Abstract
Objective
To delineate the characteristics and clinical significance of plasma inflammatory cytokines altered in COVID-19.
Design
Retrospective, single-centre cohort study.
Setting
Tongji Hospital in Wuhan, China.
Participants
Among a cohort of 308 patients with a diagnosis of COVID-19, 138 patients died while 170 patients recovered and were discharged from the hospital. The data were collected until 27 February 2020.
Primary and secondary outcome measures
Clinical characteristics and laboratory findings were obtained from electronic medical records using data collection forms.
Results
The percentage of patients with elevated interleukin 2 receptor (IL-2R), IL-6, IL-8, IL-10 and tumour necrosis factor (TNF) increased with severity of disease (p<0.0001 for all). IL-2R (p<0.0001), IL-6 (p<0.0001), IL-8 (p=0.0001), IL-10 (p<0.0001) and TNF (p<0.0001) were also twofold to 20-fold higher in patients who died compared with those who recovered. Also, IL-6 and IL-10 increased in both the progressive patient groups: moderate (p=0.0026) and severe (p<0.0001). In multivariate analysis, higher levels of IL-2R (OR 1.001, 95% CI 1.000 to 1.002, p=0.031) and IL-6 (OR 1.013, 95% CI 1.003 to 1.024, p=0.015) on admission were associated with increasing odds of in-hospital death, independent of other covariates, including severity of disease and lymphocyte count.
Conclusion
Increased proinflammatory and anti-inflammatory cytokines, including IL-2R, IL-6, IL-8, TNF and IL-10, showed an obvious association with both COVID-19 severity and in-hospital mortality. Thus, our study indicates that cytokines are valuable in predicting the severity of COVID-19 and helps in distinguishing critically ill patients from the less affected ones.
Keywords: respiratory infections, infection control, immunology, COVID-19
Strengths and limitations of this study.
This study systematically investigated the cytokine profiles among patients with COVID-19.
The major finding is that cytokine level is valuable in predicting the severity and prognosis of COVID-19.
The quality of the study is reduced by lack of observation of the relationship between dynamic cytokine levels and progression of COVID-19.
This study is a retrospective, single-centre cohort analysis based on initial inflammatory cytokine levels on admission.
Data regarding lymphocyte subsets are not available.
Introduction
The COVID-19 outbreak, caused by SARS-CoV-2, is erupting worldwide.1 2 The WHO declared COVID-19 as a pandemic.3 Although most cases were mild to moderate, increasing COVID-19 cases led to a significant number of patients developing severe symptoms and death. A lot of detailed clinical information on COVID-19 is currently known, but the characteristics of inflammatory cytokines associated with patients with COVID-19 remain largely unclear.
Severe illness, of almost any aetiology, is accompanied by a generalised host inflammatory response. This host immune response process is referred to as systemic inflammatory response syndrome. If this process is not controlled or is dysfunctional, it will lead to cytokine storm syndrome.4 Cytokine storm is one of the possible mechanisms underlying rapid disease progression.5 Recent studies have reported a relationship between serum inflammatory cytokine levels and severity or prognosis of patients with COVID-19. However, there are some inconsistent conclusions. For example, several studies reported interleukin (IL)-6 as a potential biomarker for predicting COVID-19 progression or monitoring disease severity.6–8 However, Song et al9 found no significant differences in the level of IL-6 and tumour necrosis factor (TNF) between severe and non-severe patients with COVID-19. Most of the previous studies only focused on the role of IL-6 in COVID-19. The characteristics and the role of other cytokines in COVID-19, especially the anti-inflammatory cytokine IL-10, have received little attention. IL-10 can be a parameter in predicting the clinical outcome of patients with severe community-acquired pneumonia and is also the most important anti-inflammatory cytokine in human immune response.10
Thus, the authors of this research have systematically studied cytokine profiles along with their relationship with the severity and prognosis of COVID-19. Meanwhile, objective factors of patients related to their basic level of cytokines were explored. Earlier identification of a severe or a critical case can help in providing clinical intervention on time.
Methods
Study design and participants
This was a retrospective, single-centre study which enrolled 308 patients from 10 January 2020 to 13 February 2020 at Tongji Hospital, Huazhong University of Science and Technology (Wuhan, China). As of 27 February 2020, 138 patients have died while 170 have recovered and were discharged from the hospital. Data were collected at the time of admission. Diagnosis and clinical classification of COVID-19 were made according to the clinical guidelines (version 5 trial) developed by the National Health Commission of the People’s Republic of China (http://www.nhc.gov.cn/). The clinical classification is as follows:
Moderate type: including fever, respiratory tract symptoms and imaging that shows pneumonia.
Severe type: meeting any of the following criteria: (1) respiratory distress, respiratory rate ≥30 beats/min; (2) in resting state, meaning oxygen saturation ≤93%; and (3) arterial blood oxygen partial pressure/oxygen concentration ≤300 mm Hg (1 mm Hg=0.133 kPa).
Critical type: involving one of the following conditions: (1) respiratory failure requiring mechanical ventilation; (2) presence of shock; and (3) combined organ failure requiring admission in intensive care unit.
