Insular biodiversity has aroused the curiosity of biologists in assessing ecological and evolutionary hypotheses concerning their mainland counterparts. Comparisons of particular life history traits between island and mainland populations have provided a broader view of phenotypic and behavioral plasticity at both intraspecific and interspecific levels (Meiri 2007). Body size has been one of the most important biological traits used to measure phenotypic features, as it correlates with all aspects of life history and is influenced by many abiotic and biotic factors (Peters 1983). There are some studies that explore body size variation as a measure of sexual size dimorphism (Shine 1989); this measure can be male- or female-biased and is more evident in the larger species (Abouheif and Fairbairn 1997). Conversely, some investigations have shown the effects of the island rule hypothesis on body size variation, which suggests gigantism in small animals and dwarfism in large animals as a response to competition, predation, and food availability in insular environments (Van Valen 1973; Meiri et al. 2008). Lizards of the genus Anolis (Squamata: Dactyloidae) have been used as excellent models in attempting to understand ecological and physiological processes between and within species, as well as across environmental conditions (Losos 2009). Body size comparisons between island and mainland populations have been recorded in the Clouded Anole Anolis nebulosus (Hernández-Salinas et al. 2014; Senczuk et al. 2014; Siliceo-Cantero et al. 2016). These studies have shown that sexual dimorphism is higher on the island than on the mainland; however, such comparisons were conducted in a single study area per environment, and the continental and insular sites have been the same in some of the above-referenced studies. Therefore, whether similar body size patterns are different between insular and mainland populations remains somewhat unknown.
The goal of this study is to assess the effect of the island rule hypothesis on sexual size dimorphism (SSD) in A. nebulosus. First, we compared the body size (SVL: snout-vent length) between males and females from 3 island populations. If insular populations exhibit sexual dimorphism, in concordance with previous works (e.g., Hernández-Salinas et al. 2014), initially we expected a male-biased body size. Scharf and Meiri (2013), however, observed that analyzing body size alone could lead to different conclusions and misleading hypotheses on sexual dimorphism, and so for this reason, we also compared 4 morphological traits between the sexes among the island populations. Second, we evaluated the body size of island populations and some mainland populations across most of the geographic range of A. nebulosus. The island rule could not only favor a large body size, but also the ornamentation related to sexual selection; thus, we also compared the male ornament (i.e., dewlap length and size) between the island and mainland populations. Third and finally, we calculated the SSD index to illustrate the direction of sexual dimorphism among island and mainland populations.
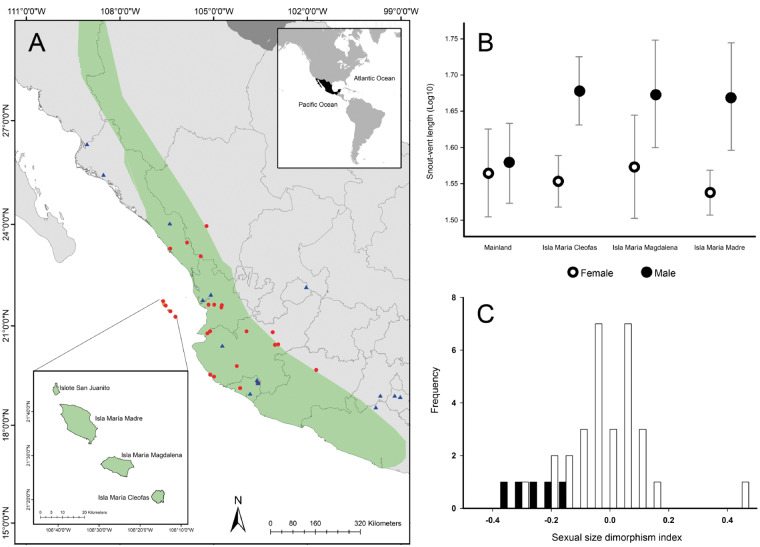
The methods and statistical analyses are described in the Supplementary Material. Briefly, we measured 6 morphological traits in 305 museum specimens, which represent 4 insular populations and 32 mainland populations of A. nebulosus (Figure 1A, Appendix 1). The number of specimens measured from each population ranged from 2 to 61. The measurements were taken with a caliper scale 150 mm to ± 0.1 mm precision. To avoid potential miscalculations, the same person measured all the museum specimens. We identified their sex by using external sex-specific characteristics. We also estimated the dewlap area of males from island and mainland populations. All of the measurements were log10 transformed before the data analysis. To calculate the SSD index, additional data on island and mainland populations were obtained from the literature.
