Abstract
With the growing incidence of new cases and the increasing prevalence of patients living longer with spine metastasis, a methodological approach to the management of patients with recurrent or progressive disease is increasing in relevance and importance in clinical practice. As a result, disease management has evolved in these patients using advanced surgical and radiotherapy technologies. Five key goals in the management of patients with spine metastases include providing pain relief, controlling metastatic disease at the treated site, improving neurologic deficits, maintaining or improving functional status, and minimizing further mechanical instability. The focus of this review is on advanced reirradiation techniques, given that the majority of patients will be treated with upfront conventional radiotherapy and further treatment on progression is often limited by the cumulative tolerance of nearby organs at risk. This review will also discuss novel surgical approaches such as separation surgery, minimally invasive percutaneous instrumentation, and laser interstitial thermal therapy, which is increasingly being coupled with spine reirradiation to maximize outcomes in this patient population. Lastly, given the complexities of managing recurrent spinal disease, this review emphasizes the importance of multidisciplinary care from neurosurgery, radiation oncology, medical oncology, neuro-oncology, rehabilitation medicine, and palliative care.
Keywords: metastasis, reirradiation, recurrence, SBRT, spine, SRS, stereotactic radiosurgery
Five thousand new cancer cases will be diagnosed each day in the United States in 2020.1 In this rapidly growing population, metastases to the spine represent a frequently encountered complication of advanced cancer. Approximately 40% of patients will present with clinical symptoms from spinal metastases during the course of their systemic disease. However, this is just the tip of the iceberg, as up to 90% have been found to have underlying micrometastatic disease based on autopsy series.2 In addition, recent advances in treatment and supportive care have led to increased survival for patients with de novo metastatic disease as well as those who progress to distant metastases, both increasing the overall prevalence of cancer patients.3 Systematic reviews of clinical trials for patients with bone metastases treated with conventional palliative radiotherapy have underscored the modest outcomes with initial treatment. For example, only one-third of patients who respond to conventional radiotherapy achieve a complete pain response, and 40% do not experience any substantial improvement in symptoms. Strikingly, 50% of patients who initially respond to treatment experience symptom relapse within a year.4–6 With the growing incidence of new cases and the increasing prevalence of patients living longer with spine metastasis, an aggressive approach to managing patients with recurrent or progressive disease is increasing in relevance and importance in clinical practice. This review provides an overview of the approaches for patients with recurrent or progressive disease with a focus on reirradiation strategies, surgical considerations, multidisciplinary care, and clinical trials and emerging concepts.
Initial Approach to the Patient
There are key patient descriptors (performance status, neurologic status, spinal stability, etc), disease characteristics (histology of primary tumor, extent of disease including epidural extension, status of metastatic disease, etc), and treatment-specific criteria (prior surgery, prior radiotherapy and ability to undergo additional invasive or noninvasive interventions, etc) that are incorporated into clinical decision making for patients with spinal metastases. Table 1 summarizes key criteria to assess in the evaluation of a patient with recurrent disease, incorporating principles from the neurologic, oncologic, mechanical, and systemic (NOMS) framework and the American College of Radiology Appropriateness Criteria evidence-based guidelines for malignant epidural spinal cord compression (MESCC) and recurrent spinal metastasis.7,8
Table 1.
Key Criteria for the Evaluation and Management of Recurrent or Progressive Spine Metastasis, Modified From the Neurologic, Oncologic, Mechanical, and Systemic Framework8 and American College of Radiology Appropriateness Criteria and Evidence-Based Guidelines7
| Recurrence factors |
|---|
| Extent of disease recurrence (local vs local and systemic) |
| Interval from prior treatment to disease relapse or progression |
| In-field vs marginal vs out-of-field failure |
| Grade of epidural disease |
| Best response and duration of response to prior treatment |
| Prior therapy |
| Prior surgery and extent of resection |
| Implanted hardware and presence of hardware failure |
| Failure after conventional external beam radiotherapy vs SBRT |
| Patient tolerance to prior radiotherapy |
| Prior dose exposed to adjacent organs at risk and remaining tolerance |
| Neurologic |
| Grade of epidural extension |
| Presence of spinal cord compression |
| Symptoms related to compression of the spinal cord/nerve roots/cauda equina |
| Oncologic |
| Expected degree of tumor response to each treatment modality |
| Anticipated durability of treatment response to each treatment modality |
| Availability of further cancer-directed therapies |
| Mechanical |
| Presence of pathologic fracture |
| Need for surgical stabilization, if not performed in the past |
| Presence of movement-related pain |
| Spinal Instability Neoplastic Score |
| Anticipated risk of vertebral body compression fracture with reirradiation (especially SBRT) |
| Systemic |
| Overall performance status of the patient |
| Extent of metastatic disease and response to prior therapies |
| Presence of brain metastases |
| Patient medical comorbidities |
| Influence of tumor biology on patient outcome |
| Life expectancy |
| Patient goals of care |
Abbreviation: SBRT, stereotactic body radiotherapy.
