Abstract
The discovery of alternative signaling pathways that regulate cell death has revealed multiple strategies for promoting cell death with diverse consequences at the tissue and organism level. Despite the divergence in the molecular components involved, membrane permeabilization is a common theme in the execution of regulated cell death. In apoptosis, the permeabilization of the outer mitochondrial membrane by BAX and BAK releases apoptotic factors that initiate the caspase cascade and is considered the point of no return in cell death commitment. Pyroptosis and necroptosis also require the perforation of the plasma membrane at the execution step, which involves Gasdermins in pyroptosis, and MLKL in the case of necroptosis. Although BAX/BAK, Gasdermins and MLKL share certain molecular features like oligomerization, they form pores in different cellular membranes via distinct mechanisms. Here, we compare and contrast how BAX/BAK, Gasdermins, and MLKL alter membrane permeability from a structural and biophysical perspective and discuss the general principles of membrane permeabilization in the execution of regulated cell death.
Keywords: apoptosis, cell death, membrane pores, necroptosis, pyroptosis
Subject Categories: Autophagy & Cell Death, Immunology
In this review, Ana Garcia‐Saez and colleagues compare and contrast the mechanisms by which proteins such as BAX/BAK, Gasdermins and MLKL can create membrane pores, and which roles these play, in different forms of regulated cell death.
Pore formation in membranes is a conserved strategy to kill cells
Biological membranes define biochemical environments and are fundamental for the existence of life (Deamer, 2016). Selective transport between these enclosed spaces is highly regulated and sustained disruption in membrane integrity is, as a consequence, a point of no return in cell death (Youle & Strasser, 2008; Kunzelmann, 2016; Broz et al, 2020). Yet, alterations in membrane permeability are relevant for a large number of biological processes including immunity, metabolism, and infection (Kagan, 2012; McCormack et al, 2013).
A membrane pore can be defined as any local membrane perturbation that allows the passive flow of molecules (Schwarz & Robert, 1992). Pore‐forming proteins (PFPs) represent a large and structurally diverse family of proteins that have the common function of altering membrane permeability by creating pores. They can act exogenously as secreted soluble proteins that permeabilize the plasma membrane of their target cells. This includes most pore‐forming toxins (PFTs), which are some of the most potent virulence factors found in nature (Ros & Garcia‐Saez, 2015; Dal Peraro & van der Goot, 2016) or perforin, which is released by cytotoxic T cells and Natural Killer cells (Voskoboinik et al, 2015; Prinz et al, 2020). PFPs can also be intracellular executioners as components of cell death signaling pathways (Espiritu et al, 2019; Flores‐Romero et al, 2020). For instance, Bcl‐2‐associated X protein (BAX) and BCL2‐antagonist/killer 1 (BAK) form pores that lead to mitochondrial outer membrane permeabilization (MOMP) during intrinsic apoptosis. Gasdermins (GSDMs) execute pyroptosis by a mechanism that culminates with pore opening at the plasma membrane. In necroptosis, mixed lineage kinase domain‐like (MLKL) induces plasma membrane permeabilization through a yet unclear mechanism that could also be linked to pore formation (Liu et al, 2016; Cosentino & Garcia‐Saez, 2017; Flores‐Romero & Garcia‐Saez, 2019a).
PFPs are usually classified in α or β, depending on the secondary structure of the protein segments forming the pores (Ros & Garcia‐Saez, 2015; Cosentino et al, 2016). In addition, PFPs can build different types of pores, as defined by the presence or absence of lipids in the pore structure (Ros & Garcia‐Saez, 2015; Gilbert, 2016). They are classified as protein‐lined if they are constituted only of proteins, as pure lipid pores, or as protein/lipid pores, when they contain both types of molecules. In protein‐lined pores, the lumen is solely covered by transmembrane segments of proteins organized into α‐ or β‐barrel “walls” (Dal Peraro & van der Goot, 2016; Gilbert, 2016). Importantly, in this type of pores, the membrane or certain membrane lipids can play a functional role in protein recruitment, assembly, and folding (Rojko & Anderluh, 2015; Gilbert, 2016). In contrast, membrane lipids together with the amphipathic regions of proteins or peptides form the edge of protein/lipid pores (Ludtke et al, 1996; Matsuzaki et al, 1996). Here, lipids rearrange from their bilayer distribution to a non‐lamellar assembly that creates a continuous surface in which the membrane bends at the pore boundary like a torus. Protein/lipid or toroidal pore opening is promoted by proteins or protein fragments that generate membrane tension. PFP accumulation at or next to the pore rim then reduces the line tension and stabilizes the open pore state (Karatekin et al, 2003; Puech et al, 2003) (Fig 1). Pure lipid pores are also toroidal, but in the absence of proteins, their opening probability and lifetime are very low. They mainly occur upon strong membrane perturbations such as mechanical and electrical tension or osmotic swelling (Tieleman et al, 2003; Tieleman & Marrink, 2006).
Figure 1. Mechanism of pore formation by cationic amphipathic peptides to exemplify the formation and stabilization of a toroidal pore.
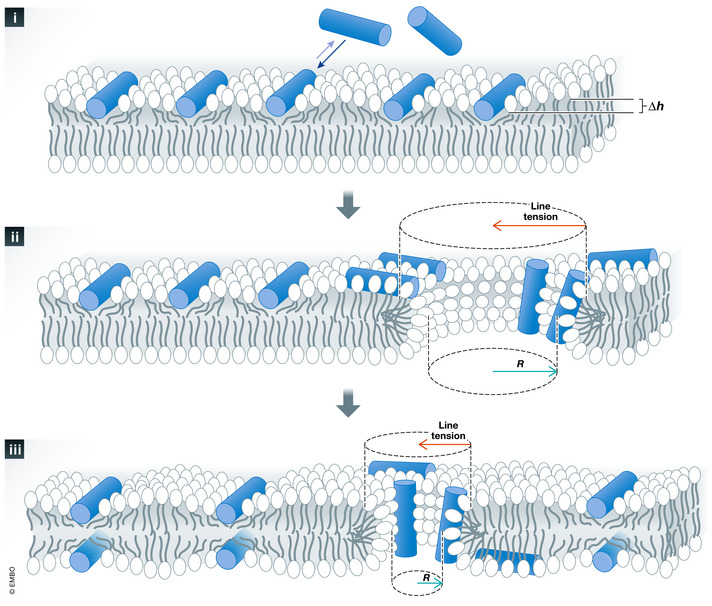
(i) Pore‐forming peptides bind avidly to the accessible interface of the lipid bilayer and occupy a volume only in the interfacial region, which causes asymmetric stretching and membrane thinning (∆h). As a consequence, the membrane is stressed and destabilized, so that defects in the lipid bilayer become more likely and eventually a pore is formed (ii). Once the pore is open, a line tension appears at the pore edge due to the extra energy cost associated with the reorientation of the lipids into a highly curved boundary. This tension increases with the pore perimeter and is therefore a line tension. The initial pore grows quickly as long as the membrane tension dominates. But as the pore size grows, so does the counterbalancing line tension too. Furthermore, with the open pore, the peptides redistribute in the membrane by diffusion through the pore to the other monolayer, which reduces the membrane tension due to asymmetric distribution of the peptides. At a certain moment, the line tension becomes predominant and the pore size starts to decrease. However, if the pore‐forming peptides bound near the pore rim are able to reduce the line tension, an equilibrium can be reached with a smaller but stable pore (iii). R = radius, ∆h = change in the thickness of the membrane (absence vs. presence of the protein/peptide mass). Adapted from (Fuertes et al, 2011).
In regulated cell death, a diverse repertoire of endogenous pro‐death effectors reveals a plethora of strategies evolved to permeabilize cellular membranes. The architecture of the resulting pores is defined by specific protein and lipid compositions, as well as by intramolecular interactions. This determines pore heterogeneity, size, and stability, and thereby the type and extent of molecules that can be released through them. As a consequence, the properties of membrane pores impact the signaling cascades that are activated downstream of membrane permeabilization.
BAX and BAK pores in apoptosis: old friends with new habits
The toroidal pore of BAX and BAK
Currently, it is well established that the BCL2 family members BAX, BAK, and perhaps BOK are the executioners of MOMP and thereby fundamental effectors of the intrinsic apoptotic pathway (Moldoveanu & Czabotar, 2019). In healthy cells, BAX and BAK exist as inactive, monomeric proteins that constitutively shuttle between cytosol and mitochondria, with BAX being mostly cytosolic and BAK mitochondria‐associated (Edlich et al, 2011; Schellenberg et al, 2013; Todt et al, 2015; Lauterwasser et al, 2016). Upon apoptosis induction, both proteins accumulate at and insert into the mitochondrial membrane, undergo conformational rearrangements, oligomerize and form pores that release pro‐apoptotic factors such as cytochrome c and SMAC/DIABLO (Nechushtan et al, 2001; Rehm et al, 2003; Zhou et al, 2005; Edlich et al, 2011). However, the relative order of events, the structural intermediates involved, as well as the molecular properties of the membrane openings mediated by BAX‐type proteins remains controversial (Moldoveanu & Czabotar, 2019; Flores‐Romero & Garcia‐Saez, 2019a).
Despite their functional heterogeneity, the 3D structures of all multidomain BCL2 proteins (including not only BAX, BAK, and BOK, but also the pro‐survival family members) present the same globular α‐helical fold, with a predominantly hydrophobic central hairpin that is flanked on both sides by pairs of amphipathic α‐helices. This peculiar folding is strikingly similar to the pore‐forming domain of bacterial α‐PFTs such as the colicins and diphtheria toxin (Muchmore et al, 1996; Suzuki et al, 2000; Petros et al, 2004; Moldoveanu et al, 2013; Ke et al, 2018). Considering these structural similarities and their ability to allow currents through artificial membrane systems known as black lipid membranes, it was initially proposed that BAX‐type proteins induce MOMP by generating membrane pores or channels (Minn et al, 1997; Sattler et al, 1997; Schlesinger et al, 1997; Basanez et al, 1999).
Different observations have since then supported the hypothesis that active BAX and BAK form long‐lived toroidal pores. In vitro, BAX decreases the lifetime of planar membranes and forms pores with variable conductance (Basanez et al, 1999) that are affected by the physical properties of the membrane and the presence of lipids with intrinsic monolayer curvature (Basanez et al, 2002; Terrones et al, 2004). A peptide derived from helix α5 of BAX forms membrane pores with lipid molecules in the lumen as demonstrated by X‐ray diffraction (Qian et al, 2008) and conductance experiments in black lipid membranes (Garcia‐Saez et al, 2005). This is in agreement with the transbilayer lipid movement coupled with membrane permeabilization induced by BAX (Epand et al, 2003; Garcia‐Saez et al, 2006) (Fig 2A and B). These lines of evidence converge into the current view of a protein/lipid pore of tunable size constituted by BAX/BAK homodimers, where the size of BAX and BAK pores is not constant, but it evolves with time and depends on protein concentration. In this model, the central hairpin of helices α5 and α6 lies on the membrane surface at the edge of the pore (Qian et al, 2008; Basanez et al, 2012; Bleicken et al, 2013; Mandal et al, 2016; Cosentino & Garcia‐Saez, 2017; Bleicken et al, 2018) (Fig 2A and B). Not only BAX/BAK molecules, but also the mechanical properties of the membrane play a role in the size and stability of the pores (Karatekin et al, 2003).
Figure 2. BAX/BAK toroidal pore.
