Abstract
Spine tumors may arise within or surrounding the spinal cord and/or vertebral column. Spinal tumors can be benign or malignant. Based on their epicenter, they may be classified as intradural-intramedullary, intradural-extramedullary, or extradural. Of these, extradural lesions are the most common, and are typically metastatic. Primary bone tumors of the spinal column comprise 5% of all primary skeletal tumors. The majority of primary spinal column tumors are benign, with malignant tumors comprising only 20%. Overall, spine metastases are the most common malignant spine tumor, and these usually arise from primaries such as lung, breast, and prostate cancers. The advent of improved systemic therapies leading to improved survival and the frequent use of imaging has positioned metastatic spine disease as the new epidemic in oncology. For spine tumors, establishing the correct diagnosis is heavily reliant on magnetic resonance imaging and histological confirmation. In this review, we will provide an overview of the epidemiology, radiological and histopathological features, and the natural history of key primary (benign and malignant) spinal cord and column tumors and metastatic spine tumors. Treatment principles for primary spinal cord or column tumors are aimed toward curative resection, whereas palliative resection forms the treatment principle for most metastatic tumors.
Keywords: spine metastases, spine neoplasm, spine tumors
Primary spine tumors are generally uncommon. However, this remains an important topic because they can cause considerable morbidity for patients by causing pain and affecting motor and sensory function. Spine tumors can arise within the spinal cord itself, or from the adjacent structures. These tumors can broadly be divided into primary and secondary (metastatic) tumors. Metastatic spine tumors spread to the vertebral column via hematogenous route (eg, via the Batson plexus). Contrary to primary spine tumors, metastatic spine disease (MSD) is extremely prevalent. In a postmortem study involving patients with breast or prostate cancer, the prevalence of MSD was between 70% and 90%.1
In general, spine tumors can be classified according to their anatomic location into intradural-intramedullary, intradural-extramedullary, and extradural (Figure 1). Intradural-intramedullary tumors are neoplasms arising within the spinal cord. They account for 20% of all intraspinal tumors in adults and 35% of all intraspinal tumors in children.2 Intradural-intramedullary tumors may arise from intrinsic cells within the spinal cord (intra-axial lesions), or via seeding/systemic spread. Most primary intra-axial tumors are either ependymomas or astrocytomas. Tumors are labeled as intradural-intramedullary if the epicenter arises at the level of C1 to the level of the conus (L1/L2). Lesions above C1 and involving the medulla are labeled as cervicomedullary. Intradural-extramedullary tumors are located within the dura but outside the spinal cord. Meningiomas and nerve sheath tumors (NSTs) are the 2 most common types of tumors.3 Extradural tumors are the most common (60% of all spine tumors). These tumors arise outside the dural sac, typically from the vertebral bodies. These are usually metastatic in nature. These tumors are particularly important because of the risk of epidural spinal cord compression.
Figure 1.
Diagram showing the various anatomical locations of spine tumor.
In this review article, we will provide a broad overview of the various types of tumors that can occur in the spine. We will discuss primary tumors of the spinal cord (benign and malignant), primary vertebral column tumors (benign and malignant), and metastatic spinal tumors separately. For each we will provide brief epidemiology, radiological and histopathological pearls, and touch on the natural history of the entity.
Primary Spinal Cord Tumors
Primary spinal cord tumors are rare conditions that comprise 3% of all primary CNS tumors in adults. Age-adjusted incidence rates are slightly higher for men than women, 0.67 compared with 0.59 per 100 000 population, respectively.4
Benign Spinal Cord Tumors
Spinal Meningioma.
