Abstract
Mesenchymal stem cells can be replaced by exosomes for the treatment of inflammatory diseases, injury repair, degenerative diseases, and tumors. Exosomes are small vesicles rich in a variety of nucleic acids [including messenger RNA, Long non-coding RNA, microRNA (miRNA), and circular RNA], proteins, and lipids. Exosomes can be secreted by most cells in the human body and are known to play a key role in the communication of information and material transport between cells. Like exosomes, miRNAs were neglected before their role in various activities of organisms was discovered. Several studies have confirmed that miRNAs play a vital role within exosomes. This review focuses on the specific role of miRNAs in MSC-derived exosomes (MSC-exosomes) and the methods commonly used by researchers to study miRNAs in exosomes. Taken together, miRNAs from MSC-exosomes display immense potential and practical value, both in basic medicine and future clinical applications, in treating several diseases.
Keywords: MicroRNA, Mesenchymal stem cells, Exosomes, Modulation of mesenchymal stem cells, Inflammatory diseases, Injury repair
Core Tip: Mesenchymal stem cell (MSC)-derived exosomes (MSC-exosomes) have obvious therapeutic effects on inflammation, organ damage, and tumors. Exosomes are membranes containing many substances, such as nucleic acids, proteins, and lipids. In many studies, microRNAs (miRNAs) have been found to be the core substance of therapeutic effect after exosomes are engulfed by cells. MiRNAs in exosomes secreted by different stem cells are diverse. In turn, different miRNAs target different genes to treat matched diseases. This point of view proves that miRNAs in MSC-exosomes have a broad research space.
INTRODUCTION
Mesenchymal stem cells (MSCs) are adult stem cells that exist in a variety of tissues (such as bone marrow, cord blood and cord tissue, placental tissue, adipose tissue, and menstrual blood) and have the potential for multidirectional differentiation[1-3]. MSCs have the potential to differentiate into multiple tissues and escape immunity, making it a good candidate for cell therapy[4]. Although MSCs have been shown to be effective in treating many refractory diseases, the ethical and safety problems of stem cells, as well as the inevitable aspect of promoting tumor growth, still pose great threats to their development[5,6].
Exosomes are small vesicles that carry a variety of substances and are important for information transmission and material communication between cells. Recent studies have shown that exosomes exert significant improvements in various diseases, such as tissue injury[7,8], neurodegenerative diseases[9,10], tissue fibrosis[11-13], diabetes[14,15], and even tumors[16,17], suggesting that exosomes have great therapeutic potential. Researchers have found that MSCs do not function through cell transplantation and inflammatory response, but through the secretion of small extracellular vesicles, the exosomes[6]. Subsequent research has further confirmed that diseases, which can be treated with MSCs, can also be treated with MSC-derived exosomes (MSC-exosomes), such as myocardial infarction[18], liver fibrosis[19], and tumors[20]. Previous studies have demonstrated that MSC-exosomes exert similar effects on myocardial repair as do MSCs, and in some cases perform better than MSCs[21].
Material transfer in stem cells mediated by exosomes has several advantages, including flexibility of use, easy preservation, stability of materials in vesicles, and substantial enrichment from culture medium. MSC-exosomes play a very important role in the treatment of diseases. There are many substances in exosomes, and exactly what substances are playing a key role is still being explored. In numerous studies, microRNA (miRNA) frequency is very high, suggesting that miRNAs in exosomes may play an important role in treatment. Therefore, this review will introduce MSC-exosomes, miRNAs, miRNA in exosomes, and how miRNA plays a crucial role in MSC-exosomes in specific diseases.
EXOSOMES
History of exosomes
Exosomes are membranous vesicles with a diameter of 30-150 nm, which are released extracellularly by the fusion of intracellular vesicles and cell membranes. Exosomes are specific extracellular vesicles, which include apoptotic bodies, micro-vesicles, and exosomes. The first exosome was discovered by Peter Wolf in 1967, who called it "platelet dust" at that time[22]. In 1987, Johnstone named it "exosome", which originated from the endosome formed by the endocytosis process and was eventually released from the inside to the outside of the cell[23]. Exosomes were initially thought of as a way for cells to excrete waste, and were ignored for about a decade. It was until 1996, when G. Raposo discovered that immune cells similar to B lymphocytes also secrete antigen-presenting exosomes, that people began to notice[24]. In 2007, Valadi et al[25] discovered that cells can exchange genetic material with each other via RNA in exosomes. In 2010, exosomes secreted by MSCs were shown to be significant in the treatment of myocardial infarction, and since then, there has been increasing research on exosomes derived from MSCs[7]. In 2013, it was found that miRNAs in exosomes in the plasma of tumor patients could be used as non-invasive biological markers of tumors, and the role of miRNAs in exosomes began to receive attention[26]. Subsequently, miRNAs in astrocyte-derived exosomes were found to promote brain tumor metastasis by targeting the gene phosphatase and tensin homolog deleted on chromosome ten (PTEN)[27]. Moreover, miRNAs in adipose-derived exosomes have also been shown to regulate gene expression in other tissues[28]. These are important discoveries proving that cells can exchange information and substances through exosomes, laying a foundation for further studies on exosomes.
Biogenesis of exosomes
Exosomes are formed by the invagination of the cell membrane into the endosome, and then into multivesicle bodies (MVB), which are finally secreted to the extracellular fluid. Late endosomal membrane invagination leads to the formation of intraluminal vesicles (ILVs) in large MVBs[29]. At present, among the biosynthesis mechanisms of exosomes, the endosomal sorting complex required for transport (ESCRT) pathway has received the most attention. It is a complex system composed of four proteins. They are ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III. The cooperation between these results in MVB synthesis. MVB then fuses with the cytoplasmic membrane, releasing ILVs outside the cell to form exosomes[30]. Not only ESCRT proteins but also other proteins, including Flotillin, TSG101, CD6, and CD63, are involved in this process. In the mechanism of exosome formation, some researchers believe that RAL-1 is not only involved in the formation of MVBs, but also in the fusion of MVBs and cell membranes, which is of great significance for further study of exosomes[31]. Although the endosomal dependent pathway is considered to be the main synthesis pathway of exosomes, some studies have shown that direct budding of the plasma membrane also plays an important role in exosome biogenesis[32]. Moreover, the transmembrane protein syndecans 1 to 4 directly regulate ILVs during exosome formation by co-accumulation with syntenin and ALG-2 interacting protein X in exosomes[33]. Additionally, neutral sphingomyelinase can also promote ILV formation by promoting the budding of MVBs[34]. Due to the small diameter of exosomes and the complexity of the synthesis process, the synthesis process of exosomes is still being discussed further.
Isolation methods of exosomes
At present, ultracentrifugation is still the gold standard for exosome isolation[35], as well as the most common method. However, the biggest challenge that ultracentrifugation faces is time consumption. If researchers want to conduct numerous experiments on exosomes, it is bound to take much time to extract. There are many ways to extract exosomes, and there will be more in future. These methods of isolating exosomes have their own advantages and disadvantages[158-161] (Table 1). The biggest problem at present is that we cannot unite these advantages, that is, easy to obtain, high purity, and less exosome damage. Among the many methods, the most advanced is microfluidic technology, which is also one of the most studied and attention-grabbing at present. It includes immunoaffinity-based microfluidics[36], size-based microfluidic technologies[37], and contact-free microfluidics-versatile tools[38]. Immunoaffinity-based microfluidics is one of the most widely used microfluidic technologies and has shown a commercialization trend. However, due to its high cost, difficulty in maintaining the integrity of exosome structure, and applicability only to specific subgroups, its development prospects are not promising[38]. The first size-dependent microfluidic system is the Exosome Total Isolation Chip[39]. The advantage of this method is that exosomes can be extracted with only a few samples, which is convenient for clinical detection. Besides, the process of this method is relatively simple. The brilliance of contact-free microfluidics-versatile tools lies in the fact that they do not require sophisticated instruments and external electric field forces, and can achieve exosome recovery up to 80% and purity up to 90% without contact[38]. This device achieves size-dependent, continuous exosome separation by applying viscoelastic forces on exosomes through a sheath-like solution consisting of a low concentration (0.1%) of biocompatible polyoxyethylene.
Table 1.
