Abstract
Objective
In earlier studies, patients with rheumatic and musculoskeletal disease (RMD) who got infected with COVID-19 had a higher risk of mechanical ventilation than comparators. We sought to determine COVID-19 outcomes among patients with RMD 6 months into the pandemic.
Methods
We conducted a cohort study at Mass General Brigham in Boston, Massachusetts, of patients with RMD matched to up to five comparators by age, sex and COVID-19 diagnosis date (between 30 January 2020 and 16 July 2020) and followed until last encounter or 18 August 2020. COVID-19 outcomes were compared using Cox regression. Risk of mechanical ventilation was compared in an early versus a recent cohort of patients with RMD.
Results
We identified 143 patients with RMD and with COVID-19 (mean age 60 years; 76% female individuals) and 688 comparators (mean age 59 years; 76% female individuals). There were no significantly higher adjusted risks of hospitalisation (HR: 0.87, 95% CI: 0.68–1.11), intensive care unit admission (HR: 1.27, 95% CI: 0.86–1.86), or mortality (HR: 1.02, 95% CI: 0.53–1.95) in patients with RMD versus comparators. There was a trend towards a higher risk of mechanical ventilation in the RMD cohort versus comparators, although not statistically significant (adjusted HR: 1.51, 95% CI: 0.93–2.44). There was a trend towards improvement in mechanical ventilation risk in the recent versus early RMD cohort (10% vs 19%, adjusted HR: 0.44, 95% CI: 0.17–1.12).
Conclusions
Patients with RMD and comparators had similar risks of poor COVID-19 outcomes after adjusting for race, smoking and comorbidities. The higher risk of mechanical ventilation in the early RMD cohort was no longer detected in a recent cohort, suggesting improved management over time.
Keywords: autoimmune diseases, epidemiology, outcome assessment, health care
Key messages.
What is already known about this subject?
Patients with rheumatic disease and providers continue to be concerned about the risks of poor outcomes from COVID-19.
Earlier studies observed a higher risk of mechanical ventilation in patients with rheumatic disease versus comparators early in the pandemic.
What does this study add?
Six months into the ongoing COVID-19 pandemic, we found that patients with rheumatic disease had no significantly higher risk of hospitalisation, intensive care unit admission or death compared with those without rheumatic disease after adjusting for race, smoking and comorbidities.
There was an association between rheumatic disease and risk of mechanical ventilation, but this was attenuated after adjusting for comorbidities.
There was a temporal trend towards a reduction in risk of mechanical ventilation in the recent versus earlier rheumatic disease cohort.
How might this impact on clinical practice or future developments?
These findings provide reassurance that rheumatic disease may not place patients at a higher risk of severe COVID-19 respiratory complications or death compared with the general population.
COVID-19 outcomes may have improved over time, possibly due to improvement in management.
Introduction
COVID-19, caused by the novel SARS-CoV-2, has become an unprecedented global health crisis, with over 36 million confirmed cases and over 1 million deaths worldwide as of October 2020.1 Especially as workplaces and schools reopen, whether patients with rheumatic disease and those on immunosuppressive medications are at a higher risk of complications of COVID-19 infection continue to be a concern to both patients and providers.2 Several case series have suggested that patients with rheumatic disease may not be at a higher risk of severe COVID-19 outcomes,3–5 although a comparative cohort study from Wuhan, China, reported higher rates of mechanical ventilation among patients with rheumatic disease versus comparators (38% vs 10%, p<0.001).6 A comparative cohort study from Spain found that having a connective tissue disease was independently associated with a trend towards higher odds of severe COVID-19 (OR: 1.82, 95% CI: 1.00–3.30).7 Lastly, disease-specific registry studies from the rheumatology and inflammatory bowel disease communities have shown a higher risk of severe COVID-19 with glucocorticoid use, although not with biologic or targeted synthetic disease-modifying antirheumatic drugs (DMARDs).8–10
During the initial crisis phase of the pandemic in Boston, Massachusetts (March and April 2020), we performed a comparative cohort study that demonstrated similar odds of hospitalisation and death but threefold higher odds of mechanical ventilation among 52 patients with rheumatic disease versus 104 matched comparators without the rheumatic disease.11 In this follow-up study, we examine COVID-19 outcomes and temporal trends in an expanded number of systematically identified patients with rheumatic disease and matched comparators 6 months into the ongoing COVID-19 pandemic.
Methods
Study population
Mass General Brigham (MGB) is a large, multi-centre healthcare system that includes tertiary care hospitals (Massachusetts General Hospital and Brigham and Women’s Hospital), community hospitals, and primary and specialty outpatient centres in the greater Boston, Massachusetts, area. We identified patients seen at MGB who were ≥18 years of age and had a positive test result for SARS-CoV-2 by PCR clinical assay between 30 January 2020 and 16 July 2020, using the MGB centralised data warehouse, Research Patient Data Registry.12 Patients diagnosed in the outpatient setting were required to have at least one follow-up encounter following the positive SARS-CoV-2 test. This study was approved by the MGB Institutional Review Board (2020P000833). Patients were not involved in the design, conduct or reporting of this study.
