Abstract
Anorexia nervosa (AN) severely impacts individual’s mental and physical health as well as quality of life. In 21% of cases no durable response to conservative treatment can be obtained. The serious course of the disease in the most severely affected patients justifies invasive treatment options. One of the treatment methods increasingly used in recent years is deep brain stimulation (DBS). A 42-year-old woman suffering from chronic AN of the bulimic subtype shows a 46.9% weight gain and a subjective increase in quality of life, 12 months after bilateral nucleus accumbens (NAcc) DBS implantation. No improvement in comorbid depression could be achieved. DBS of the NAcc is a treatment option to be considered in severe AN when conventional treatment modalities recommended by evidence-based guidelines have not been able to bring lasting relief to the patient’s suffering.
Keywords: neurosurgery, eating disorders, psychiatry
Background
Anorexia nervosa (AN) is a severe mental health disorder leading to physical and psychosocial morbidity. AN has the highest mortality rate among psychiatric disorders.1 It takes a chronic course in up to 21% of patients and is associated with critical metabolic, endocrine and electrolyte imbalances as well as psychiatric comorbidities.1 2
The reward system in the brain which is believed to be dysfunctional in AN, is composed of the midbrain/ventral tegmental area, ventral striatum (including the nucleus accumbens (NAcc)), medial prefrontal and orbitofrontal cortex. Frank et al3 have shown an altered connectivity in the circuits that regulate energy and reward homeostasis in patients with AN and bulimia nervosa. The neurocircuitry involved in AN, is believed to overlap the one involved in obsessive-compulsive disorder (OCD).1 As deep brain stimulation (DBS) of the NAcc has an efficacy in OCD, the NAcc might also be an effective DBS target in patients with chronic AN.4 We hereafter describe the case of a patient suffering from intractable AN of the bulimic subtype.
Case presentation
A 42-years-old woman suffering from chronic AN of the bulimic subtype was referred to our neurosurgical department for DBS implantation. The severity of the disease has increased over the years and eventually led to comorbid depression. At the time of presentation to our department, the patient weighed only 32 kg (body mass index (BMI) 12.8 kg/m2) and was compulsively binge eating and purging several times a day. Concurrent metabolic and endocrine disturbances have led to an amenorrhea and osteoporosis. Severe leucopenia has repeatedly led to life-threatening infections. The patient has participated in various psychiatric therapies, including behavioural therapy. None of these were able to provide lasting relief of symptoms or weight gain. After a dedicated interdisciplinary discussion, the request for the local ethics committee’s approval and the informed consent of the patient, the DBS implantation took place in October 2017.
Treatment
In means of standard frame-based DBS procedure under general anaesthesia, an Inomed ZD-frame was mounted to the patient’s head. The planning was performed on the base of CT-MRI matching with the Framelink system of the Medtronic Stealthstation S7 planning station. A single-dose antibiotic prophylaxis was administered. The skin and the periosteum of the patient were noticed to be extremely thin. After skin incisions, a burr hole was created with the trephine drill. A recess was milled into the bone to lower the burr hole caps and thus reduce the risk for skin ulceration. The burr hole rings from the Boston Set were attached to the burr holes.
Boston Scientific 8-lead electrodes were implanted after microelectrode registration. The electrodes were attached to the burr hole cap, which were covered with subgaleal tissue thereafter. To prevent ulceration of the skin, gutters were milled into the bone to accommodate the connectors. The impedances were checked by a technician of the Boston Company and approved intraoperatively.
Outcome and follow-up
Surgery was uneventful. After the surgery, the patient did not show any new neurological deficits and wound healing was regular. Postoperative location of DBS electrodes was determined computationally, as shown in figure 1, to be within the NAcc using the PACER algorithm which was created by our group.5 Inpatient psychiatric follow-up was organised in a specialised centre in Germany.
Figure 1.
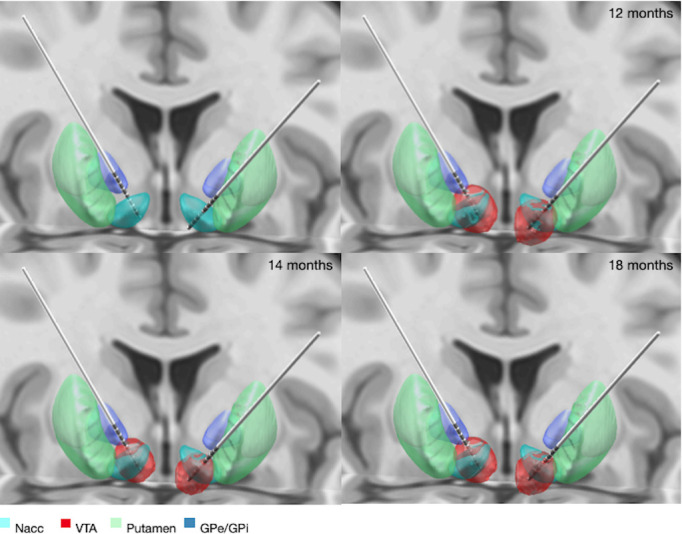
DBS location, electric fields and estimated VTA. The positioning of the electrodes and selected contacts led to a satisfying overlap-volume between VTA and NAcc, as shown in table 1. DBS, deep brain stimulation; NAcc, nucleus accumbens; VTA, volumes of tissue activated; GPe, globus pallidus externus; GPi, globus pallidus internus.