Tongji Hospital is a designated hospital for critically ill patients. In this cohort, we observed relatively high mortality. Exacerbation from moderate to severe, or severe to critical, was defined as progression. The primary outcome of the study was in-hospital mortality. The study excluded patients with secondary vasculitis and uraemia that needed maintenance on haemodialysis, along with the ones who died within 48 hours of admission. Before enrolment, written informed consent was obtained from patients involved in the study, while data were collected retrospectively.
Cytokine measurement
In order to explore the influence of COVID-19 on the secretion of cytokines, chemiluminescence immunoassay (CLIA) was performed. Cytokines including IL-1β, interleukin 2 receptor (IL-2R), IL-6, IL-8 (also known as CXCL8), IL-10 and TNF were assessed using serum samples drawn from patients shortly after hospitalisation. CLIA was performed using a fully automated analyser (Immulite 1000, DiaSorin Liaison, Italy; or Cobas e602, Roche Diagnostics, Germany) for all the patients according to the manufacturer’s instructions. IL-1β kit (#LKL11), IL-2R kit (#LKIP1), IL-8 kit (#LK8P1), IL-10 kit (#LKXP1) and TNF kit (#LKNF1) were purchased from DiaSorin (Vercelli, Italy). IL-6 kit (#05109442190) was purchased from Roche Diagnostics. The patients were divided into elevated and normal groups according to the instructions.
Statistical analysis
Continuous variables were expressed as a median with IQR, and categorical variables were described as frequency rates and percentages. We used Mann-Whitney U test or Kruskal-Wallis test, whichever was appropriate, to compare differences between groups. Proportions for categorical variables were compared using χ2 test, Fisher’s exact test or Yates’ continuity corrected χ2 test. Univariate and multivariate logistic regression models (forward: likelihood ratio (LR)) were applied to screen the risk of death. The Kaplan-Meier method was used to assess the cumulative rate of mortality based on the normal range of cytokines and was compared with the log-rank test. Receiver operating characteristic curve (ROC) analysis was performed to assess the accuracy of inflammatory cytokine levels in predicting death. The tests were two-sided, and a p value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS V.23.0.
Patient and public involvement
This was a retrospective, single-centre cohort study and no patients were involved in the study design or in setting the research questions or the outcome measures directly. No patients were asked for advice on interpretation or writing of the results.
Results
Characteristics of COVID-19 patient cohort
The demographic and clinical characteristics of 308 patients with COVID-19 are summarised in table 1. At the time of admission, 91 (30%) patients were classified as moderate type, 133 (43%) patients as severe, and 84 (27%) patients fulfilled the criteria of critical type. As per the results, 138 (45%) patients died and 170 (55%) were discharged from the hospital. Moderately ill and surviving patients were more likely to be female and younger. The ratio of comorbidity was significantly higher in critical (p<0.0001) and deceased (p<0.0001) group. In the laboratory findings, the levels of lymphocytes, platelets and total cholesterol were lower in the critical and deceased groups, whereas neutrophil count, D-dimer, alanine aminotransferase, blood urea nitrogen, creatinine, procalcitonin, lactic dehydrogenase, C reactive protein (CRP), IL-2R, IL-6, IL-8, IL-10 and TNF were significantly higher (p<0.0001 for all). Also, the critical (p<0.0001) and deceased (p<0.0001) groups were more likely to receive non-invasive ventilation or invasive ventilation. The median time from disease onset to outcome was 22 (IQR 15.8–30.0) days for non-survivors and 29 (IQR 24.0–34.0) days for survivors.
