SUMMARY
The standard of care for gastroesophageal cancer patients with hepatic or pulmonary metastases is best supportive care or palliative chemotherapy. Occasionally, patients can be selected for curative treatment instead. This study aimed to evaluate patients who underwent a resection of hepatic or pulmonary metastasis with curative intent. The Dutch national registry for histo- and cytopathology was used to identify these patients. Data were retrieved from the individual patient files. Kaplan–Meier survival analysis was performed. Between 1991 and 2016, 32,057 patients received a gastrectomy or esophagectomy for gastroesophageal cancer in the Netherlands. Of these patients, 34 selected patients received a resection of hepatic metastasis (n = 19) or pulmonary metastasis (n = 15) in 21 different hospitals. Only 4 patients received neoadjuvant therapy before metastasectomy. The majority of patients had solitary, metachronous metastases. After metastasectomy, grade 3 (Clavien–Dindo) complications occurred in 7 patients and mortality in 1 patient. After resection of hepatic metastases, the median potential follow-up time was 54 months. Median overall survival (OS) was 28 months and the 1-, 3-, and 5- year OS was 84%, 41%, and 31%, respectively. After pulmonary metastases resection, the median potential follow-up time was 80 months. The median OS was not reached and the 1-, 3-, and 5- year OS was 67%, 53%, and 53%, respectively. In selected patients with gastroesophageal cancer with hepatic or pulmonary metastases, metastasectomy was performed with limited morbidity and mortality and offered a 5-year OS of 31–53%. Further prospective studies are required.
Keywords: esophageal and gastric cancer, esophageal and gastric surgery, hepatic surgery, metastasis, pulmonary surgery
INTRODUCTION
Gastric cancer and esophageal cancer are the third (723,000 deaths annually) and sixth (400,000 deaths annually) most common causes of cancer-related deaths worldwide.1 Neoadjuvant chemo(radio)therapy or perioperative chemotherapy followed by surgical resection is the cornerstone of curative care for gastroesophageal cancer.2–4 However, up to 40% of patients with gastroesophageal cancer present with synchronous metastatic disease and are regarded incurable.5,6 Approximately another 50% will develop metachronous metastases during follow-up after surgery.7–11 The most common sites of metastases are the lung, liver, peritoneum, and bone.12,13 Currently, the standard treatment for patients with hepatic or pulmonary metastases from gastroesophageal cancer consists of best supportive care or palliative chemotherapy. This leads to a median survival of 4–5 months for esophageal and 4–11 months for gastric cancer.14,15 Resection of hepatic or pulmonary metastases has been established as a treatment option for other tumors, such as endocrine and colorectal cancer.16–22 Several small retrospective studies suggest that resection of hepatic and pulmonary metastases from gastroesophageal cancer is also feasible and may increase survival in a selected group of patients.23–27 Unfortunately, large series on metastasectomy are scarce for metastatic gastric cancer and even scarcer for esophageal cancer. According to a meta-analysis published in 2018,28 no Western series exist that report on the resection of metachronous pulmonary metastasis.
The aim of this study was to identify and evaluate all patients in the Netherlands who underwent a resection of hepatic or pulmonary metastases from gastroesophageal cancer with curative intent. Primary outcomes were progression-free survival (PFS) and overall survival (OS). Secondary outcomes included surgical complications. Lastly, we wanted to describe the factors that may influence long-term survival.
METHODS
Patients and data
All patients who underwent a resection of hepatic or pulmonary metastases from gastroesophageal cancer between January 1991 and March 2016 were identified from the Dutch national registry for histo- and cytopathology (PALGA). The PALGA database collects every pathological report generated by pathology departments in the Netherlands since 1975.29 The PALGA database was searched for ‘(stomach OR esophagus) AND (liver OR lung) AND metastases AND resection’ and synonyms. Summaries of the pathology reports were supplied by PALGA and used for further patient selection. Patients treated with curative intent, and of whom patient records were available, were included. Patient and treatment-related characteristics, as well as surgical outcome and histopathological data, were retrospectively collected from the individual patient files. The Netherlands Comprehensive Cancer Organisation supplied the total number of gastrectomies and esophagectomies performed for gastroesophageal cancer in the Netherlands between January 1991 and March 2016.