The first neurosurgical follow-up appointment took place 1 month later. By this time the patient had already gained 3.5 kg of weight. The patient reported no change in symptoms of depression under fluoxetine therapy. Postoperative stimulation parameters and weight are shown in table 1, figures 2 and 3.
Table 1.
Stimulation parameters, weight and VTA/NAcc overlap in mm3 over time
| Initial | 12 months | 14 months | 15 months | 18 months | 24 months | |||||||
| Stimulation parameters | ||||||||||||
| Amplitude (mA) | 5.5 | 5.5 | 4.5 | 4.7 | 5 | 0 | ||||||
| Frequency (Hz) |
204 | 204 | 204 | 204 | 204 | 0 | ||||||
| Pulse width (µs) |
350 | 350 | 350 | 350 | 350 | 0 | ||||||
| VTA/NAcc overlap (mm3) | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left |
| 98.1 | 125.7 | 98.1 | 125.7 | 75.1 | 92.2 | 79.6 | 99.1 | 86.5 | 1090 | 0 | 0 | |
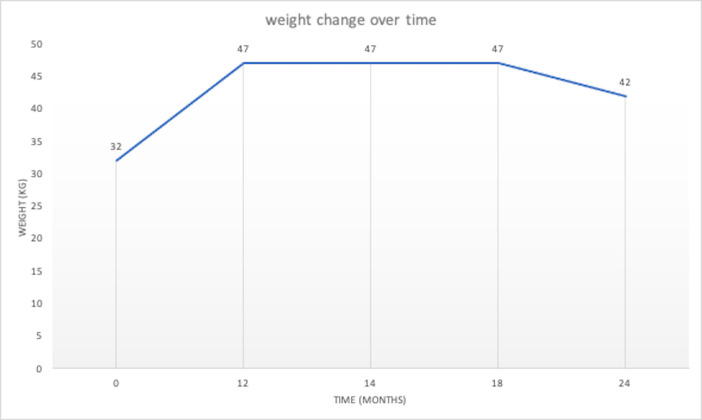
| Weight (kg) | 32 | 47 | 47 | 47 | 47 | 42 | ||||||
VTA/NAcc overlap was calculated using the FastField software.7
NAcc, nucleus accumbens; VTA, volumes of tissue activated.
Figure 2.
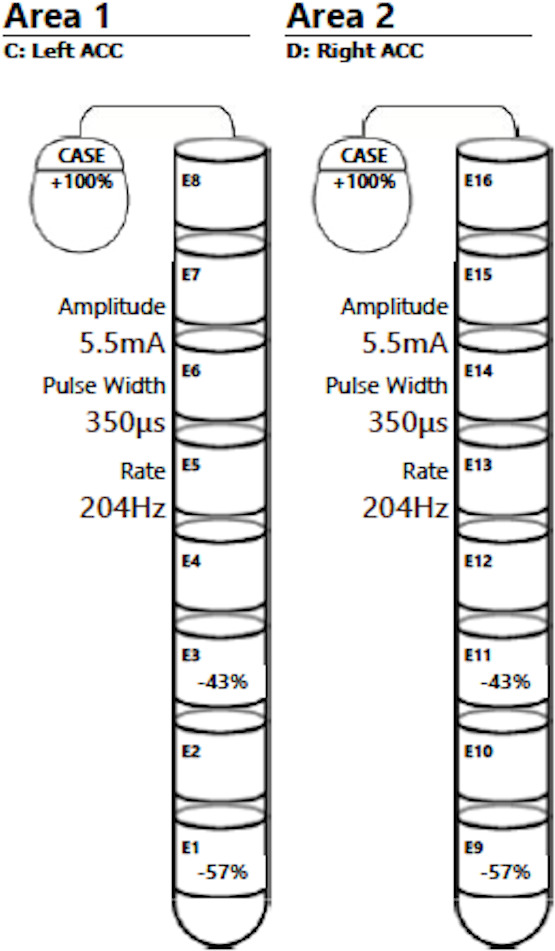
Illustration of the initial stimulation parameters applied after electrode implantation. As a good clinical response was obtained in terms of weight gain, the initially selected contacts have been maintained throughout the whole process. ACC; Nucleus Accumbens.