Exosome isolation methods
| Isolation method | Principle | Advantages | Limitations |
| Ultracentrifugation | Exosomes are purified by physical centrifugation according to their size and specific gravity | (1) The most common method; (2) Bulk extractability; and (3) Low cost | (1) The operation is complex and time-consuming; (2) Increased impurities; (3) Loss due to adsorption on the tube wall; and (4) Expensive equipment is needed[158] |
| Ultrafiltration | According to the size of exosomes, exosomes are separated by filter membrane | (1) Simple operation; (2) Rapid process; and (3) High yield | (1) Low-purity; and (2) Stress and shear forces can cause exosome damage |
| Size exclusion chromatography | The biofluid dissolves in the mobile phase and passes through the stationary phase, in which the various components of the mixture move at different speeds and are separated[159] | (1) High recovery rate; and (2) The structural integrity of exosomes is maintained | (1) Time-consuming; and (2) Low-purity |
| Precipitation[160,161] | By chemical extraction, the exosome liquid is combined with the liquid in the kit, and eventually the exosomes are deposited. | (1) Simple operation; (2) Rapid process; (3) No need for special equipment | Increased impurities |
| Immune affinity capture | Immune isolation is performed by magnetic bead-specific adsorption of exosome surface antigens | (1) Easy operation; (2) Rapid process; (3) High purity; and (4) High yield | (1) Does not apply to large-volume cell supernatant; and (2) High cost |
| Microfluidic technologies (ExoChip) | A microfluidic platform based on nano-acoustic filters, viscoelastic fluid separation, lateral displacement, and immune affinity separates exosomes from biological fluids | (1) Rapid separation; (2) High purity; and (3) Saving the sample | The research is not sufficient and is not widely used at present |
Identification of exosomes
The most commonly used identification method for exosomes is electron microscopy, which display a saucer-like structure with a clear membrane[40]. The electron microscope also includes transmission electron microscope[41], scanning electron microscope[42], cryo-electron microscope[43], and atomic force microscope[44], and the fine morphology of exosomes can be observed. Exosomes can also be identified by nanoparticle tracking analysis and dynamic light particles[45]. This method can help determine the distribution range of particle size of the detected substance and further determine whether the substance is an exosome. In addition to particle size and morphology, surface biomarkers of exosomes also need to be detected. Western blot, flow spectrometry, mass spectrometry, and enzyme-linked immunosorbent assay are also the methods used. Exosome extraction methods are developing day by day, and new exosome identification methods are also emerging. In 2019, Jeppesen et al[46] used RNA-seq techniques to analyze exosome components, and structural illumination microscope imaging techniques to observe exosome biomarkers, and defined the respective components of exosomes and non-vesicle compartments, providing a deeper insight into the heterogeneity of exosomes. In addition, the flow field-flow fractionation was also used to analyze different subpopulations of exosomes, and it was further found that proteins in exosomes were significantly correlated with enzyme metabolism and hypoxia, microtubules, and coagulation proteins, as well as specific pathways, such as glycolysis and mammalian target of rapamycin (mTOR) signaling[47]. The rapid development of exosome identification methods has enabled us to deepen our understanding of the fine structure of exosomes and the mechanism of disease treatment, thus enabling us to apply exosomes to more diseases.
Components of exosomes
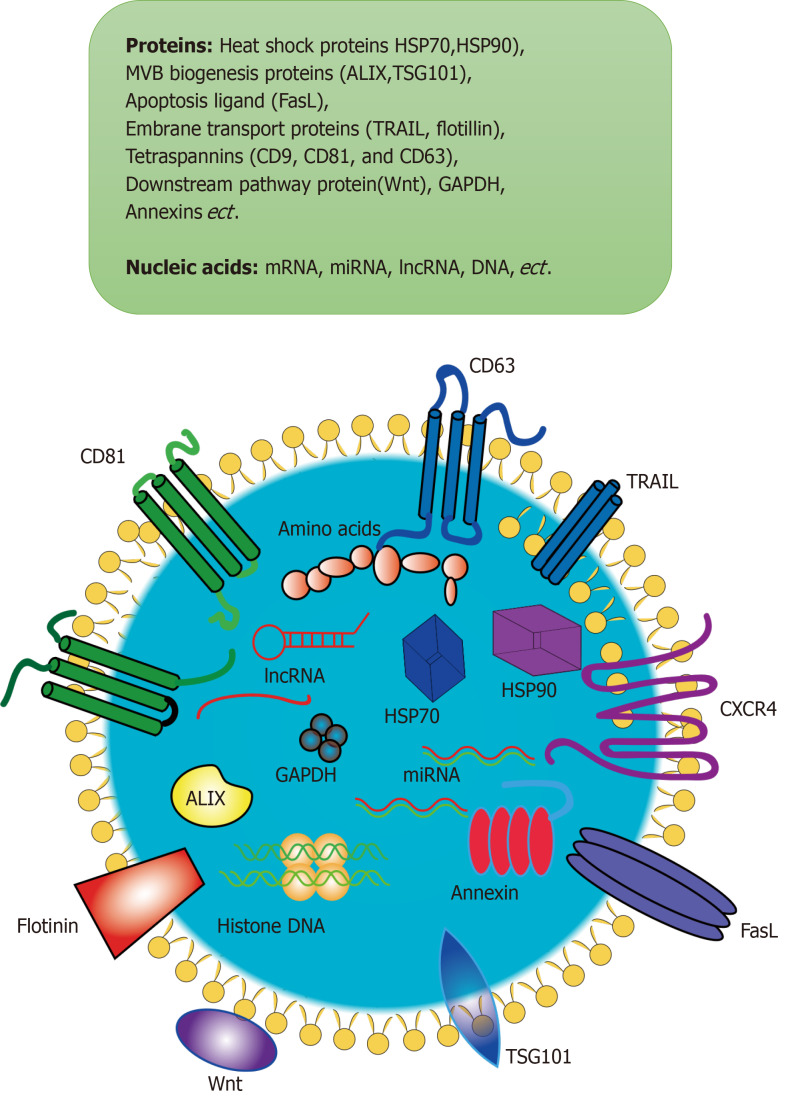
Exosomes are known to be small extracellular vesicles containing proteins, nucleic acids (DNA, miRNA, lncRNA, mRNA, tRNA, and circRNA), and cholesterol. Exosomes have a diameter of about 30-150 nm, and the surface markers are mainly CD63, CD81, CD9, tumor susceptibility gene 101 protein (TSG101), heat shock 70 kDa protein (HSP70), and heat shock 90 kDa protein (HSP90) (Figure 1). Proteomic analysis of exosomes from different sources has been performed many times by researchers[48-51]. About 1600 proteins were found in biofluid-derived exosomes, of which 300 were common in at least two groups, and only two were shared by more than four[52]. After proteomics detection of MSC-exosomes and enrichment analysis of the pathways involved, it was found that proteins in exosomes were mostly related to heparin binding, phospholipid binding, integrin, immune response, and cell adhesion functions[53]. In addition, deep sequencing of miRNAs was also performed to further understand the profiles and expression of miRNAs in human MSC-exosomes. The let-7 family of human embryonic-derived MSCs was found to be widespread, and is associated with the self-renewal characteristics of stem cells[54]. New targets and approaches for the treatment of acute leukemia have also been explored by comparing miRNA differences in exosomes derived from bone marrow MSCs (BM-MSCs) in patients with acute leukemia and exosomes derived from BM-MSCs in normal people. In addition to sequencing miRNAs, the researchers further understood the genes and pathways targeted by miRNAs through network analysis. It was found that genes targeted by miRNAs are mainly related to cardiac regeneration, repair, and angiogenesis, and the pathways involved mainly include Wnt signaling, pro-fibrotic signaling via transforming growth factor-β (TGF-β) stimulation, platelet derived growth factor (PDGF), proliferation, and apoptosis[55].
Figure 1.
Exosomes are small vesicles that are secreted by cells and wrapped in membranes made up of lipid bilayer molecules. Exosomes contain proteins, nucleic acids, and other substances. Their proteins include heat shock proteins, MVB biogenesis proteins, cytoskeleton proteins, apoptosis, ligand, embrane transport proteins and so on. Their nucleic acids include mRNA, miRNA, lncRNA, DNA, and so on. ALIX: ALG-2 interacting protein X; CXCR4: CXC-chemokine receptor 4; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; HSP70: Heat shock 70 kDa protein; TSG101: Tumor susceptibility gene 101 protein.
The research on whether there are other substances in exosomes is still in progress. In the past, research was limited due to the very small size of exosomes, but this gradually improved as science and technology developed further. As mentioned above, Jeppesen et al[46] re-evaluated exosomes by advanced methods and re-determined exosome composition. Exosomes were previously considered to be carriers of extracellular DNA secretion. Studies have shown that extracellular double-stranded DNA does not exist in exosomes and cannot be the target of liquid biopsies in cancer patients. In addition, Annexin A1 was identified as a marker protein for the formation of vesicles in the classic plasma membrane of exosomes[56]. The discovery of new substances in exosomes may provide new ideas for using them as mediators for diagnosis and treatment.
