Abstract
Geographic variation in body size is common within many animal species. The causes of this pattern, however, remain largely unexplored in most vertebrate groups. Bats are widely distributed globally owing to their ability of powered flight. Most bat species encounter a variety of climatic conditions across their distribution range, making them an ideal taxon for the study of ecogeographic patterns in body size. Here, we used adult least horseshoe bats, Rhinolophus pusillus, to test whether geographic variation in body size was determined by heat conservation, heat dissipation, climatic seasonality, or primary productivity. We measured body mass and head-body length for 246 adult bats from 12 allopatric colonies in China. We quantified the ecological conditions inhabited by each colony, including mean maximum temperature of the warmest month, mean minimum temperature of the coldest month, temperature seasonality, precipitation seasonality, and annual net primary productivity (ANPP). Body mass and head-body length, 2 of the most reliable indicators of body size, exhibited marked differences between colonies. After controlling for spatial autocorrelation, the mean minimum temperature of the coldest month explained most of the variation in body size among colonies, regardless of sex. The mean maximum temperature, climatic seasonality, and ANPP had limited power in predicting body size of males or females in comparison with mean minimum temperature. These results support the heat conservation hypothesis and suggest adaptive responses of body size to cold climates in cave-dwelling bats.
Keywords: bat, Bergmann’s rule, body size, climatic adaptation, heat conservation
Understanding the patterns and causes of phenotypic divergence has been a central theme in ecology and evolutionary biology since Darwin (Darwin 1859; Schlichting and Pigliucci 1998; Guo et al. 2016; Santana and Cheung 2016; Pato et al. 2019). Body size is strongly tied to the behavior, physiology, and ecology of animals, and ultimately affects individual survival and reproductive success (Isaac 2005; Porter and Kearney 2009; Smith et al. 2010; Tattersall et al. 2012). Ecogeographic patterns of body size have been demonstrated in many endothermic and ectothermic animals (Ashton 2002a, 2002b; Belk and Houston 2002; Ashton and Feldman 2003; Meiri and Dayan 2003). In general, body size of animals tends to increase with increasing latitude, albeit with some exceptions (Ashton et al. 2000; Meiri and Dayan 2003). In common brushtail possums Trichosurus vulpecula, for example, skull size is positively related to the latitude of sampling location (Correll et al. 2016). In gray-bellied flowerpiercers Diglossa carbonaria and hooded robins Melanodryas cucullata, wing length increases from low to high latitudes (Graves 1991; Gardner et al. 2009). Seed-feeding beetles (Stator limbatus) exhibit pronounced variation in elytron length along a latitudinal gradient (Stillwell et al. 2007). Nonetheless, the mechanisms that underlie latitudinal clines in body size of animals remain an open question.
Several combined processes may mold latitudinal variation in body size of animals, including heat retention, heat dissipation, and selective pressures imposed by climatic seasonality and food availability. The heat conservation hypothesis (i.e., Bergmann’s rule) emphasizes that animals in cooler environments evolved larger body size to conserve body heat by reducing the surface-to-volume ratio (Bergmann 1847; Blackburn and Hawkins 2004). Similarly, the heat dissipation hypothesis suggests that individuals with a small body size have a high surface-to-volume ratio, allowing efficient dissipation of body heat in hotter climates when ambient temperature is lower than the upper boundary of the thermoneutral zone (James 1970; Speakman and Król 2010). Moreover, animal body size may also be shaped by climatic seasonality, given that large individuals have greater fasting endurance during periods of food shortage in unpredictable environments (Lindsey 1966; Lindstedt and Boyce 1985; Ashton 2002b). The resource availability hypothesis argues that the areas of high primary productivity could provide abundant food resources, driving the evolution of larger body size in animals (Rosenzweig 1968; Mcnab 2010; Correll et al. 2016). These hypotheses provide adaptive explanations for the occurrence of genetically based latitudinal clines in body sizes of animals, although phenotypic plasticity may also determine intrapopulation variation in body size due to environmental change (Teplitsky et al. 2008; Watt et al. 2010; Gardner et al. 2011). Since climate and food availability may act simultaneously, the above hypotheses are not mutually exclusive (Watt et al. 2010).
Bats are one of the most species-rich groups of mammals, with great morphological and ecological diversity (Fenton and Bogdanowicz 2002; Schnitzler et al. 2003; Luo et al. 2014, 2019a). They possess the ability of powered flight, providing an opportunity for long distance dispersal over natural barriers and colonization of diverse biogeographic realms (Norberg and Rayner 1987; Luo et al. 2019b). Most bat species experience different climatic regimes within their distribution range (IUCN SSC 2019). Previous studies have shown that latitudinal clines in cranial size of some bat species can be explained by the differences in temperature, as in big brown bats Eptesicus fuscus (Burnett 1983), Daubenton’s bats Myotis daubentonii (Bogdanowicz 1990), and greater horseshoe bats Rhinolophus ferrumequinum (Budinski et al. 2015). In Pallid bats Antrozous pallidus, net primary productivity and minimum temperature of the coldest month are significant predictors of skull size (Kelly et al. 2018). Using body mass as a measure of body size, Jiang et al. (2019) failed to find a statistically significant relationship between body size and mean monthly temperature in R. ferrumequinum. These findings indicate that the causes of geographic variation in body size may depend on the species and on the use of different body size indices, thereby warranting further investigation.