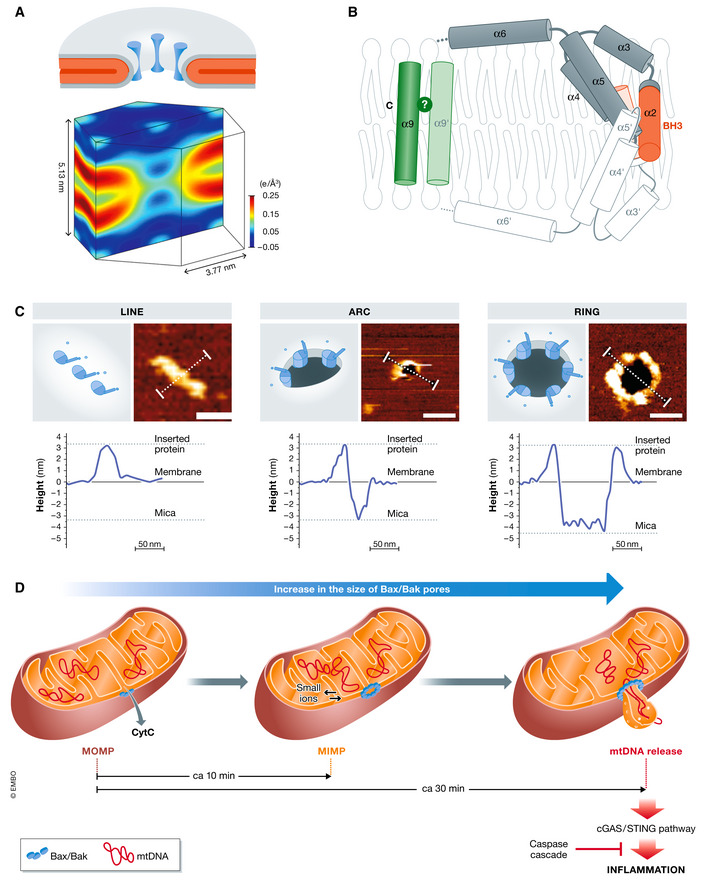
(A) Schematic representations of the protein/lipid model shown as a 3D view cut through the membrane pore. Gray layers represent lipid headgroups of the bilayer, the acyl chains are shown in red and protein helices by dark cylinders (top). The corresponding normalized electron density distributions of acyl chains in lipid bilayers containing BAX α5 (bottom). Note that, unlike in a protein channel, in a toroidal pore: (i) the surface of the pore is lined by lipid headgroups, (ii) membrane monolayers are bent at the pore edge, and (iii) the two leaflets of the bilayer become continuous. Taken from (Qian et al, 2008). (B) Structural representation of membrane‐embedded BAX in the context of a toroidal pore based on (Bleicken et al, 2014; Bleicken et al, 2018). BAX is represented with nine cylinders corresponding to its nine helices. BH3 domain and C‐terminal/tail anchoring domain are depicted in orange and green, respectively. One monomer is shown in gray (1–9) and the other is depicted in white (1ʹ–9ʹ). The relative orientation of the helices α9 remains unresolved. (C) BAX oligomers are organized into line, arc, and rings. Each panel shows the schematic representation (left) and the AFM images (right) of BAX assemblies. Both arcs and rings but not lines, reveal a circular depression (black) that spans the lipid membrane (dark orange). BAX molecules around the pore rim (yellow) protrude above the membrane plane, as confirmed by the height cross‐sections shown below each image (corresponding to the white line in the AFM images). The topography of the arc structure reveals a pore only partially surrounded by BAX molecules, while lipids alone form the rest of the pore rim. Based on (Salvador‐Gallego et al, 2016). (D) Model for the temporal control of content release during MOMP. Upon apoptotic stimuli, BAX and BAK permeabilize the MOM and induce the release of apoptotic factors, for example, cyt C. The consequent MIM permeabilization and the widening of BAX/BAK pores induce the release of mtDNA in the cytosol. In absence of caspase activity, this leads to the activation of the cGAS/STING signaling pathway. Based on (Cosentino & Garcia‐Saez, 2018).
According to the toroidal pore model, the initial asymmetric insertion of BAX α‐helices into the cytosolic leaflet of the MOM stresses the membrane and generates membrane tension. Once a threshold tension is reached (which may be locally enhanced by protein concentration or oligomerization), the energy required to reorganize lipids out of the bilayer structure becomes thermally accessible and a pore opens, which dissipates membrane tension (Lee et al, 2004). Lipids reassemble into a torus around the membrane pore to avoid exposure of the hydrophobic acyl chains to the water environment. The two membrane monolayers form a continuous surface at the pore edge with negative curvature in the plane of the membrane and positive curvature in the plane perpendicular to the membrane. The bending of the lipids at the pore rim has an energetic cost that is directly proportional to the length of the pore, giving rise to line tension (Ludtke et al, 1996; Matsuzaki et al, 1996). Line tension acts as the driving force for pore closure and opposes membrane tension. As a result, toroidal pores are metastable structures whose lifetime is governed by the balance between membrane tension and line tension (Valcarcel et al, 2001; Yang et al, 2001; Karatekin et al, 2003; Fuertes et al, 2010b). In support of this model, the rim of BAX pores is formed by both lipid headgroups and protein molecules (Kuwana et al, 2016; Salvador‐Gallego et al, 2016), with BAX molecules decreasing the line tension to maintain the pore stably open (Basanez et al, 1999; Garcia‐Saez et al, 2007; Fuertes et al, 2010a; Fuertes, 2011; Unsay et al, 2017; Bleicken et al, 2018) (Figs 1 and 2B).
BAX/BAK membrane topology in the context of the toroidal pore
Once in the membrane, activated‐BAX/BAK undergoes a rearrangement of their globular fold that implies first the opening of their N‐terminal region and then, in the case of BAX, the dislodgment and transmembrane insertion of the tail anchoring domain in helix α9 (Nechushtan et al, 1999; Griffiths et al, 2001). This is followed by exposure of their BH3 domain, which is thought to occur concomitantly with the reorganization of BAX/BAK into two functionally different regions, namely the dimerization/core (helices α2–α5) and the piercing/latch (helices α6–α8) domains (Dewson et al, 2008; Dewson et al, 2012; Czabotar et al, 2013; Moldoveanu et al, 2013; Bleicken et al, 2014; Flores‐Romero et al, 2017). Although the structural organization of BAX dimers in the membrane remains controversial (Westphal et al, 2014; Mandal et al, 2016; Cosentino & Garcia‐Saez, 2017; Bleicken et al, 2018), some of the models proposed provide an explanation for how BAX/BAK may generate the membrane stress required for pore opening and alleviate the line tension for pore stabilization (Bleicken et al, 2014; Mandal et al, 2016; Fig 2B). Partial opening and insertion of the hairpin of helices α5–α6 in BAX/BAK dimers within the lipid headgroup region of the cytosolic leaflet of the MOM would initially generate positive curvature and membrane stress leading to pore opening (Czabotar et al, 2013; Bleicken et al, 2014). The crescent‐like shape of these two α‐helices in the context of the pore rim would then act as a scaffolding chaperone that stabilizes the high lipid curvature, decreases the line tension, and maintains the open pore state (Mandal et al, 2016; Bleicken et al, 2018; Fig 2B). The formation of high‐order oligomers with such membrane disposition would potentiate the stabilizing effect (Subburaj et al, 2015).
In agreement with this, atomic force microscopy (AFM) experiments on supported bilayers, electron microscopy (EM) assays in outer membrane vesicles (OMVs) and in lipid nanodiscs containing BAX confirmed protein enrichment at the rim of membrane pores of variable sizes. Remarkably, the pore wall was not completely covered by protein molecules (Xu et al, 2013; Subburaj et al, 2015; Kuwana et al, 2016; Salvador‐Gallego et al, 2016) (Fig 2C). This evidence supports a model for BAX/BAK‐mediated MOMP where oligomerization at the MOM induces the formation of heterogeneous toroidal pore structures of tunable size, which are flexible and evolve overtime leading to the release of apoptotic factors.
BAX and BAK supramolecular structures and functions beyond MOMP
Historically, the segregation of BAX and BAK into discrete puncta at mitochondria, also known as apoptotic foci, has been linked with the apoptotic phenotype (Nechushtan et al, 2001; Karbowski et al, 2002). High‐resolution imaging techniques have recently allowed deciphering the riddles of these supramolecular structures, revealing distinct molecular architectures such as lines, arcs, and full rings (Grosse et al, 2016; Salvador‐Gallego et al, 2016). Rings and arcs of BAX similar to those found at the MOM perforated the membrane in supported lipid bilayers indicating that these higher‐order oligomerization states do not necessarily require other mitochondrial proteins (Fig 2C). BAX seems to oligomerize by subsequent addition of dimers (Subburaj et al, 2015), but the formation and evolution of these oligomers at the supramolecular level are vaguely understood. BAX molecules might first organize into linear and arc‐shaped structures, with some of them evolving to complete rings. Alternatively, lines and arcs might correspond to kinetically trapped assemblies in the process of pore formation. Several inter‐dimer binding surfaces have been described for BAX‐type proteins, including helices α6 and α9 (Dewson et al, 2009; Zhang et al, 2010) or the interface of helices α3/α5 (Mandal et al, 2016), but none of them appears to be indispensable for BAX/BAK oligomerization. Considering that BAX/BAK self‐assembly seems to be crucial for reducing line tension and pore stabilization, one could envision that the membrane itself may play a role in the organization and dynamics of BAX/BAK supramolecular arrangements by contributing to the state of minimal energy. In this scenario, the energy cost of the membrane perturbations (perhaps including initial pore opening) induced by the insertion of BAX dimers into the membrane could be reduced by the coalescence of these membrane alterations and associated BAX/BAK molecules. Such a model would explain the higher‐order assembly of BAX/BAK dimers via membrane‐mediated interactions (Harroun et al, 1999; Reynwar et al, 2007; Shlomovitz & Gov, 2009; Cowan et al, 2020) and provide a mechanistic basis for the pore growth observed during apoptosis (Riley et al, 2018; Flores‐Romero & García‐Sáez, 2020).
Connected with BAX foci, it has been proposed that BAX and BAK pores of hundreds of nanometers in diameter can also induce mitochondrial inner membrane (MIM) permeabilization and extrusion into the cytosol, leading to the release of mitochondrial DNA (mtDNA) and the activation of cGAS/STING inflammatory pathway (McArthur et al, 2018; Riley et al, 2018; Fig 2D). These discoveries have challenged our understanding of the role of BAX/BAK in apoptosis beyond that of inducing MOMP for caspase activation, which now expands to inflammation and cell‐to‐cell communication. The mechanism how BAX and BAK promote MIM poration and mtDNA release remains unknown. While some studies suggest that monomers or dimers are sufficient to form functional pores in model membranes (Kushnareva et al, 2012; Xu et al, 2013), it seems unlikely that BAX/BAK monomers or dimers would be able to induce the large membrane disruptions required for mtDNA release. One could envision a scenario in which low order oligomers of BAX/BAK induce openings at the MOM that release cytochrome c and other proteins, while large supramolecular structures may enable other cell functions including mtDNA release and inflammatory responses. The continuous growth of BAX pores upon MOMP would thereby allow a controlled release of the mitochondrial components to regulate in time these alternative functions and with them, the inflammatory outcome of apoptosis (Cosentino & Garcia‐Saez, 2018; McArthur et al, 2018; Riley et al, 2018).