Given the complexity of care, the management of patients with recurrent or progressive metastases to the spinal column requires multidisciplinary evaluation. At a minimum, a dedicated spine tumor team consisting of an oncologically oriented spine surgeon, radiation oncologist, medical physicist, medical oncologist, neuroradiologist, and physical medicine and rehabilitation physician is recommended. Key factors for evaluation include prior oncologic therapy and the potential for future treatments; prior neurosurgical intervention and current neurologic status (ie, American Spinal Injury Association [ASIA] Impairment Scale); evaluation of potential mechanical stability (ie, Spinal Instability Neoplastic Score [SINS]); tolerance of prior radiotherapy; cumulative radiation dose to the spinal cord or cauda equina; and capacity to undergo interventional procedures, all while considering the guarded prognosis of a patient with recurrent disease.
Corticosteroids should be administered to those with symptoms due to epidural extension and analgesics for pain relief. Decisions regarding the role of hormonal therapies in patients with breast or prostate cancer, cytotoxic chemotherapy, targeted therapy, immunotherapies, bone-protective or regenerative agents, and radiopharmaceuticals are similar to the management of patients with previously untreated spine metastasis. However, because patients with recurrent or progressive spine disease require interventions focused on local disease control, the multidisciplinary management of these patients with a focus on radiotherapy and surgical interventional approaches are described in detail in this report.
Radiotherapy Reirradiation Strategies
The initial evaluation of the patient with recurrent or progressive spinal metastasis for reirradiation requires an understanding of the setting of disease relapse. There are 3 potential scenarios encountered in clinical practice: 1) recurrence of symptoms after initial treatment or no initial symptom improvement, 2) partial response to initial treatment with an expectation of increased response with additional radiotherapy, and 3) radiographic disease progression at risk of causing neurologic compromise.9
Prior to embarking on another course of radiotherapy regardless of the re-treatment technique, it is always critical to evaluate the prior radiotherapy treatment plan in detail and the patient’s tolerance to radiotherapy. Multiple animal models have analyzed the extent of radiation damage repair of the spinal cord as well as documented the kinetics of neuronal recovery, establishing the basis for consideration of re-treatment in select patients.10,11 Radiotherapy treatment strategies in the recurrent setting can include a hypofractionated course, hyperfractionated schedules, conventionally fractionated radiotherapy, pulsed-reduce dose-rate radiotherapy (PRDR), and stereotactic radiosurgery (SRS) or stereotactic body radiotherapy (SBRT). A summary of selected major studies within various settings for reirradiation of spinal metastases is presented in Table 2.
Table 2.
Summary of Selected Key Studies of Reirradiation for Recurrent or Progressive Spinal Metastases
| Author, y | Study type | No. of patients (targets) | Population | RT details | Outcomes |
|---|---|---|---|---|---|
| Chow et al, 201412 | RCT of RT dose | 237a | Prior cEBRT | 8 Gy/1 fx (116) vs 20 Gy/5 fx (121) | No difference between groups; pain response 30% |
| Huisman et al, 201213 | Systematic review | 527 | Prior cEBRT | Various dose/ fractionation | Pain response 58%; complete response 16%-28% |
| Rades et al, 200514 | Case series | 62 | Prior cEBRT, recurrent spinal cord compression | 8-20 Gy/1-5 fx | Improved motor function 40% |
| Garg et al, 201115 | Case series | 59 (63) | SBRT after failed cEBRT | 27-30 Gy/3-5 fx | 1-y LC 76%; 1-y OS 76% |
| Thibault et al, 201516 | Case series | 40 (56) | SBRT after failed SBRT | 24-35 Gy/2-5 fx | 1-y LC 81%; median OS 10 mos |
| Myrehaug et al, 201717 | Systematic review | 405 (447) | SBRT after any prior RT | Various dose/ fractionation | 1-y LC 76%; risk of VCF 12% |
| Ito et al, 201818 | Case series | 28 | Postoperative SBRT after initial failed cEBRT | 24 Gy/2 fx | 1-y LC 70%; 1-y OS 63% |
Abbreviations: cEBRT, conventional external beam radiotherapy; fx, fraction; LC, local control; OS, overall survival; RCT, randomized controlled trial; RT, radiotherapy; SBRT, stereotactic body radiotherapy; VCF, vertebral compression fracture.
aTwenty-eight percent of the 850 patients in this RCT were reirradiated to spinal metastases.