Rheumatic disease case identification
From this group of patients with confirmed COVID-19, we searched the electronic health record (EHR) for International Classification of Diseases (ICD)-9 and ICD-10 codes to identify patients with a possible rheumatic disease (online supplemental table 1). Rheumatic disease diagnosis was confirmed by manual review of the EHR. Patients with only crystalline arthropathy, fibromyalgia or osteoarthritis were excluded, as these are not typically considered systemic autoimmune rheumatic diseases (or treated with systemic immunomodulators).13 The following patients were also excluded: remote polymyalgia rheumatica (last prednisone use ≥5 years prior), antiphospholipid antibody syndrome with no prior immunosuppression, and sarcoidosis with no prior immunosuppression or with prior immunosuppression ≥5 years ago. For reference, our first study regarding COVID-19 outcomes in patients with rheumatic disease at MGB included patients with rheumatic disease identified in a similar fashion with a COVID-19 diagnosis date between 30 January 2020 and 8 April 2020.11 Patients from the first study were also included in the current study.
annrheumdis-2020-219279supp001.pdf (69.6KB, pdf)
Non-rheumatic disease comparator identification
Each patient with a rheumatic disease was matched to up to five comparators without a rheumatic disease ICD code from the same COVID-19-positive MGB population, based on age, sex and the index date (the date of collection of initial positive SARS-CoV-2 test ±5 days, since testing criteria changed over time). For comparators with multiple test dates, the date of the first positive result was used.
Data collection
For patients with rheumatic disease, clinical variables of interest regarding the rheumatic disease diagnosis were extracted from the EHR by manual chart review. These included the rheumatic disease diagnosis, immunomodulatory medications (including the specific dose of any glucocorticoid when applicable), rheumatic disease duration and disease activity level (based on the global assessment from the last rheumatology provider note documented in the EHR) as determined by the reviewer.
For both patients with rheumatic disease and comparators, additional variables were extracted from the COVID-19 Data Mart,14 an EHR-based data enclave established by MGB that includes all patients who have had a lab test for SARS-CoV-2 performed. Variables extracted from the COVID-19 Data Mart included demographics (age, sex and self-identified race/ethnicity), smoking status, medical comorbidities and COVID-19 clinical outcomes (including dates of hospitalisation, intensive care admission, mechanical ventilation and death). Baseline characteristics including demographics, comorbidities, smoking history and body mass index (BMI) were assessed in the 1 year prior to the index date, and the Charlson Comorbidity Index (CCI)15 was calculated prior to the index date.
Statistical analysis
Categorical variables are presented as number (percentage), and continuous variables are reported as mean±SD or median±IQR, as appropriate. Continuous variables were compared using a two-sample t-test for continuous normally distributed variables or Mann-Whitney U test for continuous non-normally distributed variables. Categorical variables were compared using χ2 tests.
Baseline was the index date that the initial positive PCR for SARS-CoV-2 was obtained. Person-days (PD) of follow-up were determined for each subject from the index date to the first of any of the following events: occurrence of the outcome of interest, date of the last encounter at MGB or end of the study period (18 August 2020). We calculated incidence rates per 1000 days by dividing the number of events by the number of PD. Multivariable Cox proportional hazard regression models were used to estimate HRs and 95% CIs for the following outcomes in separate models: hospitalisation, intensive care unit admission, mechanical ventilation and death, comparing patients with rheumatic diseases to matched comparators. Covariates in the multivariable models were chosen due to known risk factors for COVID-19 or imbalance between patients with and without rheumatic disease at baseline, in addition to the matching factors of age, sex and date of the test. The first multivariable model adjusted for race and smoking. The second multivariable model adjusted for cardiovascular disease (coronary artery disease, hypertension, heart failure), chronic lung disease (obstructive sleep apnea, chronic obstructive pulmonary disease, asthma and interstitial lung disease) and body mass index. The third and final multivariable models adjusted for race, smoking and CCI (dichotomised as <2 or ≥2). For hospitalisation, intensive care unit admission and mechanical ventilation, death was treated as a competing risk using a cause-specific model yielding subdistribution HRs.16
To expand on our previous observations and evaluate time trends in mechanical ventilation in patients with rheumatic disease we divided our rheumatic disease cohort into early and recent cohorts (prior to and after 12 April 2020, respectively, which was the calendar midpoint of all COVID-19 diagnosis dates in the rheumatic disease cohort) and compared the risk of mechanical ventilation between the early and recent cohorts using multivariable Cox proportional hazards regression. To determine whether temporal trends in risk of mechanical ventilation might be related to more mild cases being diagnosed in the recent cohort, we also compared the risk of hospitalisation in the early versus recent cohorts. A similar analysis of temporal trends in mechanical ventilation was performed in the comparator cohort. The level of significance was set as a two-tailed p<0.05, and statistical analyses were completed using SAS statistical software (V.9.4; SAS Institute).