However, it is important to note that additional components are present at BAX/BAK foci at mitochondria, such as Dynamin‐related protein 1 (Drp1) and Mitofusins (Karbowski et al, 2002; Ugarte‐Uribe & Garcia‐Saez, 2017; Ugarte‐Uribe et al, 2018; Hertlein et al, 2020), Optic atrophy‐1(OPA1)/ Metalloendopeptidase OMA1 (OMA1) (Yamaguchi et al, 2008; Jiang et al, 2014), or Voltage‐Dependent Anion Channel (VDAC) (Lauterwasser et al, 2016; Kim et al, 2019), which might contribute to this phenomenon. As MIM permeabilization appears to occur after MOMP, the driving force that makes the mitochondrial interior squeeze out through BAX/BAK macro‐pores might involve mechanical (osmotic) forces. This could possibly be due to or happen in combination with additional mitochondrial alterations like the dismantling of mitochondrial cristae. The mitochondrion‐specific lipid cardiolipin (CL), which seems to regulate the action of several BCL2 proteins (Terrones et al, 2004; Landeta et al, 2014; Bleicken et al, 2017; Flores‐Romero et al, 2019) and is also implicated in mitochondrial functions including organelle ultrastructure (Schlame & Ren, 2009), could also play a role in MOM/MIM permeabilization. Because of its unique structural properties (e.g., two negative charges, a relatively small head group and four acyl chains), CL can form highly curved inverted hexagonal structures (Grijalba et al, 1999; Ortiz et al, 1999; Unsay et al, 2013) and laterally segregate into defined nanodomains in vitro (Kawai et al, 2004; Sorice et al, 2009). These additional elements might modulate the formation, size, shape, and kinetics of BAX/BAK assemblies in apoptotic foci.
At the functional level, it is reasonable to argue that the regulation of the extent and kinetics of BAX/BAK‐induced MOMP may elicit different scenarios, which could be: (i) genomic instability and cancer, associated with partial release of cytochrome c and minority MOMP (Ichim et al, 2015), (ii) immunologically silent apoptosis, when MOMP, caspase activation and cell removal due to “eat me signals” are fast and complete (Depraetere, 2000; Singh et al, 2019), or (iii) immunologically active apoptosis in case of prolonged apoptotic signaling leading to mtDNA release and to activation of the cGAS/STING and perhaps Mitochondrial antiviral‐signaling protein (MAVS) pathways (Cosentino & Garcia‐Saez, 2018; McArthur et al, 2018; Riley et al, 2018; Flores‐Romero & Garcia‐Saez, 2019b). The regulation of these scenarios could be mechanistically related with the structural flexibility and dynamics of assembly of BAX/BAK structures, ranging from active monomer/dimer units to supramolecular structures with different sizes and shapes. Importantly, if the different oligomeric states of BAX and BAK exert different functions in the cell, their specific targeting could expand the application of BAX and BAK for therapy.
Gasdermins are potent pore‐formers at the core of pyroptosis
The GSDM family is a new class of PFPs
GSDMs represent a family of proteins that comprises six members in humans: GSDM A, B, C, D, and E (also known as DFNA5), and PJVK (also known as DFNB59), and ten in mice (GSDM A1‐3, C1‐4, D, E, and PJVK). Some of these proteins have proved to be essential for the highly inflammatory pathway of pyroptosis (Aglietti & Dueber, 2017; Galluzzi et al, 2018; Broz et al, 2020). Pyroptotic cell death is characterized by extensive cell swelling and membrane blebbing in absence of cell detachment (Chen et al, 2016; de Vasconcelos et al, 2019), which resembles the phenotype induced by PFTs during their attack to the plasma membrane of target cells (Garcia‐Saez et al, 2011; Ros et al, 2017). This, together with the essential role of GSDMs in pyroptosis, suggested that GSDMs have an intrinsic and potent pore‐forming activity that mediates osmotic lysis in pyroptosis. Accordingly, the N‐terminal domain (GSDMNT) of GSDMs alone displays pore‐forming activity in liposomes, which has been the basis to define GSDMs as the minimal machinery for pyroptosis execution (Aglietti et al, 2016; Ding et al, 2016; Liu et al, 2016).
Intense research during the recent years has provided insight into the structure of GSDMs (Fig 3A–D). All GSDMs (except DFNB59) display a two‐domain architecture formed by an N‐terminal (GSDMNT) and a C‐terminal (GSDMCT) domain, separated by a linker region (Fig 3A; Broz et al, 2020). The crystal structure of full‐length GSDMA3 (Ding et al, 2016) and GSDMD (Kuang et al, 2017) revealed that the GSDMNT is inhibited by inter‐domain interactions with juxtaposed regions of the GSDMCT. The α‐helical fold of GSDMCT interacts with the helix α1 and a short β‐hairpin located on the concave side of the β‐sheet of GSDMNT. Additionally, the short α‐helix at the end of the β‐sheet of GSDMNT protrudes from the globular structure to interact with GSDMCT (Ding et al, 2016). For many GSDMs, caspase‐mediated proteolytic processing induces the dissociation of the GSDMNT from its auto‐inhibitory C‐domain (Fig 3A; Kuang et al, 2017; Liu et al, 2018; Liu et al, 2019). Free monomers of GSDMNT translocate then to the inner leaflet of the plasma membrane and induce pyroptotic pores. Remarkably, the sequence and 3D‐structure of all GSDMs differ significantly from any other known PFP (Ruan et al, 2018; Broz et al, 2020). Therefore, GSDMs have emerged as a new group of PFPs with a common function in pyroptosis.
Figure 3. GSDMs pores evolve from toroidal to barrel structures.
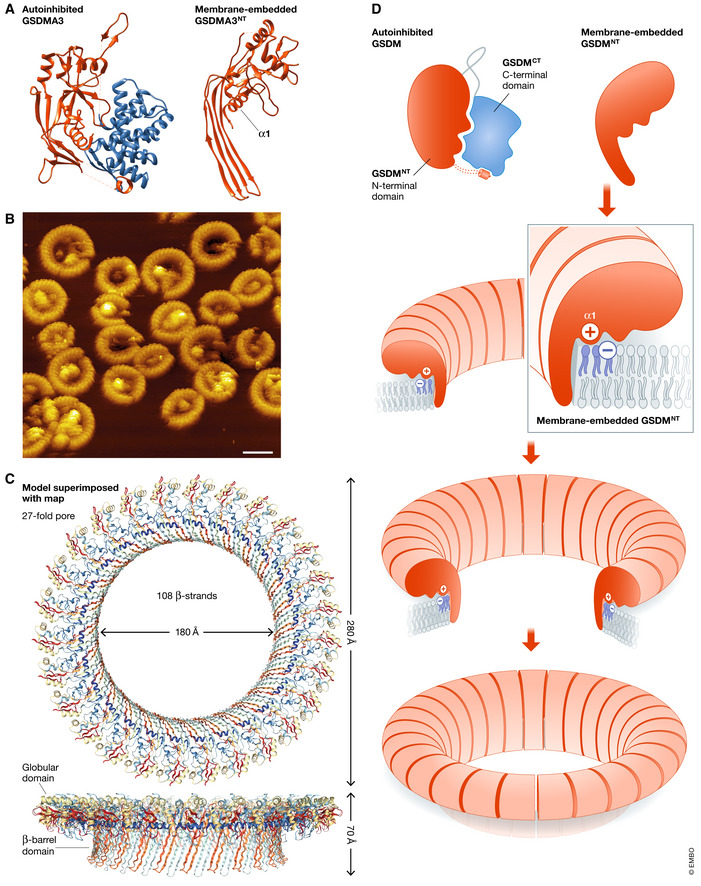
(A) Crystal structure of GSDMA3 in its auto‐inhibited form (PDB: 5B5R). The GSDMA3NT and GSDMA3CT domains are colored pink and blue, respectively. Inter‐domain interactions between the GSDMA3NT and the GSDMA3CT keep the protein in an auto‐inhibited state (Ding et al, 2016). (B) GSMDs involves the form arc‐, slit, and ring‐shaped GSDMDNT oligomers as imaged using time‐resolved AFM (Mulvihill et al, 2018). (C) Cryo‐EM structure of the GSDMA3 membrane pore (PDB: 6CB8). Atomic model of the 27‐fold symmetric GSDMA3 pore at 3.8 Å resolution (Ruan et al, 2018). (D) Model of pore formation by GSDMs. After cleavage, monomers of GSDMNT translocate to the inner leaflet of the plasma membrane and then self‐associate into arcs or slit structures that resemble toroidal pores and later evolve into ring‐shape protein‐lined pores with a β‐barrel configuration.
The structure of a GSDMA3NT pore was recently determined by cryo‐EM (Fig 3C) (Ruan et al, 2018). Together with the crystal structure of full‐length GSDMA3 (Ding et al, 2016), these findings were the basis for a model of the transformation that this protein undergoes from the auto‐inhibited form to the active membrane‐embedded state. Cleaved GSDMNT associates with negatively charged lipids at the plasma membrane via a cluster of positively charged amino acids in helix α1 (Ruan et al, 2018). Interaction with the lipid environment facilitates a conformational change that involves refolding into a new four‐stranded amphiphilic β‐sheet and generation of three oligomerization interfaces. The establishment of well‐defined protein‐protein interactions drives the stabilization of a ring‐shaped β‐barrel pore embedded in the membrane. The amphipathic β‐sheets are bundled together in a transmembrane configuration where the hydrophobic surfaces are in contact with the membrane core, while the hydrophilic ones are facing the pore lumen (Ding et al, 2016). However, the structural arrangement of the lipids in the vicinity of GSDMNT pores remains unknown.
Time‐resolved AFM studies of the pores formed by human GSDMDNT in model membranes provided new information about the dynamics of GSDMD assembly and pore opening (Fig 3B; Sborgi et al, 2016; Mulvihill et al, 2018). These data support a model where, instead of oligomer pre‐assembly in solution, GSDMDNT monomers first bind to the membrane and then self‐associate into arcs or slit structures that later evolve into ring‐shaped pores. The formation of stable pores with a defined ring structure seems to be a robust and intrinsic property of GSDMDNT, as changes in protein concentration (Ding et al, 2016; Liu et al, 2016) and membrane lipid composition (Mulvihill et al, 2018) affect protein binding to the membrane but not pore size. Arcs and slits are also able to perforate the membrane (Mulvihill et al, 2018), which indicates the direct involvement of lipids in these intermediate pore structures. As discussed for BAX, protein–lipid pores can be induced when amphipathic regions of proteins bind to or insert into the membrane, lowering the energy to form a pore (Cosentino & Garcia‐Saez, 2017). In this scenario, it is reasonable to assume that the perimeter of the pore wall would be partially covered by GSDMs molecules and partially with a semi‐toroidal disposition of the membrane in the opposite side (Fig 3B and D).
GSDMs are mechanistically similar to other β‐PFPs
GSDMs are classified as β‐PFPs based on the structural element that forms the pore wall. Compelling data obtained from orthogonal studies with different family members suggest that pores formed by GSDMs have a relatively narrow size distribution, with diameters in the order of 10–30 nm (Ding et al, 2016; Sborgi et al, 2016; Heilig et al, 2018; Ruan et al, 2018). As a result, and despite being structurally unique PFPs, GSDMs pores resemble the transmembrane β‐barrel channels formed by the membrane attack complex perforin‐like/cholesterol‐dependent cytolysin (MACPF/CDC) family (Ros & Garcia‐Saez, 2015; Gilbert, 2016). Examples of MACPF/CDC members are the bacterial cytolisins (e.g., Streptolysin O and Listeriolysin O), the complement proteins and perforin from cytotoxic T lymphocytes (Anderluh et al, 2014; Gilbert et al, 2014). Contribution of lipids to intermediate structures of different shapes is also shared between GSDMs and the MACPF/CDC families (Sonnen et al, 2014; Metkar et al, 2015; Podobnik et al, 2016; Mulvihill et al, 2018). Furthermore, although evolutionary distant, GSDMs and MACPF/CDC families have a common function in the bacterial defense systems and in immunity (Gilbert et al, 2013; Anderluh et al, 2014; Broz et al, 2020).