Table 1.
Demographics and clinical characteristics of patients with COVID-19
| Variables | Condition on admission | Outcome | |||||
| Moderate (n=91) |
Severe (n=133) |
Critical (n=84) |
P value | Survivor (n=170) |
Non-survivor (n=138) |
P value | |
| Age, years | 55.0 (39.0–67.0) | 66.0 (55.5–73.0) | 70.0 (63.3–78.8) | <0.0001 | 57.0 (43.0–68.0) | 70.0 (62.8–78.0) | <0.0001 |
| Female, n (%) | 51/91 (56) | 67/133 (50) | 24/84 (29) | 0.0003 | 95/170 (56) | 47/138 (34) | 0.0001 |
| Comorbidity, n (%) | 35/89 (39) | 82/132 (62) | 61/83 (74) | <0.0001 | 72/167 (43) | 106/137 (77) | <0.0001 |
| Hypertension | 18/89 (20) | 51/132 (39) | 41/83 (49) | <0.0001 | 43/167 (26) | 67/137 (49) | <0.0001 |
| Diabetes | 14/89 (16) | 22/132 (17) | 20/83 (24) | 0.1621 | 24/167 (14) | 32/137 (23) | 0.0443 |
| Cardiovascular disease | 5/89 (6) | 21/132 (16) | 15/83 (18) | 0.0158 | 13/167 (8) | 28/137 (20) | 0.0013 |
| Cerebrovascular disease | 2/89 (2) | 3/132 (2) | 7/83 (8) | 0.0402 | 4/167 (2) | 8/137 (6) | 0.2155 |
| Pulmonary disease | 6/89 (7) | 11/132 (8) | 9/83 (11) | 0.3380 | 10/167 (6) | 16/137 (12) | 0.0775 |
| Chronic kidney disease | 0/89 (0) | 3/132 (2) | 4/83 (5) | 0.0353 | 1/167 (1) | 6/137 (4) | 0.0715 |
| Signs and symptoms, n (%) | |||||||
| Fever | 76/90 (84) | 119/133 (90) | 77/84 (92) | 0.1318 | 145/169 (86) | 127/138 (92) | 0.0875 |
| Cough | 54/90 (60) | 101/132 (77) | 71/84 (85) | 0.0002 | 114/169 (68) | 112/137 (82) | 0.0047 |
| Laboratory parameters | |||||||
| Neutrophil count, ×109/L | 3.1 (2.5–4.1) | 4.0 (2.7–5.7) | 10.1 (7.2–14.7) | <0.0001 | 3.3 (2.4–4.3) | 8.1 (4.9–12.5) | <0.0001 |
| Lymphocyte count, ×109/L | 1.2 (0.9–1.6) | 0.9 (0.7–1.3) | 0.5 (0.4–0.8) | <0.0001 | 1.1 (0.8–1.5) | 0.6 (0.4–0.8) | <0.0001 |
| Platelet count, ×109/L | 213.0 (160.0–309.0) | 208.5 (149.0–261.3) | 156.0 (94.5–222.0) | <0.0001 | 220.0 (170.8–291.3) | 151.0 (107.0–222.5) | <0.0001 |
| D-dimer, μg/mL | 0.4 (0.3–0.8) | 1.0 (0.5–2.6) | 18.1 (2.6–21.0) | <0.0001 | 0.5 (0.3–1.0) | 7.9 (1.3–21.0) | <0.0001 |
| ALT, U/L | 18.0 (13.0–24.0) | 23.0 (16.0–39.0) | 33.5 (21.0–55.8) | <0.0001 | 20.0 (13.8–32.5) | 28.0 (18.0–46.5) | <0.0001 |
| BUN, mmol/L | 3.7 (3.1–4.7) | 5.1 (3.7–6.7) | 9.6 (7.0–16.1) | <0.0001 | 3.9 (3.1–5.2) | 8.8 (5.6–12.9) | <0.0001 |
| Creatinine, μmol/L | 66.0 (57.0–81.0) | 72.0 (56.0–93.0) | 87.5 (70.5–120.0) | <0.0001 | 65.5 (57.0–82.0) | 86.5 (66.8–120.0) | <0.0001 |
| Total cholesterol, mmol/L | 3.8 (3.2–4.5) | 3.5 (3.1–3.9) | 3.2 (2.8–3.8) | <0.0001 | 3.6 (3.2–4.3) | 3.3 (2.8–3.9) | <0.0001 |
| Procalcitonin, ng/mL | 0.05 (0.04–0.09) | 0.09 (0.05–0.23) | 0.49 (0.17–1.49) | <0.0001 | 0.05 (0.04–0.09) | 0.31 (0.14–1.09) | <0.0001 |
| Lactic dehydrogenase, U/L | 229.0 (190.0–283.0) | 315.0 (219.5–431.8) | 637.0 (490.5–872.5) | <0.0001 | 243.0 (194.8–309.8) | 524.5 (366.0–721.0) | <0.0001 |
| C reactive protein, mg/L | 7.7 (2.5–24.3) | 49.3 (8.9–93.3) | 112.3 (71.4–187.3) | <0.0001 | 10.7 (2.4–36.7) | 100.5 (62.4–161.2) | <0.0001 |
| Interleukin 1β ≥5 pg/mL, n (%) | 13/91 (14) | 17/133 (13) | 11/84 (13) | 0.8120 | 23/170 (14) | 18/138 (13) | 0.9006 |
| Interleukin 2 receptor, U/mL | 475.5 (375.8–630.8) | 799.0 (538.5–1097.0) | 1259.5 (942.3–1825.0) | <0.0001 | 553.0 (402.0–802.0) | 1137.5 (822.0–1584.3) | <0.0001 |
| ≥710 U/L, n (%) | 19/91 (21) | 74/133 (56) | 77/84 (92) | <0.0001 | 51/170 (30) | 113/138 (82) | <0.0001 |
| Interleukin 6, pg/mL | 5.6 (2.7–15.3) | 24.3 (6.7–61.7) | 64.8 (29.42–153.1) | <0.0001 | 7.9 (2.7–22.8) | 59.7 (23.6–137.4) | <0.0001 |
| ≥7 pg/mL, n (%) | 41/91 (45) | 100/133 (75) | 77/83 (92) | <0.0001 | 90/170 (53) | 132/137 (96) | <0.0001 |