Conventional Palliative Reirradiation Treatment
There is a variety of radiotherapy options for patients with poor expected survival (< 6 months) to achieve pain response and reduce neurologic deficits. A large systematic review and meta-analysis of unresponsive or recurrent bone metastasis reported the outcomes of 527 reirradiated patients in 7 studies, of which 36% of re-treatments were localized to the spine, with modest rates of complete (16%-28%) and partial (28%-45%) response using a variety of dose and fractionation schedules.13 Especially important for this population is that the time response to symptom improvement ranged from 3 to 5 weeks with a duration of response from 15 to 22 weeks. Using this basis, a multicenter trial in patients with painful bone metastasis after prior radiotherapy randomly assigned patients between 2 regimens: 8 Gy in 1 fraction or 20 Gy in 5 fractions.12 Although patients with metastasis to the spine comprised approximately 30% of the trial population, it is important to note that the prior spine treatments could include only 6 to 8 Gy in 1 fraction, 18 Gy in 4 fractions, or 20 Gy in 5 fractions. Although there was no difference in the intent-to-treat population between the tested fractionation regimens, those noninferiority findings were not confirmed in the prespecified, noninferiority margin in the per-protocol population, leading to both fractionation schedules being recommended for clinical practice. Finally, spinal reirradiation using a variety of dose and fractionation regimens (8 Gy in 1 fraction, 15 Gy in 5 fractions, and 20 Gy in 5 fractions) has been demonstrated to improve neurologic deficits in patients with recurrent MESCC after prior irradiation without occurrence of radiotherapy-induced myelopathy.14 Therefore, depending on the clinical scenario, hypofractionated radiotherapy schedules (8 Gy in 1 fraction or 20 Gy in 5 fractions) can be used for patients to provide short-term pain relief. More prolonged fractionation schedules (eg, 20-25 Gy in 10 fractions) or hyperfractionated schedules (eg, 1-1.5 Gy twice daily) may be associated with a reduced risk of developing radiation myelopathy and can be considered in select patients based on the extent of disease, performance status, prior radiotherapy with consideration of the time interval between treatments, and expected survival of the patient.19–21 Short-course repeat radiotherapy does have its limitations in patients with recurrent disease and longer expected survival (> 6 months) because overall responses are observed in only 50% of patients and complete pain responses in a mere 10%.12
Spine Stereotactic Radiosurgery/Stereotactic Body Radiotherapy
SRS and SBRT are more often used in the upfront management of patients with localized spine metastasis given the high rates of pain control, durability of pain relief, high rates of tumor control even in the setting of radioresistant disease, and ability to decompress epidural disease and improve neurologic function.22 These merits over conventional irradiation techniques become increasingly advantageous for patients with recurrent or progressive disease with longer expected survival.15 Animal SRS models initially established the safety of single-fraction re-treatment following 30 Gy in 10 fractions.23 A number of institutional series have demonstrated promising outcomes for reirradiation of spinal metastasis with SRS/SBRT. A systematic review of the literature yielded 9 published series with a 1-year local control rate of 76%, crude improvement in pain scores in 65% to 81% of treated patients, a crude vertebral body fracture rate of 12%, and a radiation myelopathy incidence of 1.2% among patients re-treated to 20 to 30 Gy in 2 to 5 fractions.17 SBRT reirradiation (24 Gy/2 fractions) has also been evaluated in the postoperative setting after 30 Gy in 10 fractions and decompression surgery with modest disease control rates and safety profiles.18 A more fractionated SBRT regimen (median, 30 Gy/4 fractions) has also been studied in patients after prior spine SBRT (median, 24 Gy/2 fractions) with a 1-year tumor control rate of 81% and no observed radiation-induced vertebral body compression fractures or cases of radiation myelopathy.16 Prospective investigations are needed to determine the effectiveness and safety of reirradiation; however, in the interim, the Hypofractionation Treatment Effects in the Clinic (HyTEC) report endorsed 4 key recommendations for safe reirradiation with spine SBRT: 1) cumulative thecal sac equivalent dose in 2 Gy fractions to a maximum of 70 Gy (α/β of 2); 2) maximum SBRT dose to the thecal sac of less than or equal to 25 Gy; 3) maximum thecal sac SBRT dose to a cumulative maximum dose ratio of 0.5 or less; and 4) minimum time interval to reirradiation of 5 or more months.24 Using this as a guide, proposed SRS and SBRT re-treatment dose and fractionation schedules with corresponding spinal cord and cauda equina constraints are provided in Table 3 and a clinical case example in Figure 1.
Table 3.
Stereotactic Radiosurgery/Stereotactic Body Radiotherapy Reirradiation Dose and Fractionation Schedules and Spinal Cord/Thecal Sac Safety Constraints With a Low Expected Incidence of Radiation Myelopathy24,26,29
| Recommended treatment dose/ fractionation | 18-24 Gy/1 fx | 24 Gy/2 fx | 27-30 Gy /3 fx | 30 Gy/4 fx | 40 Gy/5 fx | ||
|---|---|---|---|---|---|---|---|
| Prior radiotherapy | 2 Gy BED | None | 12.4-14 Gy spinal cord 16 Gy cauda equina |
17 Gy | 20.3 Gy | 23 Gy | 25.3 Gy |
| 20 Gy/5 fx (30 Gy [2/2]) | 9 Gy | 12.2 Gy | 14.5 Gy | 16.2 Gy | 18 Gy | ||
| 30 Gy/10 fx (37.5 Gy [2/2]) | 9 Gy | 12.2 Gy | 14.5 Gy | 16.2 Gy | 18 Gy | ||
| 37.5 Gy/15 fx (42 Gy [2/2]) | 9 Gy | 12.2 Gy | 14.5 Gy | 16.2 Gy | 18 Gy | ||
| 40 Gy/20 fx (40 Gy [2/2]) | NA | 12.2 Gy | 14.5 Gy | 16.2 Gy | 18 Gy | ||
| 45 Gy/25 fx (43 Gy [2/2]) | NA | 12.2 Gy | 14.5 Gy | 16.2 Gy | 18 Gy | ||
| 50 Gy/25 fx (50 Gy [2/2]) | NA | 11 Gy | 12.5 Gy | 14 Gy | 15.5 Gy |
Abbreviations: BED, biologically effective dose; fx, fraction; NA, not available.