Results
Study population
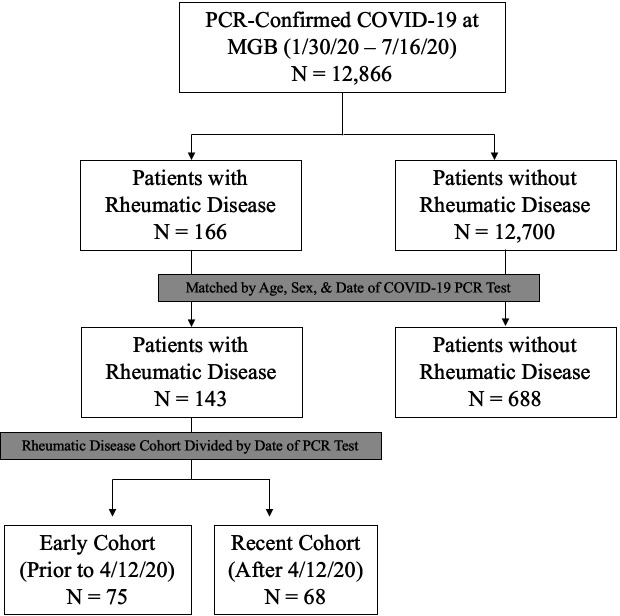
As of 16 July 2020, there were 12 866 patients with a positive test result for SARS-CoV-2 at MGB. Of these, 733 (6%) had a positive rheumatic disease screen by ICD code, and 143 (1%) had confirmed rheumatic disease on EHR review and were matched to 688 comparators (figure 1).
Figure 1.
Flow diagram of rheumatic disease. Patients and comparators with COVID-19 infection at Mass General Brigham (MGB). MGB, Mass General Brigham.
Patients with rheumatic disease and those without rheumatic disease were well matched; the mean age was 60 years in the rheumatic disease group and 59 years in the comparator group, and 76% were female individuals in each group (table 1). The distribution of race was similar between those with and without the rheumatic disease. The percent with Hispanic ethnicity was similar between groups (8% vs 12%, p=0.16). A higher proportion of patients with rheumatic disease were either former (33% vs 21%) or current (4% vs 3%) smokers (p<0.0003).
Table 1.
Clinical characteristics of patients with rheumatic disease with COVID-19 (N=143) and matched comparators (N=688) at the time of COVID-19 diagnosis
| Characteristic | Rheumatic disease (N=143) | No rheumatic disease (N=688) | P value |
| Age, years (mean±SD) | 60±16 | 59±16 | 0.75 |
| Female, n (%) | 108 (76) | 520 (76) | 1.00 |
| Race, n (%) | 0.19 | ||
| White | 68 (48) | 342 (50) | |
| Black | 35 (25) | 117 (17) | |
| Asian | 5 (4) | 26 (4) | |
| Other | 35 (25) | 203 (30) | |
| Hispanic ethnicity, n (%) | 11 (8) | 81 (12) | 0.16 |
| Body mass index, kg/m2 (mean±SD) | 30.2±6.7 | 29.5±7.0 | 0.33 |
| Smoking status, n (%) | 0.0003 | ||
| Never | 75 (52) | 341 (50) | |
| Former | 47 (33) | 146 (21) | |
| Current | 5 (4) | 20 (3) | |
| Unknown | 16 (11) | 181 (26) | |
| Comorbidities, n (%) | |||
| Hypertension | 77 (54) | 241 (35) | <0.0001 |
| Diabetes | 30 (21) | 123 (18) | 0.38 |
| Coronary artery disease | 25 (17) | 40 (6) | <0.0001 |
| Heart failure | 16 (11) | 42 (6) | 0.03 |
| Asthma | 20 (14) | 52 (8) | 0.01 |
| Chronic obstructive pulmonary disease | 11 (8) | 29 (4) | 0.08 |
| Obstructive sleep apnoea | 17 (12) | 36 (5) | 0.003 |
| Interstitial lung disease | 10 (7) | 7 (1) | <0.0001 |
| Chronic kidney disease | 26 (18) | 53 (8) | 0.0001 |
| Any neoplasm | 41 (29) | 162 (24) | 0.19 |
| Charlson comorbidity index (median, IQR) | 2.0 (1.0–4.0) | 0.0 (0.0–2.0) | <0.0001 |
COVID-19: coronavirus disease 2019.