The primary tumor was staged according to the tumor node metastasis classification system of the International Union Against Cancer (UICC). Radicality was defined according to the UICC standards; R0: complete microscopic resection, R1: microscopic residual disease, or R2: local macroscopic residual disease or distant macroscopic residual disease. Curative intent was defined as patients for whom the intention of the surgery was to perform an R0 resection. Metastases were considered synchronous when they were diagnosed within 6 months after diagnosis of the primary tumor. Locations of hepatic metastases were defined according to Couinaud's liver segments.30 Location of pulmonary metastases were defined according to the definition of the Thoracic Society.31 Hepatic resections were divided into major and minor resections. Minor resections were defined as a resection of less than four segments; every other resection was defined as a major resection. This study received ethical approval (Institutional Review Board number 16–312/C) from the Medical Ethics Review Committee of the UMCU, and the need to obtain informed consent was waived.
Outcomes
The primary outcomes were PFS and OS. ‘PFS’ was defined as the interval between metastasectomy and recurrence or progression of (residual) disease regardless of organ or tissue, or death from any cause. ‘OS’ was defined as the interval between metastasectomy and date of last follow-up or death.
Secondary outcome in this study was the safety of metastasectomy, expressed in terms of postoperative morbidity and 30-day mortality and in-hospital mortality. Postoperative complications were classified according to the Clavien–Dindo classification. Follow-up was not standardized.
Statistical considerations
Data were analyzed using SPSS for windows, version 22.0 (IBM corp., Armonk, New York). All continuous data were presented as median (range) or mean (± standard deviation (SD)) based on their distribution; all categorical data were presented as a number (percentage). Patient and treatment-related characteristics in relation to the surgical procedure were studied using descriptive statistics. Potential follow-up time was calculated with Kaplan–Meier estimate of potential follow-up (‘reverse Kaplan–Meier’).32 Kaplan–Meier curves were used to assess PFS and OS. Nonoverlapping curves were compared with the log-rank test.
RESULTS
Patients
Between January 1991 and March 2016, a total of 32,057 patients received a gastrectomy or esophagectomy for gastroesophageal cancer in the Netherlands. Our initial PALGA search yielded summaries of 309 patients that could potentially be included in the current study. A total of 138 of these patients were excluded, because based upon the summaries of the pathology reports, it was clear that a biopsy was performed instead of a metastasectomy with curative intent. For the remaining 171 patients, the patient files (if available) were reviewed. Patients were excluded who did not receive a resection of gastroesophageal metastasis with curative intent (primary tumor left in situ, biopsy instead of resection, multiple other metastases left in situ, or metastasis not of gastroesophageal origin on definitive pathology) or who had unavailable patient files. The remaining 34 patients from 21 different hospitals were included. These patients were thus treated with a resection of hepatic or pulmonary metastasis from gastroesophageal origin with curative intent.
Hepatic resection for metastases from gastroesophageal cancer
A resection of hepatic metastases was performed in 19 patients (Table 1). The (neo)adjuvant treatments are described in Tables 1 and 2. Extrahepatic metastasis prior to hepatic resection was present in 1 patient. This patient had a retrosternal metastasis that was treated with resection and radiotherapy. Metachronous metastases were diagnosed in 13 patients and synchronous metastases in 6 patients. The metastases were solitary in 16 patients and non-solitary (2 metastases) in 3 patients.
Table 1.