Figure 1.
(A) Geographic distribution of A. nebulosus (Flores-Villela and Hammerson, 2007), showing the location of the insular populations in this study. Data from red circles and blue triangles were used for statistical analyses, whereas only data from red circles were used to estimate SSD index. (B) Comparison of body size between females and males from the island and mainland populations. (C) Sexual dimorphism index in snout–vent length between insular (black) and mainland (white) populations, whereas the negative and positive values indicate male- and female-biased results, respectively.
In total, we measured 37 males and 14 females from Isla María Cleofas, 25 males and 4 females from Isla María Madre, 43 males and 18 females from Isla María Magdalena, 3 males and 1 female from Islote San Juanito. We also measured 88 males and 53 females from different mainland populations (Figure 1A). In general, the SVL of insular males ranged from 32.0 to 61.0 mm, and the SVL of insular females ranged from 31.5 to 48.7 mm. The SVL of mainland males and females ranged from 32.0 to 57.0 mm and 32.0 to 51.0 mm, respectively (Supplementary Table S1). The SVLlog10 was not affected by the interaction of the island population and their sex (two-way ANOVA test, F2,135 = 1.37, P = 0.26). However, a post hoc Tukey test showed differences in the SVLlog10 between the sexes for each island population (HSD test, P < 0.001). Based on the first component (Principal Component Analysis), the interaction between the island and their sex affected the head and limb sizes (two-way ANOVA test, F2,135 = 3.55, P = 0.03). A post hoc Tukey test showed that differences in head and limb sizes occurred between females from Isla María Madre and Isla María Magdalena (HSD test, P = 0.002), and between the sexes in Isla María Magdalena (HSD test, P = 0.028). A two-way ANOVA test also showed that the interaction between sites (island and mainland) and their sex affected the SVLlog10 (F3,291 = 16.29, P < 0.01, Figure 1B), specifically males from the mainland differed from their insular counterparts (HSD test, P < 0.001). The average dewlap length of males differed among populations (F3,183 = 41.7, P < 0.01), with the lowest average of 23.1 ± 3.9 mm in the mainland populations, whereas the insular populations showed similar averages (31.2 ± 6.4 mm to 32.1 ± 7.3 mm; Supplementary Table S1). The estimated dewlap area also was different among populations (F3,175 = 21.5, P < 0.01). The SSD index showed differences between the insular and mainland populations (Mann–Whitney U test, U = 7, P < 0.01). All 4 insular populations were male-biased, whereas 40% of the mainland population were female-biased, 50% were male-biased, and 10% were isomorphism (Figure 1C).
Our results supported the occurrence of sexual dimorphism in A. nebulosus throughout the island populations. Based on the literature and our results, this species exhibits insular gigantism biased toward males, regardless of isolation. A striking result was that our study species showed a broad range of SSD, with an extreme male bias evident on the island populations, although the mainland populations showed a continuous sexual dimorphism from male- to female-biased. These findings that the selective pressures affect intersexual body sizes might vary among populations. Evolutionary biologists have provided some ecological and adaptive hypotheses to explain sexual size dimorphism, and have suggested that a large male size could promote success in male–male competition for females and territories. When females exceed males in size, this confers an advantage in fecundity selection because the number of offspring increases with female size, while the lack of sexual dimorphism relates to resource partitioning (reviewed by Cox et al. 2007). Our results showed that the main difference in sexual dimorphism among the island populations was their SVLs, while the head and limb measurements were similar between the sexes, except for females from Isla María Magdalena. The prevalence of large males might be advantageous in male aggression and territoriality during the breeding season. Siliceo-Cantero et al. (2016) observed that males from islands spent 95% of their time conducting social interactions, while mainland males spent 44% of their time resting and hiding. These authors suggested that the absence of competitors and predators on island populations enhances the time and energy invested in other species-specific physiological and behavioral factors (Stamps et al. 1997). The above behavioral observations might be associated with differences in the male ornamentation, as males on islands showed a larger body size and larger dewlaps than their conspecifics on mainland populations. This information constitutes new evidence that the island rule hypothesis also favors the male ornamentation in the study species. Dewlap displays in males have been associated with courtship, territorial defence, predator deterrence, and species recognition (William and Rand 1977, Fitch and Hillis 1984). Alternatively, anole species with a large SSD are derived from ancestral clades with male-biased dimorphism (Poe et al. 2007); consequently, the insular conditions trigger a cascade of intrinsic and extrinsic factors that favor a large body size for insular males, thereby increasing their sexual size dimorphism. Although this study focused on island populations, the results of mainland populations suggest that fecundity selection might be advantageous in populations where females exceed the size of males. Moreover, the male ornamentation in mainland populations might vary among specific landscapes and climatic regimes. Nevertheless, these ideas must be evaluated in greater detail in future studies.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
Conflict of Interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
We are deeply thankful to Neftali Camacho (LACM), Addison Wynn (NMNH), Esther Langan (NMNH), Christina K. Sami (NMNH), David A. Kizirian (AMNH), and Lauren Vonnahme (AMNH) for logistic support to visiting each museum. Shai Meiri and anonymous reviewers provided insightful suggestions that improved the manuscript, and Louis W. Porras also improves the English of the manuscript. Finally, we thank José González and Ismael Huerta for help in preparing the figure.