There was a higher proportion of patients in the rheumatic disease group with comorbidities including hypertension (54% vs 35%, p<0.0001), coronary artery disease (17% vs 6%, p<0.0001), interstitial lung disease (7% vs 1%, p<0.0001), heart failure (11% vs 6%, p=0.03), asthma (14% vs 8%, p=0.01), chronic obstructive pulmonary disease (COPD) (8% vs 4%, p=0.08), obstructive sleep apnoea (12% vs 5%, p=0.003) and chronic kidney disease (18% vs 8%, p=0.0001). There was a similar proportion of patients in each group with diabetes and malignancy.
Among patients with rheumatic disease, the disease distribution was broad and included rheumatoid arthritis (44; 31%), systemic lupus erythematosus (27; 19%), psoriatic arthritis (10; 7%), other inflammatory arthritis (10; 7%), polymyalgia rheumatica (8; 6%), antineutrophil cytoplasmic antibody-associated vasculitis (6; 4%) and others (table 2). The disease duration was less than 1 year in 1 (1%), 1–4 years in 27 (19%), 5–10 years in 27 (19%) and greater than 10 years in 87 patients (61%). Fifty-three patients (37%) were in remission, whereas 90 (63%) had active disease at the time of COVID-19 diagnosis. Patients with rheumatic disease were on a variety of immunomodulatory medications: 30 (21%) were on hydroxychloroquine, 41 (29%) were on biologic DMARDs, 44 (31%) were on conventional synthetic DMARDs and 4 (3%) were on targeted synthetic DMARDs. Of those on oral glucocorticoids (51; 36%), the median prednisone-equivalent dose was 5 mg/day.
Table 2.
Details of rheumatic disease diagnosis and management at the time of COVID-19 diagnosis (N=143)
| Characteristic | n (%) |
| Rheumatic disease diagnosis | |
| Rheumatoid arthritis | 44 (31) |
| Systemic lupus erythematosus | 27 (19) |
| Psoriatic arthritis | 10 (7) |
| Other inflammatory arthritis | 10 (7) |
| Polymyalgia rheumatica | 8 (6) |
| ANCA-associated vasculitis | 6 (4) |
| Other vasculitis | 6 (4) |
| Axial spondyloarthritis | 5 (4) |
| Inflammatory myositis | 4 (3) |
| Systemic sclerosis | 3 (2) |
| Undifferentiated connective tissue disease | 3 (2) |
| Sarcoidosis | 2 (1) |
| Mixed connective tissue disease | 2 (1) |
| Juvenile idiopathic arthritis | 2 (1) |
| Kikuchi disease | 2 (1) |
| Giant cell arteritis | 2 (1) |
| Antiphospholipid syndrome | 1 (1) |
| Sjögren’s syndrome | 1 (1) |
| Multiple diagnoses* | 5 (4) |
| Rheumatic disease duration (years) | |
| <1 | 1 (1) |
| 1–4 | 27 (19) |
| 5–10 | 27 (19) |
| >10 | 87 (61) |
| Unknown | 1 (1) |
| Disease activity | |
| Active | 90 (63) |
| Remission | 53 (37) |
| Baseline rheumatic disease medications | |
| Biologic DMARDs† | 41 (29) |
| TNF inhibitor | 17 (12) |
| IL-6 receptor inhibitor | 3 (2) |
| B-cell activating factor inhibitor | 2 (1) |
| CD20 inhibitor | 11 (8) |
| IL-17 inhibitor | 3 (2) |
| IL-12/IL-23 inhibitor | 1 (1) |
| CTLA-4 immunoglobulin | 4 (3) |
| C5 inhibitor | 1 (1) |
| Targeted synthetic DMARDs (JAK inhibitors) | 4 (3) |
| Conventional synthetic DMARDs‡ | 44 (31) |
| Leflunomide | 9 (7) |
| Azathioprine | 6 (4) |
| Methotrexate | 18 (13) |
| Mycophenolate | 10 (7) |
| Tacrolimus | 2 (1) |
| Sulfasalazine | 1 (1) |
| Cyclophosphamide | 1 (1) |
| Hydroxychloroquine | 30 (21) |
| Oral glucocorticoid | 51 (36) |
| Prednisone-equivalent daily dose (median, IQR, mg) | 5 (5 to 10) |
*‘Multiple diagnoses’ category includes patients with overlap features of multiple primary rheumatic diseases.
†One patient was on two biologic DMARDs (rituximab and eculizumab).
‡Three patients were on multiple conventional synthetic DMARDs.
ANCA, antineutrophil cytoplasmic antibody; C5, complement component 5; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DMARD, disease-modifying antirheumatic drug; IL, interleukin; JAK, Janus kinase; TNF, tumour necrosis factor.