Gastroesophageal cancer: patient and treatment-related characteristics in relation to surgical procedure
| Characteristic | Hepatic resection (N = 19) | Pulmonary resection (N = 15) | |||
|---|---|---|---|---|---|
| Gender | Female | 4 | 21.1% | 5 | 33.3% |
| Male | 15 | 78.9% | 10 | 66.7% | |
| Mean age (years) | 59.7 | ±12.2 | 63.5 | ±8.79 | |
| Mean BMI (kg/m2) | 20.6 | ±3.49 | 24.5 | ±2.85 | |
| Missing | 7 | 36.8% | 3 | 20.0% | |
| ASA score | I | 3 | 15.8% | 1 | 6.67% |
| II | 10 | 52.6% | 6 | 40.0% | |
| III | 2 | 10.5% | 4 | 26.7% | |
| Missing | 4 | 21.1% | 4 | 26.7% | |
| Organ of primary tumor | Stomach | 8 | 42.1% | 4 | 26.7% |
| Esophagus | 11 | 57.9% | 11 | 73.3% | |
| Location of primary tumor | Middle esophagus | 0 | 2 | 13.3% | |
| Distal esophagus | 7 | 36.8% | 8 | 53.3% | |
| Cardia (esophagectomy) | 3 | 15.8% | 1 | 6.67% | |
| Cardia (gastrectomy) | 1 | 5.26% | 4 | 26.7% | |
| Corpus | 2 | 10.5% | 0 | ||
| Antrum | 4 | 21.1% | 0 | ||
| Pars pylori | 1 | 5.26% | 0 | ||
| Missing | 1 | 5.26% | 0 | ||
| Stage of primary tumor | 0 | 1 | 5.26% | 0 | |
| IA | 3 | 15.8% | 3 | 20.0% | |
| IB | 1 | 5.26% | 0 | ||
| IIA | 4 | 21.1% | 6 | 40.0% | |
| IIB | 2 | 10.5% | 1 | 6.67% | |
| IIIA | 5 | 26.3% | 5 | 33.3% | |
| IIIB | 1 | 5.26% | 0 | ||
| IV | 2 | 10.5% | 0 | ||
| Histology of primary tumor | Adenocarcinoma | 17 | 89.5% | 9 | 60.0% |
| Squamous cell carcinoma | 1 | 5.26% | 4 | 26.7% | |
| Neuroendocrine carcinoma‡ | 1 | 5.26% | 1 | 6.67% | |
| Undifferentiated | 0 | 1 | 6.67% | ||
| Differentiation of primary tumor | Moderately | 8 | 42.1% | 5 | 33.3% |
| Poorly | 4 | 21.1% | 4 | 26.7% | |
| Undifferentiated | 0 | 1 | 6.67% | ||
| Missing | 7 | 36.8% | 5 | 33.3% | |
| (Neo)adjuvant therapy of primary tumor | Neoadjuvant CRT | 6 | 31.6% | 4 | 26.7% |
| Neoadjuvant CT | 0 | 1 | 6.67% | ||
| Neoadjuvant and adjuvant CT | 2 | 10.5% | 3 | 20.0% | |
| None | 11 | 57.9% | 7 | 46.7% | |
| History of extrahepatic or | Yes | 1 | 5.26% | 0 | |
| extrapulmonary metastasis prior to metastasectomy | No | 18 | 94.7% | 15 | 100.0% |
| Interval diagnoses primary tumor | Synchronous | 6 | 31.6% | 1 | 6.7% |
| and metastases | Metachronous | 13 | 68.4% | 14 | 93.3% |
| Median interval (months) between diagnosis of primary tumor and diagnosis of metastases† | 20 | 11–27 | 30 | 18–39 | |
| Missing | 2 | 10.5% | 1 | 6.67% | |
| Number of metastases | 1 | 16 | 84.2% | 13 | 86.7% |
| 2 | 3 | 15.8% | 2 | 13.3% | |
Data are n (%), mean (±SD), and median (IQR).
†For the metachronous tumors.
‡Both patients had a high-grade neuroendocrine neoplasm with a Ki-index of >20% or >20 mitoses per 10 high power fields, which were thus classified as neuroendocrine carcinoma.40
ASA, American Society of Anesthesiologists; BMI, body mass index; CRT, chemoradiotherapy; CT, chemotherapy.
Table 2a.