Assumes maximum (0.03-cc) dose to spinal cord planning risk volume or thecal sac.
Figure 1.
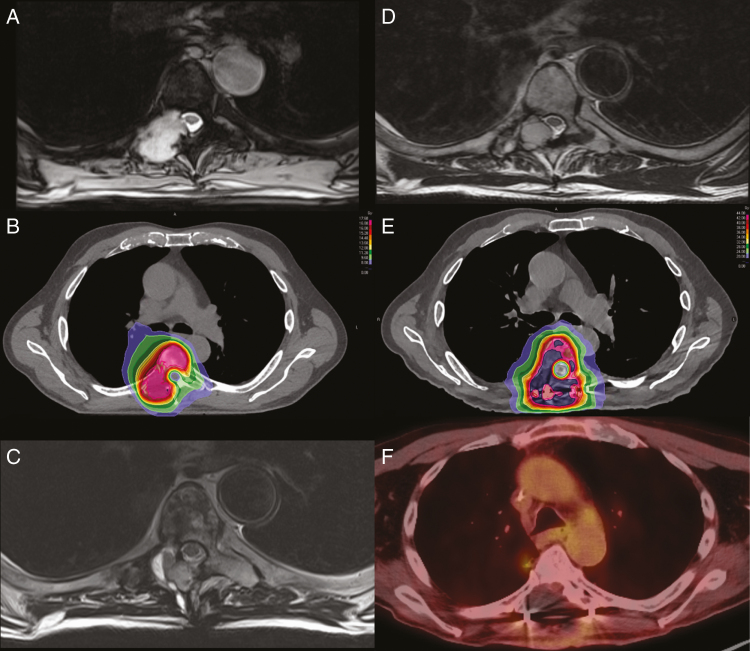
A 79-year-old man with stage IV high-grade pleomorphic myxofibroblastic sarcoma of bone with metastasis to the thoracic spine. A, Axial T2-weighted MRI demonstrates a lobulated expansile mass involving the right T6 posterior body, pedicle, lamina, transverse process, and right superior and inferior articular facets with epidural extension in the canal and thecal sac compression with mild displacement and flattening of the spinal cord. B, Axial spine stereotactic radiosurgery (SRS) CT treatment plan with corresponding isodose distribution to a prescribed dose of 16 Gy in 1 fraction. C, Axial T2-weighted MRI 6 months post-SRS reveals rapid disease recurrence with disease involvement of the T6 spinal level with epidural extension in the right posterior lateral spinal canal with thecal sac compression and mild displacement of the spinal cord anterolaterally for which the patient underwent laminectomy and tumor resection. D, Axial T2-weighted MRI reveals a recurrent heterogeneous epidural mass resulting in mild canal stenosis approximately 1 year after resection (no adjuvant therapy offered). E, Axial spine stereotactic body radiotherapy (SBRT) CT treatment plan with corresponding isodose distribution to a prescribed dose of 40 Gy in 5 fractions performed after separation surgery and tumor debulking. F) Axial PET/CT performed over 1 year following salvage separation surgery and SBRT demonstrating no radiographic evidence of recurrent disease; the patient ultimately died of systemic disease progression without any symptomatic disease recurrence or treatment-related toxicity at the treated site.
Few retrospective case series have evaluated the outcomes of patients treated with a third course of external beam radiotherapy, and decisions for these scenarios need to be highly individualized.27 For example, in a series of 23 patients who underwent 3 courses of spine radiotherapy, only 2 patients had tumors close to the spinal cord itself.28 In another series of 10 patients reirradiated for recurrent spine metastasis with fractionation schedules ranging from 20 to 30 Gy in 3 to 5 fractions, the crude local control rate was 80%; however, grade 1 and 2 neuropathies were observed.29 Although the treatment fractionation schedules were quite variable in this study, the median total dose to 5% of the spinal cord was 59.4 Gy, and the median maximum dose was 71 Gy, in normalized 2-Gy equivalents.
Pulsed-Reduced Dose-Rate Radiotherapy and Brachytherapy
PRDR radiotherapy is an alternative technique for safely reirradiating recurrent disease. This reduced dose rate is achieved by dividing the standard treatment (typically 4-6 Gy/minute) into subfractions of 0.2 Gy delivered with fixed time intervals, resulting in a pulsed treatment with an effective dose rate of 0.07 Gy/minute.30 This technique has been used in large-volume, recurrent CNS malignancies allowing for safe high-dose reirradiation with a median re-treatment dose of 54 Gy.31 With regards to tolerance of the spinal cord to reirradiation, a retrospective series demonstrated the feasibility of wide-field PRDR in recurrent spine ependymomas, with a median PRDR dose of 40 Gy (range, 30.6-54 Gy) and a median cumulative lifetime dose of 105.2 Gy (range, 90-162.4 Gy).32 Finally, it is important to note that for patients who have exhausted all external beam radiotherapy options regardless of technique or modality, consideration can be given to brachytherapy with intraoperative or image-guided percutaneous catheter placement and treatment with iridium-192.33
Surgical Considerations in the Recurrent Patient
Surgical intervention followed by radiation therapy has been shown to improve ambulation, pain, quality of life, functional status, and even survival in patients with symptomatic MESCC.34 Recurrent tumors, however, were excluded from most trials. As patients with cancer live longer, reoperation for recurrent MESCC is a challenge increasingly faced by the oncologic spine surgeon, void of randomized data.