Outcomes of COVID-19 infection in patients with rheumatic disease
In unadjusted and multivariable models, the risk of hospitalisation was similar in patients with rheumatic disease and comparators (58 (41%) vs 295 (43%), adjusted model 3, HR: 0.87, 95% CI: 0.68–1.11). The risks of intensive care unit admission (28 (20%) vs 96 (14%), adjusted model 3, HR: 1.27, 95% CI: 0.86–1.86) and death (12 (8%) vs 48 (7%), adjusted model 3, HR: 1.02, 95% CI: 0.53–1.95) were also similar in those with rheumatic diseases and comparators, respectively (table 3). The first (adjusted for race and smoking) and second (adjusted for cardiovascular disease, chronic lung disease and BMI) models yielded similar results to the third model (adjusted for race, smoking and CCI) for the outcomes of hospitalisation, intensive care unit admission and death. In contrast, there was a higher risk of mechanical ventilation in patients with rheumatic disease versus comparators in the unadjusted model (22 (15%) vs 63 (9%), HR: 1.75, 95% CI: 1.12–2.74) and the first model, which adjusted for race and smoking (HR: 1.72, 95% CI: 1.07–2.76). However, after adjusting for comorbidities, this difference was attenuated and no longer statistically significant (adjusted model 2, HR: 1.56, 95% CI: 0.97–2.50; adjusted model 3, HR: 1.51, 95% CI: 0.93–2.44).
Table 3.
COVID-19 outcomes in patients with rheumatic disease (N=143) vs matched comparators (N=688)
| Rheumatic disease (N=143) | No rheumatic disease (N=688) | |
| Hospitalisation, n (%) | 58 (41) | 295 (43) |
| Total follow-up time (person-days) | 5847 | 21 671 |
| Incidence rate/1000 days (95% CI) | 9.90 (7.40–12.50) | 13.60 (12.10–15.20) |
| Unadjusted HR (95% CI) | 0.95 (0.75–1.21) | 1.0 (Ref) |
| Adjusted model 1, HR (95% CI)* | 0.89 (0.70–1.13) | 1.0 (Ref) |
| Adjusted model 2, HR (95% CI) | 0.86 (0.68–1.09) | 1.0 (Ref) |
| Adjusted model 3, HR (95% CI) | 0.87 (0.68–1.11) | 1.0 (Ref) |
| Intensive care unit admission, n (%) | 28 (20) | 96 (14) |
| Total follow-up time (person-days) | 7502 | 29 746 |
| Incidence rate/1000 days (95% CI) | 3.70 (2.30–5.10) | 3.20 (2.60–3.90) |
| Unadjusted HR (95% CI) | 1.38 (0.95–2.00) | 1.0 (Ref) |
| Adjusted model 1, HR (95% CI) | 1.33 (0.91–1.94) | 1.0 (Ref) |
| Adjusted model 2, HR (95% CI) | 1.22 (0.83–1.79) | 1.0 (Ref) |
| Adjusted model 3, HR (95% CI) | 1.27 (0.86–1.86) | 1.0 (Ref) |
| Mechanical ventilation, n (%) | 22 (15) | 63 (9) |
| Total follow-up time (person-days) | 7812 | 31 042 |
| Incidence rate/1000 days (95% CI) | 2.80 (1.60–4.00) | 2.00 (1.50–2.50) |
| Unadjusted HR (95% CI) | 1.75 (1.12–2.74) | 1.0 (Ref) |
| Adjusted model 1, HR (95% CI) | 1.72 (1.07–2.76) | 1.0 (Ref) |
| Adjusted model 2, HR (95% CI) | 1.56 (0.97–2.50) | 1.0 (Ref) |
| Adjusted model 3, HR (95% CI) | 1.51 (0.93–2.44) | 1.0 (Ref) |
| Death, n (%) | 12 (8) | 48 (7) |
| Total follow-up time (person-days) | 8790 | 33 428 |
| Incidence rate/1000 days (95% CI) | 1.40 (0.60–2.10) | 1.40 (1.00–1.80) |
| Unadjusted HR (95% CI) | 1.16 (0.63–2.13) | 1.0 (Ref) |
| Adjusted model 1, HR (95% CI) | 1.20 (0.62–2.33) | 1.0 (Ref) |
| Adjusted model 2, HR (95% CI) | 1.03 (0.54–1.97) | 1.0 (Ref) |
| Adjusted model 3, HR (95% CI) | 1.02 (0.53 –1.95) | 1.0 (Ref) |
*Model 1 adjusted for race and smoking. Model 2 adjusted for cardiovascular disease (coronary artery disease, hypertension, heart failure), chronic lung disease (obstructive sleep apnoea, chronic obstructive pulmonary disease, asthma and interstitial lung disease) and body mass index. Model 3 adjusted for race, smoking and Charlson Comorbidity Index (dichotomised as ≤2 or >2). Matching factors were age, sex and date of initial positive PCR for SARS-CoV-2.