This stark similitude supports a conserved strategy of pore formation by large β‐PFPs and makes it reasonable to extrapolate some features of the MACPF/CDC pores to pyroptotic pores. Evidence obtained with different members of the MACPF/CDC family suggests that these proteins follow a mechanism of pore formation in which the rolling insertion of oligomers results in the flow of lipids from the pore rim back to the bilayer (Sonnen et al, 2014; Gilbert, 2016; Podobnik et al, 2016). In this process, lipids return from the semi‐toroidal edges in the pore to the bilayer structure, rather than being punched out into solution during oligomer insertion (Gilbert et al, 2013; Gilbert, 2016). Considering the dynamics of GSDMs pores, it is tempting to speculate that GSDMs could follow a similar mechanism of lipid clearance that involves the evolution of intermediate protein–lipid semi‐toroidal structures to a fully protein‐lined pore during pyroptosis (Fig 3D; Sonnen et al, 2014; Gilbert, 2016).
The inflammatory pore of GSDMs
GSDMs pores are non‐selective membrane channels that mediate the secretion of intracellular components that act as activators of the immune system (Zanoni et al, 2016; Evavold et al, 2018). Chief among them, the inflammatory cytokines IL‐1β and IL‐18 can be released via these pores through non‐conventional mechanisms in a cell death‐independent manner (Heilig et al, 2018). The passive transport of molecules through GSDMs pores also leads to osmotic imbalance, cell bursting, and final death, which allows the unspecific release of larger damage‐associated molecular patterns (DAMPS) from cells (Broz et al, 2020; Ros et al, 2020). One interesting aspect that remains a matter of debate is whether GSDMs pores could impact the events that anticipate cell death by regulating the size of the molecules that are released from pyroptotic cells. To play a significant role in controlling contents efflux, GSDMs pores should precede cell bursting with a sufficiently long lag time, which may be regulated by opposing cellular processes. Indeed, the endosomal sorting complex required for transport (ESCRT) machinery has been shown to counterbalance GSDMDNT pores during pyroptosis (Ruhl et al, 2018). This and other membrane repair mechanisms may, as a result, be relevant not only for protecting against cell death but also for regulating pore number and cell death dynamics. In this context, pore formation by GSDMs would emerge as a mechanism to tightly control the kinetics and extent of release of inflammatory molecules from pyroptotic cells.
Staying open‐minded: How does MLKL execute necroptosis?
MLKL is not similar to any other PFP
MLKL is the only effector exclusively implicated in necroptosis and the most downstream component of the pathway known so far (Galluzzi et al, 2018; Espiritu et al, 2019; Flores‐Romero et al, 2020). It is a cytosolic, monomeric pseudo‐kinase with no day‐job in healthy cells known to date, although evidence suggests that it may be implicated in membrane trafficking (Yoon et al, 2017). During necroptosis, it becomes activated by phosphorylation by receptor‐interacting protein 3 (RIP3). MLKL activation triggers its oligomerization and association with the inner leaflet of the plasma membrane, which eventually leads to membrane permeabilization and cell death (Wallach et al, 2016; Petrie et al, 2017; Petrie et al, 2018). Despite its central role in necroptosis, MLKL is a very intriguing protein and the mechanism how it induces plasma membrane permeabilization remains a topic of debate.
The 3D structure of inactive MLKL is comprised by two domains: an N‐terminal four helix bundle domain (4HB) and a C‐terminal pseudo‐kinase domain (psK) bridged by a flexible brace region (Murphy et al, 2013; Su et al, 2014; Fig 4A). Intensive research over the last years has demonstrated that the 4HB of MLKL acts as the killer domain, whereas the psK domain and the brace region are critical to keep in check the cell death‐inducing capacity of MLKL (Hildebrand et al, 2014; Su et al, 2014; Arnez et al, 2015; Tanzer et al, 2016). The 4HB domain of MLKL is arranged into a coiled‐coil of amphipathic α‐helices in which the hydrophobic faces are hidden in the core of the structure (Murphy et al, 2013; Su et al, 2014). This configuration has 3D homology with the HeLo‐like domain found in fungal Heterokaryon incompatibility protein S (HET‐S), which form pores by a mechanism involving the insertion of their N‐terminal α‐helix in the membrane (Daskalov et al, 2016).
Figure 4. MLKL induces pores by a still unclear mechanism.
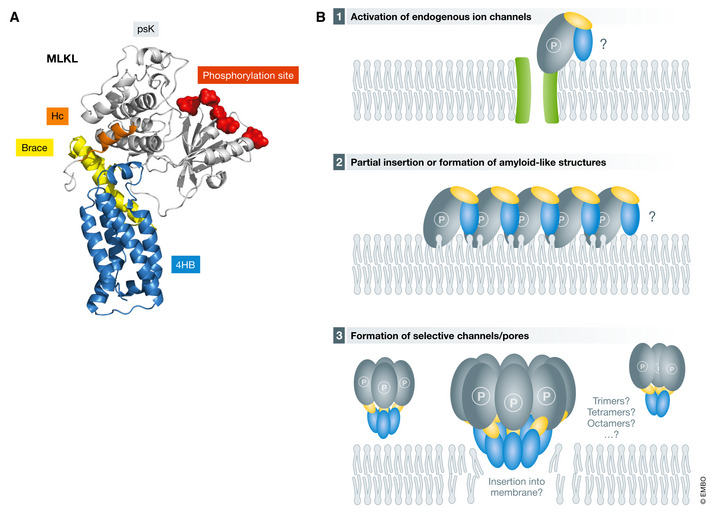
(A) Crystal structure of mouse MLKL (PDB: 4BTF). The 4HB domain, the brace region, and the psK domain are colored blue, orange, and gray, respectively. (B) Alternative models proposed for the mechanism how MLKL mediates plasma membrane permeabilization. (1) indirect mechanism via activation of endogenous ion channels, (2) partial insertion into the lipid bilayer, or (3) formation of defined channels or pores.
Upon activation, MLKL is thought to undergo a conformational change that facilitates its weak interaction with PIPs at the plasma membrane through a cluster of positively charged residues located in the 4HB (Dondelinger et al, 2014). High‐affinity sites are then exposed, which stabilizes stronger interactions with PIPs via a rolling‐over mechanism (Quarato et al, 2016). Besides this, very little is known about the assembly pathway and oligomeric state of MLKL at plasma membrane. Different studies have proposed that MLKL can form trimers, tetramers, hexamers, and even higher‐order oligomers (Cai et al, 2014; Chen et al, 2014; Huang et al, 2017; Liu et al, 2017; Petrie et al, 2018). It is reasonable to speculate that the different reported oligomers could be intermediate states of higher‐order complexes.
The supramolecular structure of membrane MLKL oligomers promoting membrane permeabilization remains undefined too. Early studies proposed that MLKL activates endogenous ion channels, which would be the actual responsible for cell death (Cai et al, 2014; Chen et al, 2014). However, this model does not seem to hold true in light of more recent data showing that specific ion channels are not essential for necroptosis (Chen et al, 2014; Xia et al, 2016; Ousingsawat et al, 2017). A more general hypothesis is that MLKL directly mediates the permeabilization of the plasma membrane either as a result of its partial insertion into the lipid bilayer (Su et al, 2014) or by forming defined channels or pores (Fig 4B; Xia et al, 2016). The evidence that membrane nanopores represent a core mechanism in necroptosis supports a possible pore‐forming function for MLKL (Ros et al, 2017). However, whether MLKL is directly or indirectly forming necroptotic pores, alone or together with other cellular components, is something that still remains to be elucidated.
Alternative models for pore formation by MLKL
One distinctive feature of pore‐forming domains is the presence of a highly hydrophobic stretch that helps partitioning into the hydrophobic core of the membrane. Amphipathic α‐helices can also function as pore‐forming domains since they are ideal for binding to membrane interfaces (Ros & Garcia‐Saez, 2015; Grage et al, 2016). To predict potential pore‐forming segments in MLKL, we built the hydropathy profile of the 4HB (Fig 5A). We found that none of the α‐helices in the 4HB of MLKL is sufficiently hydrophobic to be predicted to insert in membranes. This is in contrast to other α‐PFPs, including BAX or the HeLo‐like proteins, which questions the pore‐forming activity of MLKL. We also calculated the mean hydrophobic moment (µ) as a quantitative indicator of amphipathic nature of the individual helices in the 4HB (Fig 5B). The values obtained were comparable to those of single‐helix pore‐forming domains of other PFPs analyzed, although in MLKL, the hydrophobic patches of the four α‐helices cooperate in the assembly of the helical bundle fold and may thus not necessarily behave as pore‐forming domains.
Figure 5. Alternative models of pore formation by the amphipathic α‐helices of MLKL.
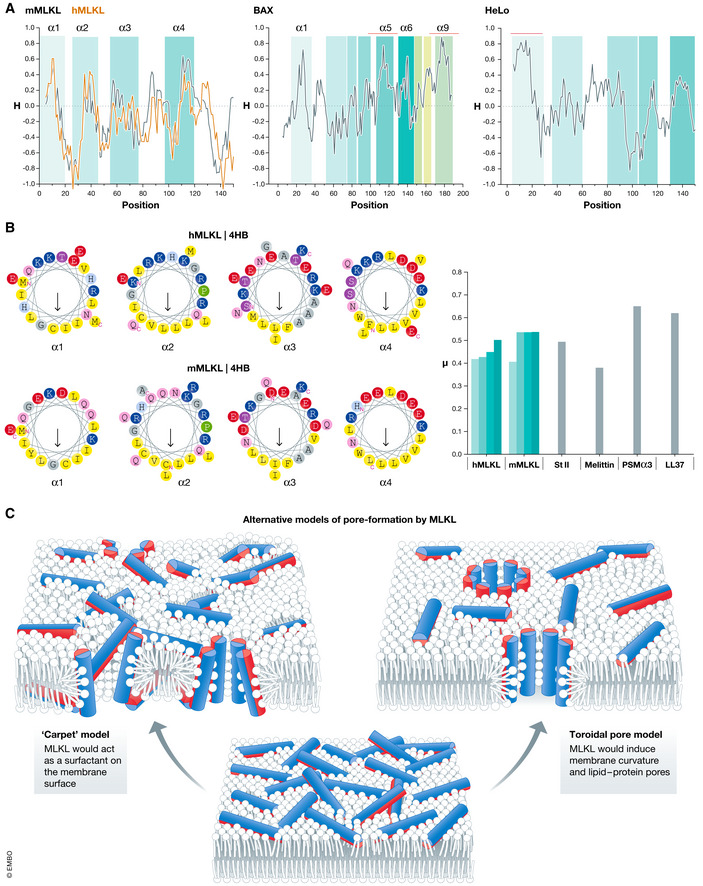
(A) Hydropathy profiles of the 4HB of MLKL, the HeLo‐like N domain‐containing protein from Chaetomium globosum and BAX. Profiles were built with the program Protscale (https://web.expasy.org/protscale/), using the Eisenberg scale with a window of 9 amino acid residues. Segments above 0 are predicted as hydrophobic and below 0 as hydrophilic. Predicted transmembrane segments are highlighted in red. Predictions were made using two different softwares: TMHMM (http://www.cbs.dtu.dk/services/TMHMM) and TCDB (http://www.tcdb.org/progs/?tool=hydro). (B) Amphipathic nature of the α‐helices of the 4HB of MLKL. Left: Wheel projections of the α‐helices of the 4HB of human (top) or mouse (bottom) MLKL. Arrows point toward the hydrophobic face of the α‐helices. Projections were built using the server Heliquest (https://heliquest.ipmc.cnrs.fr/). Right: μ of the amphipathic α‐helices of the 4HB of MLKL, the pore‐forming domain of the α‐PFT sticholysin II and the lytic peptides melittin, LL37 and PSMα3. (C) Amphipathic α‐helices of MLKL could alter membrane integrity by two alterative models. In a carpet model, MLKL would act as a surfactant on the membrane surface, while in the toroidal pore, MLKL would induce membrane curvature and lipid–protein pores. Cylinders indicate individual amphipathic helices of the 4HB of MLKL. Hydrophobic surfaces are depicted in red and hydrophilic in blue.