Meningiomas can arise from arachnoidal cap cells anywhere along the neural axis. Most of them occur intracranially, whereas approximately 10% can be found adherent to the spinal dura. The age-adjusted incidence is 0.33 per 100 000 with women in the seventh to eighth decades having the highest incidence.5 Approximately 80% of spinal meningiomas arise in the thoracic region, with the cervical region being the second most common (15%).6 Prior exposure to ionizing radiation and neurofibromatosis type 2 (NF2) syndrome are known risk factors.7 Radiologically, the majority are intradural-extramedullary in location, having a well-circumscribed appearance on MRI. They often have a broad-based dural attachment and most exhibit a dural tail sign. They can be isointense on T1 and T2 imaging and exhibit homogeneous contrast enhancement. Histologically, they show a lobulated architecture with whorls and psammoma bodies, and stain positive for vimentin. The majority are World Health Organization (WHO) grade 1, with the meningothelial subtype being the most common in the spine. WHO grade II clear cell subtypes have a predilection for the spine and are thought to arise from the denticulate ligaments.8 Surgical removal is the treatment of choice, with a consideration for adjuvant radiotherapy in higher grade, or recurrent, tumors.
Nerve sheath tumors.
NSTs are the most common intradural-extramedullary lesions. Most NSTs arise from the dorsal sensory roots. Spinal schwannomas are most common, followed by neurofibromas and ganglioneuromas. The peak incidence of schwannomas occurs in the fifth to seventh decade.3 About 35% to 45% of patients with NST have neurofibromatosis. Neurofibromas are associated with NF1, and schwannomas are associated with NF2. These are well circumscribed and indistinguishable radiologically. The majority are isointense on T1, hyperintense on T2, and all exhibit contrast enhancement. CT imaging may exhibit chronic changes such as widening of the neural foramina and scalloping of the adjacent bone. Histologically, key features are that of a biphasic tumor with a highly ordered cellular component (Antoni A) that palisades (Verocay bodies) plus myxoid hypocellular component (Antoni B). These stain strongly for S100. Management is via surgical excision, and because of their well-circumscribed nature, can be peeled away from the parent nerve easily. Most do not recur and hence do not need adjuvant radiation. A minority of cases can undergo malignant transformation into malignant peripheral nerve sheath tumor, angiosarcoma, or epithelioid malignant change, for which the prognosis is poor.
Malignant Spinal Cord Tumors
Spinal gliomas.
The majority of intramedullary spinal cord tumors are gliomas. The word glioma refers to a tumor with histological similarity to normal glial cells. The major types of spinal glioma tumors are ependymomas and astrocytomas.
Ependymomas.
Spinal ependymomas are the most common intramedullary tumors.9 Ependymomas are glial tumors arising from ependymal cells and most commonly occur adjacent to the ventricular surface, along the spinal canal, or in the film terminale.10 Though spinal cord gliomas are rare compared with cerebral lesions, ependymomas comprise approximately 60% to 80% of spinal gliomas compared with 3% of intracranial gliomas.11 Among all spinal tumors in ages 0 to 19, ependymomas are the most common histology, comprising about 20%.12
NF2 is a dominant hereditary condition and is manifested by multiple tumors of the nervous system. Imaging evidence of ependymomas is seen in one-third of these patients. In contrast to sporadic spinal cord ependymomas, those associated with NF2 tend to display an indolent growth pattern, and potentially can be observed if found incidentally.13 In the WHO classification of brain tumors, ependymal tumors are divided into 4 major groups: subependymoma (grade I), myxopapillary ependymoma (grade I), ependymoma (grade II), and anaplastic ependymoma (grade III).14 Radiologically, ependymomas present a well-circumscribed lesion with variable enhancement. These may be associated with cystic change, hemorrhage, or calcification. Key histological features include perivascular pseudorosettes and ependymal rosettes. Gross total removal should be attempted for all patients. Compared with lower-grade ependymomas, anaplastic tumors appear to have a higher recurrence rate and poorer survival.
Astrocytic tumors of the spinal cord.
Spinal astrocytomas account for approximately 6% to 8% of all spinal cord tumors.10 In children, they are the most common intramedullary tumor, and the second most common in adults. These tumors may occur throughout the spinal cord. The peak incidence is in the third decade, with males being affected more commonly. There is an increased incidence in patients with NF1. They arise from astrocytic glial cells. Compared to their intracranial counterparts, they generally have a lower histological grade (with ~ 75% being low grade). However, high-grade spinal astrocytomas have an increased risk of leptomeningeal dissemination.