| Interleukin 8, pg/mL | 15.4 (7.7–29.4) | 19.5 (12–35.5) | 30.8 (21.0–71.8) | <0.0001 | 16.3 (9.4–28.7) | 26.6 (16.4–60.40) | <0.0001 |
| ≥62 pg/mL, n (%) | 10/91 (11) | 13/133 (10) | 77/84 (92) | <0.0001 | 14/170 (8) | 33/138 (24) | 0.0001 |
| Interleukin 10, pg/mL | 5.0 (5.0–5.1) | 5.9 (5.0–10.80) | 10.9 (6.4–18.7) | <0.0001 | 5.0 (5.0–6.7) | 10.1 (5.4–16.4) | <0.0001 |
| ≥9.1 pg/mL, n (%) | 10/91 (11) | 46/133 (35) | 76/83 (92) | <0.0001 | 31/170 (18) | 76/137 (56) | <0.0001 |
| TNF, pg/mL | 7.7 (6.0–9.5) | 8.6 (6.9–11.9) | 11.2 (7.4–18.8) | <0.0001 | 7.8 (6.1–9.7) | 10.9 (7.7–15.9) | <0.0001 |
| ≥8.1 pg/mL, n (%) | 43/91 (47) | 80/133 (60) | 77/84 (92) | <0.0001 | 82/170 (48) | 101/138 (73) | <0.0001 |
| Treatment, n (%) | |||||||
| Mechanical ventilation | 4/91 (4) | 46/133 (35) | 79/84 (94) | <0.0001 | 7/170 (4) | 122/138 (88) | <0.0001 |
| Antibiotics treatment | 85/91 (93) | 128/133 (96) | 84–0/84 (100) | 0.0191 | 160/170 (94) | 137/138 (99) | 0.0343 |
| Antiviral treatment | 90/90 (100) | 124/126 (98) | 62/79 (79) | <0.0001 | 167/167 (100) | 109/128 (85) | <0.0001 |
| Corticosteroids | 48/90 (53) | 86/133 (65) | 74/84 (88) | <0.0001 | 84/169 (50) | 124/138 (90) | <0.0001 |
| Immunoglobulin | 31/91 (34) | 77–56/133 (58) | 47–37/84 (56) | 0.0031 | 69/170 (41) | 86/138 (62) | 0.0001 |
| Duration of complaint, days | 8.0 (4.8–13.0) | 10.0 (7.0–13.5) | 11.0 (7.0–15.0) | 0.0007 | 8.0 (6.0–13.0) | 10.0 (7.0–15.0) | 0.0027 |
| Hospitalisation, days | 16.0 (12.0–23.0) | 19.0 (12.0–24.0) | 8.0 (4.0–13.0) | <0.0001 | 19.5 (14.0–24.0) | 10.0 (5.0–17.0) | <0.0001 |
| Duration of disease, days | 26.0 (20.0–31.0) | 29.0 (24.0–35.0) | 19.0 (15.0–29.0) | <0.0001 | 29.0 (24.0–34.0) | 22.0 (15.8–30.0) | <0.0001 |
| Progression, n (%) | 8/45 (18) | 52/129 (40) | – | – | – | – | – |
Data are median (IQR), mean (SD) or n (%).
P values were calculated by Mann-Whitney U test, Kruskal-Wallis test, χ2 test, Fisher’s exact test or Yates’ continuity corrected χ2 test, as appropriate.
Duration of complaint: time from onset of symptom to hospital admission; duration of disease: time from onset of symptom to outcome; hospitalisation: time from hospital admission to outcome.
ALT, alanine aminotransferase; BUN, blood urea nitrogen; TNF, tumour necrosis factor.
Plasma cytokine alteration in COVID-19
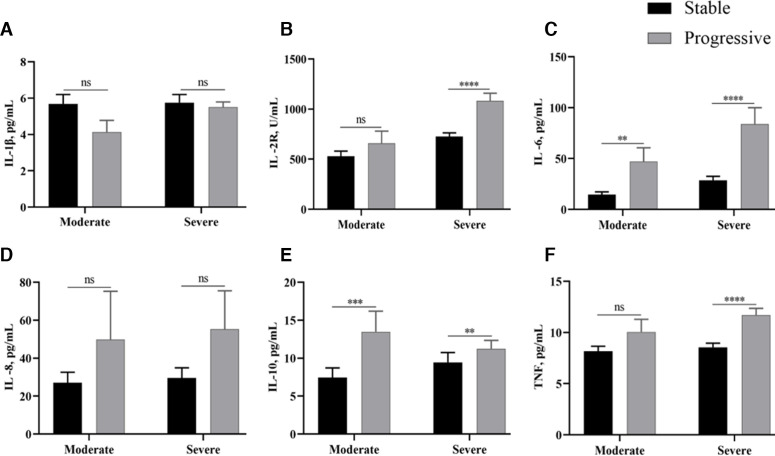
The baseline for cytokine concentrations was determined from blood obtained at the time of admission. The levels and the abnormal ratio of cytokines, including IL-2R, IL-6, IL-8, IL-10 and TNF, gradually increased as the disease progressed or resulted in poor prognosis (p<0.0001 for all). No significant difference was observed in IL-1β (p=0.8120) within each of the groups. Likewise, IL-2R (p<0.0001), IL-6 (p<0.0001), IL-8 (p=0.0001), IL-10 (p<0.0001) and TNF (p<0.0001) were also twofold to 20-fold higher in patients who died compared with those who recovered (table 1). Additionally, several cytokines, including proinflammatory and anti-inflammatory, were elevated at baseline in patients whose conditions were progressive compared with patients whose conditions were stable (figure 1). Specifically, the proinflammatory IL-2R (p<0.0001) and TNF (p<0.0001) were significantly higher in the progressive group compared with the stable group in patients of severe type. IL-6 was increased in the progressive group, both in moderate (p=0.0026) and in severe (p<0.0001) types of patients. Although numerically high, the differences in IL-8 levels between the two groups did not reach statistical significance. Similarly, the progressive group had higher levels of the anti-inflammatory cytokine IL-10 in both types of severity (p=0.0008 for moderate type, p=0.0011 for severe type). These results suggest that the progression of COVID-19 was associated with the initial levels of plasma cytokine.