Reoperation in this population is primarily for surgical site infection (42%) and hardware failure (29%), but reoperation for recurrent tumor comes in third place, accounting for as many as 16% of all reoperations.35 In patients who survived more than 24 months after their first operation, up to 23% required reoperation at the index level, of which 25% were secondary to tumor recurrence or progression.36 Most reoperations for tumor recurrence occurred in cancers with favorable survival profiles like renal cell carcinoma, prostate carcinoma, and neuroendocrine tumors, and occurred in a delayed fashion at a median time of 8.3 months after the index procedure.36 This will, however, likely change and become more common with improved targeted therapies for histologies traditionally considered with poor prognosis like lung cancer.37,38 There are many factors to consider when facing this scenario. Reoperations on their own carry a higher risk of adverse events, which is made more complex by operating in a radiated field with decreased bone quality and increased wound-healing capabilities.39 Patients are often at a more advanced stage of their disease and have a history of multiple lines of systemic therapies, which may put them at higher risk from undergoing significant spinal surgery. Higher neurological risks and adverse events should be carefully considered, which makes appropriate patient selection of the utmost importance. Because survival is very complex to predict, performance status plays a central and perhaps more important role in the decision to operate.40,41 Finally, extensive reconstruction techniques that will outlast their remaining life, as well as a plan to achieve bony union, are potential strategies to decrease the risk of reoperation in patients with longer expected survival.42
Maintaining ambulation is paramount to patients struggling with cancer,43 and is also a common inclusion criterion for several oncology trials. Reoperation in a patient with high-grade MESCC due to recurrent radioresistant tumors or who cannot receive additional radiotherapy may be the best and only way to avoid paralysis Figure 2. Encouraging outcomes following reoperation for tumor recurrence have been reported,35,36,44 and a median survival of up to 9.1 months after repeat surgery has been described in some series.36 However, others did not show survival differences after a first or second surgery.35 As for the index surgery, most patients who have revision surgery for recurrent symptomatic MESCC improve neurologically. However, neurological deterioration has been observed in some series to be higher, as noted by Quraishi et al (23% neurologic deterioration in revision surgery vs 8% in first surgery).35 Perhaps more important, functional status was shown to be maintained or improved by one Eastern Cooperative Oncology Group grade in as many as 97% of patients after revision surgery.
Figure 2.
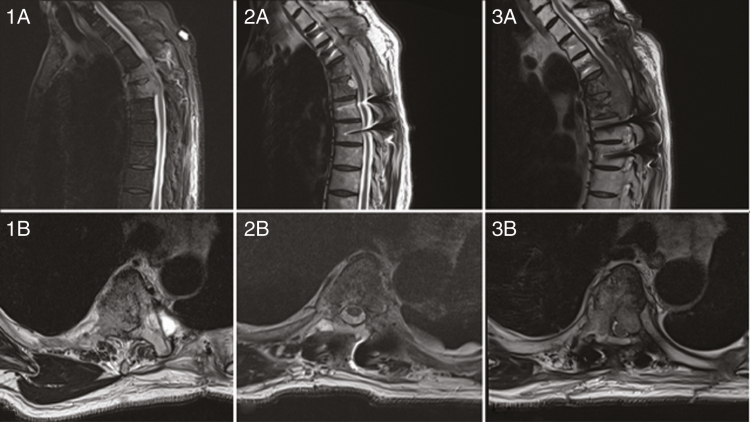
A 64-year-old man who presented with acute neurological deterioration secondary to a T4 and T5 metastatic epidural spinal cord compression from a known lung adenocarcinoma (1a/1b). He underwent surgical debulking of the lesion, separation surgery with restauration of the epidural space and spinal fixation, followed by stereotactic body radiotherapy. His postoperative MRI confirmed good decompression and removal of the epidural tumor (2a/b). Six months later, on a follow-up MRI the patient had a recurrence locally (3a/b) and required a reoperation.