Radiologically, astrocytomas arise from the cord parenchyma (as opposed to ependymomas, which arise from the central canal). They often span several segments and appear exophytic in nature, sometimes being mistaken for an extramedullary tumor. Margins are poorly defined because of their infiltrative nature. Peritumoral edema and cysts are seen in less than half of patients. They appear hyperintense on T2 imaging, and most lesions show contrast enchantment on T1 imaging. Astrocytic tumors are composed of infiltrative cells with irregular, hyperchromatic nuclei and eosinophilic, GFAP-positive cytoplasm. These tumors are graded histologically according to their most anaplastic-appearing areas.14 Molecular parameters, used in the WHO classification, are based growth pattern, behavior, and isocitrate dehydrogenase–mutation status.1,14
The mainstay of treatment for primary spinal cord astrocytoma is surgical resection, with the goal of preservation of neurologic function, guided by intraoperative neuromonitoring. Adjuvant radiotherapy and chemotherapy may be used depending on the extent of resection and tumor grade.
Primary Spinal Column Tumors
Benign Spinal Column Tumors
Primary bone tumors are very rare and only up to 5% are located in the spine, with benign spinal tumors (80%) being more common than malignant spinal tumors (20%).15,16 These tumors are often incidental findings; however, they may present with local pain, symptoms of nerve root compression, neurological deficits, or deformity. In some cases, a biopsy is required to exclude the possibility of malignancy. If the tumor is locally aggressive, it may require surgical treatment based on its growth and aggressiveness.
Hemangioma.
Hemangiomas are vascular intraosseous lesions that are hamartomatous in origin with possible extraosseous extensions.17 They most frequently occur in the thoracic and lumbar spine.17 Autopsy studies estimate that the incidence of such lesions is 10%.17 Despite their frequency, they are rarely symptomatic and are usually detected incidentally. There is greater prevalence in the middle age group with a female predominance.18,19 They have a “salt and pepper” appearance on CT imaging, are hyperintense on T1 and T2, and show significant contrast enhancement. Hemangiomas have an excellent prognosis and most can be observed.20
Osteoid osteoma and osteoblastoma.
Osteoid osteomas and osteoblastomas are bone-forming lesions that produce osteoid and woven bone. These typically occur in the younger population (children and adolescents), with a slight male predilection. They have similar histological features but vary in terms of the nidus size (osteoid osteoma being smaller). Most occur within long bones, with 10% occurring in the vertebrae, predominantly in the posterior elements.21,22 CT is the modality of choice showing a radiolucent nidus with surrounding sclerosis. Management can be conservative with nonsteroidal anti-inflammatory drugs, because a portion of these resolve spontaneously. Surgical intervention of radiofrequency ablation needs to be considered in some patients.
Malignant Spinal Column Tumors
Malignant spinal tumors are less common than benign spinal tumors, forming only 20% of total primary spinal tumors.15,16 Although these tumors are rare, they may present with local pain, symptoms of nerve root compression, neurological deficits, or deformity. These tumors are usually locally aggressive, and their surgical treatment is based on their growth and extent of local extension, which is commonly based on the Weinstein-Boriani-Biagini surgical system.23 The surgical treatment is dependent on multiple factors but is mainly guided by the primary tumor type.
Chordoma.
Chordomas represent 4% of all primary bone tumors. They originate from the embryonic remnants of the notochord (which extends from the Ratke pouch to the tip of the coccyx). As such, they can occur anywhere along the vertebral column, but are more commonly seen in the clivus and sacral regions.24 Chordomas are usually seen in adults within the third to seventh decades, and have a male predilection.25 Chordomas are generally slow growing and locally destructive with the possibility of metastasis seen in 5% to 40% of patients.26 CT and MRI both are useful to work up chordomas. CT depicts a contrast-enhancing destructive lytic lesion, with a soft-tissue component with irregular intralesional calcifications. MRI typically shows a high signal on T2 with heterogeneous contrast enhancement with a honeycomb appearance. Gross morphology shows a soft, whitish, multilobulated mass with a fibrous pseudocapsule.25 Histologically, there is a lobular arrangement of physaliphorous (bubbly) tumor cells with intervening fibrovascular septae.26,27Figure 2 illustrates MRI and pathological findings from a young patient with clival chordoma. Tumor cells stain positive for cytokeratin, epithelial membrane antigen, S100, and brachyury. Three subtypes are recognized: conventional chordoma (most common), chondroid chordoma (least aggressive), and dedifferentiated chordoma (worst prognosis). Management generally involves maximal surgical resection followed by adjuvant radiotherapy. These lesions have poorly defined margins, accounting for the high probability of recurrences postoperatively. Radiotherapy using heavy ion particles is suggested to improve control rates.28
Figure 2.