Figure 3.
Increase in weight over time.
One year after the DBS-implantation the patient showed a total weight gain of 15 kg. The patient reported an improvement in quality of life and a decrease in the frequency of binge eating and purging.
As the skin conditions were very atrophic, the patient developed a 1×1 cm large ulceration of the skin over the fixation cap of the left DBS electrode, shown in figure 4. The wound drained a clear fluid every morning. No fever or increase in blood infection parameters was noticed. The edges of the ulceration were not inflammatory and there was no pus formation. After a long discussion with the patient, we decided to continue with a close follow-up. At this point, the patient’s menstrual cycle had already normalised. After 14 months of follow-up, her weight was stable, but she presented with an increase in the frequency of binge eating and purging over the previous months. Stimulation parameters were reduced. The wound over the left burr hole cap remained unchanged.
Figure 4.
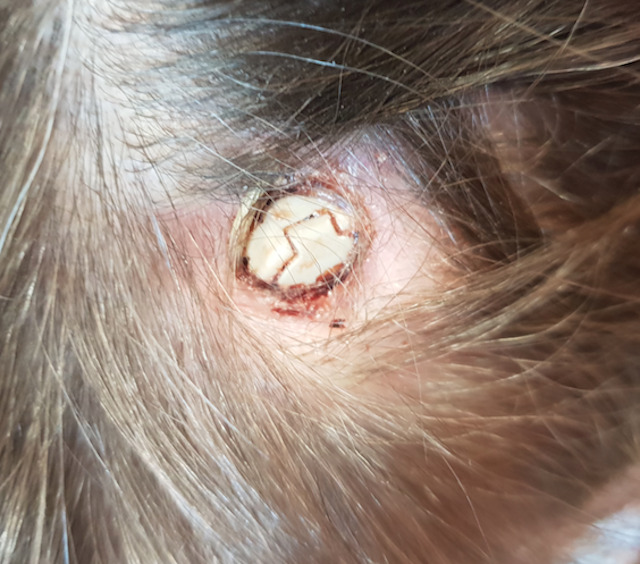
A 1×1 cm large skin ulceration over the left DBS-electrode fixation cap. Even though the material is exposed there is no sign of infection; no inflammation of the wound edges and no pus formation. This complication may be common in AN patients as the disease causes severe skin atrophy which can lead to wound healing disorders and ulcerations over the implanted material. AN, anorexia nervosa; DBS, deep brain stimulation.
Four weeks later, the patient self-reported a stable weight but a further increase in the frequency of binge eating and purging. Stimulation parameters were gradually increased by our team in the following months. As the symptoms persisted, at 19 months of follow-up the patient asked for the stimulation to be turned off. At 24 months of follow-up the patient self-reported a total weight gain of 15 kg. When checked by a scale, we found a discrepancy of 5 kg. Six weeks later, the patient had lost an additional 3 kg of weight and suffered from the persistence of bingeing and purging, with the stimulation still turned off at that point. The patient was then admitted to the emergency room with headaches and signs of infection over the subcutaneous trajectory of the cables and the wound over the left burr hole cap. An oral antibiotic therapy was started immediately (clindamycine 600 mg, three times per day) and the explantation of the DBS system was performed as soon as possible.
Discussion
The treatment of eating disorders, such as AN associated with binge eating remains challenging. DBS is a safe, minimally invasive surgical technique with low complication rates. There is limited data on the effect of DBS of the NAcc in AN.
Electrode position and follow-up
Knowledge of the exact position of the electrodes is of major interest for the postoperative programming of the stimulation fields. Lead DBS, an open-source software, was used for computational reconstruction of the exact location of the electrodes in the patient’s brain.6 In Lead DBS, PaCER algorithm was used for automated electrode trajectory and contact reconstruction as shown in figure 1.5 Electric fields of the different stimulation settings at 12 months, 14 months and 18 months after surgery where simulated using the SimBio/FieldTrip model described in Baniasadi et al.7 Note that VTAs are independent of the stimulation frequency. However, different stimulation frequencies might induce different effects within the same VTA.
Review of literature
Wu et al published a small case series (N=4, with three patients suffering from comorbid OCD) which showed an average of 65% body weight increase at 38 months follow-up in four adolescent AN patients treated by DBS of the NAcc.8 In our case, we observed an increase in body weight of 46.9% at 1-year follow-up.