Hepatic resection: perioperative and histological outcomes
| Outcomes | N = 19 | ||
|---|---|---|---|
| Neoadjuvant therapy prior to hepatic | Chemoradiotherapy† | 2 | 10.5% |
| resection | Chemotherapy‡ | 1 | 5.3% |
| Chemoradiation, followed by chemoimmunotherapy§ | 1 | 5.3% | |
| None | 14 | 73.7% | |
| Missing | 1 | 5.3% | |
| Timing of hepatic resection | Before resection of primary tumor | 1 | 5.3% |
| Combined resection (of primary and metastasis) | 3 | 15.8% | |
| After resection of primary tumor | 15 | 78.9% | |
| Year of hepatic resection | 1995–2000 | 3 | 15.8% |
| 2001–2005 | 2 | 10.5% | |
| 2006–2010 | 4 | 21.1% | |
| 2011–2014 | 10 | 52.6% | |
| Distribution | Unilobar | 17 | 89.5% |
| Bilobar | 2 | 10.5% | |
| Segments | 1 | 12 | 63.2% |
| >2 | 7 | 36.8% | |
| Mean diameter (in mm) of metastasis¶ | 34.5 | ±37.5 | |
| Missing | 2 | 10.5% | |
| Type of hepatic resection | Minor | 16 | 84.2% |
| Major | 3 | 15.8% | |
| Radicality | R0 | 17 | 89.5% |
| R1–2 | 2 | 10.5% | |
| Complications | Total | 6 | 31.6% |
| CD1 | 0 | 0.0% | |
| CD2 | 2 | 10.5% | |
| CD3 | 3 | 15.8% | |
| CD4 | 0 | 0.0% | |
| CD5 | 1 | 5.3% | |
| Reintervention | 4 | 21.1% | |
| In-hospital or 30 day postoperative mortality | 1 | 5.3% | |
| Median hospital stay (days) | 7 | 1–76 | |
| Missing | 3 | 15.8% |
Data are n (%), mean (±SD), or median (range).
†Combined resection of primary tumor and metastasis; neoadjuvant therapy was aimed at the primary tumor.
‡ Metastasis was initially too large for resection; however, after induction chemotherapy, resection could be performed.
§Metastasectomy was performed before resection of primary tumor.
¶Metastasis with largest diameter, as based upon pathology in 11 cases and computed tomography scan in 2 cases.
CD, Clavien–Dindo classification.
The operative characteristics of the hepatic resections are displayed in Table 2a. Metastases were unilobar in 17 patients and confined to one liver segment in 12 patients. An R0 resection was achieved in 17 patients. After hepatic resection, complications were encountered in 6 patients. This led to a reintervention in 4 patients and death within 30 days in 1 patient. This patient had extensive cardiovascular comorbidities. He was readmitted after a postoperative hemorrhage. After a total intensive care unit stay of 26 days, this patient died due to ventricular fibrillation.
Survival after hepatic resection
The median potential follow-up time was 54 months. Kaplan–Meier survival curves after hepatic resection for gastroesophageal cancer metastases are displayed in Fig. 1. The median progression-free survival was 16 months and the 1-, 3-, and 5-year PFS was 58%, 21%, and 21%, respectively. The median overall survival (OS) was 28 months and the 1-, 3-, and 5-year OS was 84%, 41%, and 31%, respectively.
Fig. 1.
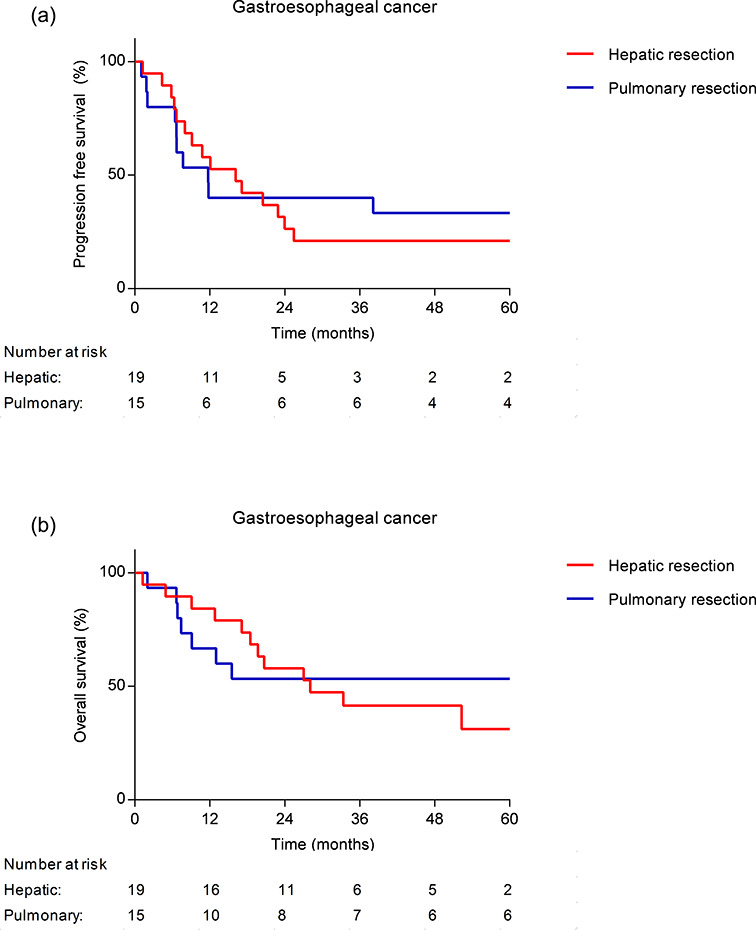
Kaplan–Meier survival curves after hepatic or pulmonary resection, displaying PFS (a) and OS (b).