The goal of this work is to assess the patterns and causes of geographic variation in body size of least horseshoe bats, Rhinolophus pusillus, a geographically widespread species in China. Rhinolophus pusillus is distributed in India, Thailand, Malaysia, and southern and central China (IUCN SSC 2019). These bats dwell in caves or mines throughout the year. Adults form colonies composed of several to several hundred individuals (Jiang et al. 2010; Wu et al. 2018). Rhinolophus pusillus is a flutter detecting forager that requires forested habitats and emerges to forage soon after local sunset (Wu et al. 2018). The mitochondrial cytochrome b (Cyt b) sequence divergence ranges from 0.2% to 1.7% across colonies in China, suggesting the absence of cryptic species within R. pusillus (Li et al. 2006). We measured body mass and head-body length for 12 wild colonies from southern and central China under different temperature conditions ranging from 14.9°C to 21.8°C (Jiang et al. 2010). We extracted the ecological conditions for each colony, including average minimum temperature of the coldest month, average maximum temperature of the warmest month, temperature seasonality, precipitation seasonality, and annual net primary productivity (ANPP). If heat conservation is a driver of body size variation among colonies, body size of bats should be predicted by average minimum temperature of the coldest month. If heat dissipation determines geographic variation in body size, bat body size should be negatively associated with average maximum temperature of the warmest month. If the seasonality hypothesis holds for bats, we expect that individual body size would be positively correlated with the seasonality of temperature and precipitation. Finally, according to the resource availability hypothesis, we expect that bat body size would scale positively with ANPP.
Materials and Methods
Morphological measurements
During September 2010 and July 2013, we captured 246 adult R. pusillus from the roost using hand nets and mist nets in 12 sites along a temperature gradient (Figure 1); the sites were Anhui (AH: 5 ♀, 7 ♂), Chongqing (CQ: 4 ♀, 9 ♂), Fujian (FJ: 10 ♀, 10 ♂), Guangxi (GX: 11 ♀, 9 ♂), Guizhou (GZ: 1 ♀, 17 ♂), Hubei (HB: 8 ♀, 2 ♂), Henan (HN: 13 ♀, 5 ♂), Jiangsu (JS: 22 ♀, 6 ♂), Sichuan (SC: 3 ♀, 20 ♂), Shandong (SD: 12 ♀, 14 ♂), Yunnan (YN: 17 ♀, 34 ♂), and Zhejiang (ZJ: 2 ♀, 5 ♂), P.R. China. The FJ, JS, and SD colonies occupied deserted man-made caves, whereas others lived in natural caves. These bats occupied the caves from spring to winter. The colony roosts were separated by 78–1896 km. We previously marked 30 individuals from the JS and SD colonies with numbered aluminum alloy bands (0.05 g, Porzana Ltd., Icklesham, UK) on their right forearm (Luo et al. 2017; Wu et al. 2018). We found no seasonal movement between the colonies based on the recapture of marked bats. Sex was determined by inspecting external genitalia (Jin et al. 2012). The pregnant and lactating females, which were characterized by an enlarged abdomen and exposed nipples, were not included in this study. We placed the bats on a soft towel and measured their head-body length from nose tip to anus using a digital display Vernier caliper (TESA-CAL IP67, TESA Tech., Renens, Switzerland; 0.01 mm). Bat body mass was quantified using a portable electronic balance (TESA-CAL IP67, TESA Tech.; 0.01 g) prior to the onset of foraging. All bats were released into their roosts after morphological data collection. This study was conducted according to the relevant laws for experiments involving vertebrates of the People’s Republic of China, and was approved by the Committee on the Use and Care of Animals at the Northeast Normal University (approval number: NENU-W-2010-101). Experimental procedures were in accordance with the ABS/ASAB guidelines for the Use of Animals in Research.
Figure 1.
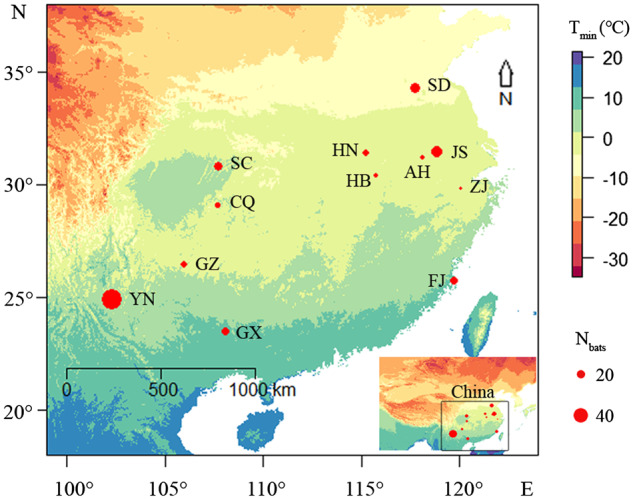
Geographic locations for captured bats in this study. AH, Anhui colony; CQ, Chongqing colony; FJ, Fujiang colony; GX, Guangxi colony; GZ, Guizhou colony; HB, Hubei colony; HN, Henan colony; JS, Jiangsu colony; SC, Sichuan colony; SD, Shandong colony; YN, Yunnan colony; ZJ, Zhejiang colony. Tmin: mean minimum temperature of the coldest month. Circle sizes are proportional to the number of bats sampled (Nbats).