References
- Abouheif E, Fairbairn DJ, 1997. A comparative analysis of allometry for sexual size dimorphism: assessing Rensch’s rule. Am Nat 149:540–562. [Google Scholar]
- Cox RM, Butler MA, John-Alder HB, 2007. The evolution of sexual size dimorphism in reptiles In: Fairbairn DJ, Blanckenhorn WU, Szekely T, editors. Sex, Size & Gender Roles: Evolutionary Studies of Sexual Size Dimorphism. Oxford, UK: Oxford University Press; 38–49. [Google Scholar]
- Fitch HS, Hillis DM, 1984. The Anolis dewlap: interspecific variability and morphological associations with habitat. Copeia 1984:315–323. [Google Scholar]
- Flores-Villela O Hammerson GA 2007. Anolis nebulosus. The IUCN Red List of Threatened Species 2007: e.T64207A1275224. [Google Scholar]
- Hernández-Salinas U, Ramírez-Bautista R, Pavón NP, Rosas Pacheco LF, 2014. Morphometric variation in island and mainland populations of two lizard species from the Pacific Coast of Mexico. Rev Chil Hist Nat 87:21. [Google Scholar]
- Losos JB, 2009. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles. Berkeley: University of California Press. [Google Scholar]
- Meiri S, 2007. Size evolution in island lizards. Glob Ecol Biogeogr 16:702–708. [Google Scholar]
- Meiri S, Cooper N, Purvis A, 2008. The island rule: made to be broken? Proc R Soc B 275:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RH, 1983. The Ecological Implications of Body Size. Cambridge: Cambridge University Press. [Google Scholar]
- Poe S, Goheen JR, Hulebak EP, 2007. Convergent exaptation and adaptation in solitary island lizards. Proc R Soc B 274:2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf I, Meiri S, 2013. Sexual dimorphism of heads and abdomens: different approaches to “being large” in female and male lizards. Biol J Linn Soc 110:665–673. [Google Scholar]
- Senczuk G, García A, Colangelo P, Annesi F, Castiglia R, 2014. Morphometric and genetic divergence in island and mainland populations of Anolis nebulosus (Squamata: Polychrotidae) from Jalisco (Mexico): an instance of insular gigantism. Ital J Zool 81:204–214. [Google Scholar]
- Shine R, 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Quart Rev Biol 64:419–461. [DOI] [PubMed] [Google Scholar]
- Siliceo-Cantero HH, García A, Reynolds RG, Pacheco G, Lister BC, 2016. Dimorphism and divergence in island and mainland anoles. Biol J Linn Soc 118:852–872. [Google Scholar]
- Stamps JA, Losos JB, Andrews RM, 1997. A comparative study of population density and sexual size dimorphism in lizards. Am Nat 149:64–90. [Google Scholar]
- Van Valen L, 1973. A new evolutionary law. Evol Theory 1:1–30. [Google Scholar]
- Williams EE, Rand AD, 1977. Species recognition, dewlap function and faunal size. Am Nat 17:261–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.