Unfortunately, we still lack the structure and topology of MLKL in membranes. It remains to be disclosed whether MLKL activation facilitates the unfolding of the 4HB and the exposure of normally hidden hydrophobic surfaces. This could lead to formation a protein‐lined transmembrane pore in which the polar surfaces of the amphipathic α‐helices face the aqueous lumen and the hydrophobic sides are in contact with the membrane core. Alternatively, the amphipathic α‐helices of the 4HB might remain lying at the membrane interface region without spanning the bilayer, as it has been proposed (Fig 5C) (Su et al, 2014; Petrie et al, 2017). In such scenario, MLKL could mediate pore formation via alternative mechanisms:
One possibility could be that the 4HB helices of MLKL behave as surfactants and create a carpet on the membrane surface(Fig 5C) , thereby destabilizing the bilayer structure and permeabilizing the membrane once a concentration threshold at the membrane is reached, like in the case of detergents or in form of amyloid fibers (Liu et al, 2017). In this regard, MLKL could follow a mechanism similar to the antimicrobial peptide LL37 or the bacterial cytotoxic PSMα3 peptide, where amphipathic α‐helices are densely packed forming amyloid fibers that induce membrane destabilization (Tayeb‐Fligelman et al, 2017; preprint: Engelberg & Landau, 2020).
MLKL may act instead as an α‐PFP by inducing membrane curvature without fully spanning the hydrophobic core of the membrane (Fig 5C). A similar mechanism has been proposed for different lytic peptides and proteins such as melittin and magainin (Sengupta et al, 2008; Lee et al, 2011) and actinoporins (Alvarez et al, 2003; Mesa‐Galloso et al, 2019; Fig 5C). In this case, the asymmetric attack of the amphipathic α‐helices of the unfolded 4HB would induce membrane thinning and tension, inducing membrane curvature and toroidal pore formation (Chen et al, 2003; Grage et al, 2016; Woo & Lee, 2017). Curvature induction could be relevant not only for the membrane permeabilizing activity of MLKL but also for potential alternative functions in endosome/exosome generation (Yoon et al, 2017; Fan et al, 2019).
Another option would be that MLKL induces membrane curvature and permeabilization due to local high concentration together with PIPs. In this case, MLKL would behave similarly to the poorly understood fibroblast growth factor (FGF2), a mediator of the unconventional secretory pathway (Steringer et al, 2012; Dimou et al, 2019) and the HIV‐1 transactivator of transcription (HIV‐Tat) (Zeitler et al, 2015).
In all these pore models, the alteration of the bilayer structure by MLKL could facilitate the flip‐flop movement of lipids via a scramblase activity, thereby providing an explanation for the exposure of phosphatidylserine (PS) to the outer leaflet of the plasma membrane in necroptotic cells (Gong et al, 2017a,b).
Is MLKL an intrinsic killer?
At a single cell level, necroptosis is a slow process in which cells round and detach with increasing osmotic pressure (Chen et al, 2016; Ros et al, 2017). Recent studies have shown that during the early phase of necroptosis, phosphorylated MLKL remains at the plasma membrane for a long time before cell death (Tanzer et al, 2015; Fan et al, 2019). These features contrast GSDMs and other PFPs, suggesting that MLKL may not have a potent membrane permeabilizing activity.
A question that remains open is whether this weak activity is an intrinsic property of MLKL or the result of tight regulation by the cell. Different evidence indicates that maintaining MLKL levels at the plasma membrane below a threshold prevents necroptosis (Gong et al, 2017b; Hildebrand et al, 2020; Petrie et al, 2020; Samson et al, 2020). Besides RIP3, several proteins and co‐factors have been identified as modulators of MLKL activity in cells. This includes the TAM kinases (Najafov et al, 2019), the heat shock protein 90 (HSP90) (Bigenzahn et al, 2016; Jacobsen et al, 2016), ATP (Petrie et al, 2018), and the highly phosphorylated versions of soluble inositol phosphates (i.e., IP4, IP5, and IP6) (Dovey et al, 2018; McNamara et al, 2019). Moreover, membrane repair mechanisms involving exosome release, endocytosis, and exocytosis play a counteracting role in necroptosis (Yoon et al, 2017; Gong et al, 2017b; Fan et al, 2019). The fact that MLKL orthologues from different species are not exchangeable among different host cells also supports the hypothesis that host‐specific factors might regulate MLKL activity in cells (Tanzer et al, 2016; Petrie et al, 2018).
During the commitment phase of necroptosis, MLKL mediates a number of cellular alterations including PS exposure, intracellular vesicle trafficking, exosome release and production of inflammatory cytokines, and DAMPs (Yoon et al, 2017; Gong et al, 2017a,b; Douanne et al, 2019; Espiritu et al, 2019). Given the multiple processes activated downstream of MLKL translocation to the plasma membrane, an interesting possibility would be that MLKL is not an intrinsic killer or that its weak activity is functionally relevant. In this scenario, the primary role of MLKL could be to promote these distinctive intracellular modifications in order to allow necroptotic cells to communicate in a unique manner with the environment. Whether the result of MLKL activation is cell death would then depend on the extension of MLKL‐dependent membrane damage and the efficiency of the cellular mechanisms that promote or counterbalance MLKL assembly at the plasma membrane.
Pores by BAX/BAK, GSDMs, and MLKL: the devil is in the detail
BAX/BAK, GSDMs, and MLKL have evolved to have a common role as executors of regulated cell death via membrane permeabilization. There are similarities in the general mechanisms of action of these proteins. With the exception of BAK, they are all produced as soluble proteins that undergo extensive conformational changes upon activation and interaction with the membrane. Membrane binding is driven, at least partially, by electrostatic interactions of positively charged residues located on the protein surface with anionic lipids in the target membranes. Hydrophobic interactions between the bilayer and amphipathic and/or transmembrane segments of these proteins also contribute to membrane partition and insertion. Once at the membrane, all of them form oligomers that are able to disrupt the bilayer, allowing the passive flux of molecules.
However, BAX/BAK, GSDMs, and MLKL strongly differ in the way they alter the structure and permeability of cellular membranes. This is reasonable, as distinct types of effectors may be required to permeabilize target membranes with different protein and lipid composition, as well as mechanical properties. To execute apoptosis, BAX is targeted to the MOM, while GSDMs and MLKL bind to the inner leaflet of the plasma membrane to mediate pyroptosis and necroptosis. The ability of BAX/BAK to open protein–lipid pores is very sensitive to the membrane lipid composition (Basanez et al, 2002; Terrones et al, 2004), which may be a regulatory mechanism during apoptosis. On the other hand, GSDMs and MLKL target the plasma membrane by a common mechanism of interaction with anionic PIPs (Parisi et al, 2018; Ros et al, 2020). These two proteins can also be found in organelles. MLKL binds to endosomes and regulates intracellular vesicle trafficking and receptor recycling (Yoon et al, 2017). Some studies have suggested that GSDMs can disrupt mitochondria via its ability to recognize CL (Ding et al, 2016; Huang et al, 2020). However, it remains unclear how GSDMs and MLKL discriminate between different organelles and which are the conditions that trigger their specific targeting to different cellular membranes.
Based on the secondary structure of the pores they form, BAX/BAK, and perhaps MLKL, are classified as α‐PFPs, while GSDMs are β‐PFPs. These structural differences strongly determine the molecular properties of the membrane pores built by these proteins. α‐helical proteins tend to form more flexible pores in which oligomeric structures are weakly associated with intra‐chain hydrogen bonds interactions. In contrast, β‐barrel pores present high stability by inter‐strand hydrogen bonds formation between juxtaposed chains (Gilbert et al, 2014; Ros & Garcia‐Saez, 2015). These differences would explain why GSDMs have well‐defined protein–protein interfaces that stabilize the β‐barrel arrangement in the membrane and are amenable for structural studies. Instead, BAX and BAK assemblies are less rigid and more heterogeneous in terms of interaction interfaces and supramolecular structures, and have escaped so far high‐resolution structural determination. It will be interesting to learn how the structure of MLKL oligomers in the membrane compares to that of BAX/BAK and GSDMs.
Along these lines, while a common feature of the mechanism of BAX/BAK and GSDMs is the formation of arc‐shaped structures that only partially cover the pore rim, the intramolecular interactions that stabilize these protein–lipid assemblies are different. In the case of GSDMs, it seems that arcs are intermediates transitioning into complete rings in which lipids are excluded. However, the BAX/BAK pores are flexible and dynamic, and their size depends on protein density at the membrane and on lipid composition (Landeta et al, 2011; Bleicken et al, 2013; Landeta et al, 2015; Subburaj et al, 2015; Salvador‐Gallego et al, 2016; Cosentino & Garcia‐Saez, 2017). This, together with the difficulty to define interaction surfaces between BAX/BAK dimers, supports a model in which protein dimers and lipids could be intermixed with each other also in the ring‐like structures. Further research is needed to confirm this distinctive property of BAX pores compared to GSDMs ones.
Why so many ways of piercing membranes to kill a cell?
Altogether, the different mechanisms of pore formation are likely to define the signaling processes activated downstream of membrane permeabilization in all forms of regulated cell death. On the one hand, the MOM is a membrane naturally permeable to molecules up to 5 kDa due to the presence of several porins (e.g., VDACs) and a singular lipid composition (Fleischer et al, 1967; Vance, 1990; Kagan et al, 2004). In this context, the growing nature of BAX pores may be key to enable temporal control of the release of mitochondrial contents ranging from small proteins like cytochrome c, to intermediate molecules like SMAC and to larger polymers like mtDNA (Kuwana et al, 2002; McArthur et al, 2018; Riley et al, 2018). The heterogeneity in BAX/BAK pores may therefore underline alternative cellular responses in apoptosis.
On the other hand, although GSDMs and MLKL target the same cellular membrane, the mechanism how they induce membrane permeabilization presents remarkable differences. In pyroptosis, GSDMDNT forms big and non‐selective pores that mediate the release of proteins, like interleukins and other DAMPs, even before cell death. In contrast, MLKL induces the formation of smaller pores of limited permeability in necroptosis. These differences could set the basis for the different kinetics of death and morphological features of pyroptotic and necroptotic cells. It is tempting to speculate that in the tissue, two outcomes could be possible. On the one hand, content efflux might be transient and highly controlled with prevalence of plasma membrane repair and recovery in the absence of cell death. Alternatively, dominance of loss of ion homeostasis and osmotic swelling would lead to plasma membrane bursting and complete exposure of the cellular components upon cell death. The possibility that these two scenarios may communicate distinct signals to the microenvironment is intriguing.
Conclusions and future perspectives
Despite decades of intense research, the structure and dynamic evolution of the apoptotic pores formed by BAX and BAK and the possible roles of other mitochondrial proteins and/or lipids remain open questions. What are the functional consequences of the structural flexibility of BAX/BAK supramolecular structures? Are there mechanistic and functional links between MOMP and MIMP? Our knowledge about necroptosis and pyroptosis is continuously expanding, but the functional impact that membrane permeabilization induced by GSDMs and MLKL is only starting to be grasped. We are still questioning whether MLKL is a PFP at all. How does the membrane repair machinery counterbalance the number and size of pores in pyroptosis and necroptosis? Is there a role for sub‐lytic activation of GSDMs and MLKL?
Pore formation has emerged as a common execution step in the signaling pathways leading to the different types of regulated cell death. By inducing or creating membrane pores of distinct nature, BAX/BAK, GSDMs, and MLKL control the movement of molecules across membranes and determine the cell’s fate. They are also directly responsible for the type and timing of contents released and their effect in the organism. Therefore, understanding how membrane pores are regulated and assembled holds a significant potential to devise new strategies to not only control cell death, but also modulate the inflammatory and immunologic effects of these processes for therapy.