Environmental variables
We determined the latitude, longitude, and elevation above sea level for each location using a handheld GPS locator (eTrex Vista). We extracted climatic conditions experienced by each colony from the WorldClim version 2 at 30 arc-s resolution (∼1 km2) using the R package raster (Hijmans and Van Etten 2013). The WorldClim database contains climatic raster layers worldwide that were compiled using data from weather stations over the last 30 years (1970–2000) (Fick and Hijmans 2017). We obtained 4 climatic variables, namely mean minimum temperature of the coldest month (Tmin), mean maximum temperature of the warmest month (Tmax), mean monthly temperature, and mean monthly precipitation. To quantify climatic seasonality per site, we calculated the annual standard deviations of mean monthly temperature and precipitation (Kelly et al. 2018). To estimate the availability of food resources for each site, we obtained ANPP (g C m−2 year−1) based on the sampling locations and Moderate Resolution Imaging Spectroradiometer (MODIS) Net Primary Productivity (NPP) dataset (MOD17A3) at a resolution of 30 arc-s (Zhao et al. 2005; http://www.ntsg.umt.edu/project/mod17). We monitored roost temperature of the JS colony via a thermohygrograph (HTC-1, Hangzhou, China) during May and August 2011. We extracted the corresponding air temperature outside the JS colony roost from a nearby weather station using the R package RNCEP (Kemp et al. 2012). The climatic data from local weather stations are equivalent to those from the WorldClim database (Fick and Hijmans 2017).
Data analyses
The data on body size and environmental variables were normally distributed (Kolmogorov–Smirnov test). We used a general linear model (GLM) to test whether body mass and head-body length showed significant differences between colonies and between sexes. We employed the ordinary linear regression (OLS) model to examine the relationship between individual body mass and head-body length. The OLS model allowed us to test latitudinal variation in body size while controlling for the effects of altitude. The OLS model was also used to assess the relationship between roost temperature of the JS colony and air temperature outside the cave. A spatial error simultaneous autoregressive model (SARerr) was applied to quantify the relative contributions of environmental variables to body size variation among colonies. SARerr represents a modification of the OLS model after incorporating spatial structure in the dependent error term and is a more robust statistical test for detecting the relationship between morphological variables and environmental factors than the OLS model (Kelly et al. 2018). Tmin, Tmax, temperature seasonality, precipitation seasonality, and ANPP were assigned as fixed variables. Body mass and head-body length of bats were assigned as dependent variables. The seasons of sampling were assigned as the covariate. There were no significant interactions between predictor variables after a likelihood-ratio test. The optimized SARerr model was selected according to Akaike’s information criterion corrected for small sample size. Statistical tests were conducted separately for males and females due to the marked sexual dimorphism in body mass (see below). All statistical analyses were carried out in R 3.6.1.
Results
Geographic variation in body size
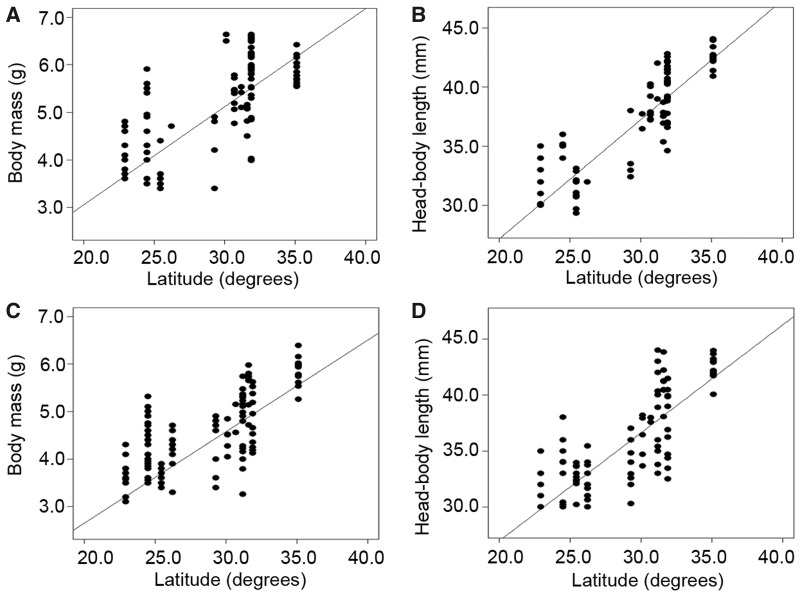
In adult least horseshoe bats, body mass ranged from 3.10 to 6.63 g, and head-body length ranged from 29.34 to 44.06 mm (Supplementary Table S1). Body mass was positively related to head-body length in both females (OLS: β = 0.19, t = 11.89, R2 = 0.57, P < 0.0001) and males (OLS: β = 0.15, t = 13.44, R2 = 0.57, P < 0.0001). There were pronounced geographic differences in body mass (GLM: F11,222 = 34.21, P < 0.0001) and head-body length (GLM: F11,222 = 78.64, P < 0.0001; Table 1). Body size of females tended to be larger than that of males (GLM: body mass: F1,222 = 31.79, P < 0.0001; head-body length: F1,222 = 1.60, P = 0.21). The OLS models detected a significant positive association between latitude and body size, regardless of sex (females: body mass: β = 0.21, t = 10.38, R2 = 0.52, P < 0.0001; head-body length: β = 1.01, t = 19.39, R2 = 0.79, P < 0.0001; males: body mass: β = 0.19, t = 12.81, R2 = 0.57, P < 0.0001; head-body length: β = 0.96, t = 13.92, R2 = 0.65, P < 0.0001; Figure 2).