Functions of MSC-exosomes
Exosomes can be secreted by almost any type of cell in the body, and the function of exosomes depends on the cell type from which they originate. For example, exosomes derived from tumor cells can accelerate tumor progression and even influence the function of normal cells at the distal location, promoting the formation of a pre-metastasis microenvironment[57]. Besides, exosomes released by neurons can repair damaged brain cells and play an important role in the development of neurons and neural circuits[58]. The difference is that MSC-exosomes mainly play a therapeutic role, such as regulating immunity[59-61] and promoting repair of tissue damage[62-64], which is basically consistent with the effect of stem cells on human tissues. MSCs have previously been used as a treatment for severe infectious diseases, mainly to neutralize destructive inflammation and promote the repair of damaged organs[65,66]. MSC-exosomes were not used to treat severe infectious diseases before 2020. Significantly, doctors treated severe coronavirus disease 2019 patients with exosomes derived from BM-MSCs during the outbreak. They observed an 83% survival rate, with three of the 24 patients remaining in critical condition and four experiencing non-treatment-related deaths[67]. This indicates that MSC-exosomes also have a good effect on the severe multi-organ damage caused by infectious diseases, suggesting a new application of MSC-exosomes. The effect of MSC-exosomes on tumors has always been controversial, and most literature shows that MSC-exosomes have a tumor promoting effect, namely, promoting tumor angiogenesis, proliferation, and migration[68,69]. In order for MSC-exosomes to inhibit and kill tumors, many scientists have modified exosomes in various ways, including loading overexpression of certain miRNAs[70,71], carrying anti-cancer drugs or suicide genes[72,73], engineering stem cells[74,75], or in combination with other anti-cancer approaches[76,77]. The extensive use of exosomes provides a promising avenue for disease treatment, especially the exosomes derived from MSCs, which are of high safety and can perform functions in place of stem cells. In general, the use of MSC-exosomes in cell-free therapy is helpful for the treatment of many diseases[78]. It regulates inflammation in inflammatory diseases, promotes tissue repair in tissue damage diseases, and kills tumors as an adjuvant tumor therapy.
Thus far, the study of exosomes derived from stem cells has reached the clinical stage[162] (Table 2). However, miRNAs have rarely been involved in these clinical trials, although many studies have been done on miRNAs in MSC-exosomes in animal models. The clinical application of miRNAs in the treatment of exosomes derived from stem cells is a very important research direction in the future. Therefore, in the table, we proposed a miRNA hypothesis that may be associated with MSC-exosome treatment of diseases combined with studies in animal experiments[79-83]. Clinical trials of MSC-exosomes have focused on diseases, such as multi-organ failure, coronavirus pneumonia, and type 1 diabetes. Some clinical studies are not specific to specific diseases, but only to explore the safety of exosome aerosol administration through the airway, which will provide possibilities for diverse exosome administration methods. A clinical study was conducted at Cairo University in Egypt to explore the important role of miR-136, miR-494, and miR-495 in the diagnosis of eclampsia with exosomes derived from umbilical cord mesenchymal stem cells. This study demonstrated the clinically important role of miRNAs in MSC-exosomes, not just in mouse models[84].
Table 2.
Representative clinical trials of mesenchymal stem cell-derived exosomes
| Exosome origin | Diseases | Administration method | Status | miRNAs that may be associated with MSC therapy for this disease |
| Allogenic mesenchymal stromal cells | Cerebrovascular disorders | Intravenous injection | Completed | MiRNA-184, miRNA-210, miR-133b, miR-17-92[81,82,162] |
| Allogenic adipose mesenchymal stem cells | COVID-19 | Aerosol inhalation | PhaseI | Has not been reported |
| Allogenic mesenchymal stromal cells | Multiple organ failure | Intravenous injection | Not yet Recruiting | Has not been reported |
| Human UC-MSCs | Macular holes | Intravitreal injection | PhaseI | Has not been reported |
| Human UC-MSCs | Dry eyes | Eye drops | Phase II | Has not been reported |
| Adipose mesenchymal stem cell | Alzheimer’s disease | Nasal drip | Phase II | MiR-146a-5p[79] |
| Human UC-MSCs | Diabetes mellitus type 1 | Intravenous infusion | Phase III | MiR-1908, miR-203a[80] |
| MSCs | COVID-19 | Inhalation | Phase II | Has not been reported |
| Human UC-MSCs | Chronic ulcer | Applying and closed by transparent dressing | Completed | Has not been reported |
UC-MSCs: Umbilical cord mesenchymal stem cells; MSCs: Mesenchymal stem cells; COVID-19: Corona virus disease 2019.
MICRORNAS
MicroRNA was discovered in 1993 by Victor Ambros and Gary Ruvkun. The first miRNA is lin-4[85,86], which is known as small molecule timing RNA. Although these two articles were published in Cell, they were not taken seriously at that time. Further, miRNAs did not receive much attention over the next 20 years. The launch of the human genome project in 1990 was helpful in the discovery of miRNAs. Finally, in October 2001, Tuschi, Bartel, and Ambro published three papers in Science that named this RNA microRNA (miRNA)[87-89]. MiRNAs are a class of non-coding RNAs with 20-22 base sequences, which can regulate gene expression through post-transcriptional regulation of target mRNA. The seed regions (nucleotide sites 2-8) of miRNA can bind to the mRNA, and then the miRNA can guide the RNA-induced silencing complex (RISC) to reach the binding site, prompting the mRNA to degrade or inhibiting its translation[90]. MiRNAs have been identified by researchers to be involved in many important life processes, including cell proliferation, apoptosis[91], cell death, fat metabolism[92], and cell differentiation[93,94].
Recent advances in miRNAs have greatly promoted both clinical diagnosis and basic research. Wang et al constructed an in vivo miRNA imaging system, consisting of cellular MnO2 nanoscale sponges and autocatalytic DNA enzymes. Since some miRNAs are closely related to tumor formation in vivo, this method is convenient for the diagnosis of tumor etiology and is of great significance for the evaluation of tumor treatment[95,96]. Iwasaki et al[97] found that urine contained miR-6807-5p and miR-6856-5p, which could be used to detect gastric cancer. In addition to the detection of Helicobacter pylori, this method is the second one that can realize the early and non-invasive detection of gastric cancer, which means that this method can be mutually verified with the detection of Helicobacter pylori and improve the accuracy of non-invasive detection. At present, the most common function of miRNAs is to target and negatively regulate mRNA levels of genes and mediate their degradation. However, in 2018, Mihnea Paul Dragomir et al[98] published a snapshot in Cell, which introduced seven other non-classical molecular mechanisms of miRNAs and summarized new ideas for miRNA research for researchers, including: (1) The precursor of miRNA, pri-miRNA, can be translated into peptides; (2) miRNA can bind to other functional proteins and change their functions; (3) miRNA can activate Toll-like receptors; (4) miRNA can upregulate protein expression; (5) miRNA can target and regulate mitochondrial related mRNA to change mitochondrial function; (6) miRNA can activate the gene transcription process; and (7) miRNA can negatively regulate other non-coding RNAs. These can broaden the thinking for researchers studying miRNAs and no longer restrict miRNAs to only regulating 3′-untranslated region (UTR) fragments of target genes.
MiRNAs differ in MSC-exosomes from different sources. Through sequencing, it was found that the miRNAs mainly contained in adipose-derived MSC-exosomes are miR-486-5p, miR-10a-5p, miR-10b-5p, miR-191-5p, and miR-222-3p, while the miRNAs mainly contained in bone marrow-derived MSC-exosomes are miR-143-3p, miR-10b-5p, miR-486-5p, miR-22-3p, and miR-21-5p[99]. MiR-21, miR-23a, miR-125b, and miR-145 are the main miRNAs contained in MSC-exosomes from the umbilical cord[100]. This means that MSC-exosomes may affect different target genes due to different sources when acting through miRNAs, thus treating different diseases.