A subgroup analysis of the 8 patients with gastric cancer hepatic metastasis showed a median OS of 23 months and the 1-, 3- and 5- year OS were 75%, 25% and 25% respectively. The 11 patients with esophageal cancer hepatic metastasis had a median OS of 52 months and the 1-, 3-, and 5-year OS was 91%, 55%, and 27%, respectively.
Pulmonary resection for metastases from gastroesophageal cancer
A resection of pulmonary metastases was performed in 15 patients (Table 1). The (neo)adjuvant treatments are described in Tables 1 and 2. None of the patients had extrapulmonary metastases. Metachronous metastases were diagnosed in 14 patients and synchronous metastases in 1 patient. The metastases were solitary in 13 patients and nonsolitary (2 metastases) in 2 patients.
The operative characteristics of the pulmonary resections are displayed in Table 2b. An R0 resection was achieved in 12 patients, an R1–2 resection in 2 patients, and data on the radicality were missing in 1 patient. After pulmonary resection, complications were encountered in 6 patients, though data on complications were missing in 2 patients. The complication led to a reintervention in 4 patients and there was no 30-day mortality or in-hospital mortality. The median hospital stay was 7 days.
Table 2b.
Pulmonary resection: perioperative and histological outcomes
| Outcomes | N = 15 | ||
|---|---|---|---|
| Neoadjuvant therapy prior to pulmonary resection | None | 15 | 100.0% |
| Timing of pulmonary resection | Before resection of primary tumor | 0 | |
| Combined resection (of primary and metastasis) | 0 | ||
| After resection of primary tumor | 15 | 100.0% | |
| Year of pulmonary resection | 1991–1995 | 2 | 13.3% |
| 1996–2000 | 0 | ||
| 2001–2005 | 1 | 6.7% | |
| 2006–2010 | 6 | 40.0% | |
| 2011–2015 | 6 | 40.0% | |
| Distribution | Left, superior lobe | 2 | 13.3% |
| Left, inferior lobe | 4 | 26.7% | |
| Right, superior lobe | 8 | 53.3% | |
| Right, inferior lobe | 0 | ||
| Middle lobe | 0 | ||
| Missing | 1 | 6.7% | |
| Mean diameter (in mm) of metastasis† | 17.3 | ±12.6 | |
| Missing | 2 | 13.3% | |
| Operative methods | Wedge resection | 11 | 73.3% |
| Segmentectomy | 0 | ||
| Lobectomy | 4 | 26.7% | |
| Radicality | R0 | 12 | 80.0% |
| R1–2 | 2 | 13.3% | |
| Missing | 1 | 6.7% | |
| Complications | Total | 6 | 40.0% |
| CD1 | 2 | 13.3% | |
| CD2 | 0 | ||
| CD3 | 4 | 26.7% | |
| CD4 | 0 | ||
| CD5 | 0 | ||
| Missing | 2 | 13.3% | |
| Reintervention | 4 | 26.7% | |
| In-hospital or 30 day postoperative mortality | 0 | ||
| Median hospital stay (days) | 12 | 3–41 | |
| Missing | 1 | 6.67% |
Data are n (%), mean (±SD), or median (IQR).
†Metastasis with largest diameter, as based upon pathology in 11 cases and computed tomography scan in 2 cases.
CD, Clavien–Dindo classification.
Survival after pulmonary resection
The median potential follow-up time was 80 months. Kaplan–Meier survival curves after pulmonary resection for gastroesophageal cancer metastases are displayed in Fig. 1. The median PFS was 12 months and the 1-, 3-, and 5- year PFS was 40%, 40%, and 33%, respectively. The median OS was not reached and the 1-, 3-, and 5-year OS was 67%, 53%, and 53%, respectively.