Figure 1.
Cytokine values for patients with COVID-19 on admission versus the progressive and stable groups in both moderate and severe types. The error bars represent mean±SEM. P values were calculated by Mann-Whitney U test: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. IL, interleukin; IL-2R, interleukin 2 receptor; TNF, tumour necrosis factor. (A) IL-1β, (B) IL-2R, (C) IL-6, (D) IL-8, (E) IL-10, and (F) TNF.
Correlation between baseline cytokine levels and physiological variables measured on admission
The relationship between plasma cytokines and various physiological variables was assessed and is presented in table 2. Except for IL-1β, other plasma cytokines were positively correlated with age, albumin, creatinine, random blood glucose, D-dimer, lactic dehydrogenase and CRP (p<0.001 for all). Also, IL-2R (p=0.002), IL-6 (p<0.001) and IL-10 (p<0.001) were negatively correlated with total cholesterol. There was no significant relationship between the levels of cytokine and of C3 and C4.
Table 2.
Correlation among baseline inflammatory biomarkers and physiological variables measured on the day of admission
| IL-1β | IL-2R | IL-6 | IL-8 | IL-10 | TNF | |
| Age | −0.002 | 0.426 | 0.356 | 0.293 | 0.261 | 0.361 |
| 0.967 | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | |
| Time from onset of symptom to hospital admission | −0.061 | 0.222 | −0.011 | 0.038 | −0.069 | −0.055 |
| 0.289 | <0.001* | 0.843 | 0.504 | 0.226 | 0.336 | |
| Albumin | 0.177 | −0.527 | −0.467 | −0.319 | −0.346 | −0.290 |
| 0.003* | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | |
| ALT | −0.047 | 0.276 | 0.250 | 0.198 | 0.167 | 0.248 |
| 0.415 | <0.001* | <0.001* | 0.001* | 0.003* | <0.001* | |
| Creatinine | −0.037 | 0.332 | 0.355 | 0.226 | 0.284 | 0.439 |
| 0.512 | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | |
| Uric acid | 0.033 | 0.170 | 0.155 | 0.128 | 0.052 | 0.331 |
| 0.578 | 0.004* | 0.054 | 0.031* | 0.387 | <0.001* | |
| Total cholesterol | −0.021 | −0.177 | −0.332 | −0.080 | −0.276 | −0.103 |
| 0.709 | 0.002* | <0.001* | 0.159 | <0.001* | 0.071 | |
| Random blood glucose | 0.004 | 0.303 | 0.276 | 0.272 | 0.336 | 0.270 |
| 0.950 | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | |
| D-dimer | −0.026 | 0.614 | 0.509 | 0.328 | 0.378 | 0.367 |
| 0.651 | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | |
| Lactic dehydrogenase | −0.065 | 0.598 | 0.562 | 0.339 | 0.472 | 0.391 |
| 0.255 | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | |
| CRP | 0.008 | 0.618 | 0.776 | 0.345 | 0.528 | 0.493 |
| 0.894 | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | |
| C3 | −0.226 0.52 |
−0.235 0.137 |
−0.026 0.882 |
−0.066 0.652 |
0.093 0.576 |
−0.262 0.075 |
| C4 | 0.62 0.053 |
0.001 0.991 |
−0.056 0.711 |
−0.156 0.295 |
0.070 0.673 |
−0.21 0.163 |
Values represent Spearman’s correlation coefficients (upper) and p values (lower).
*Denotes statistically significant correlations.
ALT, alanine aminotransferase; CRP, C reactive protein; IL, interleukin; IL-2R, interleukin 2 receptor; TNF, tumour necrosis factor.
Association of plasma cytokines with in-hospital death
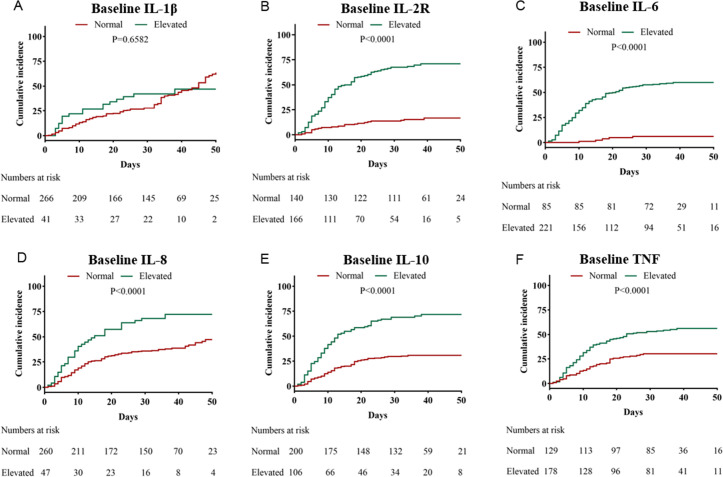
Kaplan-Meier analysis indicated a significantly higher mortality rate in patients with abnormal plasma cytokine values, including elevated IL-2R, IL-6, IL-8, IL-10 and TNF (p<0.0001 for all) (figure 2). Univariate logistic regression analysis revealed that IL-2R, IL-6, IL-8, IL-10 and TNF were related to a poor outcome (table 3). After adjusting for age, gender, comorbidities, disease severity and lymphocyte count, the levels of IL-2R and IL-6 were associated with in-hospital mortality (figure 3). Further, plasma cytokines were analysed by ROC analysis to evaluate their ability to predict in-hospital death rates (figure 4). The area under the curve was 0.82 (95% CI 0.78 to 0.87) for IL-2R, 0.85 (95% CI 0.81 to 0.89) for IL-6, 0.69 (95% CI 0.64 to 0.75) for IL-8, 0.75 (95% CI 0.69 to 0.81) for IL-10, and 0.71 (95% CI 0.65 to 0.77) for TNF (table 4).