MSC-EXOSOMES INFLUENCE DISEASES THROUGH MIRNAS
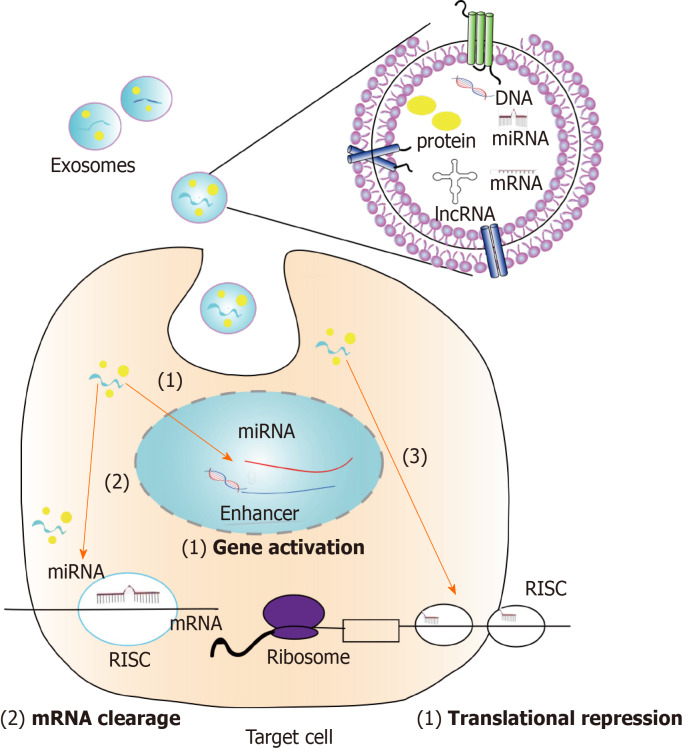
Exosomes secreted by stem cells to the outside of the cell are absorbed together with substances inside the vesicle, including nucleic acids and proteins, and finally play a role in other cells. MiRNA is currently the most studied RNA in exosomes[101]. As for the effect of miRNA on genes, there are usually two aspects: One is that miRNA directly acts on the 3'-UTR of target mRNA to degrade it; the other is that miRNA acts on the translation process of mRNA to repress its translation. Several studies have found that miRNAs can enter the nucleus and act on gene enhancers to activate genes and promote their expression[102,103]. This suggests that we may discover a new mechanism of action for miRNAs in MSC-exosomes to treat diseases in the future. Different types of miRNA target different genes and can affect multiple diseases. Previous studies have found that although both MSCs and extracellular vesicles contain miRNAs, the miRNAs contained by both are not completely equivalent, and only 44 miRNAs are co-expressed. Gene ontology analysis of miRNAs that were highly expressed in MSC-exosomes showed that these miRNAs were mainly related to the growth and development of multiple organs, cell survival, and immune regulation[104]. However, studies regarding the functional aspects of MSC-exosomes are not limited to this, but also include injury repair, treatment of degenerative diseases, and tumor intervention. In the following sections, the role of miRNAs in MSC-exosomes in these types of diseases and the genes or pathways they target will be introduced (Figure 2).
Figure 2.
Exosomes were first isolated from the cultured supernatant of stem cells and injected intravenously into the model. The exosomes then reach the damaged site, are absorbed by the cells, and enter the cell: (1) Exosome-derived microRNA binds to enhancers in the nucleus to promote gene expression; (2) miRNA in exosomes bind to the 3’-UTR of the mRNA for mRNA degradation; and (3) exosomes inhibit mRNA translation. The second and third ways have been studied, and the first way is based on the assumption of existing studies. RISC: RNA-induced silencing complex.
Inflammatory regulation
MSC-exosomes can regulate inflammation through miRNAs and reduce inflammation and anti-fibrosis[164-165] (Table 3). Stem cells come from different sources and have different miRNAs that play a regulatory role in different diseases. It has been found that stem cell-derived exosomes can use miRNAs to correct immune disorders in organ tissues, such as allergic airway, Duchenne muscular dystrophy, and myocardial ischemia-reperfusion injury[105-107]. That article shows that allergic airway miR-146a-5p downregulates the expression of interleukin-9 (IL-9) and interleukin-13 (IL-13) in Group 2 innate lymphoid cells, subsequently regulating allergic diseases[105]. This suggests that miR-146a-5p in MSC-exosomes is associated with allergic diseases, and the role of this miRNA has also been reported in previous studies[108]. In myocardial ischemia-reperfusion injury, the process of ischemia-reperfusion causes a cascade of heart inflammation. Here, the researchers showed that miRNAs in exosomes can influence macrophage transformation from M1 to M2 anti-inflammatory phenotype, thereby regulating immunity. Dil fluorescence staining showed that exosomes could be endoscopically absorbed into the cytoplasm of macrophages, thereby transforming pro-inflammatory M1 into anti-inflammatory M2, accompanied by the reduction of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α)[107]. In addition, miR-233 in MSC-exosomes protects the heart from inflammatory cytokines and reduces stimulation and damage to the heart during sepsis[109].
Table 3.
Representative articles on inflammatory regulation
| Exosome origin | Diseases | MicroRNA | Downstream molecular/pathways | MicroRNA methodology |
| Placenta-derived mesenchymal stromal cells | Duchenne muscular dystrophy | MiR-29c | TGF-β | Reporter gene assays[106] |
| Induced pluripotent stem cells | Group 2 innate lymphoid cell-dominant allergic airway | MiR-146a-5p | T helper 2 (Th2) cytokines | Anion-exchange chromatography; RNA sequencing[105] |
| Mouse BM-MSCs | Peripheral neuropathy in diabetes | MiR-17, miR-23a, and miR-125b | TLR4/NF-κB signaling pathway. | MiRNA array; ultracentrifugation[164] |
| MSCs | Myocardial ischemia-reperfusion injury | MiR-182 | TLR4 pathway | Differential centrifugation; miRNA sequencing[107] |
| Human UC-MSCs | Burn-induced excessive inflammation | MiR-181c | TLR4 pathway | PureExo Column; miRNA array analysis[164] |
| LPS-preconditioned MSCs | Wound healing | Let-7b | TLR4 pathway | Gradient centrifugation; miRNA microarray[111] |
| Human UC-MSCs | Hyperglycemia-induced retinal inflammation | MiR-126 | HMGB1 signaling pathway | Ultracentrifugation[165] |
BM-MSCs: Bone marrow MSCs; UC-MSCs: Umbilical cord mesenchymal stem cells; MSCs: Mesenchymal stem cells; TGF-β: Transforming growth factor-β; TLR4: Toll-like receptor 4; HMGB1: High-mobility group box-1.
Through a review and comparison of these articles regarding miRNAs in MSC-exosomes, we found that miRNAs in exosomes regulate inflammation mainly by regulating macrophages and T cells[107,110,111]. MiRNAs derived from MSC can reduce Toll-like receptor 4 on the surface of macrophages, promote the transformation from M1 to M2, and then secrete various anti-inflammatory factors. In the study of myocardial ischemia-reperfusion injury, miRNA-181a acts on the target gene c-fos, downregulates its expression, and promotes the polarization of Treg cells (regulatory T cells) for immune regulation[110]. Some studies have shown that miRNAs from MSC-exosomes act on diseased tissues to downregulate pro-fibrotic factors (TGF-β) and pro-inflammatory factors (TNF-α, IL-6, and IL-8). However, the exact regulatory mechanisms and target genes have not been fully elucidated, and thus deserve further research.
Injury repair
MiRNAs in MSC-exosomes also play an important role in injury repair (such as heart injury, spinal cord injury, and liver injury)[112-115]. In these diseases, miRNAs achieve injury repair by promoting cell proliferation and differentiation, and reducing necrosis and apoptosis. MiRNAs in exosomes (such as miR-144, miR-21-5p, and miR-19a) play a role in preventing apoptosis after reaching the damaged tissues. The commonly described pathway indicates that miRNA downregulates PTEN, which activates the Akt signaling pathway, then decreases the expression of apoptotic proteins (caspase 3, caspase 8, caspase 9, etc.) and the number of apoptotic cells[113,116,117]. Similarly, studies involving vaginal epithelial cells (VK2) have found that miRNAs in MSC-exosomes can promote VK2 cell proliferation and inhibit apoptosis by promoting cell cycle progression. Interestingly, some researchers have also found that miR-100-5p in MSC-exosomes can enhance autophagy in osteoarthritis by inhibiting the target gene mTOR, thus promoting bone growth and bone reconstruction. Collectively, these studies suggest that miRNAs in exosomes may exert a protective effect on cells in vital organs, such as the brain, heart, and lungs, after severe stress in the human body.
Recent studies on miRNAs in MSC-exosomes have found that an miRNA called miR-200a-3p can protect mitochondria through the KEAP1-Nrf2 signaling pathway in renal ischemia-reperfusion injury to promote the recovery of renal function[118]. The localization of miRNAs in MSC-exosomes in intracellular specific organelles will promote the understanding of the role of miRNAs and accelerate the practical promotion of the application of stem cell-derived exosomes. Currently known miRNAs function in the cytoplasm, mitochondria, and nucleus, and whether miRNAs in MSC-exosomes play a role in other organelles deserves further exploration.