A, A 7-year-old girl with neck pain. Clival chordoma on MRI axial T1 postcontrast sequence. The arrow points to a heterogeneous contrast-enhancing mass with a “honeycomb” appearance in the right craniocervical junction. B, Histopathology slide of the same patient who had undergone surgical debulking. Clusters of lobular-appearing physaliphorous tumor cells (1) are seen in a prominent myxoid stroma (2). The tumor cells display round to oval hyperchromatic nuclei with mild atypia and abundant vacuolated cytoplasm. The tumor tissues are surrounded by a fibrous pseudocapsule (3).
Chondrosarcoma.
Spinal chondrosarcomas account for 7% to 12% of all chondrosarcomas.29 Similar to chordoma, the peak prevalence is between the third and seventh decade with a male predominance; however, spinal chondrosarcomas have a predilection for the thoracic spine.30,31 Conventional chondrosarcoma constitutes more than 80%. Variants such as clear cell type (least aggressive) and high-grade mesenchymal/dedifferentiated types (poor prognosis) form the minority. Radiologically, CT demonstrates endosteal scalloping and intralesional calcification (rings and arcs calcification) associated with a soft-tissue mass in most cases. On MRI, it is usually intense on T2 with moderate to intense contrast enhancement. Although it may be challenging to differentiate chordoma from chondrosarcoma radiologically, DWI sequences appear promising.32 Histologically, appearance varies based on grade of the lesion—more aggressive chondrosarcomas have higher degrees of cellularity and nuclear pleomorphism, with the nuclei becoming large and occasionally binucleated with small nucleoli. The tumor invades the surrounding bone with cells filling marrow spaces.33,34 The absence of brachyury helps to distinguish this from chordomas. Maximal surgical resection remains the mainstay of treatment, with a consideration for adjuvant radiation for residual or high-grade disease.
Ewing sarcoma.
Ewing sarcoma starts within the medullary cavity. This condition primarily affects children and young adults, with peak incidence in people in their 20s and a slight male predilection.35 It more commonly involves the long bones and the pelvis (sacrum), with vertebral involvement seen in only 3.5% to 15% of cases.35 Radiologically, it appears as large aggressive-looking permeative tumors with onion-skin periosteal reaction. They have poorly defined edges and often extend into the adjacent soft tissue. They appear bright on T2 and exhibit heterogeneous contrast enhancement. Histologically, they appear as small, round, blue cells with uniform-appearing nuclei. CD99 is positive in almost all cases. The characteristic translocations involve the EWSR1 gene at 22q12 and either the FLI1 gene at 11q24 or the ERG gene at 21q22 (11;22) (q24;q12).36 The mainstay of treatment involves systemic chemotherapy with local treatment (surgery or radiotherapy) depending on the stage, size, and location of the tumor.
Osteosarcoma.
Osteosarcoma represents the second most common primary bone tumor. However, spinal involvement is rare, accounting for 3% to 5% of all osteosarcomas.37 Typically, osteosarcomas exhibit a bimodal incidence. Spinal osteosarcomas tend to occur at a slightly later age compared to conventional nonspinal osteosarcomas, with a slight male predominance.38 It may occur in the sacrum when associated with Paget disease.39 Thoracic and lumbar vertebrae are the most common regions of involvement in the mobile spine, with posterior elements as the most common sites.40
Plain radiographs show cortical destruction, with a wide zone of moth-eaten changes. There is prominent periosteal reaction (sunburst appearance, Codman triangle) with ill-defined tumor matrix calcification. MRI may show peritumoral edema, scattered hemorrhagic foci, and contrast-enhancing solid components. Histologically, conventional osteosarcomas show high-grade malignant spindle cells with nuclear pleomorphism, with varying amounts of osteoid production, cartilage, or fibrous tissue. The prognosis has greatly improved in recent times thanks to the use of effective systemic therapy (which can be administered before and/or after local treatment). En bloc resection is the preferred modality for local treatment but may not always be possible for spinal lesions. Adjuvant radiotherapy may be used in such cases to improve outcomes.