A subgroup analysis of the 4 patients with gastric cancer pulmonary metastasis showed a median OS of 7 months. The 1-, 3-, and 5-year OS was 25%. The 11 patients with esophageal cancer did not reach the median OS and the 1-, 3-, and 5-year OS was 82%, 64%, and 64%, respectively.
Disease-free interval subgroup analysis
Two further subgroup analyses were performed comparing OS for patients with a disease-free interval (DFI) of <24 months versus >24 months and comparing OS for patients with synchronous versus metachronous metastasis. These analyses are displayed in Online Supplementary Material 1.
DISCUSSION
This study reports on patients who underwent a resection of hepatic and pulmonary metastases from gastroesophageal cancer. According to a meta-analysis published in 2018,28 this is the first Western series on the resection of predominantly metachronous pulmonary metastasis from esophageal cancer. Metastasectomy was performed in selected patients with low postoperative morbidity and mortality rates, leading to a 5-year OS of 31–53%. The median OS was 28 months in the hepatic resection group and the median OS was not reached in the pulmonary resection group. The survival rates in these selected patients are favorable compared to the current standard treatment of best supportive care or palliative chemotherapy, which leads to a median survival of 4–11 months.14,15
Previous retrospective studies on patients with hepatic metastases from gastric cancer demonstrated a favorable survival after a hepatic resection.23,24 Only 2 series with over 5 patients exist on hepatic metachronous metastases from esophageal cancer that were treated with esophagectomy and hepatic resection.28 Liu et al. reported a 2-year OS of 21.2% in 26 patients and Adam et al. reported a 3-year OS of 12–32%.33,34 The current study demonstrates a 3-year and 5-year OS of 55% and 27%, respectively, in 11 patients after hepatic resection.
Data on pulmonary resections for gastroesophageal pulmonary metastasis are also scarce. A recent systematic review on pulmonary resections for gastric cancer pulmonary metastasis found only 44 patients reported in the literature thus far and reported a median survival of 45 months.25 In 3 small retrospective cohort series, all Japanese with predominantly squamous cell carcinoma, pulmonary resections for esophageal cancer pulmonary metastasis were found to be feasible and leading to a 5-year OS of 30–44%. They conclude that pulmonary resection could be considered in selected patients.27,35,36 The current study, which included 15 Western patients with mostly gastroesophageal adenocarcinoma, also supports this conclusion.
Although hepatic and pulmonary metastasectomies may be performed rather safely, they do carry a certain risk. This is illustrated by 1 postoperative death and 7 postoperative reinterventions in 34 patients in the current study. In the selected patient group in this study, the risk for postoperative complications and death seems acceptable as relatively favorable OS rates were achieved. In patients with metastatic gastroesophageal cancer, quality of life must be taken into account as well. Unfortunately, data on quality of life after resection of gastroesophageal metastases are lacking.
Metastasectomy of gastroesophageal hepatic or pulmonary metastases is not the standard of care in the Netherlands. The patients described in this study are highly selected. Most patients had metachronous metastasis with long intervals between diagnosis of the primary tumor and the metastasis. The metachronous presentation could be indicative of a more favorable cancer biology, as is the case for metachronous hepatic metastases from colorectal cancer.37 Furthermore, except for one patient, all patients had oligometastatic disease. The majority of metastases were solitary and radically resected and a slight majority of the primary tumors were moderately differentiated. Comparable characteristics were also reported as favorable prognostic factors in the study by Kobayashi et al. on patients who underwent pulmonary metastasectomy of esophageal cancer metastases.27 It is possible that these factors contributed to the favorable survival in the current study and these factors could be used as a means of selecting physically fit patients for curative therapy, ideally within the context of a prospective study.