Table 1.
The differences in body size between colonies and sexes
| Dependent variable | Predictors | df | F | P |
|---|---|---|---|---|
| Body mass | Colony | 11 | 34.21 | <0.0001 |
| Sex | 1 | 31.79 | <0.0001 | |
| Colony: sex | 11 | 5.56 | <0.0001 | |
| Head-body length | Colony | 11 | 78.64 | <0.0001 |
| Sex | 1 | 1.60 | 0.21 | |
| Colony: sex | 11 | 6.81 | <0.0001 |
Figure 2.
Relationship between body size and latitude in least horseshoe bats. (A) Female body mass and latitude. (B) Female head-body length and latitude. (C) Male body mass and latitude. (D) Male head-body length and latitude. Lines represent the ordinary regression models after correcting for altitude.
Relationship between body size and predictor variables
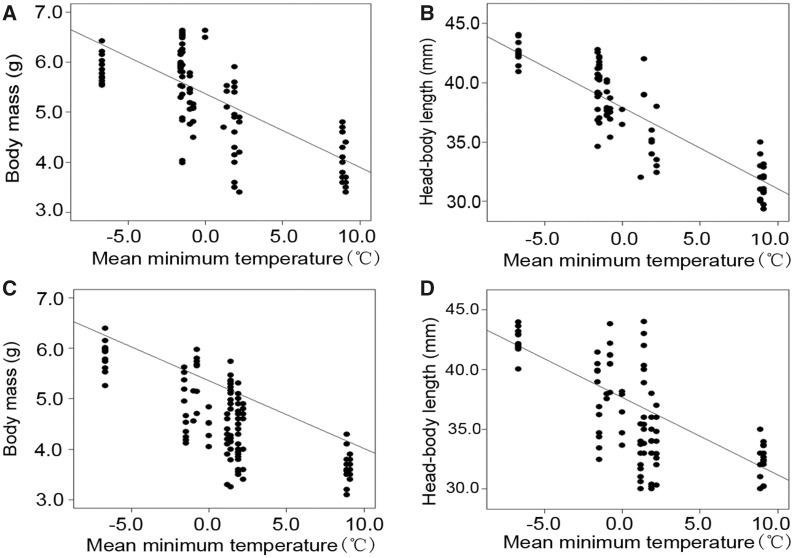
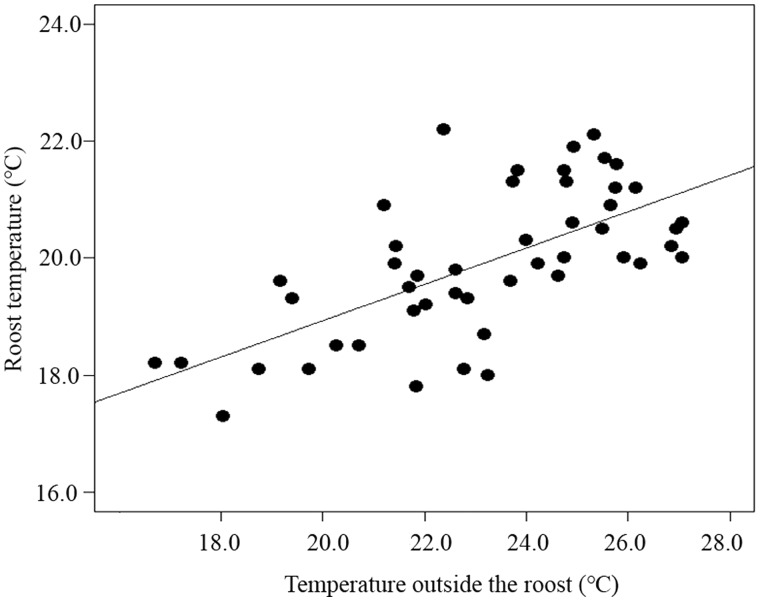
The seasonality of temperature and precipitation was not retained in the optimized SARerr models (Supplementary Table S2). By contrast, Tmin was the best predictor of body size in females and males. Tmin was negatively associated with individual body mass (SARerr: females: β = −0.15, z = −6.22, P < 0.0001; males: β = −0.13, z = −9.02, P < 0.0001; Figure 3A,C) and head-body length (SARerr: female: β = −0.53, z = −8.95, P < 0.0001; males: β = −0.62, z = −9.12, P < 0.0001; Table 2 and Figure 3B,D). Despite a significant positive association between Tmax and male head-body length (SARerr: β = 0.35, z = 2.73, P = 0.0062), the power of Tmax in predicting head-body length of females was weak (SARerr: β = 0.24, z = 1.82, P = 0.069; Table 2). ANPP was negatively related to body size of bats (SARerr: female: head-body length: β = −0.0037, z = −1.81, P = 0.070; male: body mass: β = −0.0012, z = −2.28, P = 0.023; male: head-body length: β = −0.0075, z = −2.85, P = 0.0043; Table 2). There was a marked positive association between roost temperature of the JS colony and air temperature outside the cave (OLS: β = 0.31, t = 5.92, R2 = 0.43, P < 0.0001; Figure 4).
Figure 3.