Plasma cell neoplasms.
Plasma cell neoplasms represent a spectrum of conditions that occur because of a proliferation of plasma cells, and often affect the vertebral column. Solitary plasmacytomas are localized lesions with no evidence of bone marrow involvement. More commonly, the spine is involved as part of a diffuse process (multiple myeloma) by which the bone marrow is affected. Multiple myeloma usually affects older adults, with the median age of diagnosis being in the sixth to seventh decade. Plasma cells proliferate in the bone marrow and can result in skeletal destruction. These appear radiologically as lytic lesions, osteopenia, and pathological fractures. Whole-body imaging with PET and MRI are useful for staging purposes. Biopsy of the soft-tissue mass, or bone marrow, reveals plasma cells that are identified based on morphology and immunophenotype. Mature plasma cells have abundant basophilic cytoplasm, appearing pink. Their nucleus is round and eccentrically located with cytoplasmic clearing. The nucleus contains a “clock-face” chromatin without nucleoli. Immunophenotyping usually reveals the presence of κ or λ light chains, but not both; absence of surface immunoglobulin; and expression of plasma cell markers (eg, CD38, CD138). Treatment for solitary plasmacytoma involves curative radiotherapy, whereas multiple myeloma is treated with systemic agents and a bone-marrow transplant in eligible patients.
Lymphoma.
Primary lymphoma of the spine is not common, and accounts for 1% to 3% of all lymphomas. It typically occurs in people in their 50s to 70s with a significant male preponderance of 8:1.19 Spinal involvement can occur because of a paraspinal, or vertebral, deposit extending to the spinal canal.41 Diffuse large B-cell lymphoma (non-Hodgkin lymphoma) is the most common subtype of lymphoma affecting the spine. Histologically, these are characterized by a diffuse proliferation of large neoplastic B cells (CD19, 20-positive) with a nuclear size of at least twice that of a normal lymphocyte. Systemic therapy using chemotherapy and rituximab (anti-CD20 antibody) forms the backbone, with consolidative radiotherapy used to improve outcomes.42
Metastatic Spine Tumors
MSD is the most common vertebral column tumor in adults. Based on a postmortem series, the incidence of MSD can be between 70% and 90% for patients with breast or prostate cancer. Although symptomatic spinal metastases are identified in approximately 10% of cancer patients, the frequent use of CT and MRI has led to an overall increased incidence.43 The highest incidence of spinal metastases occurs between the fourth and seventh decades, with lung, breast, prostate, and renal cell carcinomas forming the majority. In terms of pathophysiology, the metastases occur via the hematogenous route. Circulating tumor cells may extravasate and form a nidus within the vertebral body. The tumor often spreads posteriorly to involve the pedicles. In addition, the presence of factors such as RANK/RANKL, IL-1, IL-6, and TGF-β appear to influence this process.44 Spinal metastases can be categorized into extradural (intravertebral lesion with or without epidural spread) and intradural.45 Extradural lesions comprise 90% to 95% of metastatic spinal lesions.43 Intradural-extramedullary metastases (drop metastases) usually occur because of spread from intracranial deposits in the lower thoracolumbar region among the cauda equina. Intramedullary metastases are rare and may occur in the cervical cord. In terms of clinical presentation, the common symptoms are pain (95%) and neurological deficits (75%-85%), including sensory, motor, and/or sphincter dysfunction. Symptomatic spinal metastases are mostly found in the thoracic region (60%-80%) followed by the lumbosacral region (15%-30%) and the least in the cervical region (10%-15%). Radiological imaging of the entire spine is important because 10% to 20% may have multilevel spinal involvement. The appearance on CT depends on the degree of mineralization, where lytic metastases have irregular margins and a soft-tissue component.46 Sclerotic metastases, an indication of osteoblastic activity, appear hyperdense on CT and rarely extend beyond the vertebrae. Examples of lytic metastases include renal cell carcinoma, myeloma, and thyroid. Prostate cancer metastases are typically sclerotic. Breast and lung cancer metastases may exhibit a mixed pattern both of lytic and sclerotic lesions. The appearance on MRI may vary according to the degree of mineralization. However, most metastatic lesions exhibit contrast enhancement. MRI is also highly sensitive for detecting neural compression secondary to pathological fractures or epidural disease.
