Abstract
Short-term elevation of glucocorticoids (GCs) is one of the major physiological mechanisms by which vertebrates cope with challenging environmental or social factors (stressors). However, when exposure to stressors occurs repeatedly or over a prolonged period of time, animals may experience chronic elevation of GCs, which reduces the immune response efficiency and can lead to higher intensity of parasitic infection. Here, we used invasive gray squirrels Sciurus carolinensis introduced in Northern Italy and their 2 most prevalent gastrointestinal parasites, the nematode Strongyloides robustus and coccidia of the genus Eimeria, as a model to investigate relationships among macroparasite infection and concentrations of fecal glucocorticoid metabolites (FGMs), an integrated measure of circulating GCs. Our results revealed an association of FGMs with infection by St. robustus, but not with coccidia. Individuals with higher FGMs appear to be responsible for the greatest St. robustus egg shedding within gray squirrel populations, thus possibly acting as superspreaders. However, FGMs were negatively associated with adult St. robustus, suggesting that the abundance of adults of this nematode species does not induce elevation in FGMs, but is only affected by it through immune-mediated effects on its fecundity. Finally, the relationship between St. robustus (both eggs and adult parasites) and FGMs was not linear, suggesting that only high levels of physiological stress influence parasite infection. Our findings highlight that the direction and magnitude of the stress–infection relationship may depend not only on the specific host–parasite system, but also on the different life stages of the same parasite.
Keywords: fecal glucocorticoid metabolites, invasive alien species, parasites, Sciurus carolinensis, stress, tree squirrel
In natural populations, animals are exposed to fluctuating environmental and social pressures. Free-ranging vertebrates can cope with challenging environmental or social factors (stressors) through changes in behavior or life history strategies, or through activation of the hypothalamic–pituitary–adrenal (HPA) axis, which increases the secretion of glucocorticoids (GCs, i.e., the steroid hormones cortisol or corticosterone, Boonstra 2013; Creel et al. 2013; Raulo and Dantzer 2018). Other physiological mechanisms, such as the production and release of catecholamines by the adrenal medulla, can also be involved in the stress response (Koolhaas et al. 2011; MacDougall-Shackleton et al. 2019). When the HPA axis is activated by a stressor, the subsequent short-term elevation of GCs results in several physiological responses, such as increased foraging behavior, energy uptake, or stimulation of the immune system, to help individuals to cope with the adverse event (Romero 2004; Crespi et al. 2013). However, when exposure to stressors occurs repeatedly or over a prolonged period of time, animals may experience chronic elevation of GCs with negative effects on survival and reproduction, and suppression of the immune system (Romero 2004; Glaser and Kiecolt-Glaser 2005; Bonier et al. 2009; Martin 2009; Malviya et al. 2018). Indeed, GCs may have detrimental effects on several components of the immune function, including decreased proliferation and altered ratios of the lymphocyte population, impairment of the antibody-mediated and cell-mediated responses and altered activity of inflammation–modulatory molecules (Webster Marketon and Glaser 2008). A reduction in the immune response efficiency can in turn increase a host’s vulnerability to parasites and pathogens: hence high levels of physiological stress can lead to higher levels of parasitic infection (prevalence and/or intensity of infection; Brown and Fuller 2006; Corlatti et al. 2012; Arlet et al. 2015; Martínez‐Mota et al. 2017). Additionally, experimental research revealed that parasites can act as stressors by themselves, inducing an increase in HPA axis activity (Pedersen and Greives 2008; Warne et al. 2011; Pérez et al. 2019) and leading to a vicious cycle, with more stressed animals having higher parasitic loads, which in turn will further increase stress (Beldomenico and Begon 2010, 2016). However, other studies failed to find any association between GCs and parasite infection (e.g., Goldstein et al. 2005; Monello et al. 2010), or detected a significant association in one sex, but not in the other (Lobato et al. 2008; Hammond et al. 2019), suggesting that stress–infection dynamics are highly specific on the host–parasite system under study (see St. Juliana et al. 2014).
With the development of noninvasive techniques to measure variation in the levels of GCs in wild animals (Touma and Palme 2005; Sheriff et al. 2011; Palme 2019), our understanding of the relationships between physiological state and host–parasite interactions in natural populations has improved considerably (Monello et al. 2010; Gervasi et al. 2016; Puehringer-Sturmayr et al. 2018). Cortisol or corticosterone levels can be measured directly from blood samples, whereas other biological matrices (hair, feces, urine, or other excreta) can be used to measure GCs metabolites (Sheriff et al. 2011). Blood circulating GCs will reflect instantaneous stress levels, whereas their metabolites in other matrices will represent a combination of both baseline and stress-induced GC levels. For this reason, measures of GC metabolites, especially in the feces, have been used as an integrated measure of circulating GCs over a specific period of time (Sheriff et al. 2011; Palme 2019).
