Abstract
In the last few years, there has been a significant increase in younger generations using vaping devices as an alternative to smoking. Social media and celebrities have played a major role in its increased popularity. Many consumers believe it to be a relatively safer and healthier choice. We present a case of a 21-year-old, female, non-smoker with vaping exposure who developed severe acute lung injury. Her workup was negative for any other cause of acute lung injury.
Keywords: adult intensive care, interstitial lung disease, pneumonia (respiratory medicine), smoking and tobacco
Background
While cigarette smoking rates decline, the use of electronic cigarettes (e-cigarettes) and vaping has markedly increased, especially in younger populations. It is estimated that over 1 in 4 high school students and 1 in 10 middle school students used e-cigarettes in 2019.1 Proposed reasons for this dramatic rise include the introduction of flavoured tobacco products and the modification of vaping devices to be more discreet. Studies report that the most common reasons youth vape in the USA are peer influence, availability of different flavours and assumed safety as compared with other tobacco products.2 In an attempt to curb e-cigarette use among youth, regulations including restricting flavours, regulating promotion, including health warnings on packaging, and reducing nicotine levels have been proposed.3
Importantly, as the prevalence of e-cigarette use increases, evidence of e-cigarette and vaping-related lung injury (EVALI) has become apparent. In 2019, over 2050 EVALI cases were reported in 49 states, including 39 fatalities.4 The median age of those treated for EVALI was 24 years. Reported cases of EVALI have involved respiratory, gastrointestinal and constitutional manifestations. However, no clinical tests or markers that can assist in diagnosis have been identified. As the incidence of EVALI rises, it is crucial to identify signs and symptoms of the disease so early treatment can be initiated. Additionally, careful analysis of EVALI cases becomes important to identify its specific aetiology, which has remained largely unknown.5 Reporting and analysing cases of EVALI is vital to halt the growing incidence of the devastating disease and identify means for prevention.
Case presentation
A 21-year-old woman who had no significant medical history presented to a local emergency department (ED) with a 4-day history of fever, chills, cough and shortness of breath. She was not on any home medications. She had no history of recent travel, sick contacts or allergies. She reported exposure to rabbits and guinea pigs at home.
On evaluation in the ED, she was febrile at 102.7 °F, tachycardic with a pulse rate of 118 beats/minute and tachypneic with a respiratory rate of 30 breaths/minute. The rest of her physical examination was unremarkable. Laboratory evaluation was remarkable for white blood cell (WBC) count of 13.6×109/L and arterial blood gas findings of pH: 7.39, paCO2: 31, CO2: 20, PaO2:55, SaO2:84%, base excess arterial: −5. A chest X-ray revealed diffuse bilateral micronodular alveolar opacities with intrinsic air bronchograms in the left lower lobe. Chest CT scan (figure 1) revealed bilateral ground-glass opacities, interlobular septal thickening and centrilobular micronodules. She was started on empirical antibiotics with piperacillin-tazobactam and azithromycin, nebulisation and supplementary oxygen.
Figure 1.
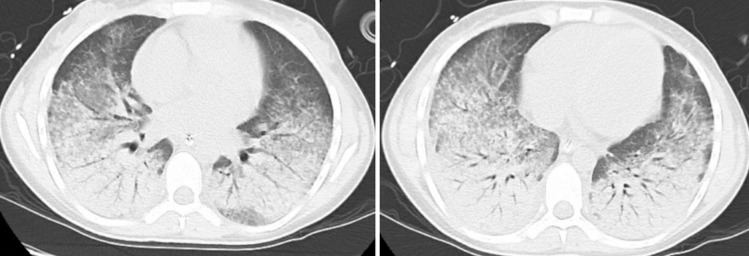
Chest CT scan showing bilateral ground-glass opacities, interlobular septal thickening, centrilobular micronodules and trace bilateral pleural fluid.
