13. Hu J, Leng X, Hu Y, et al.. The features of inflammation factors concentrations in aqueous humor of polypoidal choroidal vasculopathy. PLoS One. 2016; 11(1): e0147346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hata M, Yamashiro K, Ooto S, et al.. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58(1): 292–298. [DOI] [PubMed] [Google Scholar]
- 15. Sato T, Takeuchi M, Karasawa Y, Takayama K, Enoki T. Comprehensive expression patterns of inflammatory cytokines in aqueous humor of patients with neovascular age-related macular degeneration. Sci Rep. 2019; 9(1): 19447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou H, Zhao X, Yuan M, Chen Y. Comparison of cytokine levels in the aqueous humor of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration patients. BMC Ophthalmology. 2020; 20(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nozaki M, Raisler BJ, Sakurai E, et al.. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006; 103(7): 2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009; 46(14): 2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long Q, Cao X, Bian A, Li Y. C3a increases VEGF and decreases PEDF mRNA levels in human retinal pigment epithelial cells. Biomed Res Int. 2016; 2016: 6958752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308(5720): 421–424. [DOI] [PubMed] [Google Scholar]
- 21. Biro A, Prohaszka Z, Fust G, Blasko B. Determination of complement factor H functional polymorphisms (V62I, Y402H, and E936D) using sequence-specific primer PCR and restriction fragment length polymorphisms. Mol Diagn Ther. 2006; 10(5): 303–310. [DOI] [PubMed] [Google Scholar]
- 22. Gold B, Merriam JE, Zernant J, et al.. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006; 38(4): 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaumberg DA, Hankinson SE, Guo Q, Rimm E, Hunter DJ. A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch Ophthalmol. 2007; 125(1): 55–62. [DOI] [PubMed] [Google Scholar]
- 24. Kondo N, Honda S, Kuno S, Negi A. Coding variant I62V in the complement factor H gene is strongly associated with polypoidal choroidal vasculopathy. Ophthalmology. 2009; 116(2): 304–310. [DOI] [PubMed] [Google Scholar]
- 25. Schick T, Steinhauer M, Aslanidis A, et al.. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye (Lond). 2017; 31(5): 810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altay L, Sitnilska V, Schick T, et al.. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye (Lond). 2019; 33(12): 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omori T, Oguchi Y, Machida T, et al.. Evidence for activation of lectin and classical pathway complement components in aqueous humor of neovascular age-related macular degeneration. Ophthalmic Res. 2020; 63(3): 252–258. [DOI] [PubMed] [Google Scholar]
- 28. Yanagi Y. Pachychoroid disease: a new perspective on exudative maculopathy. Jpn J Ophthalmol. 2020; 64(4): 323–337. [DOI] [PubMed] [Google Scholar]
- 29. Ferris FL, Davis MD, Clemons TE, et al.. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Archives of Ophthalmology. 2005; 123(11): 1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mead A. Review of the development of multidimensional scaling methods. J Roy Stat Soc D-Sta. 1992; 41(1): 27–39. [Google Scholar]
- 31. Klein RJ, Zeiss C, Chew EY, et al.. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308(5720): 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakurada Y, Nakamura Y, Yoneyama S, et al.. Aqueous humor cytokine levels in patients with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Ophthalmic Res. 2015; 53(1): 2–7. [DOI] [PubMed] [Google Scholar]
- 33. Ng DS, Yip YW, Bakthavatsalam M, et al.. Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci Rep. 2017; 7: 45081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Terao N, Koizumi H, Kojima K, et al.. Association of upregulated angiogenic cytokines with choroidal abnormalities in chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2018; 59(15): 5924–5931. [DOI] [PubMed] [Google Scholar]
- 35. Agrawal R, Balne PK, Wei X, et al.. Cytokine profiling in patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2019; 60(1): 376–382. [DOI] [PubMed] [Google Scholar]
- 36. Mimura T, Funatsu H, Noma H, et al.. Aqueous humor levels of cytokines in patients with age-related macular degeneration. Ophthalmologica. 2019; 241(2): 81–89. [DOI] [PubMed] [Google Scholar]
- 37. Ozturk BT, Bozkurt B, Kerimoglu H, Okka M, Kamis U, Gunduz K. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol Vis. 2009; 15: 1906–1914. [PMC free article] [PubMed] [Google Scholar]
- 38. Sarks JP, Sarks SH, Killingsworth MC. Morphology of early choroidal neovascularisation in age-related macular degeneration: correlation with activity. Eye (Lond). 1997; 11(pt 4): 515–522. [DOI] [PubMed] [Google Scholar]
- 39. Green WR, Key SN 3rd. Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977; 75: 180–254. [PMC free article] [PubMed] [Google Scholar]
- 40. Knott RM, Robertson M, Muckersie E, et al.. A model system for the study of human retinal angiogenesis: activation of monocytes and endothelial cells and the association with the expression of the monocarboxylate transporter type 1 (MCT-1). Diabetologia. 1999; 42(7): 870–877. [DOI] [PubMed] [Google Scholar]
- 41. Fernandez-Godino R, Pierce EA. C3a triggers formation of sub-retinal pigment epithelium deposits via the ubiquitin proteasome pathway. Sci Rep. 2018; 8(1): 9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lechner J, Chen M, Hogg RE, et al.. Higher plasma levels of complement C3a, C4a and C5a increase the risk of subretinal fibrosis in neovascular age-related macular degeneration: complement activation in AMD. Immun Ageing. 2016; 13: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sørensen J, Subhi Y, Molbech CR, Krogh Nielsen M, Sørensen TL. Plasma levels of inflammatory chemokines in patients with polypoidal choroidal vasculopathy. Acta Ophthalmol. 2020; 98(4): 384–389. [DOI] [PubMed] [Google Scholar]
- 44. Subhi Y, Krogh Nielsen M, Molbech CR, Sørensen TL. Altered proportion of CCR2+ and CX3CR1+ circulating monocytes in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Clin Exp Ophthalmol. 2018; 46(6): 661–669. [DOI] [PubMed] [Google Scholar]
- 45. Oguchi Y, Sekiryu T, Omori T, et al.. Anaphylatoxin concentration in aqueous and vitreous humor in the eyes with vitreoretinal interface abnormalities. Exp Eye Res. 2020; 195: 108025. [DOI] [PubMed] [Google Scholar]