Here, we used fecal glucocorticoids metabolites (hereafter FGMs) as a measure of long-term physiological stress (Boonstra 2013; Dantzer et al. 2014; Palme 2019; Santicchia et al. 2020a), to explore whether individual variation in FGMs was associated with variation in endoparasite infection in invasive populations of the Eastern gray squirrel Sciurus carolinensis in Northern Italy. Characterization of the physiological stress response in non-native animals is essential to invasion biology because physiological processes underpin how alien species cope with new environmental conditions in the introduction range. Therefore, in a previous study, we had investigated the relationship between gray squirrels’ FGM levels and personality (Santicchia et al. 2020a). Concerning parasites, alien species often have an impoverished parasite community compared with their native range (Torchin et al. 2003; Prenter et al. 2004; Dunn et al. 2012; Romeo et al. 2014b). They may, however, also introduce alien parasites or acquire local ones, leading to spillover and spillback processes (Kelly et al. 2009; Strauss et al. 2012) that may in turn affect interactions with native species through apparent competition (Hudson and Greenman 1998). Consequently, gaining a better understanding of the relationships between physiological stress and parasitic infection in invasive species is paramount, as they might differ from those documented for native species and may ultimately affect interspecific competition as well. For instance, invasive gray squirrels in Italy have fewer macroparasites (both ecto- and endoparasites) than in their native range and only one dominant gastrointestinal helminth Strongyloides robustus, which they introduced from North America and whose prevalence (% of infected individuals) in Italian populations ranges from 57% to 73% (Romeo et al. 2014b; Santicchia et al. 2019). Previous studies showed that this nematode likely mediates the competition between gray squirrels and Eurasian red squirrels S. vulgaris in Italy, since it spills over to the native species, causing a significant reduction in red squirrels’ activity (Romeo et al. 2015; Santicchia et al. 2020b). At the gastrointestinal level, gray squirrels are also frequently infected by coccidian protozoa (genus Eimeria), with prevalence in Italian populations well above 90% (Hofmannová et al. 2016). The control of gray squirrels for native species conservation is currently mandatory in the European Union (Regulation EU No. 1143/2014) and gives the opportunity to obtain direct measures of endoparasitic infections, which are essential to understand the impact of macroparasites on their hosts (Anderson and May 1979; Woolhouse 1992).
Here, we explored relationships between different indices of endoparasitic infection and FGMs to test the main hypothesis that individual variation in physiological stress enhances infection via immunosuppressive mechanisms that limit a host’s ability to cope with it. In detail, we expect higher levels of physiological stress (defined here as higher FGMs) to be associated with 1) higher amounts of St. robustus eggs and 2) higher amounts of coccidian oocysts shed with squirrel feces. However, we expect 3) no relationship between FGMs and St. robustus intensity of infection (number of adult worms/host), as the immune response against Strongyloides spp. nematodes is mainly directed at reducing worms’ size and fecundity, and not their survival (Wilkes et al. 2004; Romeo et al. 2014a). We monitored gray squirrels by capture–mark–recapture (CMR) and collected fresh fecal samples from marked individuals to measure FGMs, and to count helminth eggs and coccidian oocysts as indirect indices of infection by gastrointestinal parasites (Predictions 1 and 2). We also obtained direct counts of adult St. robustus after dissection of the intestinal tract from a smaller sample of animals culled in areas where the invasive species is controlled through culling (Prediction 3).
Materials and Methods
Study areas, trapping, and handling squirrels
We trapped gray squirrels in 5 study areas in Piedmont, Northern Italy, between November 2014 and December 2016. Four areas are small woodlands or parks (2.6–5.9 ha), the 5th is a larger woodland (37 ha) with mature broadleaf trees and few ornamental conifers, surrounded by agricultural landscapes (details in Santicchia et al. 2020a). The choice of areas with comparable habitat type and food availability allowed us to avoid potential confounding effects of food resource abundance on the FGMs–parasite relationship. We estimated squirrel population density in the different study areas using the minimum number of animals known to be alive during each trapping session, as described in Wauters et al. (2008).
Once every 2 months, in each study area, we carried out 2 (1 area) to 3 (other 4 areas) CMR sessions lasting 4 days, to collect fecal samples for the extraction of FGMs and parasitological analysis (see “Parasitological examination” and “Extraction and quantification of FGMs” sections). We trapped squirrels using Tomahawk live traps (model 202, Tomahawk Live Trap Co., WI, USA) with a fine mesh added underneath to prevent contamination between urine and feces. Traps were baited with hazelnuts and checked 3 times/day to minimize time in trap and time since defecation (maximum 3 h). After the collection of samples, we cleaned traps and fine mesh, removing any fecal remains. We marked each captured squirrel individually using numbered metal ear tags (type 1003 S, National Band and Tag, Newport, KY, USA). Each squirrel was sexed based on external genitalia and body mass was taken to the nearest 5 g using a Pesola spring balance. Additionally, we made use of individuals culled in winter 2015 during invasive species control activities. These gray squirrels were euthanized by CO2 inhalation following international animal welfare guidelines (Close et al. 1996, 1997; Leary et al. 2013), and carcasses were stored at −20°C until examination. Trapping and handling squirrels complied with current laws on animal research in Italy and were carried out with permit of the authorities for wildlife research and management of Turin and Cuneo Provinces (respectively, D.D. 294-34626 of 2014 and Prot. n. 0002624 of 13 January 2014) and of the Italian Institute for Environmental Protection and Research (ISPRA). All of these procedures abided by American Society of Mammalogists guidelines (Sikes and Gannon 2011).
Parasitological examination
We stored fecal samples from gray squirrels for parasitological analysis dry at 4°C and examined them within 3 days from collection to avoid eggs hatching. Samples were analyzed through quantitative McMaster technique (MAFF) by diluting feces with saturated NaCl solution (1,200 g/L) and counting the number of St. robustus eggs (n = 194 samples, dilution 1:10) and coccidia oocysts (n = 77 samples, dilution 1:40) in the 2 chambers of a McMaster slide (Romeo et al. 2014a). We further examined 47 culled squirrels, for which we also had FGM measures, for adult helminths following standard parasitological procedures (Romeo et al. 2013, 2014b). Briefly, the intestinal tract from duodenum to rectum was removed from carcasses, dissected longitudinally, washed with tap water, and its content was flushed through 2 sieves (lumen 0.40 and 0.038 mm, respectively). The content of each tract was then examined under a stereomicroscope (×10 magnification), and adult St. robustus were counted (Romeo et al. 2014b; Santicchia et al. 2019).