Due to her worsening respiratory distress, she was admitted to the intensive care unit (ICU), intubated and mechanically ventilated. The aetiology of her acute respiratory distress syndrome (ARDS) was thought to be due to infection or autoimmune disease. Therefore, she was started on systemic steroids along with antibiotics. Her infectious workup of blood and sputum cultures, urinary legionella antigen, mycoplasma antibody and streptococcal antigen were negative. A comprehensive viral panel including influenza PCR was negative. Fungal serologies, including Aspergillus, Blastomyces, coccidiosis, Histoplasma, Pneumocystis jiroveci, were negative as well. Workup for autoimmune disorders showed positive antinuclear antibody (ANA) with low titers, anti-Ro/anti–Sjögren's-syndrome-related antigen A autoantibodies (anti-SSA) antibodies>8, anti-La/anti–Sjögren's-syndrome-related antigen B autoantibodies (anti-SSB) antibodies>8, negative anti-dsDNA, and normal C3 and C4 complement levels. Screening for antineutrophilic cytoplasmic antibody (ANCA), Scl70, cryoglobulin, antiphospholipid, anti-glomerular basement membrane (anti-GBM), anti-Smith (anti-SM), rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) was negative.
Bronchoscopy was performed on day 3 of admission following initial treatment, infectious studies and rheumatologic workup. It showed normal mucosa with no oedema and diffuse trace amounts of thick clear-yellow secretions. Bronchial alveolar lavage (BAL) revealed increased WBC with neutrophilic predominance. A lower lip biopsy of minor salivary glands was completed and showed no evidence of Sjogren’s syndrome. Given her high risk and unstable condition at that time, a lung biopsy was not performed.
The patient’s overall condition improved (figure 2) with steroids and she was weaned off the intubation. On further questioning, the patient revealed she was using Juice USB Lighting (JUUL) vaping products daily throughout the day for the last 4 months before admission. Although she denied knowledge of any illicit substances in the vape products, her urine screen was positive for marijuana.
Figure 2.
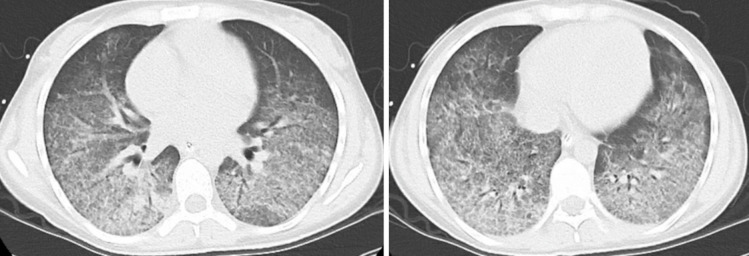
Interval chest CT after 3 days showing improvement in the diffuse bilateral opacities.
Differential diagnosis
Atypical pneumonia
Mycoplasma pneumoniae is a common cause of upper respiratory infection and community-acquired pneumonia (CAP). Diseases caused by M. pneumoniae have a variable clinical presentation, ranging from acute bronchitis and pneumonia to non-respiratory manifestations. The so-called ‘atypical pneumonia’ tends to present gradually with constitutional symptoms, cough and pleuritic chest pain. Hypoxemia and dyspnoea are less commonly seen in atypical pneumonia, as compared to other causes of CAP.6
Aspiration pneumonia
Both chemical and bacterial sources are important aetiologies of aspiration pneumonia and subsequent pneumonitis. Predisposing conditions for aspiration pneumonia include neurological defects leading to dysphagia or altered consciousness, protracted vomiting, gastric reflux and oesophageal procedures. Rapid-onset of fever, cough and dyspnoea are important manifestations of this disease. Often there will be infiltrates in the dependent portions of the lungs on imaging and prominent crackles on auscultation.7
Acute eosinophilic pneumonia
For patients who present with acute, progressive respiratory distress and diffuse parenchymal opacities of unknown aetiology, acute eosinophilic pneumonia should be considered. This rare disease may represent a hypersensitivity reaction. Diagnosis criteria include respiratory failure lasting less than 1 month, diffuse lung opacities on radiography and at least 25% eosinophils on BAL without other causes for pulmonary eosinophilia.8
Acute hypersensitivity pneumonitis
Exposure to a variety of lung irritants can cause acute, subacute or chronic hypersensitivity pneumonitis. This interstitial lung disease should be considered in all patients with a history of exposure to agricultural dust, bioaerosols, other reactive chemicals and certain microorganisms. Dyspnoea and crackles are commonly seen in the acute form of the disease, and it is often confused with acute infection. Removing irritant exposure generally leads to resolution of symptoms within days and radiographic changes within weeks.9
Lipid pneumonia
Accumulation of lipids in alveoli can be endogenous or exogenous in origin. Endogenous lipid pneumonia often follows bronchial obstruction, chronic infection, alveolar proteinosis or lipid storage diseases. The source of fat determines the severity of the disease, with animal fats often causing a severe inflammatory reaction. Symptoms include cough, dyspnoea and constitutional symptoms that improve with supportive therapy. Radiographic findings can include opacities in a variety of patterns, nodules, pneumatoceles, pneumothorax and pleural effusions.10
Cryptogenic organising pneumonia