Figure 2.
Cumulative incidence of in-hospital mortality in patients with COVID-19 subgrouped by cytokines: (A) IL-1β, (B) IL-2R, (C) IL-6, (D) IL-8, (E) IL-10 and (F) TNF. IL, interleukin; IL-2R, interleukin 2 receptor; TNF, tumour necrosis factor.
Table 3.
Logistic regression analysis of independent factors for predicting mortality of patients with COVID-19
| Cytokines | P value | OR | 95% CI |
| IL-1β, pg/mL* | 0.242 | 1.028 | 0.982 to 1.077 |
| IL-2R, U/mL* | <0.001 | 1.003 | 1.002 to 1.004 |
| IL-6, pg/mL* | <0.001 | 1.031 | 1.022 to 1.040 |
| IL-8, pg/mL* | <0.001 | 1.010 | 1.004 to 1.015 |
| IL-10, pg/mL* | <0.001 | 1.066 | 1.032 to 1.101 |
| TNF, pg/mL* | <0.001 | 1.205 | 1.131 to 1.285 |
*Per one-unit increase.
IL, interleukin; IL-2R, interleukin 2 receptor; TNF, tumour necrosis factor.;
Figure 3.
Association of inflammatory cytokines with in-hospital mortality in patients with COVID-19. ORs of each variable were obtained using multivariate logistic regression models after adjustment for age, gender, comorbidities, disease severity and lymphocyte count. Severity was staged based on the guidelines for diagnosis and treatment of COVID-19 (trial fifth edition) published by the Chinese National Health Commission on 4 February 2020. IL, interleukin; IL-2R, interleukin 2 receptor; TNF, tumour necrosis factor.
Figure 4.

Receiver operating characteristic curve of IL-2R, IL-6, IL-8, IL-10 and TNF for in-hospital death. IL, interleukin; IL-2R, interleukin 2 receptor; TNF, tumour necrosis factor.
Table 4.
Analysis of receiver operating characteristics curve for predicting in-hospital mortality of patients with COVID-19
| Variables | P value | AUC (95% CI) | Sensitivity | Specificity | Cut-off value | PPV | NPV |
| IL-2R | <0.001 | 0.82 (0.78 to 0.87) | 0.80 | 0.73 | 755.50 | 0.72 | 0.55 |
| IL-6 | <0.001 | 0.85 (0.81 to 0.89) | 0.77 | 0.75 | 22.80 | 0.71 | 0.80 |
| IL-8 | <0.001 | 0.69 (0.64 to 0.75) | 0.66 | 0.62 | 20.50 | 0.58 | 0.55 |
| IL-10 | <0.001 | 0.75 (0.69 to 0.81) | 0.80 | 0.66 | 5.15 | 0.65 | 0.55 |
| TNF | <0.001 | 0.71 (0.65 to 0.77) | 0.56 | 0.79 | 10.05 | 0.68 | 0.55 |
AUC, area under curve; IL, interleukin; IL-2R, interleukin 2 receptor; NPV, negative predictive value; PPV, positive predictive value; TNF, tumour necrosis factor.
Discussion
COVID-19 has rapidly spread throughout the world and has been labelled a pandemic by WHO. Both clinical features and serum markers associated with the severity of patients with COVID-19 have been reported.11–14 However, we do not know the exact reasons for the specific alterations in cytokine levels; it might be due to the immune response triggering the rapid disease progression. In the present study, we systematically analysed immunological characteristics, particularly cytokine profiles, and their relationship with severity, mortality and prognosis of COVID-19.