Extraction and quantification of FGMs
Fecal samples collected for GC metabolite analysis (n = 282 samples) were stored dry at −20°C within 2 h from collection and until laboratory analysis. We used a 5α-pregnane-3β, 11β, 21-triol-20-one enzyme immunoassay (EIA) to measure FGM concentrations (ng/g dry feces; Touma et al. 2003). This EIA detects GC metabolites with a 5α-3β, 11β-diol structure (for cross-reactivity see Touma et al. 2003, 2004) and has been successfully validated for use in gray squirrels (Bosson et al. 2013). We analyzed samples in duplicate. Pools of gray squirrel feces extracts were used as intra-assay controls at dilutions of 1:50 (∼30% binding) and 1:400 (∼70% binding). Average intra-assay coefficients of variation (CVs) were 8.7% and 14.8%, respectively, for pools diluted 1:50 and 1:400. Inter-assay CVs were estimated from standards of known concentration with a high (n = 25 plates, 12.4% binding) and low (n = 25 plates, 80.9% binding) concentration that had inter-assay CVs of 15.2% and 9.1%, respectively. A previous study that validated the EIA for this species showed that fecal samples collected in this species represent an integrated measure of cortisol secreted between 12 and 24 h before defecation (Bosson et al. 2013), hence we sampled only squirrels that had not been trapped or handled within 72 h prior to capture to exclude effects of capture stress on FGM concentrations.
Statistical analyses
To test our predictions, we explored the effect of FGMs on 3 different parameters of parasite infection used as response variables in separate models: 1) St. robustus egg shedding (number of St. robustus eggs from gray squirrel feces counted in the McMaster slide, n = 194 samples from 135 different animals); 2) coccidia oocyst shedding (number of oocysts from gray squirrel feces counted in the McMaster slide, n = 77 samples, 1 per individual); and 3) the intensity of infection by adult St. robustus (number of worms per examined host, n = 47, 1 per individual) from direct counts of worms. For Models 2 and 3, we used generalized linear models (GLMs) with a negative binomial error distribution to account for the aggregate distribution of parasites within the host population (Shaw et al. 1998). In Model 1, we included host identity as a random term to account for repeated measures from the same individual, but the mixed model failed to converge when using a negative binomial error structure, hence egg counts were log-transformed (ln + 1) and analyzed through a linear mixed model (LMM) with Gaussian error distribution.
For all the 3 analyses, we started from a saturated model including all the explanatory variables listed below, and selected a minimal adequate model through log-likelihood ratio tests on nested models (Lewis et al. 2011). Full model outputs are reported in Supplementary Table S1. To consider possible curvilinear effects of FGM concentration, its second-order orthogonal polynomial effect was included in all full models. In all models, we also added gray squirrel body mass and host population density as covariates, since they are known to influence parasite abundance and/or intensity of infection in mammals (Arneberg et al. 1998; Wilson et al. 2002) and specifically in gray squirrels (Romeo et al. 2014b; Santicchia et al. 2019). Sampling season was included only in Models 1 and 2, because adult helminth counts in Model 3 were obtained exclusively from animals culled in winter. In all full models (Supplementary Table S1), we considered first-order interactions between polynomial FGMs and all the covariates, except for Model 2, where the interaction between FGMs and season was not included to avoid overparametrization. Finally, in Models 1 and 2, we included sampling daytime (i.e., morning or afternoon) to account for potential daily variability in eggs and oocysts shedding (Villanúa et al. 2006; López et al. 2007). All the explanatory continuous variables were standardized ([x − mean] / SD) prior to analysis to reduce multicollinearity in the presence of a polynomial term (in all full models and for all predictors VIF < 3.4, Zuur et al. 2010).
Egg and oocyst counts in figures are reported as number/gram of feces, after multiplication of the McMaster counts for a dilution factor (x × 33 and x × 133, respectively). Unless otherwise specified, all values and parameter estimates are reported as mean (±SE). All the statistical analyses were carried out using the software RStudio 1.2.5001 (R Studio Team 2019) in R 3.6.1 (R Development Core Team 2019), using the function poly for orthogonal polynomial effects and MASS and lme4 packages to fit GLMs and the LMM, respectively.
Results
We obtained 282 FGM measures from 172 different gray squirrels (77 males and 95 females). Totally, 90 samples were collected in spring, 68 in autumn, and 124 in winter. Sample size in each of the 5 study areas ranged from a minimum of 25 to a maximum of 85 squirrels. Overall mean FGM concentration (±SE) was 11,993 ± 534 ng/g dry feces (range = 1,226–50,988; CV = 74.7%; Figure 1). Strongyloides robustus was the main gastrointestinal helminth infecting gray squirrels, as the infection (either adult worms or eggs) was detected in 229/282 samples (81.2%). Other helminths were rarely found (16/282 samples, 5.7%) and were thus not considered in further analyses. Although counts of coccidia oocysts were carried out on a subset of 77 animals, they were detected in 249/252 samples examined through copromicroscopy (98.8%). Detailed results of parasitological analysis with a description of St. robustus and coccidia infection parameters in the sampled population are presented in Table 1. Population density in our sampling sites ranged from 0.9 to 13.4 squirrels/ha and squirrels’ body mass from 365 to 695 g. FGM levels were higher during autumn (Kruskal–Wallis test: χ22=17.2; P = 0.0002) and were positively correlated with host body mass (Kendall’s τ = 0.095; z = 2.55; P = 0.01), but not with host density (P = 0.29).
Figure 1.
Frequency distribution of FGM concentration in fecal samples (n = 282) from Eastern gray squirrels Sciurus carolinensis. Dashed line indicates the 80th percentile.
Table 1.
Endoparasite infection parameters in invasive gray squirrels S. carolinensis: sample size, prevalence (percent of positive samples/examined samples) and mean intensity of infection in positive samples
| Variable | N | Prevalence ± SE (n) | Intensity of infection |
|
|---|---|---|---|---|
| Mean ± SE | Range | |||
| Adult St. robustus | 47 | 72.3 ± 6.5 (34) | 8.4 ± 1.3a | 1–26a |
| Strongyloides robustus eggs | 194c | 84.0 ± 2.6 (163) | 421 ± 34b | 33–2,409b |
| Coccidian oocysts | 77 | 98.7 ± 1.1 (76) | 259,330 ± 41,398d | 5,775–2,029,580d |
Worms/host.