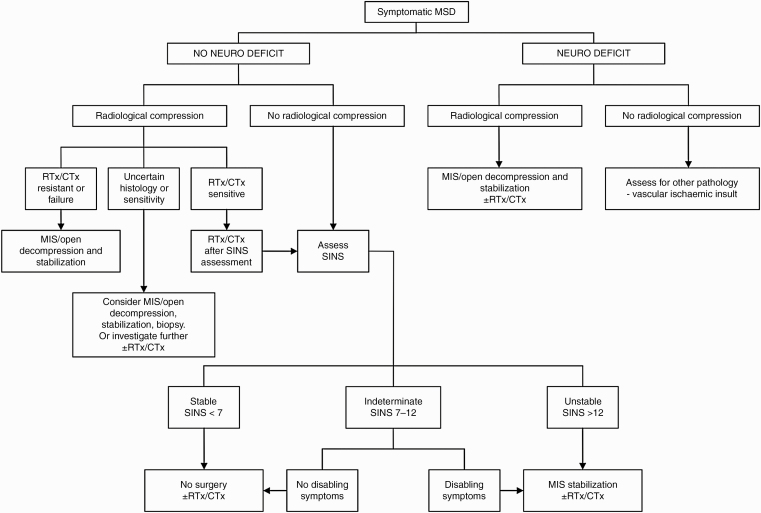
Surgical management is usually indicated for pain control, neurological decompression, and in cases of spinal instability. The Spinal Instability Neoplastic Score has been applied for decades to assess the severity of spinal instability, which is one of the indications for surgical intervention. Other factors that are taken into consideration for a surgical approach include prognosis, ambulatory status, number of involved vertebrae, and systemic burden of cancer. One of the widely used prognostic scales is the Tokuhashi scoring system.47 It assists clinical decision making to estimate survival and stratify surgical treatment based on established parameters. Patients are generally treated with postoperative radiotherapy and systemic therapy to improve cancer control. Advancements in surgical techniques and radiotherapy techniques are now being used to manage patients with MSD. For example, minimally invasive surgery shortens the recovery time for patients, and newer techniques in radiotherapy, such as stereotactic body radiotherapy, allow the use of higher radiation doses, which translates to improved control rates (Figure 3).
Figure 3.
Algorithm for management of metastatic spine disease. Adapted from Kumar et al.48 CTx, chemotherapy; MIS: minimally invasive surgery; MSD: metastatic spine disease; Neuro: neurological; RTx: radiotherapy; SINS: Spinal Instability Neoplastic Score.
Conclusion
Although primary spinal tumors are rare, it is important to classify them accurately because management depends on an accurate diagnosis. In general, patient demographics, presenting symptoms, and radiological and histological features should be considered before assigning a diagnosis. For malignant primary tumors, stage and grade of the lesion influence management. MSD forms the majority of spine tumors, and the management of this is evolving. Improvements in surgical techniques, radiotherapy delivery, and systemic therapeutics work hand in hand to improve tumor outcomes.
Acknowledgments
We would like to acknowledge Keith Gerard Lopez and Sirisha Madhu for their editorial support.
Funding
None declared.
Conflict of interest statement.
None declared.
