Abstract
Primary symptoms of Crohn’s disease usually include, among others, abdominal pain and cramping, recurrent diarrhea, fever and weight loss. The aim of this study was to show the effectiveness of ultrasound in the diagnosis and assessment of the extent of perianal complications in Crohn’s disease. Five patients (four boys and one girl) aged from 13 to 16 years, with prolonged pain in the perianal area, which worsened when sitting, were admitted to the Department of Pediatric Gastroenterology between 2017 and 2019. Each patient underwent intestinal ultrasound with a high frequency 7–12 MHz linear probe and transperineal ultrasound to assess the anal canal and the surrounding soft tissues. In each case, the echogenicity of the bowel wall was assessed for mural stratification and possible thickening. Anorectal fistulas were detected in all the five patients. Three patients (12 y.o. male, 12 y.o. female and 16 y.o. male) presented with perianal abscesses drained by fistulas. In the first patient the fistula was limited to the perianal soft tissue (22 × 23 mm); in the second patient it was localized within the ischioanal region (40 × 50 mm); and in the third patient (5–6 mm abscess) the fistula was with a hypoechoic fistulous tract reaching the sphincter. All of the patients were eventually diagnosed with Crohn’s disease as a result of further clinical workup. Ultrasound examination is a non-invasive, well-tolerated modality for the evaluation of Crohn’s disease activity and its possible complications, e.g. fistulas and abscesses. Ultrasound is especially useful in patients who require repeated follow-up investigations.
Keywords: Crohn’s disease, ultrasound examination, anorectal fistula
Introduction
Crohn’s disease (CD) is one of chronic inflammatory bowel diseases (IBD) characterized by a relapsing and remitting course. Its worldwide prevalence is increasing, especially in young population. In Europe, it comes to 322 per 100 000(1). Of all locations, ileocecal involvement is the most common. Changes in the esophagus, stomach and duodenum occur in a small percentage of cases. In terms of distribution of the disease, colitis is the only symptom in 25% of patients, 25% present only with ileitis, and 50% develop ileocolitis(2).
CD limited to the colon is more common in children than adults, which makes it difficult to distinguish CD and ulcerative colitis (UC) in some patients(3). Perianal abscesses, fistulas and ulcerations reportedly occur in 27% patients(4), most often as a manifestation of the disease exacerbation, and very rarely as the first signs of CD. The three main etiological factors are individual (genetic) susceptibility, intestinal microflora and the patient’s immune response. The disease develops when impairment of the mechanisms underlying the immune response to internal microorganisms occurs in susceptible persons. Typical primary symptoms of the disease usually include abdominal pain and cramping, recurrent diarrhea, fever and weight loss. Fatigue and anorexia are sometimes also present, as well as rectal bleeding or bloody diarrhea, especially in patients with colonic involvement. Clinical forms of the disease depend on the location of the lesions. There are also extraintestinal manifestations of CD, like arthritis, uveitis, and erythema nodosum, which are diagnosed in almost half of the patients(5).
Diagnostic treatment
Typical laboratory findings include thrombocytosis, increased acute phase proteins (particularly C-reactive protein), and anemia. C-reactive protein is a biomarker used to monitor disease activity, but correlates poorly with endoscopic findings, and a third of patients never present with its increased levels(6). Hypoalbuminemia and vitamin deficiencies might be present, especially in extensive small bowel disease.
Endoscopy remains the gold standard for diagnosis. Segmental inflammation, apthoid, longitudinal and serpiginous ulcerations are typical findings(1). One of the key diagnostic elements is to distinguish Crohn’s disease from other, similar pathologies, such as ulcerative colitis, irritable bowel syndrome, infectious colitis, etc. Therefore, the diagnosis must be based on the clinical picture, physical findings and basic laboratory abnormalities.
Diagnostic imaging is needed to confirm disease locations, extent and intestinal complications. US seems to be the most convenient and fastest modality.
Materials and methods
Five patients (four boys and one girl) aged from 13 to 16 years, with prolonged pain in the perianal area, which worsened when sitting, were admitted to the Department of Pediatric Gastroenterology, Medical University of Lublin between 2017 and 2019. Each patient underwent intestinal ultrasound using Canon Aplio i700 with a high frequency 7–12 MHz linear probe, using B-mode presentation and Dopper options and transperineal ultrasound approach to assess the anal canal and the surrounding soft tissues. In each case, the echogenicity of the bowel wall was assessed for mural stratification and possible thickening. Peri-intestinal fat was evaluated as well – for signs of inflammatory infiltration and lymphadenopathy. All abdominal structures were screened for the possible presence of fistulous tracts.