Eggs/gram of feces.
From 135 individuals.
Oocysts/g of feces.
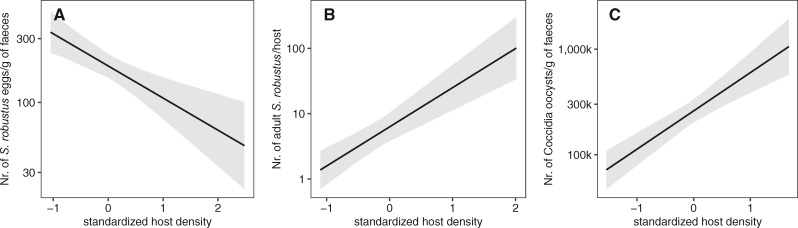
The amount of St. robustus eggs shed by gray squirrels (Model 1) varied curvilinearly with FGM concentration (see Table 2 for effect sizes and P-values). In particular, at low FGM values, there was no clear association between FGM levels and egg counts, whereas at higher FGM levels we observed a marked increase in St. robustus eggs shedding with increasing FGM levels (Figure 2A). Additionally, egg counts were significantly influenced by season (with higher values in autumn than in spring and winter) and negatively affected by host population density (Table 2, Figure 3A).
Table 2.
Minimum selected models explaining observed variation in endoparasite infection parameters in gray squirrels Sc. carolinensis
| Response variable | Explanatory variables | Parameter estimate(±SE) | Analysis of variance | P-value |
|---|---|---|---|---|
| Strongyloides robustus egg counts (log-transformed) | FGMs | −1.75 ± 3.74 | ||
| FGMs2 | 2.30 ± 4.06 | F 2,177 = 5.1a | 0.0067 | |
| Host density | −0.52 ± 0.12 | F 1,164 = 19.1 | <0.0001 | |
| Seasonb | ||||
| Spring | −0.016 ± 0.14 | |||
| Winter | −0.66 ± 0.13 | F 2,157 = 13.1 | <0.0001 | |
| Polynomial FGMs2: season | F 4,179 = 0.6a | 0.66 | ||
| Strongyloides robustus adult worm counts | FGMs | −4.90 ± 1.52 | ||
| FGMs2 | −2.73 ± 1.32 | Χ 2 2 = 12.9a | 0.0016 | |
| Body mass | 0.43 ± 0.20 | Χ 2 1 = 4.7 | 0.029 | |
| Host density | 1.38 ± 0.23 | Χ 2 1 = 35.2 | <0.0001 | |
| Coccidia oocyst counts | Body mass | −0.24 ± 0.12 | Χ 2 1 = 4.2 | 0.041 |
| Host density | 0.83 ± 0.14 | Χ 2 1 = 33.4 | <0.0001 | |
| Seasonb | ||||
| Spring | 0.48 ± 0.19 | |||
| Winter | 1.04 ± 0.20 | Χ 2 2 = 31.4 | <0.0001 |
All the continuous variables were standardized prior to analysis. The egg counts model includes squirrels’ IDs as a random term to account for repeated measures.
Combined test for the quadratic polynomial function of standardized FGM.
Autumn held as reference level.
Figure 2.
Marginal predicted effect of standardized FGMs on (A) St. robustus egg counts and (B) adult St. robustus worm counts in Eastern gray squirrels Sciurus carolinensis. Bands indicate 95% CI.
Figure 3.
Marginal predicted effect (log-scale) of standardized host density on (A) St. robustus egg counts, (B) adult St. robustus worm counts and (C) coccidia oocyst counts in Eastern gray squirrels (Sciurus carolinensis). Bands indicate 95% CI.
The number of adult St. robustus infecting squirrel hosts (Model 3) varied curvilinearly with FGMs as well (Table 2). However, in this case, the relationship was negative: low FGMs had a negligible effect on St. robustus abundance, whereas above a certain threshold, FGM levels were associated with a significant decrease in the number of parasites (Figure 2B). Strongyloides robustus abundance was also influenced positively by host density and host body mass (Table 2, Figure 3B).
Finally, we found no relationship between oocysts counts and FGMs (Model 2): the shedding of coccidia oocysts only increased with host density (Figure 3C), decreased with body mass and was influenced by season, with lower values in autumn than in winter and spring (Table 2).
Discussion
We explored whether differences in measure of physiological stress levels (i.e., FGMs) among Eastern gray squirrels were associated with individual variation in endoparasite infection, measured with both direct (i.e., intensity of infection by adult helminths) and indirect parasitic counts (i.e., helminth eggs and coccidian oocysts shed with feces). Our data revealed that in this host species FGM levels do not influence coccidia infection, but were curvilinearly related with infection by the nematode St. robustus. However, the direction of the association with this parasite varied depending on St. robustus life stage, with FGMs showing a positive association with egg counts and an opposite, inverse relationship with adult, parasitic worms.