Acknowledgements
This work has been funded by the European Research Council (grant number 817758) and the Deutsche Forschungsgesellschaft (CRC1403, subprojects A02 and B07). Open access funding enabled and organized by ProjektDEAL.
The EMBO Journal (2020) 39: e105753.
References
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC (2016) GsdmD p30 elicited by caspase‐11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA 113: 7858–7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglietti RA, Dueber EC (2017) Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol 38: 261–271 [DOI] [PubMed] [Google Scholar]
- Alvarez C, Casallanovo F, Shida CS, Nogueira LV, Martinez D, Tejuca M, Pazos IF, Lanio ME, Menestrina G, Lissi E et al (2003) Binding of sea anemone pore‐forming toxins sticholysins I and II to interfaces–modulation of conformation and activity, and lipid‐protein interaction. Chem Phys Lipids 122: 97–105 [DOI] [PubMed] [Google Scholar]
- Anderluh G, Kisovec M, Krasevec N, Gilbert RJ (2014) Distribution of MACPF/CDC proteins. Subcell Biochem 80: 7–30 [DOI] [PubMed] [Google Scholar]
- Arnez KH, Kindlova M, Bokil NJ, Murphy JM, Sweet MJ, Guncar G (2015) Analysis of the N‐terminal region of human MLKL, as well as two distinct MLKL isoforms, reveals new insights into necroptotic cell death. Biosci Rep 36: e00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J, Youle RJ (1999) Bax, but not Bcl‐xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci USA 96: 5492–5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J (2002) Bax‐type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem 277: 49360–49365 [DOI] [PubMed] [Google Scholar]
- Basanez G, Soane L, Hardwick JM (2012) A new view of the lethal apoptotic pore. PLoS Biol 10: e1001399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigenzahn JW, Fauster A, Rebsamen M, Kandasamy RK, Scorzoni S, Vladimer GI, Muller AC, Gstaiger M, Zuber J, Bennett KL et al (2016) An inducible retroviral expression system for tandem affinity purification mass‐spectrometry‐based proteomics identifies mixed lineage kinase domain‐like protein (MLKL) as an heat shock protein 90 (HSP90) client. Mol Cell Proteomics 15: 1139–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Landeta O, Landajuela A, Basanez G, Garcia‐Saez AJ (2013) Proapoptotic Bax and Bak proteins form stable protein‐permeable pores of tunable size. J Biol Chem 288: 33241–33252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Jeschke G, Stegmueller C, Salvador‐Gallego R, Garcia‐Saez AJ, Bordignon E (2014) Structural model of active Bax at the membrane. Mol Cell 56: 496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Hantusch A, Das KK, Frickey T, Garcia‐Saez AJ (2017) Quantitative interactome of a membrane Bcl‐2 network identifies a hierarchy of complexes for apoptosis regulation. Nat Commun 8: 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Assafa TE, Stegmueller C, Wittig A, Garcia‐Saez AJ, Bordignon E (2018) Topology of active, membrane‐embedded Bax in the context of a toroidal pore. Cell Death Differ 25: 1717–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Pelegrin P, Shao F (2020) The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol 20: 143–157 [DOI] [PubMed] [Google Scholar]
- Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF‐induced necroptosis. Nat Cell Biol 16: 55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FY, Lee MT, Huang HW (2003) Evidence for membrane thinning effect as the mechanism for peptide‐induced pore formation. Biophys J 84: 3751–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P et al (2014) Translocation of mixed lineage kinase domain‐like protein to plasma membrane leads to necrotic cell death. Cell Res 24: 105–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He WT, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, Han J (2016) Pyroptosis is driven by non‐selective gasdermin‐D pore and its morphology is different from MLKL channel‐mediated necroptosis. Cell Res 26: 1007–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino K, Ros U, Garcia‐Saez AJ (2016) Assembling the puzzle: oligomerization of alpha‐pore forming proteins in membranes. Biochem Biophys Acta 1858: 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino K, Garcia‐Saez AJ (2017) Bax and Bak pores: are we closing the circle? Trends Cell Biol 27: 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino K, Garcia‐Saez AJ (2018) MIM through MOM: the awakening of Bax and Bak pores. EMBO J 37: e100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan AD, Smith NA, Sandow JJ, Kapp EA, Rustam YH, Murphy JM, Brouwer JM, Bernardini JP, Roy MJ, Wardak AZ et al (2020) BAK core dimers bind lipids and can be bridged by them. Nat Struct Mol Biol 10.1038/s41594-020-0494-5 [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ et al (2013) Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152: 519–531 [DOI] [PubMed] [Google Scholar]
- Dal Peraro M, van der Goot FG (2016) Pore‐forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol 14: 77–92 [DOI] [PubMed] [Google Scholar]
- Daskalov A, Habenstein B, Sabate R, Berbon M, Martinez D, Chaignepain S, Coulary‐Salin B, Hofmann K, Loquet A, Saupe SJ (2016) Identification of a novel cell death‐inducing domain reveals that fungal amyloid‐controlled programmed cell death is related to necroptosis. Proc Natl Acad Sci USA 113: 2720–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M (2019) Single‐cell analysis of pyroptosis dynamics reveals conserved GSDMD‐mediated subcellular events that precede plasma membrane rupture. Cell Death Differ 26: 146–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D (2016) Membranes and the origin of life: a century of conjecture. J Mol Evol 83: 159–168 [DOI] [PubMed] [Google Scholar]
- Depraetere V (2000) "Eat me" signals of apoptotic bodies. Nat Cell Biol 2: E104 [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM (2008) To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell 30: 369–380 [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM (2009) Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol Cell 36: 696–703 [DOI] [PubMed] [Google Scholar]
- Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, Kluck RM (2012) Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ 19: 661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou E, Cosentino K, Platonova E, Ros U, Sadeghi M, Kashyap P, Katsinelos T, Wegehingel S, Noe F, Garcia‐Saez AJ et al (2019) Single event visualization of unconventional secretion of FGF2. J Cell Biol 218: 683–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F (2016) Pore‐forming activity and structural autoinhibition of the gasdermin family. Nature 535: 111–116 [DOI] [PubMed] [Google Scholar]
- Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW et al (2014) MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7: 971–981 [DOI] [PubMed] [Google Scholar]
- Douanne T, Andre‐Gregoire G, Trillet K, Thys A, Papin A, Feyeux M, Hulin P, Chiron D, Gavard J, Bidere N (2019) Pannexin‐1 limits the production of proinflammatory cytokines during necroptosis. EMBO Rep 20: e47840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey CM, Diep J, Clarke BP, Hale AT, McNamara DE, Guo H, Brown NW Jr, Cao JY, Grace CR, Gough PJ et al (2018) MLKL requires the inositol phosphate code to execute necroptosis. Mol Cell 70: 936–948.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang CX, Neutzner A, Tjandra N, Youle RJ (2011) Bcl‐x(L) Retrotranslocates Bax from the mitochondria into the cytosol. Cell 145: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberg Y, Landau M (2020) The human LL‐37(17–29) antimicrobial peptide reveals a functional supramolecular nanostructure. bioRxiv 10.1101/2020.02.04.933432 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand RF, Martinou JC, Montessuit S, Epand RM (2003) Transbilayer lipid diffusion promoted by Bax: implications for apoptosis. Biochemistry 42: 14576–14582 [DOI] [PubMed] [Google Scholar]
- Espiritu RA, Pedrera L, Ros U (2019) Chapter Seven – Tuning the way to die: implications of membrane perturbations in necroptosis, In Advances in biomembranes and lipid self‐assembly, Iglič A, Rappolt M, García‐Sáez AJ. (eds), pp 201–247. Cambridge, MA: Academic Press; [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC (2018) The pore‐forming protein gasdermin D regulates interleukin‐1 secretion from living macrophages. Immunity 48: 35–44.e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Guo J, Gao B, Zhang W, Ling L, Xu T, Pan C, Li L, Chen S, Wang H et al (2019) Flotillin‐mediated endocytosis and ALIX‐syntenin‐1‐mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci Signal 12: eaaw3423 [DOI] [PubMed] [Google Scholar]
- Fleischer S, Rouser G, Fleischer B, Casu A, Kritchevsky G (1967) Lipid composition of mitochondria from bovine heart, liver, and kidney. J Lipid Res 8: 170–180 [PubMed] [Google Scholar]
- Flores‐Romero H, Garcia‐Porras M, Basanez G (2017) Membrane insertion of the BAX core, but not latch domain, drives apoptotic pore formation. Sci Rep 7: 16259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Romero H, Garcia‐Saez AJ (2019a) The incomplete puzzle of the BCL2 proteins. Cells 8: 1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Romero H, Garcia‐Saez AJ (2019b) MAVS‐induced mitochondrial membrane remodeling. FEBS J 286: 1540–1542 [DOI] [PubMed] [Google Scholar]
- Flores‐Romero H, Landeta O, Ugarte‐Uribe B, Cosentino K, Garcia‐Porras M, Garcia‐Saez AJ, Basanez G (2019) BFL1 modulates apoptosis at the membrane level through a bifunctional and multimodal mechanism showing key differences with BCLXL. Cell Death Differ 26: 1880–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Romero H, Ros U, García‐Sáez AJ (2020) A lipid perspective on regulated cell death In International review of cell and molecular biology, Galluzzi L, Spetz J. (eds), Vol. 351, pp. 197–236. Cambridge, MA: Academic Press; [DOI] [PubMed] [Google Scholar]
- Flores‐Romero H, García‐Sáez AJ (2020) Lipids glue BAK dimers together. Nat Struct Mol Biol 10.1038/s41594-020-00516-y [DOI] [PubMed] [Google Scholar]
- Fuertes G, Garcia‐Saez AJ, Esteban‐Martin S, Gimenez D, Sanchez‐Munoz OL, Schwille P, Salgado J (2010a) Pores formed by Baxalpha5 relax to a smaller size and keep at equilibrium. Biophys J 99: 2917–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes G, Gimenez D, Esteban‐Martin S, Garcia‐Saez A, Sanchez O, Salgado J (2010b) Role of membrane lipids for the activity of pore forming peptides and proteins. Adv Exp Med Biol 677: 31–55 [DOI] [PubMed] [Google Scholar]
- Fuertes G (2011) Baxα5 at lipid membranes: structure, assembly and pore formation. Universitat de València. Ph.D thesis [Google Scholar]
- Fuertes G, Gimenez D, Esteban‐Martin S, Sanchez‐Munoz OL, Salgado J (2011) A lipocentric view of peptide‐induced pores. Eur Biophys J 40: 399–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25: 486–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Saez AJ, Coraiola M, Dalla Serra M, Mingarro I, Menestrina G, Salgado J (2005) Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full‐length proteins. Biophys J 88: 3976–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Saez AJ, Coraiola M, Serra MD, Mingarro I, Muller P, Salgado J (2006) Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J 273: 971–981 [DOI] [PubMed] [Google Scholar]
- Garcia‐Saez AJ, Chiantia S, Salgado J, Schwille P (2007) Pore formation by a Bax‐derived peptide: effect on the line tension of the membrane probed by AFM. Biophys J 93: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Saez AJ, Buschhorn SB, Keller H, Anderluh G, Simons K, Schwille P (2011) Oligomerization and pore formation by equinatoxin II inhibit endocytosis and lead to plasma membrane reorganization. J Biol Chem 286: 37768–37777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, Mikelj M, Dalla Serra M, Froelich CJ, Anderluh G (2013) Effects of MACPF/CDC proteins on lipid membranes. Cell Mol Life Sci 70: 2083–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, Dalla Serra M, Froelich CJ, Wallace MI, Anderluh G (2014) Membrane pore formation at protein‐lipid interfaces. Trends Biochem Sci 39: 510–516 [DOI] [PubMed] [Google Scholar]