Adverse events following spine surgery in cancer patients can be quite high.45,46 Reoperation through a radiated field carries a higher risk of dural tear, wound infection, and wound dehiscence. These complications may have catastrophic consequences, often necessitating another surgery, and imposing significant delay in resuming systemic therapies. A posterior approach in this context is often preferred, allowing normal to abnormal dural planes to be developed, multilevel circumferential decompression, and solid 360° fixation. A very effective strategy to decrease these complications is the liberal use of plastic surgery closure,47 which has resulted in decreased wound complications for revision surgeries in radiated fields. This has also been used in initial surgeries in high-risk locations like the cervicothoracic junction.46 Minimally invasive techniques, including percutaneous fixation, laser interstitial thermal therapy, cryotherapy, and cement augmentation can also be very helpful in selected cases to decrease the surgical footprint and potential wound complications.48
Clinical Trials and Emerging Treatment Concepts
At present, the only randomized trials comparing the efficacy of spine SBRT to conventional radiotherapy are Radiation Therapy Oncology Group (RTOG) 0631, which has been reported in abstract form only,49 and the Canadian SC24 trial (NCT02512965), which has accrued and is pending results. Importantly, both of these studies excluded patients who had received prior radiotherapy. With respect to prospective studies specific to this recurrent or progressive patient population, we are eagerly awaiting the results of a phase 1 feasibility study of single-session spine SRS in patients with inoperable, previously irradiated MESCC (NCT01256554). Additionally, a phase 2 trial is currently evaluating single-fraction SBRT to multifraction SBRT as salvage treatment for previously irradiated spinal metastases (NCT03028337). Prospective evaluations of reirradiation strategies, including spine SRS/SBRT, with specific reporting of pain scores using standardized pain scales, quality of life outcomes using multidimensional testing, oncologic control, and treatment-related toxicities specific to the recurrent setting, are needed to standardize retreatment practices.
With respect to emerging concepts, there are several hypotheses that are in need of prospective studies to either validate clinical concepts to improve outcomes or test innovative initial pilot data. For example, given that epidural failure is the most common pattern of disease progression, aggressive management of epidural disease is a trend in the literature. This is highly relevant to the reirradiation population given that the spinal cord dose limit is further constrained, thereby limiting efficacy. As a result, high-level evidence is needed to evaluate separation surgery for low-grade, neurologically intact epidural disease followed by reirradiation SBRT to justify the surgical resource. Means to improve spinal stability with prophylactic cement augmentation or percutaneous instrumentation also require high-level evidence before routine adoption can be advocated for because these are additional invasive procedures in a fragile patient population. Evaluation of high-dose preoperative SRS (18 Gy in 1 fraction) with planned spine stabilization within 24 hours has been performed in limited series, providing encouraging data for new management paradigms to be tested in the clinic.50 This technique requires careful adoption in the recurrent setting, given the proportion of patients who have MESCC or significant mechanical instability. However, for patients with recurrent or progressive disease detected early, and in the absence of significant epidural extension, reirradiation with preoperative spine SRS/SBRT and stabilization may be a feasible approach and warrants evaluation. Lastly, treatment with localized nonradiotherapeutic modalities for spinal metastases are emerging, and is highly relevant to those patients previously radiated because the spinal cord is eventually limited by the exposed cumulative dose. For example, photodynamic therapy, either as a sole modality or in conjunction with SBRT, is a novel development. Following promising results from a phase 1 clinical trial demonstrating the feasibility and safety of this technique in patients with vertebral body metastases, a phase 2 study is currently being planned for accrual.25 Ultimately and encouragingly, several options are evolving for this population, which has been traditionally deemed too complex for aggressive intervention.
Conclusions
In summary, there are a number of key criteria to assess in a patient with recurrent or progressive disease, including the extent and timing of disease recurrence, prior therapy, neurologic status, extent of prior tumor-directed therapy, and future oncologic treatment options, mechanical stability, and overall patient status. Incorporating these fundamental principles into the evaluation and management of this complex patient population is key to optimizing the functional, mechanical, and oncologic outcomes. These patients should be reviewed and discussed at a multidisciplinary tumor conference with appropriate expertise and experience from all key members. Referral to a specialized center of excellence with experience in treating recurrent disease should be considered if the patient has received prior therapy close to spinal cord tolerance, advanced radiotherapy treatment options are to be used such as PRDR or SRS/SBRT, complex surgical interventions are required in the setting of multiple prior therapies, or advanced minimally invasive surgical techniques are needed. Ultimately, enrollment in ongoing clinical trials will allow for the development of prospective evidence to guide decision making, test emerging concepts, and evaluate innovative multimodal approaches to better serve our patients.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement.