Strongyloides robustus egg counts in gray squirrels’ feces varied with physiological stress as predicted (Prediction 1), with higher FGM levels associated with increased eggs shedding. Previous research on gray squirrels showed that individual fecundity of St. robustus is negatively related to the number of worms infecting the host (Romeo et al. 2014a). This density dependence in fecundity is due to the host immune response, which increases with intensity of infection, inducing a reduction in individual worms’ size and eggs output, as experimentally observed in St. ratti infecting rodents (Paterson and Viney 2002; Wilkes et al. 2004; Bleay et al. 2007). Consequently, high physiological stress levels might enhance St. robustus fecundity through immunosuppressive mechanisms and “stressed” hosts may thus be less efficient at controlling helminths’ size and reproductive output. On the contrary, although we expected no association (Prediction 3) between FGMs and adult St. robustus counts, the 2 variables were inversely related. It is not clear why higher physiological stress levels should be associated with reduced nematode intensity and, in contrast with our findings, the congeneric nematode St. ratti showed both higher intensity of infection and increased fecundity in experimentally infected rats following administration of exogenous corticosteroids (Wilkes et al. 2004). However, negative relationships between measures of parasitism and stress levels were observed in other host–parasite systems, indicating that the direction of the association between stress and infection is highly dependent on the specific system under study. For instance, in females of 2 chipmunk species, Tamias alpinus and T. speciosus, FGMs were negatively correlated with flea abundance (Hammond et al. 2019). In male sand lizards Lacerta agilis, individuals in good body condition were found to have lower corticosterone and lower numbers of ectoparasites than low-condition individuals, but in contrast, there was an opposite, negative relationship between corticosterone titer and endoparasite load (Lindsay et al. 2016). Similarly, experimentally stressed capybaras Hydrochoerus hydrochaeris had higher coccidia loads, but, conversely, fewer nematodes than control individuals (Eberhardt et al. 2013); and in a multispecies host–parasite experiment, St. Juliana et al. (2014) found that infestation by some flea species, but not by others, caused a significant decrease of FGMs in some, but not in other rodent species. In any case, having lower St. robustus intensity of infection does not necessarily mean that squirrels with high FGMs suffer a lower-parasitic impact, since mounting a less efficient immune response (as a consequence to high levels of circulating GCs) may relax the constraints on worms’ size (Viney et al. 2006), which is directly related to pathogenicity in most nematode species (Skorping et al. 1991).
Although we did not explicitly test for the hypothesis that nematode infection induces an increase in GC levels, this negative association between FGMs and adult worm counts suggests that St. robustus does not act as a stressor, at least when parasitic load is low to medium. Indeed, prevalence of infection with St. robustus in our study populations was similar to previous studies in Italy (Romeo et al. 2014b; Santicchia et al. 2019) and in the gray squirrel’s natural range (Davidson 1976), but the intensities of infection documented here were lower (8.4 ± 1.3 helminths per infected host in this study; 16.9 ± 2.1 in a larger sample from gray squirrels in Northern Italy, Romeo et al. 2014b). Moreover, in gray squirrels from the Southeastern United States (native range), severe clinical signs of St. robustus infection were recorded only in hosts infected with more than 150 helminths (Davidson 1976). It is thus possible that in this host–parasite system severe pathogenic effects—and induction of elevated GCs—do occur, but with intensities of infection higher than we recorded here.
Despite the associations FGM–adult worms and FGM–egg counts had opposite directions, both relationships were curvilinear, indicating that elevated FGMs is associated to St. robustus infection only above a certain FGMs threshold. Interindividual variation in FGM levels among gray squirrels was high, suggesting that at least some squirrels were experiencing physiological stress. The frequency distribution of FGM levels within our sample was however highly skewed; hence, it might be that most of the individuals experienced “baseline” levels of GCs, with only a few squirrels experiencing GC levels that were high enough to influence infection.
Similarly to FGMs, also host density had a differential effect on the 2 St. robustus life stages; whereas adult worms abundance increased with gray squirrels’ density, egg counts, in contrast, decreased. The former result is consistent with previous studies and is probably a consequence of an overall higher environmental contamination leading to an higher chance for squirrels to encounter parasite infective stages and become infected or reinfected (Romeo et al. 2014b; Santicchia et al. 2019). On the contrary, where host density is high, we observed on average lower amounts of eggs in squirrels’ feces. A tentative explanation for this opposite effect may lie again in the immune response against this nematode: individuals living in high-density areas have on average higher parasite loads and may mount therefore a more vigorous immune response that will in turn lead to reduced St. robustus individual fecundity and possibly to lower total egg shedding per animal. In any case, neither the association between FGMs and St. robustus worms and eggs was dependent from squirrel density, nor were FGM concentrations correlated with it. Consequently, in both low- and high-density sites, individuals exhibiting higher FGM levels appear to be the ones responsible for the greatest egg shedding within the population, thus potentially acting as superspreaders (Galvani and May 2005; Stein 2011; VanderWaal and Ezenwa 2016). This relationship might have potential implications on parasite transmission dynamics involving alien species under enforced control. For instance, disturbance caused by invasive culling methods such as shooting, might increase chronic stress and consequently egg shedding by invaders, with repercussions on native species. Or, for example, control through trapping might artificially select for bolder, less stressful individuals and in turn favor shyer, more stressful animals which may contribute more to environmental contamination by parasite infective stages. However, the latter does not seem to be the case for gray squirrels, since in this species FGMs levels are not related to individual personality (Santicchia et al. 2020a).
Finally, we had expected a positive association between FGM levels and coccidian oocysts, (Prediction 2), but no significant relationship was found. Coccidian protozoa are highly species-specific and in most host species are known to be mildly to severely pathogenic (Yun et al. 2000). Consequently, coccidiosis normally induces a strong response and a long-lasting immunity such that the highest prevalence and intensity are observed in juveniles (Yun et al. 2000; Daugschies and Najdrowski 2005). Indeed, high-GC levels have been found to be positively associated with coccidia infection in several systems (e.g., Eberhardt et al. 2013 on capybaras; Lindsay et al. 2016 on sand lizards). However, coccidia infection in tree squirrels appears to be more of a chronic nature, since in several species, including gray squirrels, we observe prevalence values well above 80% in all age classes and infections are mostly subclinical (Bertolino et al. 2003; Ball et al. 2014; Hofmannová et al. 2016). This suggests that coccidia do not elicit a strong immune response in tree squirrels, either because the pathogen evolved a reduced virulence or because hosts adopt a tolerance–strategy (Råberg et al. 2009), and this would explain the lack of association with physiological stress levels observed in this study. However, it must be underlined that FGMs represent only one possible measure of the stress response, which is indeed multifaceted (MacDougall-Shackleton et al. 2019) and we cannot therefore exclude that other components of the HPA axis are associated with this infection.