Only 7 patients in this study had synchronous oligometastasis and it is thus difficult to use the data in the current study to discuss a procedure to select these patients for curative therapy. For these patients, a tailored approach is required, which could include any combination of systemic therapy, resection of the primary tumor, and local treatment of the metastasis by resection or (stereotactic body) radiotherapy. Recently, the AIO-FLOT3 trial also demonstrated favorable results: a median OS of 31 months with perioperative chemotherapy and combined resection of the primary tumor and oligometastasis in patients with limited synchronous metastatic diseases.38 The AIO-FLOT5 trial is currently investigating this approach in a randomized setting.39
A strength of this study is the use of the Dutch national registry for histo- and cytopathology, which guaranteed the inclusion of all patients who underwent a hepatic or pulmonary resection with curative intent for metastatic gastroesophageal cancer (with available patient files) in the Netherlands within a time frame of 25 years. A limitation of this study is its retrospective nature. Especially in old patient files, some variables were missing and the reason why a resection was or was not performed was not always explicitly mentioned. The main limitation of this study is selection bias. Nevertheless, one could speculate that the favorable survival is unlikely to be the result of selection alone, but rather of selection in combination with metastasectomy.
In conclusion, in selected patients with gastroesophageal cancer with hepatic or pulmonary metastasis, metastasectomy was performed with limited morbidity and mortality and offered a 5-year OS of 31–53%. These results justify the conduction of prospective studies with strict inclusion criteria, which can evaluate whether or not more patients can be selected for a metastasectomy and if an improvement in survival and quality of life is observed in a randomized setting.
Acknowledgments
The authors would like to thank the Dutch national registry for histo- and cytopathology (PALGA) for identification of the patients. Furthermore, the authors would like to thank the Netherlands Comprehensive Cancer Organisation (IKNL) for supplying the total number of gastrectomies and esophagectomies performed for gastroesophageal cancer in the Netherlands between January 1991 and March 2016.
Conflicts of interest: Authors have no conflicts of interest or financial ties to disclose.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher's website:
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. van Hagen P, Hulshof M C C M, van Lanschot J J B, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. [DOI] [PubMed] [Google Scholar]
- 3. Shapiro J, van Lanschot J J B, Hulshof M C C M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16: 1090–8. [DOI] [PubMed] [Google Scholar]
- 4. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet 2017; 390: 2383–96. [DOI] [PubMed] [Google Scholar]
- 5. Thomassen I, Van Gestel Y R, Van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014; 134: 622–8. [DOI] [PubMed] [Google Scholar]
- 6. Koppert L B, Lemmens V E P P, Coebergh J W W, et al. Impact of age and co-morbidity on surgical resection rate and survival in patients with oesophageal and gastric cancer. Br J Surg 2012; 99: 1693–700. [DOI] [PubMed] [Google Scholar]
- 7. Hulscher J B F, Van Sandick J W, Tijssen J G P, Obertop H, Van Lanschot J J B. The recurrence pattern of esophageal carcinoma after transhiatal resection11No competing interests declared. J Am Coll Surg 2000; 191: 143–8. [DOI] [PubMed] [Google Scholar]
- 8. Nakagawa S, Kanda T, Kosugi S I, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004; 198: 205–11. [DOI] [PubMed] [Google Scholar]
- 9. Parry K, Visser E, van Rossum P S N, Mohammad N H, Ruurda J P, van Hillegersberg R. Prognosis and treatment after diagnosis of recurrent esophageal carcinoma following esophagectomy with curative intent. Ann Surg Oncol 2015; 22: 1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dresner S M, Griffin S M. Pattern of recurrence following radical oesophagectomy with two-field lymphadenectomy. Br J Surg 2000; 87: 1426–33. [DOI] [PubMed] [Google Scholar]
- 11. Cunningham D, Allum W H, Stenning S P, et al. Ives for the MTP. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 12. Shaheen O, Ghibour A, Alsaid B. Esophageal cancer metastases to unexpected sites: A systematic review. Gastroenterol Res Pract 2017; 2017: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roviello F, Marrelli D, De Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 2003; 90: 1113–9. [DOI] [PubMed] [Google Scholar]