R.K. has received honoraria from Elekta AB, Accuray Inc, and Novocure, and research support from Medtronic Inc. N.D. has a consulting agreement with Stryker and Baxter, and has received speaker fees from and is a stockholder in Medtronic. J.S.D. has nothing to declare. A.S. has served as an advisor/consultant for Abbvie, Merck, Roche, Varian (Medical Advisory Group), Elekta (Gamma Knife Icon), BrainLAB, and VieCure (Medical Advisory Board); is an ex officio board member of the International Stereotactic Radiosurgery Society (ISRS); has conducted past educational seminars with Elekta AB, Accuray Inc, Varian (CNS Teaching Faculty), BrainLAB, and Medtronic Kyphon; has received a research grant from Elekta AB and travel accommodations/expenses from Elekta, Varian, and BrainLAB; and belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Lee CS, Jung CH. Metastatic spinal tumor. Asian Spine J. 2012;6(1):71–87. PMCID: PMC3302920. PMID: 22439092. doi: 10.4184/asj.2012.6.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–1436. [DOI] [PubMed] [Google Scholar]
- 5. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;2002(2):CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101–109. [DOI] [PubMed] [Google Scholar]
- 7. Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Expert Panel on Radiation Oncology-Bone Metastases; Lo SS, Ryu S, Chang EL, et al. ACR Appropriateness Criteria metastatic epidural spinal cord compression and recurrent spinal metastasis. J Palliat Med. 2015;18(7):573–584. [DOI] [PubMed] [Google Scholar]
- 9. Chow E, Hoskin P, Mitera G, et al. ; International Bone Metastases Consensus Working Party. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):1730–1737. [DOI] [PubMed] [Google Scholar]
- 10. Ang KK, Jiang GL, Feng Y, et al. Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys. 2001;50(4):1013–1020. [DOI] [PubMed] [Google Scholar]
- 11. Ang KK, Price RE, Stephens LC, et al. The tolerance of primate spinal cord to re-irradiation. Int J Radiat Oncol Biol Phys. 1993;25(3):459–464. [DOI] [PubMed] [Google Scholar]
- 12. Chow E, van der Linden YM, Roos D, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164–171. [DOI] [PubMed] [Google Scholar]
- 13. Huisman M, van den Bosch MAJ, Wijlemans JW, et al. Effectiveness of reirradiation for painful bone metastases: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2012;84(1):8–14. [DOI] [PubMed] [Google Scholar]
- 14. Rades D, Stalpers LJ, Veninga T, Hoskin PJ. Spinal reirradiation after short-course RT for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;63(3):872–875. [DOI] [PubMed] [Google Scholar]
- 15. Garg AK, Wang XS, Shiu AS, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117(15):3509–3516. [DOI] [PubMed] [Google Scholar]
- 16. Thibault I, Campbell M, Tseng CL, et al. Salvage stereotactic body radiotherapy (SBRT) following in-field failure of initial SBRT for spinal metastases. Int J Radiat Oncol Biol Phys. 2015;93(2):353–360. [DOI] [PubMed] [Google Scholar]
- 17. Myrehaug S, Sahgal A, Hayashi M, et al. Reirradiation spine stereotactic body radiation therapy for spinal metastases: systematic review. J Neurosurg Spine. 2017;27(4):428–435. [DOI] [PubMed] [Google Scholar]
- 18. Ito K, Nihei K, Shimizuguchi T, et al. Postoperative re-irradiation using stereotactic body radiotherapy for metastatic epidural spinal cord compression. J Neurosurg Spine. 2018;29(3):332–338. [DOI] [PubMed] [Google Scholar]
- 19. Grosu AL, Andratschke N, Nieder C, Molls M. Retreatment of the spinal cord with palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52(5):1288–1292. [DOI] [PubMed] [Google Scholar]
- 20. Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10(3):200–209. [DOI] [PubMed] [Google Scholar]
- 21. Nieder C, Grosu AL, Andratschke NH, Molls M. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys. 2006;66(5):1446–1449. [DOI] [PubMed] [Google Scholar]
- 22. Moraes FY, Chen X, Yan M, et al. Evolving role of stereotactic body radiation therapy in the management of spine metastases: defining dose and dose constraints. Neurosurg Clin N Am. 2020;31(2):167–189. [DOI] [PubMed] [Google Scholar]
- 23. Medin PM, Foster RD, van der Kogel AJ, Sayre JW, McBride WH, Solberg TD. Spinal cord tolerance to reirradiation with single-fraction radiosurgery: a swine model. Int J Radiat Oncol Biol Phys. 2012;83(3):1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sahgal A, Chang JH, Ma L, et al. Spinal cord dose tolerance to stereotactic body radiation therapy [published online ahead of print October 10, 2019]. Int J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 25. Fisher C, Ali Z, Detsky J, et al. Photodynamic therapy for the treatment of vertebral metastases: a phase I clinical trial. Clin Cancer Res. 2019;25(19):5766–5776. [DOI] [PubMed] [Google Scholar]
- 26. Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):107–116. [DOI] [PubMed] [Google Scholar]
- 27. Nieder C, Gaspar LE, De Ruysscher D, et al. Repeat reirradiation of the spinal cord: multi-national expert treatment recommendations. Strahlenther Onkol. 2018;194(5):365–374. [DOI] [PubMed] [Google Scholar]
- 28. Abusaris H, Storchi PRM, Brandwijk RP, Nuyttens JJ. Second re-irradiation: efficacy, dose and toxicity in patients who received three courses of radiotherapy with overlapping fields. Radiother Oncol. 2011;99(2):235–239. [DOI] [PubMed] [Google Scholar]