Results/Case presentation
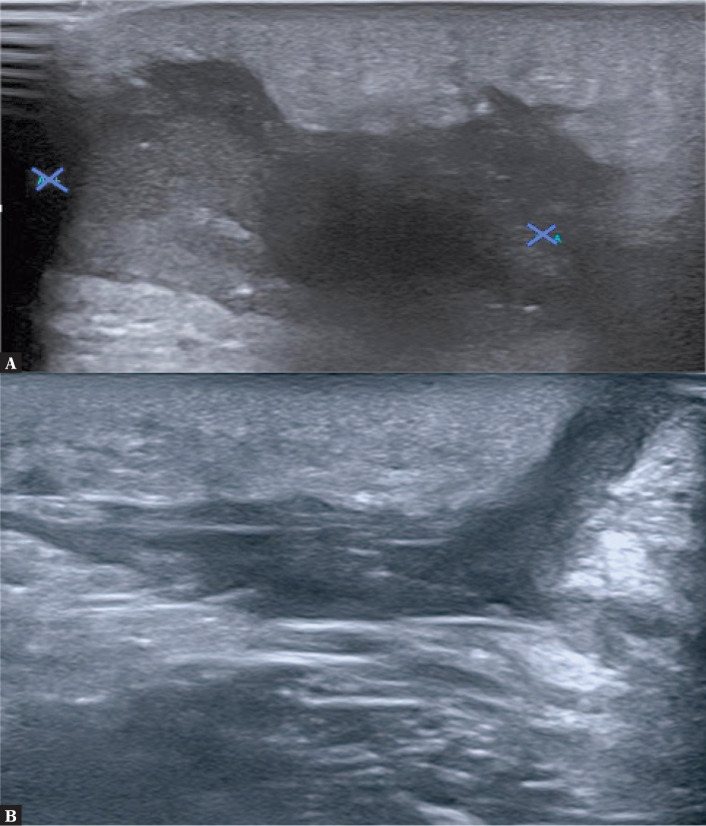
Case 1 concerns a 12-year-old boy. B-mode grayscale ultrasound image showed irregular, heterogeneous, partially anechoic area at the left side of the anus, which was consistent with inflammatory infiltration, and abscess formation. The second slide showed a fistulous tract originating from the previously mentioned abscess, and reaching the skin level (Fig. 1).
Fig. 1.
A. A 12-year-old boy underwent US examination. On the left side of the anus in soft tissues of the buttock there is an area with irregular, echogenically heterogeneous tissue sized about 22 × 23 mm with features of increased vascularization. The lesion has characteristics of an inflammatory infiltration. An abscess is beginning to form. B. US examination showed a fistulous tract originating from the abscess and reaching the skin
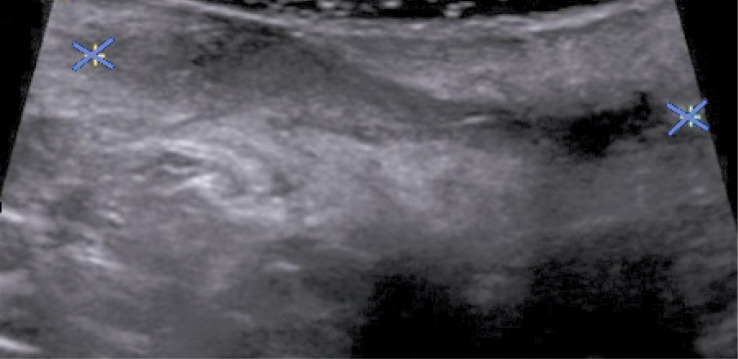
Another patient, a 12-year-old girl, underwent ultrasound, which showed a heterogeneous hypoechoic area (41 × 27 mm) and fluid with heterogeneously echogenic “thick” content in the area of the intergluteal fistula. Ultrasound scan showed pathologic area of inflammatory infiltration with a forming abscess at the level of the intergluteal fistula (Fig. 2).
Fig. 2.
A 12-year-old girl underwent US examination, which showed a heterogeneous hypoechoic area (41 × 27 mm) and fluid with heterogeneously echogenic „thick” content in the area of the intergluteal fistula. There is an inflammatory infiltration of the surrounding tissues and a visible perianal abscess
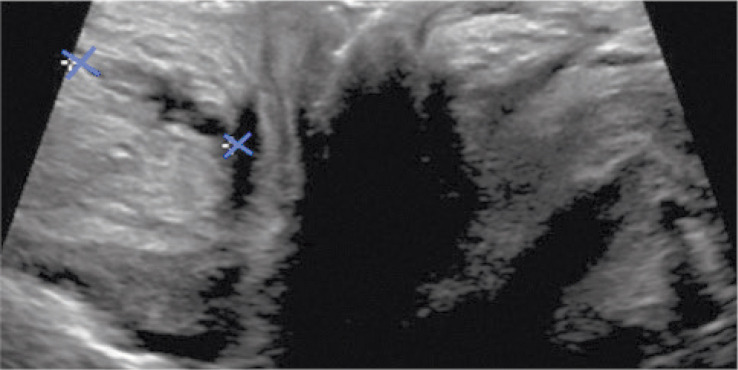
Endorectal US in a 15-year-old boy showed a 18-mm long and 3–4 mm thick fistulous tract reaching the sphincter (Fig. 3).
Fig. 3.
Endorectal US in a 15-year-old boy showed a 18-mm long and 3–4 mm thick fistula reaching the sphincter
Another US examination of 16-year-old boy showed an anechoic area in perianal soft tissues on the left side corresponding to a 5–6 mm abscess with hypoechoic fistulous canal reaching the sphincter with the length of 25 mm and width of 5 mm. Color Doppler option showed no vascular flow signal.