- 14. Janmaat V T, Bruno M J, Peppelenbosch M P, et al. Palliative chemotherapy and targeted therapies for esophageal and gastro-esophageal junction cancer. Cochrane Database Syst Rev 2017; 28: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner A D, Syn N, Moehler M, et al. Chemotherapy for advanced gastric cancer (Review). Cochrane Database of Systematic Reviews 2017; CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fong Y, Fortner J, Sun R L, Brennan M F, Blumgart L H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230: 309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases. Ann Surg 2003; 238: 871–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Association Française de Chirurgie. Br J Surg 1997; 84: 977–80. [DOI] [PubMed] [Google Scholar]
- 19. Riquet M, Foucault C, Cazes A, et al. Pulmonary resection for metastases of colorectal adenocarcinoma. Ann Thorac Surg 2010; 89: 375–80. [DOI] [PubMed] [Google Scholar]
- 20. Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 2007; 84: 324–38. [DOI] [PubMed] [Google Scholar]
- 21. McCormack P M, Burt M E, Bains M S, Martini N, Rusch V W, Ginsberg R J. Lung resection for colorectal metastases. 10-year results. Arch Surg 1992; 127: 1403–6. [DOI] [PubMed] [Google Scholar]
- 22. Chan M Y, Ma K W, Chan A. Surgical management of neuroendocrine tumor-associated liver metastases: a review. Gland Surg 2018; 7: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markar S R, Mikhail S, Malietzis G, et al. Influence of surgical resection of hepatic metastases from gastric adenocarcinoma on long-term survival: systematic review and pooled analysis. Ann Surg 2016; 263: 1092–101. [DOI] [PubMed] [Google Scholar]
- 24. Markar S R, Mackenzie H, Mikhail S, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer 2017; 20: 379–86. [DOI] [PubMed] [Google Scholar]
- 25. Aurello P, Petrucciani N, Giulitti D, Campanella L, D’Angelo F, Ramacciato G. Pulmonary metastases from gastric cancer: is there any indication for lung metastasectomy? A systematic review. Med Oncol 2016; 33: 9. [DOI] [PubMed] [Google Scholar]
- 26. Huddy J R, Thomas R L, Worthington T R, Karanjia N D. Liver metastases from esophageal carcinoma: is there a role for surgical resection? Dis Esophagus 2015; 28: 483–7. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi N, Kohno T, Haruta S, et al. Pulmonary metastasectomy secondary to esophageal carcinoma: long-term survival and prognostic factors. Ann Surg Oncol 2014; 21: 365–9. [DOI] [PubMed] [Google Scholar]
- 28. Schizas D, Lazaridis II, Moris D, et al. The role of surgical treatment in isolated organ recurrence of esophageal cancer—a systematic review of the literature. World J Surg Onc 2018; 16: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007; 29: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer L, Cardenas C, Thorn M, et al. Limits of Couinaud's liver segment classification: a quantitative computer-based three-dimensional analysis. J Comput Assist Tomogr 2002; 26: 962–7. [DOI] [PubMed] [Google Scholar]
- 31. The Thoracic Society The Nomenclature of broncho-pulmonary anatomy: an international nomenclature accepted by the Thoracic Society. Thorax 1950; 5: 222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schemper M, Smith T L. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–6. [DOI] [PubMed] [Google Scholar]
- 33. Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1452 patients and development of a prognostic model. Ann Surg 2006; 244: 524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu J, Wei Z, Wang Y, Xia Z, Zhao G. Hepatic resection for post-operative solitary liver metastasis from oesophageal squamous cell carcinoma. ANZ J Surg 2018; 88: E252–6. [DOI] [PubMed] [Google Scholar]
- 35. Ichikawa H, Kosugi S, Nakagawa S, Kanda T. Operative treatment for metachronous pulmonary metastasis from esophageal carcinoma. Surgery 2011; 149: 164–70. [DOI] [PubMed] [Google Scholar]
- 36. Shiono S, Kawamura M, Sato T, et al. Disease-free interval length correlates to prognosis of patients who underwent metastasectomy for esophageal lung metastases. J Thorac Oncol 2008; 3: 1046–9. [DOI] [PubMed] [Google Scholar]
- 37. Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 2015; 41: 729–41. [DOI] [PubMed] [Google Scholar]
- 38. Al-Batran S E, Homann N, Pauligk C, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA Oncol 2017; 3: 1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Al-Batran S E, Goetze T O, Mueller D W, et al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction—a phase III tri. BMC Cancer 2017; 17: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Veen A, Seesing M F J, Wijnhoven B P L, et al. Management of resectable esophageal and gastric (mixed adeno) neuroendocrine carcinoma: A nationwide cohort study. Eur J Surg Oncol. 2018; 44: 1955–62. [DOI] [PubMed] [Google Scholar]