Granulation tissue proliferation in alveolar spaces leads to a diffuse interstitial lung disease in patients with cryptogenic organising pneumonia. Symptoms are generally subacute in onset with cough, dyspnoea and constitutional symptoms appearing within a couple of months. Patients are generally middle aged with distinctive bilateral, ground-glass opacities on a radiograph.11
Diffuse alveolar haemorrhage
Acute respiratory distress syndrome resulting from diffuse alveolar damage can lead to haemorrhage within alveoli. Patients can present with a variety of symptoms and signs, but generally with haemoptysis or dyspnoea. Symptoms generally manifest within a few days. Radiographic findings include ground-glass opacities in central portions of the lung. Histological patterns include pulmonary capillaritis, bland pulmonary haemorrhage and diffuse alveolar damage.12
Toxic inhalation syndrome
Irritant inhalation can affect all portions of the pulmonary tract and can lead to disease of any severity. Occupational, home and environmental exposures have been implicated in this disease. Water-soluble vapours and large particles often affect upper airways, while smaller particles tend to deposit in the lower respiratory tract. Different toxins may result in specific clinical symptoms, though most cases are characterised by burning in mucous membranes and upper respiratory symptoms.13
Primary pulmonary vasculitis
Small-vessel vasculitides commonly manifest with pulmonary involvement. Diseases in this category include granulomatosis with polyangiitis, Churg-Strauss syndrome and microscopic polyangiitis. These diseases are notoriously associated with ANCA. Symptoms common to this entire category of the disease include fever, malaise, myalgias and arthralgias. Nodular or cavitary lesions are often seen on imaging. Primary vasculitides can occur in all ages and are an important differential diagnosis in unexplained acute hypoxemia and shortness of breath.14
Sjogren’s syndrome
Pulmonary manifestations of Sjogren’s syndrome may involve both the airway and interstitium. Often interstitial lung disease develops years after developing Sjogren’s syndrome, but it may be the presenting symptom. Dyspnoea, cough, sputum production, chest pain and constitutional symptoms are the most common initial symptoms. Imaging of pulmonary disease secondary to Sjogren’s syndrome typically displays nodular or reticular infiltrates with basilar predominance. Serological testing and/or a salivary gland biopsy can be used to confirm the diagnosis of Sjogren’s syndrome.15
Treatment
The patient completed 7 days of piperacillin/tazobactam and doxycycline. Intravenous vancomycin was discontinued after 48 hours. Leucocytosis and elevated procalcitonin continued to trend to normal limits. Serial chest X-rays and CT imaging revealed initial improvement, stabilisation for 2–3 days and continued improvement. Extubation was attempted on the 10th day and was done successfully with bilevel positive airway pressure bridging. The patient experienced mild dyspnoea after extubation but denied any chest or abdominal pain.
Outcome and follow-up
The patient was breathing room air 3 days postextubation. She was discharged 6 days postextubation, which was 15 days from initial admission. She was sent home on a 3-week prednisone taper, along with plans for physical therapy and speech therapy. At the time of writing, she had completed 3 months of follow-up care. Subsequent follow-up with her primary care physician was encouraging, as she reported feeling better with improvement in her breathing. She was strongly encouraged to avoid vape pens and marijuana. Continuation of physical therapy was advised until her exercise tolerance improved. Follow-up pulmonology appointments revealed that her functional capacity had improved since discharge from the hospital. Pulmonology plans for a repeat chest CT scan and formal pulmonary function testing. Follow-up with rheumatology was planned to determine the significance of the positive ANA, SS-B and SS-A detected during her hospitalisation.
Discussion
With over 2000 reported cases, EVALI is becoming an important modern health issue.4 The need for more information about potential EVALI cases becomes increasingly important as e-cigarette use continues to rise among youth in the USA.1 Though tachycardia, tachypnoea and hypoxia are commonly found in these cases,5 the wide-ranging physical manifestations of the disease present a diagnostic dilemma.4 Proposed diagnostic criteria for EVALI includes the use of the vaping product in the past 90 days, lung opacities on chest X-ray or CT, exclusion of lung infection and lack of probable alternative diagnosis.16
The chest CT findings of ground-glass opacities can correlate to a variety of differential diagnoses. It is unclear whether the aetiology of this patient’s ARDS was secondary to an infection, autoimmune disorder or inhalation. Empiric treatment for pneumonia was completed, but no definitive organism was identified from cultures. It is possible the patient may have suffered from eosinophilic pneumonia or hypersensitivity pneumonitis as she responded quickly to steroids, although no eosinophils were identified on BAL.