Relationship between body size and mean minimum temperature of the coldest month (Tmin) in least horseshoe bats. (A) Female body mass and Tmin. (B) Female head-body length and Tmin. (C) Male body mass and Tmin. (D) Male head-body length and Tmin. Lines represent the best-fitting spatial error simultaneous autoregressive models.
Table 2.
Summary of spatial error simultaneous autoregressive models
| Sex | Dependent variable | Predictor | Estimate | z | P-value |
|---|---|---|---|---|---|
| Female | Body mass | T min | −0.15 | −6.22 | <0.0001 |
| Female | Head-body length | T min | −0.53 | −8.95 | <0.0001 |
| Female | Head-body length | T max | 0.24 | 1.82 | 0.069 |
| Female | Head-body length | ANPP | −0.0037 | −1.81 | 0.070 |
| Male | Body mass | T min | −0.13 | −9.02 | <0.0001 |
| Male | Body mass | ANPP | −0.0012 | −2.28 | 0.023 |
| Male | Head-body length | T min | −0.62 | −9.12 | <0.0001 |
| Male | Head-body length | T max | 0.35 | 2.73 | 0.0062 |
| Male | Head-body length | ANPP | −0.0075 | −2.85 | 0.0043 |
T min, mean minimum temperature of the coldest month; Tmax, mean maximum temperature of the warmest month; ANPP, annual net primary productivity. Estimate denotes the coefficient of optimized spatial simultaneous autoregressive error models. Data in bold are statistically significant.
Figure 4.
Relationship between roost temperature in the JS colony and air temperature outside the cave. Lines represent the ordinary regression model.
Discussion
In this study, we used adult least horseshoe bats as a model to understand the potential mechanisms underlying ecogeographic patterns of body size. As expected, these bats exhibited significant latitudinal variation in body size, with elevated body mass and head-body length toward higher latitudes. We found that body size of females and males was poorly predicted by average maximum temperature of the warmest month and climatic seasonality. However, the latitudinal cline in body size of least horseshoe bats was primarily explained by the differences in average minimum temperature of the coldest month. Together, these results provide evidence supporting the hypothesis that heat conservation drives geographic variation in body size of bats.
The mean minimum temperature of the coldest month was a significant explanatory variable of ecogeographic variation in body size of least horseshoe bats, even after controlling for spatial autocorrelation. This supports the prediction of Bergmann’s rule, which emphasizes that individuals should be larger in cooler environments in order to reduce the rate of heat loss per unit of body mass (Bergmann 1847; James 1970; Meiri and Dayan 2003; Watt et al. 2010). Support for Bergmann’s rule has been found in some endotherms and ectotherms. Within the order Chiroptera, patterns of geographic variation in skull size or principal components of tibia length and hand-wing length are significantly predicted by temperature conditions experienced by bats such as E. fuscus (Burnett 1983), M. daubentonii (Bogdanowicz 1990), Cynopterus sphinx (Storz et al. 2001), and R. ferrumequinum (Budinski et al. 2015). Similarly, body size is also negatively linked to annual minimum temperature in some other endotherms, including the cerulean warbler Dendroica cerulea (Jones et al. 2005), tree sparrows Passer montanus (Lan et al. 2018), rock hyrax Procavia capensis (Yom-Tov 2008), female koala Phascolarctos cinereus (Briscoe et al. 2015), and Japanese sika deer Cervus nippon (Kubo and Takatsuki 2015). The negative relationship between body size and environmental temperature exists even in some amphibians and reptiles (Ashton 2002a; Ashton and Feldman 2003; Jin and Liao 2015). Moreover, physiological experiments reveal that big brown bats and eastern red bats Lasiurus borealis increase their torpid metabolic rate when ambient temperature declines, and low-latitude individuals with smaller body size sustain higher metabolic rates required for thermoregulation at cool temperatures compared with those from high latitude (Dunbar and Brigham 2010). In some birds and mammals, basal metabolic rate scales negatively with environmental temperature (Broggi et al. 2007; Clarke et al. 2010). These previous findings, together with the results presented here, indicate that conservation of body heat acts as an important shaping force for generating body size diversity within a species.
The mean maximum temperature of the warmest month explained a minor part of the variation in body size across colonies compared with mean minimum temperature of the coldest month. Three possible explanations may account for the observed phenomenon. First, most bats initiate foraging activity after sunset (Speakman 1991), which reduces the possibility of exposure to extreme heat events. Second, many bat species dwell gregariously in natural and artificial shelters such as caves, enabling them to avoid heat stress during extremely hot weather (Kunz and Fenton 2003). Indeed, our field monitoring showed that roost temperature of the JS colony varied from 18°C to 22°C in summer, even with air temperature outside the roost reaching 28°C. This confirms that least horseshoe bats and other cave-dwelling bats can be largely buffered from summer maximum temperature by staying in the roost. Third, in addition to altering heat exchange via the body surface, bats can dissipate their body heat from highly vascular wing membranes (Lancaster et al. 1997; Reichard and Fellows 2010). It has been shown that small island flying foxes Pteropus hypomelanus increased their exposed wing surface and the frequency of wing spreading in response to elevated ambient temperatures (Ochoa-Acuña and Kunz 1999). In free-flying Brazilian free-tailed bats Tadarida brasiliensis, the surfaces of wings can release 0.85 W of body heat through radiative flux into the surrounding air (Reichard and Fellows 2010). These findings suggest that heat dissipation is not a crucial determinant of latitudinal clines of body size in bats, presumably owing to roosting habit and thermoregulatory behavior.