- Gilbert RJ (2016) Protein‐lipid interactions and non‐lamellar lipidic structures in membrane pore formation and membrane fusion. Biochem Biophys Acta 1858: 487–499 [DOI] [PubMed] [Google Scholar]
- Gong YN, Guy C, Crawford JC, Green DR (2017a) Biological events and molecular signaling following MLKL activation during necroptosis. Cell Cycle 16: 1748–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, Green DR (2017b) ESCRT‐III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169: 286–300.e216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grage SL, Afonin S, Kara S, Buth G, Ulrich AS (2016) Membrane thinning and thickening induced by membrane‐active amphipathic peptides. Front Cell Dev Biol 4: 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GJ, Corfe BM, Savory P, Leech S, Esposti MD, Hickman JA, Dive C (2001) Cellular damage signals promote sequential changes at the N‐terminus and BH‐1 domain of the pro‐apoptotic protein Bak. Oncogene 20: 7668–7676 [DOI] [PubMed] [Google Scholar]
- Grijalba MT, Vercesi AE, Schreier S (1999) Ca2+‐induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+‐stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry 38: 13279–13287 [DOI] [PubMed] [Google Scholar]
- Grosse L, Wurm CA, Bruser C, Neumann D, Jans DC, Jakobs S (2016) Bax assembles into large ring‐like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J 35: 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harroun TA, Heller WT, Weiss TM, Yang L, Huang HW (1999) Experimental evidence for hydrophobic matching and membrane‐mediated interactions in lipid bilayers containing gramicidin. Biophys J 76: 937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P (2018) The Gasdermin‐D pore acts as a conduit for IL‐1beta secretion in mice. Eur J Immunol 48: 584–592 [DOI] [PubMed] [Google Scholar]
- Hertlein V, Flores‐Romero H, Das KK, Fischer S, Heunemann M, Calleja‐Felipe M, Knafo S, Hipp K, Harter K, Fitzgerald JC et al (2020) MERLIN: a novel BRET‐based proximity biosensor for studying mitochondria‐ER contact sites. Life Sci Alliance 3: e201900600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier JM, Dobson RC, Webb AI et al (2014) Activation of the pseudokinase MLKL unleashes the four‐helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA 111: 15072–15077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JM, Kauppi M, Majewski IJ, Liu Z, Cox AJ, Miyake S, Petrie EJ, Silk MA, Li Z, Tanzer MC et al (2020) A missense mutation in the MLKL brace region promotes lethal neonatal inflammation and hematopoietic dysfunction. Nat Commun 11: 3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Zheng X, Wang ZA, Chen X, He WT, Zhang Y, Xu JG, Zhao H, Shi W, Wang X et al (2017) The MLKL channel in necroptosis is an octamer formed by tetramers in a dyadic process. Mol Cell Biol 37: e00497‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LS, Hong Z, Wu W, Xiong S, Zhong M, Gao X, Rehman J, Malik AB (2020) mtDNA activates cGAS signaling and suppresses the YAP‐mediated endothelial cell proliferation program to promote inflammatory injury. Immunity 52: 475–486.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, Haller M, Riley JS, Mason SM, Athineos D et al (2015) Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell 57: 860–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, van Delft MF, Liu Z, Conos SA, Zhang JG et al (2016) HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis 7: e2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Jiang H, Shen Z, Wang X (2014) Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc Natl Acad Sci USA 111: 14782–14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y (2004) Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med 37: 1963–1985 [DOI] [PubMed] [Google Scholar]
- Kagan BL (2012) Membrane pores in the pathogenesis of neurodegenerative disease. Prog Mol Biol Transl Sci 107: 295–325 [DOI] [PubMed] [Google Scholar]
- Karatekin E, Sandre O, Guitouni H, Borghi N, Puech PH, Brochard‐Wyart F (2003) Cascades of transient pores in giant vesicles: line tension and transport. Biophys J 84: 1734–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F, Shoda M, Harashima R, Sadaie Y, Hara H, Matsumoto K (2004) Cardiolipin domains in Bacillus subtilis marburg membranes. J Bacteriol 186: 1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke FFS, Vanyai HK, Cowan AD, Delbridge ARD, Whitehead L, Grabow S, Czabotar PE, Voss AK, Strasser A (2018) Embryogenesis and adult life in the absence of intrinsic apoptosis effectors BAX, BAK, and BOK. Cell 173: 1217–1230.e1217 [DOI] [PubMed] [Google Scholar]
- Kim J, Gupta R, Blanco LP, Yang S, Shteinfer‐Kuzmine A, Wang K, Zhu J, Yoon HE, Wang X, Kerkhofs M et al (2019) VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus‐like disease. Science 366: 1531–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Zheng J, Yang H, Li S, Duan S, Shen Y, Ji C, Gan J, Xu XW, Li J (2017) Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci USA 114: 10642–10647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K (2016) Ion channels in regulated cell death. Cell Mol Life Sci 73: 2387–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnareva Y, Andreyev AY, Kuwana T, Newmeyer DD (2012) Bax activation initiates the assembly of a multimeric catalyst that facilitates Bax pore formation in mitochondrial outer membranes. PLoS Biol 10: e1001394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331–342 [DOI] [PubMed] [Google Scholar]
- Kuwana T, Olson NH, Kiosses WB, Peters B, Newmeyer DD (2016) Pro‐apoptotic Bax molecules densely populate the edges of membrane pores. Sci Rep 6: 27299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeta O, Landajuela A, Gil D, Taneva S, Di Primo C, Sot B, Valle M, Frolov VA, Basanez G (2011) Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the BAK‐driven membrane permeabilization process. J Biol Chem 286: 8213–8230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeta O, Garcia Valero J, Flores‐Romero H, Bustillo‐Zabalbeitia I, Landajuela A, Garcia‐Porras M, Terrones O, Basanez G (2014) Lipid‐dependent bimodal MCL1 membrane activity. ACS Chem Biol 9: 2852–2863 [DOI] [PubMed] [Google Scholar]
- Landeta O, Landajuela A, Garcia‐Saez A, Basanez G (2015) Minimalist model systems reveal similarities and differences between membrane interaction modes of MCL1 and BAK. J Biol Chem 290: 17004–17019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterwasser J, Todt F, Zerbes RM, Nguyen TN, Craigen W, Lazarou M, van der Laan M, Edlich F (2016) The porin VDAC2 is the mitochondrial platform for Bax retrotranslocation. Sci Rep 6: 32994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Chen FY, Huang HW (2004) Energetics of pore formation induced by membrane active peptides. Biochemistry 43: 3590–3599 [DOI] [PubMed] [Google Scholar]
- Lee CC, Sun Y, Qian S, Huang HW (2011) Transmembrane pores formed by human antimicrobial peptide LL‐37. Biophys J 100: 1688–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J (2016) Inflammasome‐activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu H, Johnston A, Hanna‐Addams S, Reynoso E, Xiang Y, Wang Z (2017) MLKL forms disulfide bond‐dependent amyloid‐like polymers to induce necroptosis. Proc Natl Acad Sci USA 114: E7450–E7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Rathkey JK, Yang J, Dubyak GR, Abbott DW, Xiao TS (2018) Structures of the gasdermin D C‐terminal domains reveal mechanisms of autoinhibition. Structure 26: 778–784.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Yang J, Zhou B, Yang R, Ramachandran R, Abbott DW, Xiao TS (2019) Crystal structures of the full‐length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51: 43–49.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW (1996) Membrane pores induced by magainin. Biochemistry 35: 13723–13728 [DOI] [PubMed] [Google Scholar]
- Mandal T, Shin S, Aluvila S, Chen HC, Grieve C, Choe JY, Cheng EH, Hustedt EJ, Oh KJ (2016) Assembly of Bak homodimers into higher order homooligomers in the mitochondrial apoptotic pore. Sci Rep 6: 30763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Murase O, Fujii N, Miyajima K (1996) An antimicrobial peptide, magainin 2, induced rapid flip‐flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 35: 11361–11368 [DOI] [PubMed] [Google Scholar]
- McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, Chappaz S, Davidson S, San Chin H et al (2018) BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359: eaao6047 [DOI] [PubMed] [Google Scholar]
- McCormack R, de Armas L, Shiratsuchi M, Podack ER (2013) Killing machines: three pore‐forming proteins of the immune system. Immunol Res 57: 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara DE, Dovey CM, Hale AT, Quarato G, Grace CR, Guibao CD, Diep J, Nourse A, Cai CR, Wu H et al (2019) Direct activation of human MLKL by a select repertoire of inositol phosphate metabolites. Cell Chem Biol 26: 863–877.e867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa‐Galloso H, Valiente PA, Valdes‐Tresanco ME, Epand RF, Lanio ME, Epand RM, Alvarez C, Tieleman DP, Ros U (2019) Membrane remodeling by the lytic fragment of sticholysin II: implications for the toroidal pore model. Biophys J 117: 1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkar SS, Marchioretto M, Antonini V, Lunelli L, Wang B, Gilbert RJ, Anderluh G, Roth R, Pooga M, Pardo J et al (2015) Perforin oligomers form arcs in cellular membranes: a locus for intracellular delivery of granzymes. Cell Death Differ 22: 74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB (1997) Bcl‐x(L) forms an ion channel in synthetic lipid membranes. Nature 385: 353–357 [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Grace CR, Llambi F, Nourse A, Fitzgerald P, Gehring K, Kriwacki RW, Green DR (2013) BID‐induced structural changes in BAK promote apoptosis. Nat Struct Mol Biol 20: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Czabotar PE (2019) BAX, BAK, and BOK: a coming of age for the BCL‐2 family effector proteins. Cold Spring Harb Perspect Biol 12: a036319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL et al (1996) X‐ray and NMR structure of human Bcl‐xL, an inhibitor of programmed cell death. Nature 381: 335–341 [DOI] [PubMed] [Google Scholar]
- Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Muller DJ (2018) Mechanism of membrane pore formation by human gasdermin‐D. EMBO J 37: e98321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez‐Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI et al (2013) The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39: 443–453 [DOI] [PubMed] [Google Scholar]
- Najafov A, Mookhtiar AK, Luu HS, Ordureau A, Pan H, Amin PP, Li Y, Lu Q, Yuan J (2019) TAM kinases promote necroptosis by regulating oligomerization of MLKL. Mol Cell 75: 457–468.e4 [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ (1999) Conformation of the Bax C‐terminus regulates subcellular location and cell death. EMBO J 18: 2330–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ (2001) Bax and Bak coalesce into novel mitochondria‐associated clusters during apoptosis. J Cell Biol 153: 1265–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A, Killian JA, Verkleij AJ, Wilschut J (1999) Membrane fusion and the lamellar‐to‐inverted‐hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys J 77: 2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousingsawat J, Cabrita I, Wanitchakool P, Sirianant L, Krautwald S, Linkermann A, Schreiber R, Kunzelmann K (2017) Ca(2+) signals, cell membrane disintegration, and activation of TMEM16F during necroptosis. Cell Mol Life Sci 74: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi LR, Morrow LM, Visser MB, Atilla‐Gokcumen GE (2018) Turning the spotlight on lipids in non‐apoptotic cell death. ACS Chem Biol 13: 506–515 [DOI] [PubMed] [Google Scholar]
- Petrie EJ, Hildebrand JM, Murphy JM (2017) Insane in the membrane: a structural perspective of MLKL function in necroptosis. Immunol Cell Biol 95: 152–159 [DOI] [PubMed] [Google Scholar]