Consistent with a previous report, we noted lymphocytopaenia and increased inflammatory cytokine concentration in the majority of severe and critical cases, which were markedly worse when compared with the moderate cases. This indicates that an impaired immune system and a cytokine storm might be associated with COVID-19 severity. Further, the plasma concentration of cytokines in the progressive group, was higher than the stable group in severe cases, although differences in IL-2R, IL-8 and TNF between the two groups did not reach statistical significance in moderate cases, which might be partly due to the limited sample size; a recent study has shown that elevated cytokines were dynamically correlated with disease severity.5 15 16 Additionally, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) infections were also characterised by elevated levels of inflammatory cytokines with severe lung injury.17–20
Cytokines are proteins, glycoproteins or signalling peptides with potent various biological functions at picomolar concentration.21 IL-2R, IL-6, IL-8 and TNF were major proinflammatory factors required to initiate a series of effective immune cascade events to an infection or a tissue injury site. The anti-inflammatory IL-10 was found to inhibit the monocyte inflammatory response directly and negatively regulate the cascade of proinflammatory cytokines, which induced monocyte hyporesponsiveness in sepsis and multiple organ dysfunction.22 It is reported that coronavirus infection, its rapid replication, as well as the delayed type I interferon (IFN-I)signalling activate inflammatory monocyte-macrophage, resulting in an increased cytokine concentration, vascular leakage and pathogenic T cell response.23 Besides, lymphocytopaenia observed in severe and critical patients may impair T cells, which dampen the overactive innate immune response and further aggravate the inflammatory response.24 In line with the previous study, we suggest that excessive cytokine secretion may be associated with the progressive group. Nevertheless, the underlying cellular source and the mechanism involving cytokine accumulation remain to be determined.
Meanwhile, at the time of admission, serum levels of both proinflammatory and anti-inflammatory cytokines, including IL-2R, IL-6, IL-8, TNF and IL-10, were significantly higher in the case of non-survivors compared with that of the survivors. Previous studies have shown that proinflammatory cytokines predict mortality in patients with sepsis, acute respiratory distress syndrome and the severe infection seen after burn injuries.20 25 Also, elevated cytokines are a concern as an independent risk factor for poor outcome in acute renal failure.26 In line with previous research,27 univariate logistic regression analysis suggested that IL-2R, IL-6, IL-8, IL-10 and TNF were related to in-hospital mortality incidents in the present cohort. The association between IL-2R, IL-6 and in-hospital mortality was maintained after adjusting for demographics and other confounders. A similar conclusion of elevated cytokines related to in-hospital mortality has been found in SARS-CoV or MERS-CoV infection.18–20 IL-1 is released after the binding of SARS-CoV-2 to the Toll-like receptor (TLR), then mediates lung inflammation, fever and fibrosis, and provokes severe respiratory problems.28 29 However, both concentration and proportion of IL-1β, in contrast, were not increased in most of the patients, with only 13% of non-survivors showing elevated levels of IL-1β. Meanwhile, the secondary inflammatory cytokine IL-6, considered more distal than IL-1 in the inflammatory cascade, was a significant predictor of survival and had a higher area under the curve value. The specific immune cascade response and the cellular origin of cytokines in COVID-19 deserve further exploration.
At present, many drugs with variable efficacies have been proposed for the treatment of COVID-19-induced cytokine storm.28–31 The interplay of the main proinflammatory IL-6 and TNF contributes to the cytokine storm. Thus, the targeted therapy of IL-6 and TNF should not be neglected in patients with COVID-19. Tocilizumab, a specific monoclonal antibody that blocks IL-6, has been used for patients with severe COVID-19 with confirmed elevated levels of IL-6.30 Previous reports have shown that the use of anti-IL-6 treatment with tocilizumab led to a reduction in fever and lung lesion opacity, and recovered the percentage of lymphocytes in peripheral blood.32 Chloroquine and hydroxychloroquine can suppress the production of various cytokines, such as IL-1, IL-6 and TNF, via TLR signalling and cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS) stimulation of interferon genes.33 However, the therapeutic benefit of chloroquine in patients with COVID-19 remains controversial.34 35 Anti-TNF therapy is used in some patients with COVID-19 with autoimmune diseases. A case report showed that treatment with anti-TNF seems to have a protective effect on the evolution of severe types, thereby preventing the damaging effects of cytokine storm.36 However, anti-TNF has been associated with an increased risk of respiratory complications or death.37 Overall, immunomodulatory agents with good safety profiles for severe COVID-19 remain limited.
Our study had some limitations. First, this study is a retrospective analysis based on the initial inflammatory cytokines on admission. We did not describe the kinetic change in cytokine profiles of patients with COVID-19. The function of cytokine and the role of cytokine accumulation for pulmonary and other organs remain to be elucidated. Therefore, the relationship between prognosis significance and time-dependent changes in cytokines remains unknown. Second, since data regarding the lymphocyte subsets are not available, further studies are needed to analyse the correlation between the change in lymphocyte subsets and humoral immune response. Third, Tongji Hospital was a designated hospital for severely or critically ill patients with COVID-19, so there was bias in critical patient selection for prognostic research. Thus, the case fatality ratio in our study cannot reflect the true mortality of COVID-19. Last but not least, this was a retrospective, observational, single-centre study. Whether the results of the present study are applicable to other regions is questionable due to the potential differences in treatment protocol and time for patients to receive treatment.
In conclusion, both proinflammatory and anti-inflammatory cytokine alterations, including IL-2R, IL-6, IL-8, TNF and IL-10, show an obvious association with severity and in-hospital mortality in patients with COVID-19.
Supplementary Material
Acknowledgments
We thank all patients involved in the study. We are really grateful to all the health workers around the world. Their expertise and humanity are fundamental to stop SARS-CoV-2 from spreading further.
Footnotes
QQL and AC contributed equally.
Contributors: QQL, AC, XZ, LH and HL performed the data collection. QQL and AC wrote the first draft of the manuscript. AC, YW and QQL analysed the data and provided edits to the first draft of the manuscript. TW and FH participated in the revision of the manuscript and approved the final version.