Among patients with rheumatic disease, there was a trend towards a lower risk of mechanical ventilation in the recent cohort compared with the early cohort (7 (10%) vs 14 (19%), unadjusted HR: 0.45, 95% CI: 0.19–1.10) (figure 2). This trend was similar after adjusting for age, sex and CCI (adjusted HR: 0.44, 95% CI: 0.17–1.12). Indeed, the risk of mechanical ventilation among patients with rheumatic disease versus comparators was significantly elevated in the early cohort (adjusted HR: 1.88, 95% CI: 1.00–3.51) but similar in the recent cohort (adjusted HR: 0.99, 95% CI: 0.40–2.46). In contrast, the risk of hospitalisation was stable in the recent and early cohorts (27 (40%) vs 28 (37%); unadjusted HR: 0.99, 95% CI: 0.63–1.59; adjusted HR: 0.94, 95% CI: 0.59–1.49) (figure 3). In the matched comparators, there was lower unadjusted risk of mechanical ventilation in the recent cohort compared with the early cohort (HR: 0.58, 95% CI: 0.34–0.97). After adjusting for age, sex and CCI, there was a trend towards a lower risk of mechanical ventilation although not statistically significant (adjusted HR: 0.63, 95% CI: 0.38–1.07).
Figure 2.
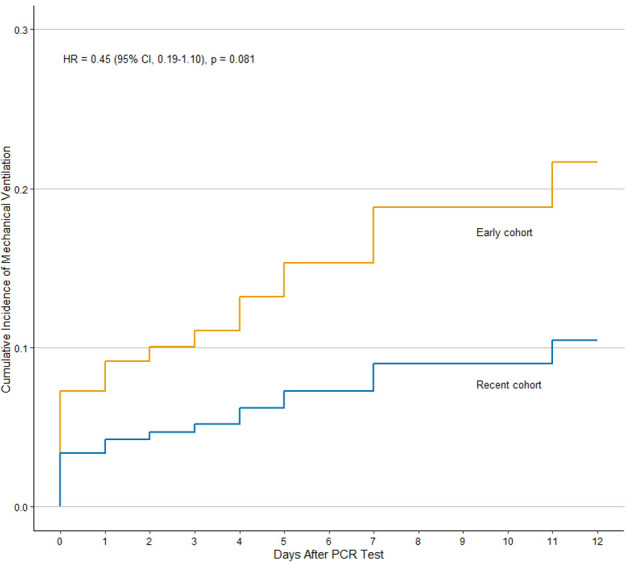
Cumulative incidence of mechanical ventilation in patients with COVID-19 and rheumatic disease in the recent (n=68) vs early (n=75) cohorts.
Figure 3.
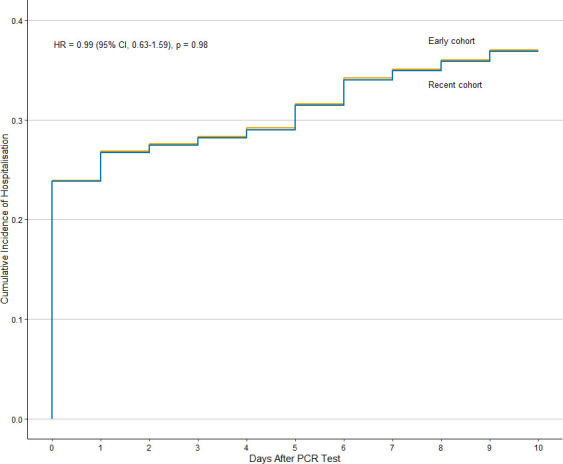
Cumulative incidence of hospitalisation in patients with COVID-19 and rheumatic disease in the recent (n=68) vs early (n=75) cohorts.
Discussion
In this large cohort study from a multicentre healthcare system in Boston, Massachusetts, patients with COVID-19 infection and rheumatic disease had similar risks of hospitalisation, intensive care unit admission and mortality versus matched comparators. Patients with rheumatic disease had a higher unadjusted risk of mechanical ventilation versus comparators, although after adjusting for race, smoking and comorbidities, the risk of mechanical ventilation was attenuated and no longer statistically significant. There was a trend towards a lower risk of mechanical ventilation in patients with rheumatic disease diagnosed later in the pandemic versus earlier in the pandemic. Outcomes of COVID-19 infection in patients with rheumatic disease may have improved over time due to improved COVID-19 management, less stress on the healthcare system due to capacity issues during the early surge or increased testing capacity allowing detection of milder cases. Larger cohort studies are needed to fully understand the temporal trends in COVID-19 outcomes in this population.