References
- 1. Posner JB. Neurologic complications of systemic cancer. Dis Mon. 1978;25(2):1–60. [DOI] [PubMed] [Google Scholar]
- 2. Smith AB, Soderlund KA, Rushing EJ, Smirniotopolous JG. Radiologic-pathologic correlation of pediatric and adolescent spinal neoplasms: part 1, intramedullary spinal neoplasms. AJR Am J Roentgenol. 2012;198(1):34–43. [DOI] [PubMed] [Google Scholar]
- 3. Koeller KK, Shih RY. Intradural extramedullary spinal neoplasms: radiologic-pathologic correlation. Radiographics. 2019;39(2):468–490. [DOI] [PubMed] [Google Scholar]
- 4. Siker ML, Bovi J, Alexander B. Chapter 30—spinal cord tumors. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. 4th ed. Philadelphia: Elsevier; 2016:521–540.e525. [Google Scholar]
- 5. Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Benzel EC, Barnholtz-Sloan JS. Descriptive epidemiology of spinal meningiomas in the United States. Spine (Phila Pa 1976). 2015;40(15):E886–E 889. [DOI] [PubMed] [Google Scholar]
- 6. Osborn AG. Diagnostic Neuroradiology. St Louis, MO: Mosby Inc; 1994. [Google Scholar]
- 7. Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg. 2003;98(3 Suppl):258–263. [DOI] [PubMed] [Google Scholar]
- 8. Abul-Kasim K, Thurnher MM, McKeever P, Sundgren PC. Intradural spinal tumors: current classification and MRI features. Neuroradiology. 2008;50(4):301–314. [DOI] [PubMed] [Google Scholar]
- 9. Grossman RI, Yousem DM. Neuroradiology: the Requisites. 2nd ed. St Louis, MO: Mosby Inc; 2003. [Google Scholar]
- 10. Chang EL, Brown P, Lo SS, Sahgal A, Suh J, eds. Adult CNS Radiation Oncology: Principles and Practice. Cham, Switzerland: Springer International Publishing; 2018. [Google Scholar]
- 11. Shrivastava RK, Epstein FJ, Perin NI, Post KD, Jallo GI. Intramedullary spinal cord tumors in patients older than 50 years of age: management and outcome analysis. J Neurosurg Spine. 2005;2(3):249–255. [DOI] [PubMed] [Google Scholar]
- 12. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plotkin SR, O’Donnell CC, Curry WT, Bove CM, MacCollin M, Nunes FP. Spinal ependymomas in neurofibromatosis type 2: a retrospective analysis of 55 patients. J Neurosurg Spine. 2011;14(4):543–547. [DOI] [PubMed] [Google Scholar]
- 14. WHO Classification of Tumours of the Central Nervous System. Vol 1 4th ed. Lyon, France: IARC Publications; 2016. [Google Scholar]
- 15. Inwards CY, Krishnan Unni K. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 16. Dang L, Liu X, Dang G, et al. Primary tumors of the spine: a review of clinical features in 438 patients. J Neuro-Oncol. 2015;121(3):513–520. [DOI] [PubMed] [Google Scholar]
- 17. Thakur N, Daniels A, Schiller J, et al. Benign tumors of the spine. J Am Acad Orthop Surg 2012;20(11):715–724. [DOI] [PubMed] [Google Scholar]
- 18. Krueger EG, Sobel GL, Weinstein C. Vertebral hemangioma with compression of spinal cord. J Neurosurg 1961;18(3):331–338. [DOI] [PubMed] [Google Scholar]
- 19. Rodallec MH, Feydy A, Larousserie F, et al. Diagnostic imaging of solitary tumors of the spine: what to do and say. Radiographics. 2008;28(4):1019–1041. [DOI] [PubMed] [Google Scholar]
- 20. Pastushyn AI, Slin’ko EI, Mirzoyeva GM. Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998;50(6):535–547. [DOI] [PubMed] [Google Scholar]
- 21. Jackson RP, Reckling FW, Mants FA. Osteoid osteoma and osteoblastoma. Similar histologic lesions with different natural histories. Clin Orthop Relat Res. 1977;( 128):303–313. [PubMed] [Google Scholar]
- 22. Kransdorf MJ, Stull MA, Gilkey FW, Moser RP Jr. Osteoid osteoma. Radiographics. 1991;11(4):671–696. [DOI] [PubMed] [Google Scholar]
- 23. Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976). 1997;22(9):1036–1044. [DOI] [PubMed] [Google Scholar]