In conclusion, in squirrels of the genus Sciurus, we already know that infection may be influenced by phenotypic parameters such as host body mass or personality (Romeo et al. 2013, 2014a; Santicchia et al. 2019) and/or by extrinsic factors such as seasonality, host density, food availability, or even the degree of habitat fragmentation (Romeo et al. 2013, 2014a; Santicchia et al. 2015). Here we added the effect of one measure of the physiological stress response, showing that squirrels with higher FGM levels are those responsible for the greatest helminth egg shedding. However, our initial hypotheses were only partially confirmed, as FGM levels were negatively associated with the abundance of adult helminths and not associated with coccidia oocysts. These findings highlight how the effects of physiological stress on infection are extremely heterogeneous and vary not only with the specific parasite considered, but also with different life stages of the same parasite, depending in turn on the complex immune mechanisms governing the host–parasite relationship. Finally, in the context of biological invasions, our results may raise interesting questions about the potential role of control activities in altering stress–parasite dynamics in invading hosts, affecting in turn parasites transmission dynamics between alien and native species.
Supplementary Material
Acknowledgments
Thanks to Zainab Almusawi and Teera Losch for helping in laboratory analysis, Candice Gagnaison, Laure Vanlauwe, and Mattia Panzeri for assistance with the fieldwork. We are grateful to the private land owners for access to their estates. Three anonymous reviewers provided constructive comments that helped us to improve the article.
Funding
This research is part of the project “Immune response of invasive species as a driver of parasite dynamics” funded by the Department of Veterinary Medicine, Università degli Studi di Milano (Piano di Sostegno alla Ricerca, Linea 2, to N.F.). Funds of University of Michigan to B.D.
Authors’ Contributions
C.R., L.A.W., and N.F. designed the study and the analyses. Fieldwork and data collection were done by F.S., L.A.W., C.R., and A.M. F.S. carried out laboratory analyses and B.D. supplied laboratory space, equipment, and coordinated laboratory analyses. R.P. produced and supplied reagents for lab analyses. C.R. carried out statistical analyses with the contribution of N.F. The manuscript was drafted by C.R. and L.A.W. All the authors contributed to improve the manuscript and gave approval for publication.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
- Anderson RM, May RM, 1979. Population biology of infectious diseases: part I. Nature 280:361–367. [DOI] [PubMed] [Google Scholar]
- Arlet ME, Chapman CA, Isbell LA, Molleman F, Mänd R et al. , 2015. Social and ecological correlates of parasitic infections in adult male gray-cheeked mangabeys (Lophocebus albigena). Int J Primatol 36:967–986. [Google Scholar]
- Arneberg P, Skorping A, Grenfell B, Read AF, 1998. Host densities as determinants of abundance in parasite communities. Proc Biol Sci 265:1283–1289. [Google Scholar]
- Ball SJ, Daszak P, Sainsbury AW, Snow KR, 2014. Coccidian parasites of red squirrels Sciurus vulgaris and grey squirrels (Sciurus carolinensis) in England. J Nat Hist 48:1225–1230. [Google Scholar]
- Beldomenico PM, Begon M, 2010. Disease spread, susceptibility and infection intensity: vicious circles?. Trends Ecol Evol 25:21–27. [DOI] [PubMed] [Google Scholar]
- Beldomenico PM, Begon M, 2016. Stress-host-parasite interactions: a vicious triangle? doi: 10.14409/favecv.v14i1/2.5160.
- Bertolino S, Wauters LA, De Bruyn L, Canestri-Trotti G, 2003. Prevalence of coccidia parasites (Protozoa) in red squirrels Sciurus vulgaris: effects of host phenotype and environmental factors. Oecologia 137:286–295. [DOI] [PubMed] [Google Scholar]
- Bleay C, Wilkes CP, Paterson S, Viney ME, 2007. Density-dependent immune responses against the gastrointestinal nematode Strongyloides ratti. Int J Parasitol 37:1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC, 2009. Do baseline glucocorticoids predict fitness?. Trends Ecol Evol 24:634–642. [DOI] [PubMed] [Google Scholar]
- Boonstra R, 2013. The ecology of stress: a marriage of disciplines. Funct Ecol 27:7–10. [Google Scholar]
- Bosson CO, Palme R, Boonstra R, 2013. Assessing the impact of live-capture, confinement, and translocation on stress and fate in eastern gray squirrels. J Mammal 94:1401–1411. [Google Scholar]
- Brown TT, Fuller CA, 2006. Stress and parasitism of white-footed mice Peromyscus leucopus in dry and floodplain environments. Can J Zool 84:1833–1839. [Google Scholar]
- Close B, Banister K, Baumans V, Bernoth E-M, Bromage N et al. , 1996. Recommendations for euthanasia of experimental animals: part 1. Lab Anim 30:293–316. [DOI] [PubMed] [Google Scholar]
- Close B, Banister K, Baumans V, Bernoth E-M, Bromage N et al. , 1997. Recommendations for euthanasia of experimental animals: part 2. Lab Anim 31:1–32. [DOI] [PubMed] [Google Scholar]
- Corlatti L, Béthaz S, von Hardenberg A, Bassano B, Palme R et al. , 2012. Hormones, parasites and male mating tactics in Alpine chamois: identifying the mechanisms of life history trade-offs. Anim Behav 84:1061–1070. [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR, 2013. The ecology of stress: effects of the social environment. Funct Ecol 27:66–80. [Google Scholar]
- Crespi EJ, Williams TD, Jessop TS, Delehanty B, 2013. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals?. Funct Ecol 27:93–106. [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ, 2014. Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species?. Conserv Physiol 2. doi: 10.1093/conphys/cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugschies A, Najdrowski M, 2005. Eimeriosis in cattle: current understanding. J Vet Med B Infect Dis Vet Public Health 52:417–427. [DOI] [PubMed] [Google Scholar]