Tumor progression
Tumor treatment with MSC-exosomes is controversial, with some studies reporting that MSC-exosomes can promote tumor development, while others believe that they can inhibit tumors[166] (Table 4). A study regarding osteosarcoma indicated that miR-208a in MSC-exosomes promoted tumor progression by downregulating programmed cell death 4 (PDCD4) and activating the ERK1/2 pathway[119]. In addition, miR-142-3p and miR-146a from MSC-exosomes have been proved to promote tumor growth through various pathways[120,121]. In the osteosarcoma study, it is worth noting that MSCs are derived from bone marrow, and the role of stem cells in tumor promotion may be due to similar origins. Similarly, BM-MSCs can also promote the progression of multiple myeloma through miR-146a, further confirming this hypothesis[121]. In these tumor-promoting processes, these miRNAs without exception promote the proliferation, migration, and invasion of tumors, but in other studies of MSC-exosomes, it also promotes angiogenesis. However, the role of miRNAs in promoting angiogenesis in tumors has not been well verified[122].
Table 4.
Representative studies in which MSC-derived exosomes affect tumors through miRNAs
| Exosome origin | Disease | MiRNA | Downstream molecular/pathway(s) | Outcome |
| BM-MSCs | Osteosarcoma | MiR-208a | Downregulation of PDCD4 and activation of the ERK1/2 pathway | Promoting tumor progression[119] |
| BM-MSCs | Multiple myeloma | MiR-146a | The Notch pathway | Promoting tumor progression[121] |
| BM-MSCs | Colon cancer | MiR-142-3p | Downregulation of Numb | Promoting tumor progression |
| BM-MSCs | Breast cancer | MiR-23b | Decreased MARCKS expression | Inhibiting tumor progression[128] |
| MiR-122-transfected AMSCs | HCC | MiR-122 | without research | Inhibiting tumor progression[129] |
| BM-MSCs | Prostate cancer | MiR-143 | TFF3 | Inhibiting tumor progression[70] |
| MSCs | Breast cancer | MiR-100 | VEGF | Inhibiting tumor progression[166] |
BM-MSCs: Bone marrow-derived mesenchymal stem cells; AMSCs: Adipose-derived mesenchymal stem cells; MSCs: Mesenchymal stem cells; HCC: Hepatocellular carcinoma; TFF3: Trefoil factor 3; VEGF: Vascular endothelial growth factor.
Currently, miRNAs in MSC-exosomes have been mainly studied for tumor inhibition in tumor studies. In MSC-exosomes, an miRNA called miR-146a is a widely published tumor suppressor, which plays a significant role in tumor inhibition in multiple myeloma[123-127]. MiR-23b in MSC-exosomes can inhibit tumor proliferation, maintain a dormant tumor state, improve life quality, and prolong the life of patients[128]. In a study of hepatocellular carcinoma, MSC-exosomes did not directly contain anti-tumor miRNAs, but transfected miR-122, which can enhance drug sensitivity, into stem cells through plasmids, and secreted into tumor cells through exosomes to enhance the sensitivity of anti-tumor drug sorafenib[129]. In addition to improving chemical sensitivity, studies have shown that miR-34c in MSC can improve tumor sensitivity to radiotherapy[77]. This suggests that MSC-exosomes can be combined with other therapies, such as chemotherapy drugs and radiotherapy, when treating tumors. Since stem-cell derived exosomes have been reported to promote tumors, it should be safer to overexpress certain known anticancer miRNAs with stem cells or to secrete anticancer drugs with exosomes. Previous studies, for example, have shown that paclitaxel is added to MSCs, then the cells target tumor tissue and release paclitaxel[130].
Other diseases
MSC-exosomes have been shown to play the same role as MSCs in many diseases. This indicates that the diseases that can be treated with MSCs can be further studied with exosomes from their sources, because exosomes themselves have advantages that stem cells do not, such as easy storage and higher safety. In some studies, researchers have found that Alzheimer's disease can be improved through stem cell therapy, and miR-146a has been proved to play an important role in this. It is also worth further exploring whether exosomes from this stem cell source contain this miRNA and whether exosomes can replace stem cells to affect brain degenerative diseases[131]. One of the first diseases treated with stem cells is graft-versus-host disease (GVHD)[132]. In recent years, studies have found that GVHD can be improved with the extracellular vesicles derived from BM-MSCs through miRNAs, which is related to the preservation of naive T cells[133]. Exosomes are the most famous subgroup of extracellular vesicles. Extracellular vesicles display functions of exosomes, which have been studied further due to their extensive functions[134]. In addition to degenerative diseases, MSCs reportedly play an important role in organ fibrosis, including liver fibrosis[135], pulmonary fibrosis, and myocardial fibrosis. MSCs are involved in fibrosis, and can reverse fibrosis and restore the original functions of damaged organs and tissues[136-138]. Regarding MSC-exosomes, which play a role in disease, the miRNAs that they contain function in a therapeutic capacity in various diseases. More critically, MSC-exosome transplantation into the body and prolonging the efficacy of this treatment require further research, as well as investigating specificity with regard to disease sites.
METHODS TO STUDY ASSOCIATION BETWEEN EXOSOMES AND MIRNAS
When exploring the distribution and function of miRNAs in exosomes, different researchers often choose different research methods according to their own conditions. The following will introduce the common methods used to study specific miRNAs in exosomes, so that researchers could choose the best methods for future work. Methods to study the association between exosomes and miRNAs mainly include small RNA sequence, RNA microarray, nanostring miRNA assay, and database bioinformatics analysis to find appropriate miRNAs in exosomes. The first and most common method is high-throughput sequencing, or next generation sequencing. The process of small RNA sequencing is to extract exosomes from the cell supernatant or the plasma and then isolate miRNAs for next generation sequencing. Thereafter, target genes and related functions of miRNAs can be determined by combining with bioinformatics analysis. Perhaps a new and undiscovered miRNA can even be obtained for further functional analysis[54,55,99,139]. Microarray is also a good high-throughput method to understand the miRNAs contained within exosomes[133,140,141]. The disadvantage of microarray is that the sequence must be known, and probes are primarily designed for miRNA transcripts, hybridizing the probes with reverse-transcribed cDNA. After obtaining data using these two methods, qPCR, Northern blot, and miRNA-FISH techniques must be used for verification. Moreover, the nanostring miRNA assay could be used to analyze the content and species of miRNAs in exosomes, with accurate experimental results, but it is not widely used and the study cost is high[142-144]. In addition, some researchers studied MSC-exosome biology not through high-throughput detection, but through databases, including miRBase (http://www.mirbase.org/), TargetScan (http://www.targetscan.org/vert_71/), and MiRDB (http://www.mirdb.org/miRDB/policy.html), to find a suitable miRNA, then apply qPCR, Western blot, or luciferase reporter gene assays for validations. These methods combined with KEGG analysis or GO analysis can also reveal the downstream pathway corresponding to miRNAs, which can further expand the research.
CONCLUSION AND PROSPECTION
The discovery that MSC-exosomes can perform a similar function to that of stem cells has led to a growing interest in the use of cell-free therapies for stem cells[78,145]. Exosomes are less tumorigenic and immunogenic than stem cells, because they are very small and do not self-replicate. Exosomes have the unique advantages of being safer, more direct, and more effective than stem cells. This means that exosomes have a wide range of applications and can be mass-produced into drugs with excellent therapeutic effects for many diseases[146]. As more and more studies have been conducted on MSC-exosomes, it has been found that in many cases, miRNAs play a crucial role in the therapeutic effect of MSC-exosomes on diseases. MiRNAs in MSC-exosomes have been reported to have significant effects on inflammatory diseases, trauma, degenerative diseases, and tumors. However, the miRNAs and their downstream pathway/target genes differ amongst different diseases. Some researchers sequenced miRNAs in MSC-exosomes, performed network analysis, and found that these were associated with Wnt signaling, TGF-β and PDGF stimulation, proliferation, and apoptosis, and function to oppose fibrosis and apoptosis, while promoting regeneration[55]. This also provides scope and ideas for subsequent research into miRNAs in MSC-exosomes. It is well known that MSC-exosomes can treat diseases, but if we want to further understand the mechanism behind disease treatment, we also need to know which components in exosomes play a crucial role. Therefore, researchers highlight specific miRNAs in their studies that regulate inflammation, promote repair, and target tumors[147]. Of course, once we understand which miRNA is at work, researchers can overexpress specific miRNA in cell culture and exosome collection to achieve targeted therapy of refractory diseases.
The use of MSC and MSC-exosomes for tumor treatment has always been controversial. Due to the strong proliferation and regeneration capacity of stem cells and the presence of tumor stem cells, it has always been a controversial topic whether stem cells can promote tumors or not. However, loading anticancer drugs or suicide genes from exosomes or overexpression of anticancer miRNAs is a safer approach[72]. The combination of MSC-exosomes with other anticancer methods presents great potential for cancer treatment[148].