- 24. Erdem E, Angtuaco EC, Van Hemert R, Park JS, Al-Mefty O. Comprehensive review of intracranial chordoma. Radiographics. 2003;23(4):995–1009. [DOI] [PubMed] [Google Scholar]
- 25. Smolders D, Wang X, Drevelengas A, Vanhoenacker F, De Schepper AM. Value of MRI in the diagnosis of non-clival, non-sacral chordoma. Skeletal Radiol. 2003;32(6):343–350. [DOI] [PubMed] [Google Scholar]
- 26. Bjornsson J, Wold LE, Ebersold MJ, Laws ER. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;71(3):735–740. [DOI] [PubMed] [Google Scholar]
- 27. Crapanzano JP, Ali SZ, Ginsberg MS, Zakowski MF. Chordoma: a cytologic study with histologic and radiologic correlation. Cancer. 2001;93(1):40–51. [PubMed] [Google Scholar]
- 28. Schulz-Ertner D, Karger CP, Feuerhake A, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68(2):449–457. [DOI] [PubMed] [Google Scholar]
- 29. Dorfman HD, Vanel D, Czerniak B, et al. WHO classification of tumours of bone: introduction. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2002:277–232. [Google Scholar]
- 30. Boriani S, De Iure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976). 2000;25(7):804–812. [DOI] [PubMed] [Google Scholar]
- 31. Murphey MD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: variants and complications with radiologic-pathologic correlation. Radiographics. 2000;20(5):1407–1434. [DOI] [PubMed] [Google Scholar]
- 32. Yeom KW, Lober RM, Mobley BC, et al. Diffusion-weighted MRI: distinction of skull base chordoma from chondrosarcoma. AJNR Am J Neuroradiol. 2013;34(5):1056–1061, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fletcher CDM. Diagnostic Histopathology of Tumors. Vol 1 1st ed. Edinburgh, Scotland: Churchill Livingstone; 1995. [Google Scholar]
- 34. Kumar V, Abbas AK, Fausto N, et al. Robbins Basic Pathology. 8th ed. Philadelphia, PA: Saunders/Elsevier; 2007. [Google Scholar]
- 35. Ilaslan H, Sundaram M, Unni KK, Dekutoski MB. Primary Ewing’s sarcoma of the vertebral column. Skeletal Radiol. 2004;33(9): 506–513. [DOI] [PubMed] [Google Scholar]
- 36. Lessnick SL, Ladanyi M. Molecular pathogenesis of Ewing sarcoma: new therapeutic and transcriptional targets. Annu Rev Pathol. 2012;7:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katonis P, Datsis G, Karantanas A, et al. Spinal osteosarcoma. Clin Med Insights Oncol. 2013;7:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. [DOI] [PubMed] [Google Scholar]
- 39. Scottish Bone Tumor Registry; Sharma H, Mehdi SA, MacDuff E, Reece AT, Jane MJ, Reid R. Paget sarcoma of the spine: Scottish Bone Tumor Registry experience. Spine (Phila Pa 1976). 2006;31(12):1344–1350. [DOI] [PubMed] [Google Scholar]
- 40. Ilaslan H, Sundaram M, Unni KK, Shives TC. Primary vertebral osteosarcoma: imaging findings. Radiology. 2004;230(3):697–702. [DOI] [PubMed] [Google Scholar]
- 41. Patnaik S, Jyotsnarani Y, Uppin S, Susarla R. Imaging features of primary tumors of the spine: a pictorial essay. Indian J Radiol Imaging. 2016;26(2):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol. 2013;31(32):4115–4122. [DOI] [PubMed] [Google Scholar]
- 43. Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010;13(1):94–108. [DOI] [PubMed] [Google Scholar]
- 44. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. [DOI] [PubMed] [Google Scholar]
- 45. Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58(4):245–259. [DOI] [PubMed] [Google Scholar]
- 46. Shah LM, Salzman KL. Imaging of spinal metastatic disease. Int J Surg Oncol. 2011;2011:769753–769753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30(19):2186–2191. [DOI] [PubMed] [Google Scholar]
- 48. Kumar N, Malhotra R, Zaw AS, et al. Evolution in treatment strategy for metastatic spine disease: presently evolving modalities. Eur J Surg Oncol. 2017;43(9):1784–1801. [DOI] [PubMed] [Google Scholar]