Alternative DBS targets have been described for the treatment of AN. Lipsman et al9 published the results of a phase I pilot trial of subcallosal cingulate (SCC) DBS in 16 patients with treatment-refractory AN. They found SCC DBS to be well tolerated and associated with sustained improvements in affective symptoms, BMI and changes in neural circuitry at 12 months after surgery.9 Other studies showed an increase in BMI in comorbid AN in patients with a major depression or OCD, after subgenual cingulate cortex and ventral capsule/ventral striatum stimulation, respectively.10 11 The stimulation of Globus Pallidus internus (GPi) and Subthalamic Nucleus (STN) in patients suffering from Parkinson’s disease has been described to also result in weight gain.12 Hypothalamic areas and the rostromedial tegmental nucleus are other areas of the brain known to be involved in modulation of food intake besides the reward circuitry.12 13 One study showed an improvement in comorbid depression reflected by a decline in Hamilton Depression Scale and Beck Depression Inventory scores, an improvement in obsessive behaviour reflected by a decline in the Yale-Brown Obsessive Compulsive Scale score, a reduction in scores for eating disorder rituals and an increase in the quality of life in three out of six patients, following surgery.9 A similar effect could be observed in our patient, as she reported a subjective increase in quality of life and a decrease in the frequency of binge eating and purging during the first year after surgery. Nevertheless, a reduction in anti-depressant medication could not be achieved.
In 2018, Lee et al2 reviewed non-invasive brain stimulation as an interesting treatment option for AN, but showed that impact on self-reported symptoms may not always translate into weight gain. Ablative neurosurgical interventions such as Magnetic Resonance Tomography (MRT)-guided stereotactic anterior capsulotomy and anterior cingulotomy have been reported as successful treatment methods for eating disorders in several cases, but larger studies are needed to assess for the effect and safety of these procedures.4
Disease-specific complications
Disease-specific postoperative complications include postoperative refeeding syndrome, wound healing disorders and a predilection for infections. Our patient experienced typical problems of wound healing due to the severe atrophy of her skin which eventually led to infection. Ablative methods could avoid complications due to the implantation of foreign material.
Final considerations
Some concerns have been expressed by the scientific community on the ability of severely anorexic patients to give informed consent to DBS, as the only alternative to avoid starvation might be forced feeding.14 Furthermore, disease-specific postoperative complications have to be kept in mind. Compared with ablative methods, DBS implantation comes with an increased risk of infection and disrupted wound healing. On the other hand, DBS is an adjustable and reversible treatment option, allows the patient to maintain control of the recovery process and thus is a dynamic process in which the patient is actively involved in making on-going decisions during follow-up.15
We therefore advocate that, in the current state of knowledge, if the patient’s life is at risk, there is a potential indication for NAcc DBS when conventional treatment modalities recommended by evidence-based guidelines have not been able to durably alleviate the patient’s suffering. Strong collaboration between psychiatrists and neurosurgeons is of great importance in assessing potential candidates for surgical intervention.
Patient’s perspective.
I have been suffering from an eating disorder (anorexia/bulimia) since I was 16 years old. Despite various therapies and hospital stays, my state of health has deteriorated gradually. In 2015 my psychiatrist told me about the possibility of deep brain stimulation. A personal conversation with the neurosurgeon convinced me and I agreed to an operation.
I underwent the operation without fear. It was the last hope I had, as I was convinced that I would not be able to overcome the disease any other way. I was physically and mentally at the end of my rope at that time. Physical complaints (dental problems, osteoporosis, abnormal blood test results, …) were the result. At times my weight was under 30 kg. Everyday life and work made me tired and I had no joy of life. I would just as gladly have been dead. I had no suicidal thoughts but dying would have been OK to me.
The operation went according to plan. I recovered quickly and had no pain. After the operation I had a 4-week stay at a specialised psychiatric centre where the handling of the stimulator (settings, charging, …) was explained to me. The following months my weight increased gradually but the symptoms remained unchanged. The stimulation parameters were adjusted several times but there were no changes in my behaviour.
In the year after the operation I gained weight. My body recovered slowly; I had energy and strength again, could be active and did not freeze anymore. The joy of life came back. In June 2019 I switched off the stimulation in consultation with my neurosurgeon. My weight remained stable; the symptoms unchanged. In October 2019 the open wound on my head became inflamed and the stimulator had to be removed. This operation also went without complications. The wounds healed well thereafter. Since then, I still keep my weight.