Least horseshoe bats living in highly productive areas tend to have a smaller body size than those from sites of low productivity. This fails to support the resource availability hypothesis that predicts limited food conditions constrain the evolution of larger body size in animals (Rosenzweig 1968; Mcnab 2010). Kelly et al. (2018) demonstrated that ANPP could explain the majority of geographic variation in skull size in the Pallid bats, a geographically widespread species across western North America. In common brushtail possums, condylobasal length is also positively associated with primary productivity during the least productive season (Correll et al. 2016). The discrepancy between our findings and those of previous investigations may be attributed to the use of different body size indices as well as species-specific responses to seasonal food shortages. In the winter season, food availability for least horseshoe bats appears to be low at high latitudes due to cold climates (Jiang et al. 2010). To cope with the cold and starvation stress, these bats store substantial amounts of fat in autumn and hibernate in the caves throughout the winter (Chen et al. 2007). In this circumstance, the hibernation strategy employed by least horseshoe bats may counteract the effects of food availability on body size.
To conclude, our results show remarkable latitudinal variation in body size of adult least horseshoe bats after correcting for altitude. There is a tight link between individual body size and average minimum temperature of the coldest month, even after accounting for the effects of spatial autocorrelation. These results provide correlative evidence for the role of heat conservation in shaping geographic variation in body size of cave-roosting bats. Coupled with previous studies (Gardner et al. 2011, 2017), our findings imply that chronic stresses from cold events drive the evolution of body size of animals. It should be noted that other ecological processes such as sexual selection and food competition between sympatric species may also underlie geographic clines in body size (Székely et al. 2004; Postawa et al. 2012). Investigating these processes was beyond the scope of this study. Despite the increasing interest in the evolution of bat body size (Tomassini et al. 2014; Jiang et al. 2019), it is still unclear whether warming climates are causing some bat species to shift their body size as a result of genetic adaptation or phenotypic plasticity during postnatal development. Future research is critically needed to explore intraspecific variation in body size and associated genes in the face of climate change.
Authors’ Contributions
B.L. and J.F. designed the study. B.L., M.W., D.G., W.W., and H.G. collected the data. B.L., M.W., and Y.L. conducted the analyses. M.W., K.C., and B.L. wrote the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We are grateful to Guanjun Lu, Rong Xu, Zhen Wang, and Xiaobin Huang for their assistance with the field work. We acknowledge the anonymous reviewers for valuable advices and comments on the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31800323), Project funded by the China Postdoctoral Science Foundation (Grant No. 2019M661188), open project program of Jilin Provincial Key Laboratory of Animal Resource Conservation and Utilization at Northeast Normal University (Grant No. 1300289102), and Scientific Research Foundation of China West Normal University (Grant Nos 18B024, 17E066).
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
- Ashton KG, 2002a. Do amphibians follow Bergmann’s rule?. Can J Zool 80:708–716. [Google Scholar]
- Ashton KG, 2002b. Patterns of within-species body size variation of birds: strong evidence for Bergmann’s rule. Glob Ecol Biogeogr 11:505–523. [Google Scholar]
- Ashton KG, Feldman CR, 2003. Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57:1151–1163. [DOI] [PubMed] [Google Scholar]
- Ashton KG, Tracy MC, Queiroz A, 2000. Is Bergmann’s rule valid for mammals? Am Nat 156:390–415. [DOI] [PubMed] [Google Scholar]
- Belk MC, Houston DD, 2002. Bergmann’s rule in ectotherms: a test using freshwater fishes. Am Nat 160:803–808. [DOI] [PubMed] [Google Scholar]
- Bergmann C, 1847. Uber die verhaltnisse der warmeokonomie der thiere zu ihrer grosse. Göttinger Studien 3:595–708. [Google Scholar]
- Blackburn TM, Hawkins BA, 2004. Bergmann’s rule and the mammal fauna of northern North America. Ecography 27:715–724. [Google Scholar]
- Bogdanowicz W, 1990. Geographic variation and taxonomy of Daubenton’s bat Myotis daubentoni in Europe. J Mammal 71:205–218. [Google Scholar]
- Briscoe NJ, Krockenberger A, Handasyde KA, Kearney MR, 2015. Bergmann meets Scholander: geographical variation in body size and insulation in the koala is related to climate. J Biogeogr 42:791–802. [Google Scholar]
- Broggi J, Hohtola E, Koivula K, Orell M, Thomson RL et al. , 2007. Sources of variation in winter basal metabolic rate in the great tit. Funct Ecol 21:528–533. [Google Scholar]
- Budinski I, Jojić V, Jovanović VM, Bjelić-Čabrilo O, Paunović M et al. , 2015. Cranial variation of the greater horseshoe bat Rhinolophus ferrumequinum (Chiroptera: Rhinolophidae) from the central balkans. Zool Anz 254:8–14. [Google Scholar]