Prior comparative cohort studies of patients with rheumatic disease from early in the pandemic reported higher odds of mechanical ventilation in patients with rheumatic disease versus comparators,6 11 and that having a connective tissue disease was associated with a trend towards higher odds of severe COVID-19 (intensive care unit admission, mechanical ventilation and/or death).7 Additionally, a cohort study using an EHR database including >52 million patients across 35 healthcare organisations showed that patients with SARS had a higher risk of hospitalisation, intensive care unit admission and mechanical ventilation versus comparators matched on age, sex and race, but this study did not adjust for comorbidities and relied on different definitions for the exposure and outcome.17 Our study extends through the first 6 months of the COVID-19 pandemic in the greater Boston area and shows no higher risks of hospitalisation, intensive care unit admission, mechanical ventilation or death in patients with rheumatic disease versus comparators after adjusting for comorbidities. Overall, the hospitalisation rate among patients with rheumatic disease in our cohort is similar to that reported in the Global Rheumatology Alliance (GRA) Physician-Reported Registry (46% in the GRA8 vs 41% in our study), and the case fatality rate of 8% in each group is similar to the overall reported case fatality rate in Massachusetts of 7.4%.18
In unadjusted analyses, we observed similar results as in our prior comparative cohort study, which showed threefold higher odds of mechanical ventilation among patients with rheumatic disease versus comparators during the first 2 months of the COVID-19 pandemic in Boston.11 Ye et al also found higher rates of mechanical ventilation among patients with rheumatic disease (n=21) compared with those without, but were unable to adjust for comorbidities.6 After extending the study period to 6 months and adjusting for comorbidities, we observed no statistically significant higher risk of severe COVID-19 outcomes including mechanical ventilation, in contrast to prior studies. Our current analyses show a trend towards a lower risk of mechanical ventilation among patients with rheumatic disease in the recent cohort as opposed to the early cohort, suggesting possible improvement in COVID-19 outcomes over calendar time.
The trend in improvement in outcomes at MGB mirrors the trends in the USA, where the COVID-19 case fatality rate has improved over time.19 This improvement in COVID-19 outcomes is likely multifactorial, including potential detection of milder cases with increased testing availability, lower volume of seriously ill patients for hospitals and providers after the initial surge of cases, or improvements in COVID-19 management over time.20–22 Of note, the risk of hospitalisation remained stable in our rheumatic disease population during the early and recent cohorts, suggesting that the improvement in mechanical ventilation risk is not related to increased testing alone.
Our study has several strengths. As Boston became a hot spot of COVID-19 infection early in the pandemic, there were a relatively large number of confirmed cases within our multicentre healthcare system. We identified patients with confirmed COVID-19 infection based on positive COVID-19 PCR testing, we confirmed the diagnosis of rheumatic disease by manual chart review, and we selected comparators who had never received a diagnostic code for rheumatic disease, thus reducing the risk of misclassification. The limitations of our study deserve comment. Some of the included covariates in the CCI, such as chronic kidney disease, may be causal intermediates. Collider bias may exist as the outcomes are conditioned on the diagnosis of COVID-19 and this may bias our results towards the null.23 We were unable to capture outcomes that may have occurred outside of MGB. However, we required patients with rheumatic disease and comparators to have at least one follow-up encounter within our healthcare system to reduce the risk of missed outcomes due to loss to follow-up. Our cohort was assembled from MGB, which includes two tertiary care facilities, in Boston, Massachusetts, and may not be generalisable to the entire USA. However, patients from primary care clinics and community hospitals affiliated with MGB were also included. We were unable to perform subgroup analyses by specific rheumatic diseases or medication classes such as oral glucocorticoids given small sample sizes and low event rates. It remains possible that patients with specific diseases or on specific medications may be at a higher risk of poor outcomes of COVID-19 infection. Last, given that MGB was a major site for many randomised placebo-controlled trials evaluating COVID-19 therapies, we are unable to assess the impact of study drugs such as remdesivir.
In conclusion, we found that patients with rheumatic disease had a similar risk of hospitalisation; intensive care unit admission; and death after adjusting for race, smoking and comorbidities. Although prior studies have shown a higher risk of mechanical ventilation in patients with rheumatic disease with COVID-19 versus comparators, our results show a temporal trend towards improvement in risk of mechanical ventilation in patients with rheumatic disease. These results may provide reassurance to patients with rheumatic disease and their providers during the ongoing COVID-19 pandemic. As in the general population, close monitoring of patients with rheumatic disease with risk factors such as pulmonary and cardiovascular comorbidities is warranted, as these patients may be at a higher risk of poor outcomes from COVID-19 infection.
Footnotes
Handling editor: Josef S Smolen
Twitter: @kmdsilvaMD, @jeffsparks, @zach_wallace_md
Contributors: NS-B, KMD, JAS and ZSW designed the study, were responsible for the acquisition, analysis and interpretation of data, and drafted and revised the article. TH and RW were involved in data acquisition and revision of the manuscript. XF was involved in data analysis and interpretation and revision of the manuscript. EMG, AMJ, YZ and HC were involved in data analysis and interpretation and revision of the manuscript. All authors approved the final version of the article.