Currently, the common source of exosomes is BM-MSCs, but BM-MSCs faces problems of invasive injury to volunteers (patients) and rapid aging after in vitro amplification, making itself an imperfect source for mass production of exosomes[105]. Although MSC-exosomes from the umbilical cord are abundant and have proven therapeutic effects on multiple liver fibrosis, brain injury and other diseases, they are difficult to obtain due to low availability of sources[19,149]. In contrast, human menstrual blood-derived stem cells have the advantages of easy access, strong proliferation capacity, and no ethical controversy, and can be used as a good source of exosomes[150-154].
In the future, the research trend of miRNAs lies in competing endogenous RNAs[149], which are a new regulation model of gene expression. Currently, it is known that miRNAs can cause gene silencing by binding mRNA, while ceRNAs (lncRNAs and circRNAs) can regulate gene expression through competitive binding of miRNAd, thereby affecting cell function[155-157]. Not only miRNAs, but lncRNAs and circRNAs are found in MSC-exosomes. If the relationship among miRNAs, mRNAs, lncRNAs, and circRNAs in exosomes derived from MSCs can be explored, the mechanism of MSC-exosomes in the treatment of diseases will be more thoroughly explored and exosomes can be rapidly applied to the human body.
ACKNOWLEDGEMENTS
The authors thank Di-Jun Xu from the College of Foreign languages of East China Jiaotong University for assistance with English language editing.
Footnotes
Conflict-of-interest statement: The authors declare no competing financial interests.
Manuscript source: Unsolicited manuscript
Peer-review started: June 21, 2020
First decision: August 9, 2020
Article in press: September 18, 2020
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Biondi A, Tanaka Y S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Xing YX
Contributor Information
Hui-Kang Xu, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Li-Jun Chen, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Si-Ning Zhou, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Yi-Fei Li, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Charlie Xiang, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China. cxiang@zju.edu.cn.
References
- 1.Li H, Yue B. Effects of various antimicrobial agents on multi-directional differentiation potential of bone marrow-derived mesenchymal stem cells. World J Stem Cells. 2019;11:322–336. doi: 10.4252/wjsc.v11.i6.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Qu J, Cheng T, Chen X, Xiang C. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. 2019;10:406. doi: 10.1186/s13287-019-1503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Blanc K, Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36–45. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, Li J, Zhang G, Huang J, Lin Z, Xiong N, Wang T. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016;2016:7653489. doi: 10.1155/2016/7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Yin S, Ji C, Wu P, Jin C, Qian H. Human umbilical cord mesenchymal stem cells and exosomes: bioactive ways of tissue injury repair. Am J Transl Res. 2019;11:1230–1240. [PMC free article] [PubMed] [Google Scholar]
- 9.Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR, Lay PA, Sue CM, Cooper AA. Parkinson's disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23:2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- 10.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11:600–7.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, Brigstock DR. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014;156:548–555. doi: 10.1016/j.surg.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB. Correction for Devhare et al., "Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells". J Virol. 2017:91. doi: 10.1128/JVI.02225-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, Hirsova P, Cao S, Mukhopadhyay D, Huebert RC, Shah VH. Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration. J Biol Chem. 2015;290:30684–30696. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano. 2018;12:7613–7628. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- 15.Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119:9433–9443. doi: 10.1002/jcb.27260. [DOI] [PubMed] [Google Scholar]
- 16.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 17.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rébé C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem Cells Int. 2015;2015:761643. doi: 10.1155/2015/761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, Isenalumhe LL, Greco SJ, Ayer S, Bryan M, Kumar R, Ponzio NM, Rameshwar P. Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res. 2016;76:5832–5844. doi: 10.1158/0008-5472.CAN-16-1092. [DOI] [PubMed] [Google Scholar]
- 21.Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, Xiao P, Meng Q, Geng YJ, Yu XY, Li Y. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed Res Int. 2017;2017:4150705. doi: 10.1155/2017/4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 24.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 26.Cazzoli R, Buttitta F, Di Nicola M, Malatesta S, Marchetti A, Rom WN, Pass HI. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J Thorac Oncol. 2013;8:1156–1162. doi: 10.1097/JTO.0b013e318299ac32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, Goetz JG, Labouesse M. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211:27–37. doi: 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casado S, Lobo MDVT, Paíno CL. Dynamics of plasma membrane surface related to the release of extracellular vesicles by mesenchymal stem cells in culture. Sci Rep. 2017;7:6767. doi: 10.1038/s41598-017-07265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo BB, Bellingham SA, Hill AF. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem. 2015;290:3455–3467. doi: 10.1074/jbc.M114.605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-'t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013:2. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–1900. doi: 10.1039/c4lc00136b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Wu HJ, Fine D, Schmulen J, Hu Y, Godin B, Zhang JX, Liu X. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13:2879–2882. doi: 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y, Wei J, Hu G, Nie G, Sun J. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano. 2017;11:6968–6976. doi: 10.1021/acsnano.7b02277. [DOI] [PubMed] [Google Scholar]
- 39.Pang B, Zhu Y, Ni J, Thompson J, Malouf D, Bucci J, Graham P, Li Y. Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics. 2020;10:2309–2326. doi: 10.7150/thno.39486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 41.Manda SV, Kataria Y, Tatireddy BR, Ramakrishnan B, Ratnam BG, Lath R, Ranjan A, Ray A. Exosomes as a biomarker platform for detecting epidermal growth factor receptor-positive high-grade gliomas. J Neurosurg. 2018;128:1091–1101. doi: 10.3171/2016.11.JNS161187. [DOI] [PubMed] [Google Scholar]
- 42.Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatischeff I, Larquet E, Falcón-Pérez JM, Turpin PY, Kruglik SG. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J Extracell Vesicles. 2012:1. doi: 10.3402/jev.v1i0.19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montoro Bustos AR, Purushotham KP, Possolo A, Farkas N, Vladár AE, Murphy KE, Winchester MR. Validation of Single Particle ICP-MS for Routine Measurements of Nanoparticle Size and Number Size Distribution. Anal Chem. 2018;90:14376–14386. doi: 10.1021/acs.analchem.8b03871. [DOI] [PubMed] [Google Scholar]
- 45.Sitar S, Kejžar A, Pahovnik D, Kogej K, Tušek-Žnidarič M, Lenassi M, Žagar E. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal Chem. 2015;87:9225–9233. doi: 10.1021/acs.analchem.5b01636. [DOI] [PubMed] [Google Scholar]
- 46.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of Exosome Composition. Cell. 2019;177:428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhães A, Ferreira JA, Osório H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y, Huo C, Qiao Z, Shang Z, Uzzaman A, Liu S, Jiang X, Fan LY, Ji L, Guan X, Cao CX, Xiao H. Comparative Proteomic Analysis of Exosomes and Microvesicles in Human Saliva for Lung Cancer. J Proteome Res. 2018;17:1101–1107. doi: 10.1021/acs.jproteome.7b00770. [DOI] [PubMed] [Google Scholar]
- 49.Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–3364. [Google Scholar]
- 50.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dang VD, Jella KK, Ragheb RRT, Denslow ND, Alli AA. Lipidomic and proteomic analysis of exosomes from mouse cortical collecting duct cells. FASEB J. 2017;31:5399–5408. doi: 10.1096/fj.201700417R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koh W, Sheng CT, Tan B, Lee QY, Kuznetsov V, Kiang LS, Tanavde V. Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMC Genomics. 2010;11 Suppl 1:S6. doi: 10.1186/1471-2164-11-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8:1419. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pluchino S, Smith JA. Explicating Exosomes: Reclassifying the Rising Stars of Intercellular Communication. Cell. 2019;177:225–227. doi: 10.1016/j.cell.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz-López L, Blancas I, Garrido JM, Mut-Salud N, Moya-Jódar M, Osuna A, Rodríguez-Serrano F. The role of exosomes on colorectal cancer: A review. J Gastroenterol Hepatol. 2018;33:792–799. doi: 10.1111/jgh.14049. [DOI] [PubMed] [Google Scholar]