- Burnett CD, 1983. Geographic and climatic correlates of morphological variation in Eptesicus fuscus. J Mammal 64:437–444. [Google Scholar]
- Chen Z, Han WW, Zhang SC, Li YC, 2007. Sperm storage in least horseshoe bat during hibernation on Hainan island. Acta Theriol Sin 27:229–233. [Google Scholar]
- Clarke A, Rothery P, Isaac NJB, 2010. Scaling of basal metabolic rate with body mass and temperature in mammals. J Anim Ecol 79:610–619. [DOI] [PubMed] [Google Scholar]
- Correll RA, Prowse TAA, Prideaux GJ, 2016. Lean-season primary productivity and heat dissipation as key drivers of geographic body-size variation in a widespread marsupial. Ecography 39:77–86. [Google Scholar]
- Darwin C, 1859. The Origin of Species. London: John Murry. [Google Scholar]
- Dunbar MB, Brigham RM, 2010. Thermoregulatory variation among populations of bats along a latitudinal gradient. J Comp Physiol B 180:885–893. [DOI] [PubMed] [Google Scholar]
- Fenton MB, Bogdanowicz W, 2002. Relationships between external morphology and foraging behaviour: bats in the genus Myotis. Can J Zool 80:1004–1013. [Google Scholar]
- Fick SE, Hijmans RJ, 2017. Worldclim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. [Google Scholar]
- Gardner JL, Heinsohn R, Joseph L, 2009. Shifting latitudinal clines in avian body size correlate with global warming in Australian passerines. Proc R Soc B 276:3845–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R, 2011. Declining body size: a third universal response to warming?. Trends Ecol Evol 26:285–291. [DOI] [PubMed] [Google Scholar]
- Gardner JL, Rowley E, Rebeira P, Rebeira A, Brouwer L, 2017. Effects of extreme weather on two sympatric Australian passerine bird species. Philos Trans R Soc B 372:20160148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves GR, 1991. Bergmann’s rule near the equator: latitudinal clines in body size of an Andean passerine bird. Proc Natl Acad Sci U S A 88:2322–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BC, Lu D, Liao WB, Merilä J, 2016. Genomewide scan for adaptive differentiation along altitudinal gradient in the Andrew’s toad Bufo andrewsi. Mol Ecol 25:3884–3900. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Van Etten J, 2013. Raster: Geographic Data Analysis and Modeling. R package version 2.1-49. Available from: http://CRAN.R-project.org/package=raster.
- Isaac JL, 2005. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mammal Rev 35:101–115. [Google Scholar]
- IUCN SSC, 2019. The IUCN Red List of Threatened Species. Available from: http://wwwiucnredlistorg (accessed July 2019).
- James FC, 1970. Geographic size variation in birds and its relationship to climate. Ecology 51:365–390. [Google Scholar]
- Jiang TL, Metzner W, You YY, Liu S, Lu GJ et al. , 2010. Variation in the resting frequency of Rhinolophus pusillus in mainland China: effect of climate and implications for conservation. J Acoust Soc Am 128:2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TL, Wang J, Wu H, Csorba G, Puechmaille SJ et al. , 2019. The patterns and possible causes of global geographical variation in the body size of the greater horseshoe bat Rhinolophus ferrumequinum. J Biogeogr 46:2363–2377. [Google Scholar]
- Jin LR, Luo B, Sun KP, Liu Y, Ho JP et al. , 2012. Postnatal growth and age estimation in Marshall’s horseshoe bat Rhinolophus marshalli. Acta Chiropterol 14:105–110. [Google Scholar]
- Jin YT, Liao PH, 2015. An elevational trend of body size variation in a cold-climate agamid lizard Phrynocephalus theobaldi. Curr Zool 61:444–453. [Google Scholar]
- Jones J, Gibb CE, Millard SC, Barg JJ, Katharine Girvan M et al. , 2005. Multiple selection pressures generate adherence to Bergmann’s rule in a neotropical migratory songbird. J Biogeogr 32:1827–1833. [Google Scholar]
- Kelly RM, Friedman R, Santana SE, 2018. Primary productivity explains size variation across the pallid bat’s western geographic range. Funct Ecol 32:1520–1530. [Google Scholar]
- Kemp MU, Emiel van Loon E, Shamoun-Baranes J, Bouten W, 2012. RNCEP: global weather and climate data at your fingertips. Methods Ecol Evol 3:65–70. [Google Scholar]
- Kubo MO, Takatsuki S, 2015. Geographical body size clines in sika deer: path analysis to discern amongst environmental influences. Evol Biol 42:115–127. [Google Scholar]
- Kunz TH, Fenton MB, 2003. Bat Ecology. Chicago (IL: ): University of Chicago Press. [Google Scholar]
- Lan MM, Fan LM, Liu FQ, Wen LY, Jing X et al. , 2018. The adaptive evolution of the tree sparrow Passer montanus phenotype to environment factors. Acta Ecol Sin 38:1392–1400. [Google Scholar]
- Lancaster WC, Thomson SC, Speakman JR, 1997. Wing temperature in flying bats measured by infrared thermography. J Therm Biol 22:109–116. [Google Scholar]
- Li G, Jones G, Rossiter SJ, Chen SF, Parsons S et al. , 2006. Phylogenetics of small horseshoe bats from East Asia based on mitochondrial DNA sequence variation. J Mammal 87:1234–1240. [Google Scholar]
- Lindsey CC, 1966. Body sizes of poikilotherm vertebrates at different latitudes. Evolution 20:456–465. [DOI] [PubMed] [Google Scholar]