- 29. Katsoulakis E, Riaz N, Cox B, et al. Delivering a third course of radiation to spine metastases using image-guided, intensity-modulated radiation therapy. J Neurosurg Spine. 2013;18(1):63–68. [DOI] [PubMed] [Google Scholar]
- 30. Cannon GM, Tomé WA, Robins HI, Howard SP. Pulsed reduced dose-rate radiotherapy: case report: a novel re-treatment strategy in the management of recurrent glioblastoma multiforme. J Neurooncol. 2007;83(3):307–311. [DOI] [PubMed] [Google Scholar]
- 31. Murphy ES, Rogacki K, Godley A, et al. Intensity modulated radiation therapy with pulsed reduced dose rate as a reirradiation strategy for recurrent central nervous system tumors: an institutional series and literature review. Pract Radiat Oncol. 2017;7(6):e391–e399. [DOI] [PubMed] [Google Scholar]
- 32. Mohindra P, Robins HI, Tomé WA, Hayes L, Howard SP. Wide-field pulsed reduced dose rate radiotherapy (PRDR) for recurrent ependymoma in pediatric and young adult patients. Anticancer Res. 2013;33(6):2611–2618. [PubMed] [Google Scholar]
- 33. Folkert MR, Bilsky MH, Cohen GN, et al. Intraoperative and percutaneous iridium-192 high-dose-rate brachytherapy for previously irradiated lesions of the spine. Brachytherapy. 2013;12(5):449–456. [DOI] [PubMed] [Google Scholar]
- 34. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. [DOI] [PubMed] [Google Scholar]
- 35. Quraishi NA, Rajabian A, Spencer A, et al. Reoperation rates in the surgical treatment of spinal metastases. Spine J. 2015;15(3 suppl):S37–S43. [DOI] [PubMed] [Google Scholar]
- 36. Laufer I, Hanover A, Lis E, Yamada Y, Bilsky M. Repeat decompression surgery for recurrent spinal metastases. J Neurosurg Spine. 2010;13(1):109–115. [DOI] [PubMed] [Google Scholar]
- 37. Batista N, Tee J, Sciubba D, et al. Emerging and established clinical, histopathological and molecular parametric prognostic factors for metastatic spine disease secondary to lung cancer: helping surgeons make decisions. J Clin Neurosci. 2016;34:15–22. [DOI] [PubMed] [Google Scholar]
- 38. Goodwin CR, Abu-Bonsrah N, Rhines LD, et al. Molecular markers and targeted therapeutics in metastatic tumors of the spine: changing the treatment paradigms. Spine (Phila Pa 1976). 2016;41(suppl 20):S218–S223. [DOI] [PubMed] [Google Scholar]
- 39. Ghogawala Z, Mansfield FL, Borges LF. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine (Phila Pa 1976). 2001;26(7):818–824. [DOI] [PubMed] [Google Scholar]
- 40. Dea N, Versteeg AL, Sahgal A, et al. Metastatic spine disease: should patients with short life expectancy be denied surgical care? An international retrospective cohort study. Neurosurgery. 2020;87(2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verlaan JJ, Choi D, Versteeg A, et al. Characteristics of patients who survived < 3 months or > 2 years after surgery for spinal metastases: can we avoid inappropriate patient selection? J Clin Oncol. 2016;34(25):3054–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Altaf F, Weber M, Dea N, et al. Evidence-based review and survey of expert opinion of reconstruction of metastatic spine tumors. Spine (Phila Pa 1976). 2016;41(suppl 20):S254–S261. [DOI] [PubMed] [Google Scholar]
- 43. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life”. JAMA. 2008;299(8):937–946. [DOI] [PubMed] [Google Scholar]
- 44. Rajah G, Rapp A, Discolo E, Eltahawy H. Surgical decompression for recurrent cord compression in cancer: a case series and review of the literature. Neurol Res. 2018;40(7):549–554. [DOI] [PubMed] [Google Scholar]
- 45. Dea N, Versteeg A, Fisher C, et al. Adverse events in emergency oncological spine surgery: a prospective analysis. J Neurosurg Spine. 2014;21(5):698–703. [DOI] [PubMed] [Google Scholar]
- 46. Mesfin A, Sciubba DM, Dea N, et al. Changing the adverse event profile in metastatic spine surgery: an evidence-based approach to target wound complications and instrumentation failure. Spine (Phila Pa 1976). 2016;41(suppl 20):S262–S270. [DOI] [PubMed] [Google Scholar]
- 47. Leary OP, Liu DD, Boyajian MK, et al. Complex wound closure by plastic surgery following resection of spinal neoplasms minimizes postoperative wound complications in high-risk patients [published online ahead of print February 28, 2020]. J Neurosurg Spine. doi:10.3171/2019.12.SPINE191238. [DOI] [PubMed] [Google Scholar]
- 48. Zuckerman SL, Laufer I, Sahgal A, et al. When less is more: the indications for MIS techniques and separation surgery in metastatic spine disease. Spine (Phila Pa 1976). 2016;41(suppl 20):S246–S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ryu S, Deshmukh S, Timmerman RD, et al. Radiosurgery compared to external beam radiotherapy for localized spine metastasis: phase III results of NRG oncology/RTOG 0631. Int J Radiat Oncol Biol Phy. 2019;105(1):S2–S3. [Google Scholar]
- 50. Steverink JG, Willems SM, Philippens MEP, et al. Early tissue effects of stereotactic body radiation therapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2018;100(5):1254–1258. [DOI] [PubMed] [Google Scholar]
- 51. Katsoulakis E, Jackson A, Cox B, Lovelock M, Yamada Y. A detailed dosimetric analysis of spinal cord tolerance in high-dose spine radiosurgery. Int J Radiat Oncol Biol Phys. 2017;99(3):598–607. [DOI] [PubMed] [Google Scholar]