- Davidson WR, 1976. Endoparasites of selected populations of gray squirrels Sciurus carolinensis in the southeastern United States. Proc Helminthol Soc Wash 43:211–217. [Google Scholar]
- Dunn AM, Torchin ME, Hatcher MJ, Kotanen PM, Blumenthal DM et al. , 2012. Indirect effects of parasites in invasions. Funct Ecol 26:1262–1274. [Google Scholar]
- Eberhardt AT, Costa SA, Marini MR, Racca A, Baldi CJ et al. , 2013. Parasitism and physiological trade-offs in stressed capybaras. PLoS ONE 8:e70382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani AP, May RM, 2005. Dimensions of superspreading. Nature 438:293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi SS, Burkett-Cadena N, Burgan SC, Schrey AW, Hassan HK et al. , 2016. Host stress hormones alter vector feeding preferences, success, and productivity. Proc Biol Sci 283:20161278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, 2005. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5:243–251. [DOI] [PubMed] [Google Scholar]
- Goldstein EJ, Millspaugh JJ, Washburn BE, Brundige GC, Raedeke KJ, 2005. Relationships among fecal lungworm loads, fecal glucocorticoid metabolites, and lamb recruitment in free-ranging rocky mountain bighorn sheep. J Wildl Dis 41:416–425. [DOI] [PubMed] [Google Scholar]
- Hammond TT, Hendrickson CI, Maxwell TL, Petrosky AL, Palme R et al. , 2019. Host biology and environmental variables differentially predict flea abundances for two rodent hosts in a plague-relevant system. Int J Parasitol Parasit Wildl 9:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmannová L, Romeo C, Štohanzlová L, Jirsová D, Mazzamuto MV et al. , 2016. Diversity and host specificity of coccidia (Apicomplexa: Eimeriidae) in native and introduced squirrel species. Eur J Protistol 56:1–14. [DOI] [PubMed] [Google Scholar]
- Hudson P, Greenman J, 1998. Competition mediated by parasites: biological and theoretical progress. Trends Ecol Evol 13:387–390. [DOI] [PubMed] [Google Scholar]
- Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM, 2009. Parasite spillback: a neglected concept in invasion ecology. Ecology 90:2047–2056. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G et al. , 2011. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35:1291–1301. [DOI] [PubMed] [Google Scholar]
- Leary SL, Underwood W, Anthony R, Cartner S, Corey D et al. , 2013. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Schaumburg, IL: American Veterinary Medical Association.
- Lewis F, Butler A, Gilbert L, 2011. A unified approach to model selection using the likelihood ratio test. Methods Ecol Evol 2:155–162. [Google Scholar]
- Lindsay WR, Wapstra E, Silverin B, Olsson M, 2016. Corticosterone: a costly mediator of signal honesty in sand lizards. Ecol Evol 6:7451–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato E, Merino S, Moreno J, Morales J, Tomás G et al. , 2008. Corticosterone metabolites in blue tit and pied flycatcher droppings: effects of brood size, ectoparasites and temperature. Horm Behav 53:295–305. [DOI] [PubMed] [Google Scholar]
- López G, Figuerola J, Soriguer R, 2007. Time of day, age and feeding habits influence coccidian oocyst shedding in wild passerines. Int J Parasitol 37:559–564. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Bonier F, Romero LM, Moore IT, 2019. Glucocorticoids and “stress” are not synonymous. Integr Org Biol 1. doi: 10.1093/iob/obz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya M, Kumar V, Mandal D, Shekhar Sarkar M, Nigam P et al. , 2018. Correlates of physiological stress and habitat factors in reintroduction-based recovery of tigers Panthera tigris. Hystrix 29:195–201. [Google Scholar]
- Martin LB, 2009. Stress and immunity in wild vertebrates: timing is everything. Gen Comp Endocrinol 163:70–76. [DOI] [PubMed] [Google Scholar]
- Martínez-Mota R, Garber PA, Palme R, Gillespie TR, 2017. The relative effects of reproductive condition, stress, and seasonality on patterns of parasitism in wild female black howler monkeys Alouatta pigra. Am J Primatol 79:e22669. [DOI] [PubMed] [Google Scholar]
- Monello RJ, Millspaugh JJ, Woods RJ, Gompper ME, 2010. The influence of parasites on faecal glucocorticoid metabolite levels in raccoons: an experimental assessment in a natural setting: parasites and faecal glucocorticoid metabolites in raccoons. J Zool 282:100–108. [Google Scholar]
- Palme R, 2019. Non-invasive measurement of glucocorticoids: advances and problems. Physiol Behav 199:229–243. [DOI] [PubMed] [Google Scholar]
- Paterson S, Viney ME, 2002. Host immune responses are necessary for density dependence in nematode infections. Parasitology 125:283–292. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Greives TJ, 2008. The interaction of parasites and resources cause crashes in a wild mouse population. J Anim Ecol 77:370–377. [DOI] [PubMed] [Google Scholar]
- Pérez JM, Molina L, Ureña-Gutiérrez B, Espinosa J, López-Montoya AJ et al. , 2019. Individual stress responses to Sarcoptes scabiei infestation in Iberian ibex, Capra pyrenaica. Gen Comp Endocrinol 281:1–6. [DOI] [PubMed] [Google Scholar]
- Prenter J, MacNeil C, Dick JTA, Dunn AM, 2004. Roles of parasites in animal invasions. Trends Ecol Evol 19:385–390. [DOI] [PubMed] [Google Scholar]
- Puehringer-Sturmayr V, Wascher CAF, Loretto M-C, Palme R, Stoewe M et al. , 2018. Seasonal differences of corticosterone metabolite concentrations and parasite burden in northern bald ibis Geronticus eremita: the role of affiliative interactions. PLoS ONE 13:e0191441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Graham AL, Read AF, 2009. Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc Lond B Biol Sci 364:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulo A, Dantzer B, 2018. Associations between glucocorticoids and sociality across a continuum of vertebrate social behavior. Ecol Evol 8:7697–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species, 2014. OJ L 317.