In conclusion the deep brain stimulation was the right decision for me. It was the only way I could gain weight. This was accompanied by a significant increase in my quality of life. If my general condition does improve, I would like to have the stimulator reimplanted. However, the intervention did not bring about any change in my behaviour. I still suffer from anorexia/bulimia and need medication and psychiatric treatment.
Learning points.
Anorexia nervosa severely impacts individuals mental and physical health and has the highest mortality rate among psychiatric disorders. In total, 21% of cases take a chronic course and do not respond to conventional treatment options.
Deep brain stimulation (DBS) should be considered as a treatment option for intractable cases. Our team was able to obtain an impressive weight gain after DBS implantation and thus avoid a fatal outcome in a patient suffering from a severe form of bulimic anorexia nervosa.
Disease-specific disorders which might lead to postoperative complications must be kept in mind, such as atrophic skin conditions and an impaired immune response.
Acknowledgments
We thank Dr Tichomirowa Maria A for providing us with important patient data.
Footnotes
Twitter: @IArroteia
Contributors: IFA contributed to review of literature, reconstruction of electrode position and VTA using lead DBS v2 software (Figure 1), close follow-up of the patient, adaptation of stimulation parameters. AH involved in article revision, IT support for the use of lead DBS v2 software and Simbio/Fieldtrip Model for the reconstruction of electrode positioning and VTA. MB contributed to IT support in use of FastField software and article revision. FH is the Chair of the Neurosurgical Department of the Centre Hospitalier de Luxembourg and guarantor of the case report. FH monitored data collection, review of literature and writing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Oudijn MS, Storosum JG, Nelis E, et al. . Is deep brain stimulation a treatment option for anorexia nervosa? BMC Psychiatry 2013;13:277. 10.1186/1471-244X-13-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DJ, Elias GJB, Lozano AM. Neuromodulation for the treatment of eating disorders and obesity. Ther Adv Psychopharmacol 2018;8:73–92. 10.1177/2045125317743435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank GKW, Shott ME, Riederer J, et al. . Altered structural and effective connectivity in anorexia and Bulimia nervosa in circuits that regulate energy and reward homeostasis. Transl Psychiatry 2016;6:e932. 10.1038/tp.2016.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun B, Liu W. Stereotactic surgery for eating disorders. Surg Neurol Int 2013;4:164–9. 10.4103/2152-7806.110668 [DOI] [Google Scholar]
- 5.Husch A, V Petersen M, Gemmar P, et al. . PaCER - A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. Neuroimage Clin 2018;17:80–9. 10.1016/j.nicl.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn A, Li N, Dembek TA, et al. . Lead-DBS V2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019;184:293–316. 10.1016/j.neuroimage.2018.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baniasadi M, Proverbio D, Gonçalves J, et al. . FastField: an open-source toolbox for efficient approximation of deep brain stimulation electric fields. Neuroimage 2020;223:117330. 10.1016/j.neuroimage.2020.117330 [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Van Dyck-Lippens PJ, Santegoeds R, et al. . Deep-Brain stimulation for anorexia nervosa. World Neurosurg 2013;80:S29.e1–S29.e10. 10.1016/j.wneu.2012.06.039 [DOI] [PubMed] [Google Scholar]
- 9.Lipsman N, Lam E, Volpini M, et al. . Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry 2017;4:285–94. 10.1016/S2215-0366(17)30076-7 [DOI] [PubMed] [Google Scholar]
- 10.Israël M, Steiger H, Kolivakis T, et al. . Deep brain stimulation in the subgenual cingulate cortex for an intractable eating disorder. Biol Psychiatry 2010;67:e53–4. 10.1016/j.biopsych.2009.11.016 [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin NCR, Didie ER, Machado AG, et al. . Improvements in anorexia symptoms after deep brain stimulation for intractable obsessive-compulsive disorder. Biol Psychiatry 2013;73:e29–31. 10.1016/j.biopsych.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Prinz P, Stengel A. Deep brain Stimulation-Possible treatment strategy for pathologically altered body weight? Brain Sci 2018;8. 10.3390/brainsci8010019. [Epub ahead of print: 22 01 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prinz P, Kobelt P, Scharner S, et al. . Deep brain stimulation alters light phase food intake microstructure in rats. J Physiol Pharmacol 2017;68:345–54. [PubMed] [Google Scholar]
- 14.Maslen H, Pugh J, Savulescu J. The ethics of deep brain stimulation for the treatment of anorexia nervosa. Neuroethics 2015;8:215–30. 10.1007/s12152-015-9240-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugh J, Tan J, Aziz T, et al. . The moral obligation to prioritize research into deep brain stimulation over brain lesioning procedures for severe enduring anorexia nervosa. Front Psychiatry 2018;9:523. 10.3389/fpsyt.2018.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]