- 58.Sharma P, Mesci P, Carromeu C, McClatchy DR, Schiapparelli L, Yates JR, 3rd, Muotri AR, Cline HT. Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci USA. 2019;116:16086–16094. doi: 10.1073/pnas.1902513116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heo JS, Choi Y, Kim HO. Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int. 2019;2019:7921760. doi: 10.1155/2019/7921760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Negi N, Griffin MD. Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells. 2020;38:596–605. doi: 10.1002/stem.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019:8. doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, Zhao C, Li H, Li YM, Zhao J. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22:261–276. doi: 10.1111/jcmm.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ouyang X, Han X, Chen Z, Fang J, Huang X, Wei H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther. 2018;9:246. doi: 10.1186/s13287-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–2422. doi: 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Fu J, Xu X, Wang S, Xu R, Zhao M, Nie W, Wang X, Zhang J, Li T, Su L, Wang FS. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS. 2013;27:1283–1293. doi: 10.1097/QAD.0b013e32835fab77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S, Su X, Xu M, Xiao X, Li X, Li H, Keating A, Zhao RC. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther. 2019;10:117. doi: 10.1186/s13287-019-1220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavon LF, Sibov TT, de Souza AV, da Cruz EF, Malheiros SMF, Cabral FR, de Souza JG, Boufleur P, de Oliveira DM, de Toledo SRC, Marti LC, Malheiros JM, Paiva FF, Tannús A, de Oliveira SM, Chudzinski-Tavassi AM, de Paiva Neto MA, Cavalheiro S. Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res Ther. 2018;9:310. doi: 10.1186/s13287-018-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Che Y, Shi X, Shi Y, Jiang X, Ai Q, Shi Y, Gong F, Jiang W. Exosomes Derived from miR-143-Overexpressing MSCs Inhibit Cell Migration and Invasion in Human Prostate Cancer by Downregulating TFF3. Mol Ther Nucleic Acids. 2019;18:232–244. doi: 10.1016/j.omtn.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Lou G, Chen L, Xia C, Wang W, Qi J, Li A, Zhao L, Chen Z, Zheng M, Liu Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39:4. doi: 10.1186/s13046-019-1512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pastorakova A, Jakubechova J, Altanerova U, Altaner C. Suicide Gene Therapy Mediated with Exosomes Produced by Mesenchymal Stem/Stromal Cells Stably Transduced with HSV Thymidine Kinase. Cancers (Basel) 2020:12. doi: 10.3390/cancers12051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melzer C, Rehn V, Yang Y, Bähre H, von der Ohe J, Hass R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers (Basel) 2019:11. doi: 10.3390/cancers11060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shamili FH, Bayegi HR, Salmasi Z, Sadri K, Mahmoudi M, Kalantari M, Ramezani M, Abnous K. Corrigendum to "Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model" [International Journal of Pharmaceutics 549 (2018) 218-229] Int J Pharm. 2019;558:441. doi: 10.1016/j.ijpharm.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 75.Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7:1522236. doi: 10.1080/20013078.2018.1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Araujo Farias V, O'Valle F, Serrano-Saenz S, Anderson P, Andrés E, López-Peñalver J, Tovar I, Nieto A, Santos A, Martín F, Expósito J, Oliver FJ, de Almodóvar JMR. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol Cancer. 2018;17:122. doi: 10.1186/s12943-018-0867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wan FZ, Chen KH, Sun YC, Chen XC, Liang RB, Chen L, Zhu XD. Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma. J Transl Med. 2020;18:12. doi: 10.1186/s12967-019-02203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 79.Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, Ren W, Guo H, Zhang L, Wang H, Chen Z, Guo AY, Li Q. Microvesicles as Potential Biomarkers for the Identification of Senescence in Human Mesenchymal Stem Cells. Theranostics. 2017;7:2673–2689. doi: 10.7150/thno.18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen YS, Kang XR, Zhou ZH, Yang J, Xin Q, Ying CT, Zhang YP, Tao J. MiR-1908/EXO1 and MiR-203a/FOS, regulated by scd1, are associated with fracture risk and bone health in postmenopausal diabetic women. Aging (Albany NY) 2020;12:9549–9584. doi: 10.18632/aging.103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen H, Yao X, Li H, Li X, Zhang T, Sun Q, Ji C, Chen G. Role of Exosomes Derived from miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J Mol Neurosci. 2018;64:421–430. doi: 10.1007/s12031-018-1041-2. [DOI] [PubMed] [Google Scholar]
- 82.Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke. 2017;48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones Buie JN, Zhou Y, Goodwin AJ, Cook JA, Vournakis J, Demcheva M, Broome AM, Dixit S, Halushka PV, Fan H. Application of Deacetylated Poly-N-Acetyl Glucosamine Nanoparticles for the Delivery of miR-126 for the Treatment of Cecal Ligation and Puncture-Induced Sepsis. Inflammation. 2019;42:170–184. doi: 10.1007/s10753-018-0882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Motawi TMK, Sabry D, Maurice NW, Rizk SM. Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch Biochem Biophys. 2018;659:13–21. doi: 10.1016/j.abb.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 85.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 86.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 87.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 88.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 89.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 90.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 92.Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790–795. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 93.Kawasaki H, Taira K. Hes1 is a target of microRNA-23 during retinoic-acid-induced neuronal differentiation of NT2 cells. Nature. 2003;423:838–842. doi: 10.1038/nature01730. [DOI] [PubMed] [Google Scholar]
- 94.Kawasaki H, Taira K. Retraction: Hes1 is a target of microRNA-23 during retinoic-acid-induced neuronal differentiation of NT2 cells. Nature. 2003;426:100. doi: 10.1038/nature02141. [DOI] [PubMed] [Google Scholar]
- 95.Wei J, Wang H, Gong X, Wang Q, Wang H, Zhou Y, Wang F. A proteinase-free DNA replication machinery for in vitro and in vivo amplified MicroRNA imaging. Nucleic Acids Res. 2020;48:e60. doi: 10.1093/nar/gkaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H, Yahaya BH, Ng WH, Yusoff NM, Lin J. Conditioned Medium of Human Menstrual Blood-Derived Endometrial Stem Cells Protects Against MPP+-Induced Cytotoxicity in vitro. Front Mol Neurosci. 2019;12:80. doi: 10.3389/fnmol.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iwasaki H, Shimura T, Yamada T, Okuda Y, Natsume M, Kitagawa M, Horike SI, Kataoka H. A novel urinary microRNA biomarker panel for detecting gastric cancer. J Gastroenterol. 2019;54:1061–1069. doi: 10.1007/s00535-019-01601-w. [DOI] [PubMed] [Google Scholar]
- 98.Dragomir MP, Knutsen E, Calin GA. SnapShot: Unconventional miRNA Functions. Cell. 2018;174:1038–1038.e1. doi: 10.1016/j.cell.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 99.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, Liu H, Xing X. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl Med. 2016;5:1425–1439. doi: 10.5966/sctm.2015-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiao M, Li J, Li W, Wang Y, Wu F, Xi Y, Zhang L, Ding C, Luo H, Li Y, Peng L, Zhao L, Peng S, Xiao Y, Dong S, Cao J, Yu W. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017;14:1326–1334. doi: 10.1080/15476286.2015.1112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, Peng YQ, Xu ZB, Fan XL, Wu ZJ, Chen D, Zheng L, Zheng SG, Fu QL. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles. 2020;9:1723260. doi: 10.1080/20013078.2020.1723260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bier A, Berenstein P, Kronfeld N, Morgoulis D, Ziv-Av A, Goldstein H, Kazimirsky G, Cazacu S, Meir R, Popovtzer R, Dori A, Brodie C. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials. 2018;174:67–78. doi: 10.1016/j.biomaterials.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 107.Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lyu B, Wei Z, Jiang L, Ma C, Yang G, Han S. MicroRNA-146a negatively regulates IL-33 in activated group 2 innate lymphoid cells by inhibiting IRAK1 and TRAF6. Genes Immun. 2020;21:37–44. doi: 10.1038/s41435-019-0084-x. [DOI] [PubMed] [Google Scholar]
- 109.Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B, Fan GC. Exosomal miR-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci Rep. 2015;5:13721. doi: 10.1038/srep13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z, Dai Q, Kang L, Xu B. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019;232:116632. doi: 10.1016/j.lfs.2019.116632. [DOI] [PubMed] [Google Scholar]