- Lindstedt SL, Boyce MS, 1985. Seasonality, fasting endurance, and body size in mammals. Am Nat 125:873–878. [Google Scholar]
- Luo B, Huang XB, Li YY, Lu GJ, Zhao JL et al. , 2017. Social call divergence in bats: a comparative analysis. Behav Ecol 28:533–540. [Google Scholar]
- Luo B, Leiser-Miller L, Santana SE, Zhang L, Liu T et al. , 2019a. Echolocation call divergence in bats: a comparative analysis. Behav Ecol Sociobiol 73:154. [Google Scholar]
- Luo B, Santana SE, Pang YL, Wang M, Xiao YH et al. , 2019b. Wing morphology predicts geographic range size in vespertilionid bats. Sci Rep 9:4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JH, Koselj K, Zsebők S, Siemers BM, Goerlitz HR, 2014. Global warming alters sound transmission: differential impact on the prey detection ability of echolocating bats. J R Soc Interface 11:20130961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcnab BK, 2010. Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164:13–23. [DOI] [PubMed] [Google Scholar]
- Meiri S, Dayan T, 2003. On the validity of Bergmann’s rule. J Biogeogr 30:331–351. [Google Scholar]
- Norberg UM, Rayner JMV, 1987. Ecological morphology and flight in bats (Mammalia: Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc B 316:337–419. [Google Scholar]
- Ochoa-Acuña H, Kunz TH, 1999. Thermoregulatory behavior in the small island flying fox Pteropus hypomelanus (Chiroptera: Pteropodidae). J Therm Biol 24:15–20. [Google Scholar]
- Pato J, Illera JC, Obeso JR, Laiolo P, 2019. The roles of geography, climate and sexual selection in driving divergence among insect populations on mountaintops. J Biogeogr 46:784–795. [Google Scholar]
- Porter WP, Kearney M, 2009. Size, shape, and the thermal niche of endotherms. Proc Natl Acad Sci U S A 106:19666–19672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postawa T, Zagorodniuk I, Bachanek J, 2012. Patterns of cranial size variation in two sibling species Plecotus auritus and P. austriacus (Chiroptera: Vespertilionidae) in a contact zone. J Zool 288:294–302. [Google Scholar]
- Reichard JD, Fellows SR, 2010. Thermoregulation during flight: body temperature and sensible heat transfer in free-ranging Brazilian free-tailed bats Tadarida brasiliensis. Physiol Biochem Zool 83:885–897. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ML, 1968. The strategy of body size in mammalian carnivores. Am Midl Nat 80:299–315. [Google Scholar]
- Santana SE, Cheung E, 2016. Go big or go fish: morphological specializations in carnivorous bats. Proc R Soc B 283:20160615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting CD, Pigliucci M, 1998. Phenotypic Evolution: A Reaction Norm Perspective. Sunderland: Sinauer Associates. [Google Scholar]
- Schnitzler HU, Moss CF, Denzinger A, 2003. From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol 18:386–394. [Google Scholar]
- Smith FA, Boyer AG, Brown JH, Costa DP, Dayan T et al. , 2010. The evolution of maximum body size of terrestrial mammals. Science 330:1216–1219. [DOI] [PubMed] [Google Scholar]
- Speakman JR, 1991. Why do insectivorous bats in Britain not fly in daylight more frequently?. Funct Ecol 5:518–524. [Google Scholar]
- Speakman JR, Król E, 2010. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J Anim Ecol 79:726–746. [DOI] [PubMed] [Google Scholar]
- Stillwell R, Morse G, Fox C, 2007. Geographic variation in body size and sexual size dimorphism of a seed-feeding beetle. Am Nat 170:358–369. [DOI] [PubMed] [Google Scholar]
- Storz JF, Balasingh J, Bhat HR, Nathan PT, Doss DPS et al. , 2001. Clinal variation in body size and sexual dimorphism in an Indian fruit bat Cynopterus sphinx (Chiroptera: Pteropodidae). Biol J Linn Soc 72:17–31. [Google Scholar]
- Székely T, Freckleton RP, Reynolds JD, 2004. Sexual selection explains Rensch’s rule of size dimorphism in shorebirds. Proc Natl Acad Sci U S A 101:12224–12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F et al. , 2012. Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr Physiol 2:2151–2202. [DOI] [PubMed] [Google Scholar]
- Teplitsky C, Mills JA, Alho JS, Yarrall JW, Merilä J, 2008. Bergmann’s rule and climate change revisited: disentangling environmental and genetic responses in a wild bird population. Proc Natl Acad Sci U S A 105:13492–13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini A, Colangelo P, Agnelli P, Jones G, Russo D, 2014. Cranial size has increased over 133 years in a common bat, Pipistrellus kuhlii: a response to changing climate or urbanization?. J Biogeogr 41:944–953. [Google Scholar]
- Watt C, Mitchell S, Salewski V, 2010. Bergmann’s rule: a concept cluster? Oikos 119:89–100. [Google Scholar]
- Wu X, Pang YL, Luo B, Wang M, Feng J, 2018. Function of distress calls in least horseshoe bats: a field study using playback experiments. Acta Chiropterol 20:455–464. [Google Scholar]
- Yom-Tov Y, 2008. Does the rock hyrax Procavia capensis conform with Bergmann’s rule?. Zool J Linn Soc 108:171–177. [Google Scholar]
- Zhao M, Heinsch FA, Nemani RR, Running SW, 2005. Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens Environ 95:164–176. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.