In the last case of a 16-year-old boy, ultrasound also showed an anechoic area in perianal soft tissues on the left side, but with dense liquid collection of 23 mm × 9 mm, indicating pus/abscess. Marginally increased vascular flow signals were found in color Doppler imaging.
All of the patients were eventually diagnosed with Crohn’s disease as a result of further clinical workup.
Discussion
Crohn’s disease manifests at any age. It most often develops in people aged 15 to 35 years, but may also occur in young children. It affects males and females equally. Classic symptoms of CD in children may include abdominal pain, often in the lower right area, diarrhea (sometimes bloody), rectal bleeding, weight loss, fever, delayed growth, joint pain, and rashes. Most of these symptoms affect patients’ quality of life and social functioning(7,8). Children with IBD have higher rates of depressive and anxiety disorders compared with healthy individuals. Additionally, patients with UC and CD involving the colon have an increased risk of colon cancer(3). Some children with CD may stay asymptomatic for a long time, even years. This is referred to as remission. On the other hand, CD in pediatric patients may manifest atypically, as an unexplained poor growth or anemia. Previous reports regarding natural history of Crohn’s disease have shown rates of complicated disease ranging from 48% to 52% 5 years after the diagnosis(9). Factors associated with complicated disease behaviors include age at diagnosis, ileal involvement, serological responses to various microbial antigens, and possibly cumulative genetic risk(10). Therefore, early diagnosis of Crohn’s disease is fundamental for ensuring proper quality of social and mental life, especially in pediatric patients. Both laboratory tests and imaging techniques are used in the diagnosis of CD. Imaging techniques include: endoscopy, colonoscopy, biopsy, upper and lower GI series or barium swallow, CT or MR enterography or capsule endoscopy. Esophagogastroduodenoscopy and ileocolonoscopy with biopsy remain the criterion standard for the diagnosis and classification of IBD in children(11). Video capsule endoscopy may be indicated to evaluate the proximal small intestine when a high suspicion for CD exists and the diagnosis cannot be confirmed by means of conventional endoscopy and imaging(3). Cross-sectional enterography, including CT and MR enterography, has replaced fluoroscopic small-bowel follow-through as the modality of choice. Both modes of enterography permit assessment of the lumen, the mucosa, the bowel wall, and intra-abdominal complications. Pelvic magnetic resonance imaging and rectal endoscopic ultrasonography in centers with expertise are the preferred modalities for evaluating perianal location(12). However, none of these imaging tests is optimal for the patient. Other imaging modalities mentioned above are invasive, require contrast administration or have high complication rates. Ultrasound examination is a non-invasive, well-tolerated modality for the evaluation of CD activity and its possible complications, as well as for assessing treatment outcomes.
Conclusion
First manifestation of Crohn’s disease may be non-specific. Ultrasound examination is a non-invasive, well-tolerated modality for the evaluation of CD activity and its possible complications, fistulas and abscesses in particular. The imaging technique is especially useful in patients who require repeated follow-up investigations. However, it cannot be used as the only tool for the diagnosis of Crohn’s disease.
Conflict of interest
Authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L: Crohn’s disease. Lancet 2017; 389: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Gajendran M, Loganathan P, Catinella AP, Hashash JG: A comprehensive review and update on Crohn’s disease. Dis Mon 2018; 64: 20–57. [DOI] [PubMed] [Google Scholar]
- 3.Rosen M, Dhawan A, Saeed A: Inflammatory bowel disease in children and adolescent. JAMA Pediatr 2015; 169: 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz DA, Loftus EV, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR et al. : The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002; 122: 875–80. [DOI] [PubMed] [Google Scholar]
- 5.Laass MW, Roggenbuck D, Conrad K: Diagnosis and classification of Crohn’s disease. Autoimmun Rev 2014; 13: 467–471. [DOI] [PubMed] [Google Scholar]
- 6.Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, Sandborn WJ et al. : C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015; 110: 802–819. [DOI] [PubMed] [Google Scholar]
- 7.Greenley RN, Hommel KA, Nebel J, Raboin T, Shun-Hwa L, Simpson P et al. : A meta-analytic review of the psychosocial adjustment of youth with inflammatory bowel disease. J Pediatr Psychol 2010; 35: 857–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackner LM, Greenley RN, Szigethy E, Herzer M, Deer K, Hommel KA: Psychosocial issues in pediatric inflammatory bowel disease: report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2013; 56: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ: Crohn’s disease complicated by strictures: a systematic review. Gut 2013; 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ: Crohn’s disease complicated by strictures: a systematic review. Gut 2013; 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD et al. : Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007; 44: 653–674. [DOI] [PubMed] [Google Scholar]
- 12.Rosen MJ, Moulton DE, Koyama T, Morgan WM, Morrow SE, Herline AJ et al. : Endoscopic ultrasound to guide the combined medical and surgical management of pediatric perianal Crohn’s disease. Inflamm Bowel Dis 2010; 16: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]