- 111.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ji W, Jiang W, Li M, Li J, Li Z. miR-21 deficiency contributes to the impaired protective effects of obese rat mesenchymal stem cell-derived exosomes against spinal cord injury. Biochimie. 2019;167:171–178. doi: 10.1016/j.biochi.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D, Wang J. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:36. doi: 10.1186/s13287-020-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, Chen M, Zhang B, Zhou Y, Yao R, Shi Y, Bu H. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11:37. doi: 10.1186/s13287-020-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. doi: 10.1186/s12974-020-1726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 117.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349–360. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cao H, Cheng Y, Gao H, Zhuang J, Zhang W, Bian Q, Wang F, Du Y, Li Z, Kong D, Ding D, Wang Y. In Vivo Tracking of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improving Mitochondria! Function in Renal Ischemia- Reperfusion Injury. ACS Nano. 2020;14:4014–4026. doi: 10.1021/acsnano.9b08207. [DOI] [PubMed] [Google Scholar]
- 119.Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235:4734–4745. doi: 10.1002/jcp.29351. [DOI] [PubMed] [Google Scholar]
- 120.Li H, Li F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br J Cancer. 2018;119:744–755. doi: 10.1038/s41416-018-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Veirman K, Wang J, Xu S, Leleu X, Himpe E, Maes K, De Bruyne E, Van Valckenborgh E, Vanderkerken K, Menu E, Van Riet I. Induction of miR-146a by multiple myeloma cells in mesenchymal stromal cells stimulates their pro-tumoral activity. Cancer Lett. 2016;377:17–24. doi: 10.1016/j.canlet.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 122.Liang B, Liang JM, Ding JN, Xu J, Xu JG, Chai YM. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10:335. doi: 10.1186/s13287-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gopalan V, Ebrahimi F, Islam F, Vider J, Qallandar OB, Pillai S, Lu CT, Lam AK. Tumour suppressor properties of miR-15a and its regulatory effects on BCL2 and SOX2 proteins in colorectal carcinomas. Exp Cell Res. 2018;370:245–253. doi: 10.1016/j.yexcr.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 124.Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356:568–578. doi: 10.1016/j.canlet.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 125.Youness RA, Hafez HM, Khallaf E, Assal RA, Abdel Motaal A, Gad MZ. The long noncoding RNA sONE represses triple-negative breast cancer aggressiveness through inducing the expression of miR-34a, miR-15a, miR-16, and let-7a. J Cell Physiol. 2019;234:20286–20297. doi: 10.1002/jcp.28629. [DOI] [PubMed] [Google Scholar]
- 126.Kang W, Tong JH, Lung RW, Dong Y, Zhao J, Liang Q, Zhang L, Pan Y, Yang W, Pang JC, Cheng AS, Yu J, To KF. Targeting of YAP1 by microRNA-15a and microRNA-16-1 exerts tumor suppressor function in gastric adenocarcinoma. Mol Cancer. 2015;14:52. doi: 10.1186/s12943-015-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, Anderson KC, Scadden DT, Ghobrial IM. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 129.Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 131.Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N, Fujimiya M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer's disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10:10772. doi: 10.1038/s41598-020-67460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 133.Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, Tahara H, Takaori-Kondo A, Ichinohe T, Maekawa T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells. 2018;36:434–445. doi: 10.1002/stem.2759. [DOI] [PubMed] [Google Scholar]
- 134.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsuchiya A, Takeuchi S, Watanabe T, Yoshida T, Nojiri S, Ogawa M, Terai S. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as "conducting cells" for improvement of liver fibrosis and regeneration. Inflamm Regen. 2019;39:18. doi: 10.1186/s41232-019-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin L, Zhang J, Deng Z, Liu J, Han W, Chen G, Si Y, Ye P. Mesenchymal stem cells ameliorate myocardial fibrosis in diabetic cardiomyopathy via the secretion of prostaglandin E2. Stem Cell Res Ther. 2020;11:122. doi: 10.1186/s13287-020-01633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mazhari S, Gitiara A, Baghaei K, Hatami B, Rad RE, Asadirad A, Joharchi K, Tokhanbigli S, Hashemi SM, Łos MJ, Aghdaei HA, Zali MR, Ghavami S. Therapeutic potential of bone marrow-derived mesenchymal stem cells and imatinib in a rat model of liver fibrosis. Eur J Pharmacol. 2020;882:173263. doi: 10.1016/j.ejphar.2020.173263. [DOI] [PubMed] [Google Scholar]
- 138.Felix RG, Bovolato ALC, Cotrim OS, Leão PDS, Batah SS, Golim MA, Velosa AP, Teodoro W, Martins V, Cruz FF, Deffune E, Fabro AT, Capelozzi VL. Adipose-derived stem cells and adipose-derived stem cell-conditioned medium modulate in situ imbalance between collagen I- and collagen V-mediated IL-17 immune response recovering bleomycin pulmonary fibrosis. Histol Histopathol. 2020;35:289–301. doi: 10.14670/HH-18-152. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, Goldberg LR, Baird GL, Ventetuolo CE, Quesenberry PJ, Klinger JR. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li W, Han Y, Zhao Z, Ji X, Wang X, Jin J, Wang Q, Guo X, Cheng Z, Lu M, Wang G, Wang Y, Liu H. Oral mucosal mesenchymal stem cell‑derived exosomes: A potential therapeutic target in oral premalignant lesions. Int J Oncol. 2019;54:1567–1578. doi: 10.3892/ijo.2019.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhagirath D, Yang TL, Bucay N, Sekhon K, Majid S, Shahryari V, Dahiya R, Tanaka Y, Saini S. microRNA-1246 Is an Exosomal Biomarker for Aggressive Prostate Cancer. Cancer Res. 2018;78:1833–1844. doi: 10.1158/0008-5472.CAN-17-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Armstrong DA, Green BB, Seigne JD, Schned AR, Marsit CJ. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer. 2015;14:194. doi: 10.1186/s12943-015-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gheinani AH, Vögeli M, Baumgartner U, Vassella E, Draeger A, Burkhard FC, Monastyrskaya K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci Rep. 2018;8:3945. doi: 10.1038/s41598-018-22142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 146.Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol Med. 2018;24:242–256. doi: 10.1016/j.molmed.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 147.Barbagallo C, Brex D, Caponnetto A, Cirnigliaro M, Scalia M, Magnano A, Caltabiano R, Barbagallo D, Biondi A, Cappellani A, Basile F, Di Pietro C, Purrello M, Ragusa M. LncRNA UCA1, Upregulated in CRC Biopsies and Downregulated in Serum Exosomes, Controls mRNA Expression by RNA-RNA Interactions. Mol Ther Nucleic Acids. 2018;12:229–241. doi: 10.1016/j.omtn.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guo Y, Zhang Z, Xu X, Xu Z, Wang S, Huang D, Li Y, Mou X, Liu F, Xiang C. Menstrual Blood-Derived Stem Cells as Delivery Vehicles for Oncolytic Adenovirus Virotherapy for Colorectal Cancer. Stem Cells Dev. 2019;28:882–896. doi: 10.1089/scd.2018.0222. [DOI] [PubMed] [Google Scholar]
- 149.Thomi G, Joerger-Messerli M, Haesler V, Muri L, Surbek D, Schoeberlein A. Intranasally Administered Exosomes from Umbilical Cord Stem Cells Have Preventive Neuroprotective Effects and Contribute to Functional Recovery after Perinatal Brain Injury. Cells. 2019:8. doi: 10.3390/cells8080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu Y, Chen X, Zhao Y, Wang Y, Li Y, Xiang C. Genome-wide DNA methylation and hydroxymethylation analysis reveal human menstrual blood-derived stem cells inhibit hepatocellular carcinoma growth through oncogenic pathway suppression via regulating 5-hmC in enhancer elements. Stem Cell Res Ther. 2019;10:151. doi: 10.1186/s13287-019-1243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, Ni C, Li Q, Xiang C, Zhang L, Wu R, Zhu W, Yu H, Hu X, Wang J. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl Med. 2017;6:209–222. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, Guo Y, Mou X, Yuan L, Chen B, Wang J, Xiang C. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl Med. 2017;6:272–284. doi: 10.5966/sctm.2015-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. doi: 10.1186/s12967-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song YX, Sun JX, Zhao JH, Yang YC, Shi JX, Wu ZH, Chen XW, Gao P, Miao ZF, Wang ZN. Non-coding RNAs participate in the regulatory network of CLDN4 via ceRNA mediated miRNA evasion. Nat Commun. 2017;8:289. doi: 10.1038/s41467-017-00304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J. 2017;12 doi: 10.1002/biot.201600699. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, Liao WT, Ding YQ, Liang L. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18:91. doi: 10.1186/s12943-019-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cao F, Gao Y, Chu Q, Wu Q, Zhao L, Lan T, Zhao L. Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis. 2019;40:3092–3098. doi: 10.1002/elps.201900295. [DOI] [PubMed] [Google Scholar]
- 162.Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP, Cha JM, Bang OY. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke: Biodistribution and MicroRNA Study. Transl Stroke Res. 2019;10:509–521. doi: 10.1007/s12975-018-0668-1. [DOI] [PubMed] [Google Scholar]
- 163.Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, Chopp M, Zhang ZG, Liu XS. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63:431–443. doi: 10.1007/s00125-019-05043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang W, Wang Y, Kong Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60:294–303. doi: 10.1167/iovs.18-25617. [DOI] [PubMed] [Google Scholar]
- 166.Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr) 2017;40:457–470. doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]