Funding: This study was supported by the National Natural Science Foundation of PR China (grant number no 81800609) and the Frontier Application Basic Project of the Wuhan Science and Technology Bureau (grant number 2020020601012235).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Research Ethics Committee of Tongji Hospital (cord number: TJ-IRB20200353).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Please contact the corresponding author for all data requests.
References
- 1.Wang L, Wang Y, Ye D, et al. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents 2020;55:105948. 10.1016/j.ijantimicag.2020.105948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C, Alsafi Z, O'Neill N, et al. World Health organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg 2020;76:71–6. 10.1016/j.ijsu.2020.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Accessed 11 Mar 2020].
- 4.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017;39:517–28. 10.1007/s00281-017-0639-8 [DOI] [PubMed] [Google Scholar]
- 5.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong J, Dong H, Xia SQ, et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. medRxiv 2020:2020.02.25.20025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect 2020;50:382–3. 10.1016/j.medmal.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Zhang J, Yang Y, et al. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv 2020:2020.03.01.20029769. [Google Scholar]
- 9.Song C-Y, Xu J, J-Q H, et al. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv 2020:2020.03.05.20031906. [Google Scholar]
- 10.Wu C-L, Chan M-C, Chang G-C, et al. Etiology and cytokine expression in patients requiring mechanical ventilation due to severe community-acquired pneumonia. J Formos Med Assoc 2006;105:49–55. 10.1016/s0929-6646(09)60108-x [DOI] [PubMed] [Google Scholar]
- 11.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020;323:1488. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–41. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 2020. 10.1093/cid/ciaa449. [Epub ahead of print: 17 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Xu J, Zhou C, et al. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med 2005;171:850–7. 10.1164/rccm.200407-857OC [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Chu H, Li C, et al. Active replication of middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis 2014;209:1331–42. 10.1093/infdis/jit504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien J-Y, Hsueh P-R, Cheng W-C, et al. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 2006;11:715–22. 10.1111/j.1440-1843.2006.00942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong SL, Chui P, Lim B, et al. Elucidating the molecular physiopathology of acute respiratory distress syndrome in severe acute respiratory syndrome patients. Virus Res 2009;145:260–9. 10.1016/j.virusres.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bienvenu J, Monneret G, Fabien N, et al. The clinical usefulness of the measurement of cytokines. Clin Chem Lab Med 2000;38:267–85. 10.1515/CCLM.2000.040 [DOI] [PubMed] [Google Scholar]
- 22.Sfeir T, Saha DC, Astiz M, et al. Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit Care Med 2001;29:129–33. 10.1097/00003246-200101000-00026 [DOI] [PubMed] [Google Scholar]
- 23.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016;19:181–93. 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KD, Zhao J, Auh S, et al. Adaptive immune cells temper initial innate responses. Nat Med 2007;13:1248–52. 10.1038/nm1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraft R, Herndon DN, Finnerty CC, et al. Predictive value of IL-8 for sepsis and severe infections after burn injury: a clinical study. Shock 2015;43:222–7. 10.1097/SHK.0000000000000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons EM, Himmelfarb J, Sezer MT, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 2004;65:1357–65. 10.1111/j.1523-1755.2004.00512.x [DOI] [PubMed] [Google Scholar]
- 27.Cheng A, Hu L, Wang Y, et al. Diagnostic performance of initial blood urea nitrogen combined with D-dimer levels for predicting in-hospital mortality in COVID-19 patients. Int J Antimicrob Agents 2020;56:106110. 10.1016/j.ijantimicag.2020.106110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020;34:327–31. 10.23812/CONTI-E [DOI] [PubMed] [Google Scholar]
- 29.Conti P, Gallenga CE, Tetè G, et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents 2020;34:333–8. 10.23812/Editorial-Conti-2 [DOI] [PubMed] [Google Scholar]
- 30.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents 2020;55:105982. 10.1016/j.ijantimicag.2020.105982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Wang BJ, Yang JC, et al. [Research advances in the mechanism of pulmonary fibrosis induced by coronavirus disease 2019 and the corresponding therapeutic measures]. Zhonghua Shao Shang Za Zhi 2020;36:691–7. 10.3760/cma.j.cn501120-20200307-00132 [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970–5. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020;16:155–66. 10.1038/s41584-020-0372-x [DOI] [PubMed] [Google Scholar]
- 34.Fiolet T, Guihur A, Rebeaud ME, et al. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect 2020. 10.1016/j.cmi.2020.08.022. [Epub ahead of print: 26 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castelnuovo AD, Costanzo S, Antinori A, et al. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: findings from the observational multicentre Italian CORIST study. Eur J Intern Med 2020. 10.1016/j.ejim.2020.08.019. [Epub ahead of print: 25 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duret P-M, Sebbag E, Mallick A, et al. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann Rheum Dis 2020;79:1251–2. 10.1136/annrheumdis-2020-217362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brito CA, Paiva JG, Pimentel FN, et al. COVID-19 in patients with rheumatological diseases treated with anti-TNF. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-218171. [Epub ahead of print: 16 Jun 2020]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.