Funding: NS-B and KMD are supported by the National Institutes of Health Ruth L. Kirschstein Institutional National Research Service Award [T32-AR-007258]. AMJ is supported by the Rheumatology Research Foundation Scientist Development Award. HC is funded by National Institutes of Health [P50-AR-060772]. JAS is funded by NIH/NIAMS (grant numbers K23 AR069688, R03 AR075886, L30 AR066953, P30 AR070253 and P30 AR072577), the Rheumatology Research Foundation R Bridge Award, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. ZSW is funded by NIH/NIAMS [K23AR073334 and L30 AR070520].
Competing interests: EMG reports editor position at New England Journal of Medicine and royalties from the textbook Rheumatology. HC reports research support from AstraZeneca and consultancy fees from Takeda, Selecta, GlaxoSmithKline, and Horizon. JAS reports research support from Amgen and Bristol-Myers Squibb and consultancy fees from Bristol-Myers Squibb, Gilead, Inova, Janssen, Optum, and Pfizer. ZSW reports research support from Bristol-Myers Squibb and consulting fees from Viela Bio.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. This study includes deidentified patient data from Mass General Brigham. All data relevant to the study are included in the article or uploaded as supplementary information.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Coronavirus disease 2019 dashboard World Health organization. Available: https://covid19.who.int/ [Accessed 10 Apr 20].
- 2.Larochelle MR. “Is It Safe for Me to Go to Work?” Risk Stratification for Workers during the Covid-19 Pandemic. N Engl J Med Overseas Ed 2020;383:e28–3. 10.1056/NEJMp2013413 [DOI] [PubMed] [Google Scholar]
- 3.Monti S, Balduzzi S, Delvino P, et al. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020;79:667–8. 10.1136/annrheumdis-2020-217424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomelleri A, Sartorelli S, Campochiaro C, et al. Impact of COVID-19 pandemic on patients with large-vessel vasculitis in Italy: a monocentric survey. Ann Rheum Dis 2020;79:1–2. 10.1136/annrheumdis-2020-217600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases - Case series from New York. N Engl J Med 2020;383:85–8. 10.1056/NEJMc2009567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye C, Cai S, Shen G, et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis 2020;79:1–8. 10.1136/annrheumdis-2020-217627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020;79:1544–9. 10.1136/annrheumdis-2020-218296 [DOI] [PubMed] [Google Scholar]
- 8.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:481–91. 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberman RH, Castillo R, Chen A, et al. COVID‐19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and DMARDs on clinical outcomes. Arthritis Rheum 2020:1–25. 10.1002/art.41456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot'. Ann Rheum Dis 2020;79:1156–62. 10.1136/annrheumdis-2020-217888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalichowski R, Keogh D, Chueh HC, et al. Calculating the benefits of a research patient data Repository. AMIA Annu Symp Proc 2006;1044:1044. [PMC free article] [PubMed] [Google Scholar]
- 13.Jorge AM, Lu N, Keller SF, et al. The effect of statin use on mortality in systemic autoimmune rheumatic diseases. J Rheumatol 2018;45:1689–95. 10.3899/jrheum.171389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New COVID-19 Tools for Researchers Mass General Brigham. Available: https://rc.partners.org/about/projects-initiatives/new-covid-19-research-tools-researchers [Accessed 18 Jun 20].
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 17.D’Silva KM, Jorge AM, Lu N. Outcomes of coronavirus disease 2019 infection among patients living with rheumatic diseases: a matched cohort study from a US multi-center research network. American College of Rheumatology Convergence: Arthritis Rheumatol, 2020. [Google Scholar]
- 18.COVID-19 dashboard Massachusetts department of public health, 2020. https://www.mass.gov/doc/covid-19-dashboard-august-30-2020/download [Google Scholar]
- 19.United States COVID-19 case fatality rate. Available: https://ourworldindata.org/coronavirus/country/united-states?country=~USA#how-did-confirmed-deaths-and-cases-change-over-time [Accessed 23 Sep 2020].
- 20.COVID-19 cases are rising, so why are deaths flatlining? the Atlantic, 2020. Available: https://www.theatlantic.com/ideas/archive/2020/07/why-covid-death-rate-down/613945/
- 21.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med Overseas Ed 2020;383:1813–26. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22., Horby P, Lim WS, et al. , RECOVERY Collaborative Group . Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 2020;0:1–11. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 23.Choi HK, Nguyen U-S, Niu J, et al. Selection bias in rheumatic disease research. Nat Rev Rheumatol 2014;10:403–12. 10.1038/nrrheum.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-219279supp001.pdf (69.6KB, pdf)