- Romeo C, Pisanu B, Ferrari N, Basset F, Tillon L et al. , 2013. Macroparasite community of the Eurasian red squirrel Sciurus vulgaris: poor species richness and diversity. Parasitol Res 112:3527–3536. [DOI] [PubMed] [Google Scholar]
- Romeo C, Wauters LA, Cauchie S, Martinoli A, Matthysen E et al. , 2014. a. Faecal egg counts from field experiment reveal density dependence in helminth fecundity: Strongyloides robustus infecting grey squirrels Sciurus carolinensis. Parasitol Res 113:3403–3408. [DOI] [PubMed] [Google Scholar]
- Romeo C, Wauters LA, Ferrari N, Lanfranchi P, Martinoli A et al. , 2014. b. Macroparasite fauna of alien grey squirrels Sciurus carolinensis: composition, variability and implications for native species. PLoS ONE 9:e88002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo C, Ferrari N, Lanfranchi P, Saino N, Santicchia F et al. , 2015. Biodiversity threats from outside to inside: effects of alien grey squirrel Sciurus carolinensis on helminth community of native red squirrel Sciurus vulgaris. Parasitol Res 114:2621–2628. [DOI] [PubMed] [Google Scholar]
- Romero LM, 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255. [DOI] [PubMed] [Google Scholar]
- Santicchia F Romeo C Martinoli A Lanfranchi P Wauters LA et al., 2015. Effects of habitat quality on parasite abundance: do forest fragmentation and food availability affect helminth infection in the Eurasian red squirrel? J. Zool. 296:38–44. [Google Scholar]
- Santicchia F, Romeo C, Ferrari N, Matthysen E, Vanlauwe L et al. , 2019. The price of being bold? Relationship between personality and endoparasitic infection in a tree squirrel. Mamm Biol 97:1–8. [Google Scholar]
- Santicchia F, Wauters LA, Dantzer B, Westrick SE, Ferrari N et al. , 2020. a. Relationships between personality traits and the physiological stress response in a wild mammal. Curr Zool 66:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicchia F, Wauters LA, Piscitelli AP, Van Dongen S, Martinoli A et al. , 2020. b. Spillover of an alien parasite reduces expression of costly behaviour in native host species. J Anim Ecol. doi: 10.1111/1365-2656.13219. [DOI] [PubMed] [Google Scholar]
- Shaw DJ, Grenfell BT, Dobson AP, 1998. Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117:597–610. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R, 2011. Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887. [DOI] [PubMed] [Google Scholar]
- Sikes RS, Gannon WL, 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 92:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorping A, Read AF, Keymer AE, 1991. Life history covariation in intestinal nematodes of mammals. Oikos 60:365–372. [Google Scholar]
- St. Juliana JR, Khokhlova IS, Wielebnowski N, Kotler BP, Krasnov BR, 2014. Ectoparasitism and stress hormones: strategy of host exploitation, common host-parasite history and energetics matter. Cotter S (ed.). J Anim Ecol 83:1113–1123. [DOI] [PubMed] [Google Scholar]
- Stein RA, 2011. Super-spreaders in infectious diseases. Int J Infect Dis 15:e510–e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A, White A, Boots M, 2012. Invading with biological weapons: the importance of disease-mediated invasions. Funct Ecol 26:1249–1261. [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM, 2003. Introduced species and their missing parasites. Nature 421:628–630. [DOI] [PubMed] [Google Scholar]
- Touma C, Palme R, 2005. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Sci 1046:54–74. [DOI] [PubMed] [Google Scholar]
- Touma C, Palme R, Sachser N, 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22. [DOI] [PubMed] [Google Scholar]
- Touma C, Sachser N, Möstl E, Palme R, 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278. [DOI] [PubMed] [Google Scholar]
- VanderWaal KL, Ezenwa VO, 2016. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct Ecol 30:1606–1622. [Google Scholar]
- Villanúa D, Pérez-Rodríguez L, Gortázar C, Höfle U, Viñuela J, 2006. Avoiding bias in parasite excretion estimates: the effect of sampling time and type of faeces. Parasitology 133:251–259. [DOI] [PubMed] [Google Scholar]
- Viney ME, Steer MD, Wilkes CP, 2006. The reversibility of constraints on size and fecundity in the parasitic nematode Strongyloides ratti. Parasitology 133:477–483. [DOI] [PubMed] [Google Scholar]
- Warne RW, Crespi EJ, Brunner JL, 2011. Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct Ecol 25:139–146. [Google Scholar]
- Wauters LA, Githiru M, Bertolino S, Molinari A, Tosi G et al. , 2008. Demography of alpine red squirrel populations in relation to fluctuations in seed crop size. Ecography 31:104–114. [Google Scholar]
- Webster Marketon JI, Glaser R, 2008. Stress hormones and immune function. Cell Immunol 252:16–26. [DOI] [PubMed] [Google Scholar]
- Wilkes CP, Thompson FJ, Gardner MP, Paterson S, Viney ME, 2004. The effect of the host immune response on the parasitic nematode Strongyloides ratti. Parasitology 128:661–669. [DOI] [PubMed] [Google Scholar]
- Wilson K, Bjørnstad ON, Dobson AP, Merler S, Poglayen G et al. , 2002. Heterogeneities in macroparasite infections: patterns and processes In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek JAP, Dobson AP, editors. The Ecology of Wildlife Diseases. Oxford: Oxford University Press; 6–44. [Google Scholar]
- Woolhouse MEJ, 1992. A theoretical framework for the immunoepidemiology of helminth infection. Parasite Immunol 14:563–578. [DOI] [PubMed] [Google Scholar]
- Yun CH, Lillehoj HS, Lillehoj EP, 2000. Intestinal immune responses to coccidiosis. Dev Comp Immunol 24:303–324. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS, 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.