- Petrie EJ, Sandow JJ, Jacobsen AV, Smith BJ, Griffin MDW, Lucet IS, Dai W, Young SN, Tanzer MC, Wardak A et al (2018) Conformational switching of the pseudokinase domain promotes human MLKL tetramerization and cell death by necroptosis. Nat Commun 9: 2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EJ, Birkinshaw RW, Koide A, Denbaum E, Hildebrand JM, Garnish SE, Davies KA, Sandow JJ, Samson AL, Gavin X et al (2020) Identification of MLKL membrane translocation as a checkpoint in necroptotic cell death using Monobodies. Proc Natl Acad Sci USA 117: 8468–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AM, Olejniczak ET, Fesik SW (2004) Structural biology of the Bcl‐2 family of proteins. Biochem Biophys Acta 1644: 83–94 [DOI] [PubMed] [Google Scholar]
- Podobnik M, Savory P, Rojko N, Kisovec M, Wood N, Hambley R, Pugh J, Wallace EJ, McNeill L, Bruce M et al (2016) Crystal structure of an invertebrate cytolysin pore reveals unique properties and mechanism of assembly. Nat Commun 7: 11598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz D, Klein K, List J, Knab VM, Menzl I, Leidenfrost N, Heller G, Polic B, Putz EM, Witalisz‐Siepracka A et al (2020) Loss of NKG2D in murine NK cells leads to increased perforin production upon long‐term stimulation with IL‐2. Eur J Immunol 50: 880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puech PH, Borghi N, Karatekin E, Brochard‐Wyart F (2003) Line thermodynamics: adsorption at a membrane edge. Phys Rev Lett 90: 128304 [DOI] [PubMed] [Google Scholar]
- Qian S, Wang W, Yang L, Huang HW (2008) Structure of transmembrane pore induced by Bax‐derived peptide: evidence for lipidic pores. Proc Natl Acad Sci USA 105: 17379–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarato G, Guy CS, Grace CR, Llambi F, Nourse A, Rodriguez DA, Wakefield R, Frase S, Moldoveanu T, Green DR (2016) Sequential engagement of distinct MLKL phosphatidylinositol‐binding sites executes necroptosis. Mol Cell 61: 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm M, Dussmann H, Prehn JH (2003) Real‐time single cell analysis of Smac/DIABLO release during apoptosis. J Cell Biol 162: 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynwar BJ, Illya G, Harmandaris VA, Muller MM, Kremer K, Deserno M (2007) Aggregation and vesiculation of membrane proteins by curvature‐mediated interactions. Nature 447: 461–464 [DOI] [PubMed] [Google Scholar]
- Riley JS, Quarato G, Cloix C, Lopez J, O'Prey J, Pearson M, Chapman J, Sesaki H, Carlin LM, Passos JF et al (2018) Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J 37: e99238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojko N, Anderluh G (2015) How lipid membranes affect pore forming toxin activity. Acc Chem Res 48: 3073–3079 [DOI] [PubMed] [Google Scholar]
- Ros U, Garcia‐Saez AJ (2015) More than a pore: the interplay of pore‐forming proteins and lipid membranes. J Membr Biol 248: 545–561 [DOI] [PubMed] [Google Scholar]
- Ros U, Pena‐Blanco A, Hanggi K, Kunzendorf U, Krautwald S, Wong WW, Garcia‐Saez AJ (2017) Necroptosis execution is mediated by plasma membrane nanopores independent of calcium. Cell Rep 19: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros U, Pedrera L, Garcia‐Saez AJ (2020) Partners in crime: the interplay of proteins and membranes in regulated necrosis. Int J Mol Sci 21: 2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, Wu H (2018) Cryo‐EM structure of the gasdermin A3 membrane pore. Nature 557: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P (2018) ESCRT‐dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362: 956–960 [DOI] [PubMed] [Google Scholar]
- Salvador‐Gallego R, Mund M, Cosentino K, Schneider J, Unsay J, Schraermeyer U, Engelhardt J, Ries J, Garcia‐Saez AJ (2016) Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J 35: 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson AL, Zhang Y, Geoghegan ND, Gavin XJ, Davies KA, Mlodzianoski MJ, Whitehead LW, Frank D, Garnish SE, Fitzgibbon C et al (2020) MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat Commun 11: 3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ et al (1997) Structure of Bcl‐xL‐Bak peptide complex: recognition between regulators of apoptosis. Science 275: 983–986 [DOI] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, Hiller S (2016) GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35: 1766–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg B, Wang P, Keeble JA, Rodriguez‐Enriquez R, Walker S, Owens TW, Foster F, Tanianis‐Hughes J, Brennan K, Streuli CH et al (2013) Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell 49: 959–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Ren M (2009) The role of cardiolipin in the structural organization of mitochondrial membranes. Biochem Biophys Acta 1788: 2080–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ (1997) Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL‐2. Proc Natl Acad Sci USA 94: 11357–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G, Robert CH (1992) Kinetics of pore‐mediated release of marker molecules from liposomes or cells. Biophys Chem 42: 291–296 [DOI] [PubMed] [Google Scholar]
- Sengupta D, Leontiadou H, Mark AE, Marrink SJ (2008) Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta 1778: 2308–2317 [DOI] [PubMed] [Google Scholar]
- Shlomovitz R, Gov NS (2009) Membrane‐mediated interactions drive the condensation and coalescence of FtsZ rings. Phys Biol 6: 046017 [DOI] [PubMed] [Google Scholar]
- Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL‐2 family proteins. Nat Rev Mol Cell Biol 20: 175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen AF, Plitzko JM, Gilbert RJ (2014) Incomplete pneumolysin oligomers form membrane pores. Open Biol 4: 140044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorice M, Manganelli V, Matarrese P, Tinari A, Misasi R, Malorni W, Garofalo T (2009) Cardiolipin‐enriched raft‐like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett 583: 2447–2450 [DOI] [PubMed] [Google Scholar]
- Steringer JP, Bleicken S, Andreas H, Zacherl S, Laussmann M, Temmerman K, Contreras FX, Bharat TA, Lechner J, Muller HM et al (2012) Phosphatidylinositol 4,5‐bisphosphate (PI(4,5)P2)‐dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J Biol Chem 287: 27659–27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Quade B, Wang H, Sun L, Wang X, Rizo J (2014) A plug release mechanism for membrane permeation by MLKL. Structure 22: 1489–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subburaj Y, Cosentino K, Axmann M, Pedrueza‐Villalmanzo E, Hermann E, Bleicken S, Spatz J, Garcia‐Saez AJ (2015) Bax monomers form dimer units in the membrane that further self‐assemble into multiple oligomeric species. Nat Commun 6: 8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N (2000) Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103: 645–654 [DOI] [PubMed] [Google Scholar]
- Tanzer MC, Tripaydonis A, Webb AI, Young SN, Varghese LN, Hall C, Alexander WS, Hildebrand JM, Silke J, Murphy JM (2015) Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J 471: 255–265 [DOI] [PubMed] [Google Scholar]
- Tanzer MC, Matti I, Hildebrand JM, Young SN, Wardak A, Tripaydonis A, Petrie EJ, Mildenhall AL, Vaux DL, Vince JE et al (2016) Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ 23: 1185–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeb‐Fligelman E, Tabachnikov O, Moshe A, Goldshmidt‐Tran O, Sawaya MR, Coquelle N, Colletier JP, Landau M (2017) The cytotoxic Staphylococcus aureusPSMalpha3 reveals a cross‐alpha amyloid‐like fibril. Science 355: 831–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, Herrmann A, Basanez G (2004) Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem 279: 30081–30091 [DOI] [PubMed] [Google Scholar]
- Tieleman DP, Leontiadou H, Mark AE, Marrink SJ (2003) Simulation of pore formation in lipid bilayers by mechanical stress and electric fields. J Am Chem Soc 125: 6382–6383 [DOI] [PubMed] [Google Scholar]
- Tieleman DP, Marrink SJ (2006) Lipids out of equilibrium: energetics of desorption and pore mediated flip‐flop. J Am Chem Soc 128: 12462–12467 [DOI] [PubMed] [Google Scholar]
- Todt F, Cakir Z, Reichenbach F, Emschermann F, Lauterwasser J, Kaiser A, Ichim G, Tait SW, Frank S, Langer HF et al (2015) Differential retrotranslocation of mitochondrial Bax and Bak. EMBO J 34: 67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte‐Uribe B, Garcia‐Saez AJ (2017) Apoptotic foci at mitochondria: in and around Bax pores. Philos Trans R Soc B Biol Sci 372: 20160217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte‐Uribe B, Prevost C, Das KK, Bassereau P, Garcia‐Saez AJ (2018) Drp1 polymerization stabilizes curved tubular membranes similar to those of constricted mitochondria. J Cell Sci 132: jcs208603 [DOI] [PubMed] [Google Scholar]
- Unsay JD, Cosentino K, Subburaj Y, Garcia‐Saez AJ (2013) Cardiolipin effects on membrane structure and dynamics. Langmuir 29: 15878–15887 [DOI] [PubMed] [Google Scholar]
- Unsay JD, Cosentino K, Sporbeck K, Garcia‐Saez AJ (2017) Pro‐apoptotic cBid and Bax exhibit distinct membrane remodeling activities: An AFM study. Biochim Biophys Acta 1859: 17–27 [DOI] [PubMed] [Google Scholar]
- Valcarcel CA, Dalla Serra M, Potrich C, Bernhart I, Tejuca M, Martinez D, Pazos F, Lanio ME, Menestrina G (2001) Effects of lipid composition on membrane permeabilization by sticholysin I and II, two cytolysins of the sea anemone Stichodactyla helianthus. Biophys J 80: 2761–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265: 7248–7256 [PubMed] [Google Scholar]
- Voskoboinik I, Whisstock JC, Trapani JA (2015) Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 15: 388–400 [DOI] [PubMed] [Google Scholar]
- Wallach D, Kang TB, Dillon CP, Green DR (2016) Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352: aaf2154 [DOI] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Menard M, Frederick P, Iyer S, Bartolo R, Gibson L, Czabotar PE, Smith BJ, Adams JM et al (2014) Apoptotic pore formation is associated with in‐plane insertion of Bak or Bax central helices into the mitochondrial outer membrane. Proc Natl Acad Sci USA 111: E4076–E4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SY, Lee H (2017) Effect of lipid shape on toroidal pore formation and peptide orientation in lipid bilayers. Phys Chem Chem Phys 19: 21340–21349 [DOI] [PubMed] [Google Scholar]
- Xia B, Fang S, Chen X, Hu H, Chen P, Wang H, Gao Z (2016) MLKL forms cation channels. Cell Res 26: 517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XP, Zhai D, Kim E, Swift M, Reed JC, Volkmann N, Hanein D (2013) Three‐dimensional structure of Bax‐mediated pores in membrane bilayers. Cell Death Dis 4: e683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD (2008) Opa1‐mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell 31: 557–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Harroun TA, Weiss TM, Ding L, Huang HW (2001) Barrel‐stave model or toroidal model? A case study on melittin pores. Biophys J 81: 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Kovalenko A, Bogdanov K, Wallach D (2017) MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 47: 51–65.e7 [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A (2008) The BCL‐2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59 [DOI] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR et al (2016) An endogenous caspase‐11 ligand elicits interleukin‐1 release from living dendritic cells. Science 352: 1232–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler M, Steringer JP, Muller HM, Mayer MP, Nickel W (2015) HIV‐Tat protein forms phosphoinositide‐dependent membrane pores implicated in unconventional protein secretion. J Biol Chem 290: 21976–21984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhu W, Lapolla SM, Miao Y, Shao Y, Falcone M, Boreham D, McFarlane N, Ding J, Johnson AE et al (2010) Bax forms an oligomer via separate, yet interdependent, surfaces. J Biol Chem 285: 17614–17627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LL, Zhou LY, Luo KQ, Chang DC (2005) Smac/DIABLO and cytochrome c are released from mitochondria through a similar mechanism during UV‐induced apoptosis. Apoptosis 10: 289–299 [DOI] [PubMed] [Google Scholar]