The patient tested positive for marijuana use and later reported using vaping devices, which is possible to be the cause of her respiratory compromise. Importantly, many components of aerosols and liquids within e-cigarettes have known poisonous or carcinogenic effects.3 Glycerol and propylene glycol are ingredients frequently encountered in e-cigarettes and have been linked to squamous metaplasia. Additionally, established carcinogens including heavy metals, acrolein, formaldehyde and acetaldehyde have been reported in e-cigarette vapour and aerosol.17 Marijuana has been reported in vaping products.3 The link between smoking marijuana and certain cancers and lung disease is currently being investigated.18 19
Similar published case reports, according to the Center for Disease Control (CDC), suggest that the clinical presentation for the majority of patients with EVALI begins with respiratory, gastrointestinal or constitutional symptoms.4 EVALI is a diagnosis of exclusion and most patients are treated with antibiotics and steroids, as was the patient in this case. The CDC also reports that based on two cohort studies involving 395 patients in the USA, more than 40% of patients with EVALI required ICU admission and more than 20% required mechanical ventilation.20
A possible treatment approach for EVALI includes the initiation of empiric antibiotics for CAP. Systemic glucocorticoids should be given for those with severe disease or evidence of cryptogenic organising pneumonia or eosinophilia pneumonia. Care should focus on maintaining oxygen saturation of 88–92 perfect, starting with a nasal cannula and continuing standardised treatment for ARDS. In a minority of patients, mechanical ventilation has been necessary and rarely extracorporeal membrane oxygenation (ECMO) has been used.16 Consider pulmonary function testing, exercise oximetry and chest CT within 8 weeks of discharge.21
Learning points.
E-cigarette and vaping-related lung injury (EVALI) does not have a clear clinical course, but typically presents as respiratory, gastrointestinal or constitutional symptoms.
Educating and counselling patients on tobacco, e-cigarette, vaping and cannabis use is vital to preventing associated lung injury morbidity and mortality.
When considering EVALI as a diagnosis, it is imperative to elicit e-cigarette or other vaping product use during patient history to assist in narrowing the differential diagnosis.
Strongly consider admitting suspected EVALI cases as symptoms can rapidly worsen.
Footnotes
Contributors: AP and DV prepared the manuscript. GW performed literature review, added images and provided extensive proofreading.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cullen KA, Gentzke AS, Sawdey MD, et al. E-Cigarette use among youth in the United States, 2019. JAMA 2019;322:2095–103. 10.1001/jama.2019.18387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai J, Walton K, Coleman BN, et al. Reasons for electronic cigarette use among middle and high school students - national youth tobacco survey, United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:196–200. 10.15585/mmwr.mm6706a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services U.S. department of health and human services. e-cigarette use among youth and young adults. A report of the surgeon general, United States, 2016. Available: https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/pdfs/2016_sgr_entire_report_508.pdf [Accessed 27 Oct 2020].
- 4.Center for Disease Control and Prevention Outbreak of lung injury associated with e-cigarettes use, or vaping. Available: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html [Accessed 12 Nov 2019].
- 5.Siegel DA, Jatlaoui TC, Koumans EH, et al. Update: interim guidance for health care providers evaluating and caring for patients with suspected E-cigarette, or vaping, product use associated lung injury. MMWR Morb Mortal Wkly Rep 2019;68:919–27. 10.15585/mmwr.mm6841e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez JA. Overview of community-acquired pneumonia in adults [Internet], 2019. Available: https://www.uptodate.com/contents/overview-of-community-acquired-pneumonia-in-adults?search=community%20acquired%20pneumonia&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 [Accessed 12 Nov 2019].
- 7.Bartlett JG. Aspiration pneumonia in adults [Internet], 2019. Available: https://www.uptodate.com/contents/aspiration-pneumonia-in-adults [Accessed 20 Nov 2019].
- 8.King JTE. Idiopathic acute eosinophilic pneumonia [Internet], 2020. Available: https://www.uptodate.com/contents/idiopathic-acute-eosinophilic-pneumonia?search=acute%20eosinophilic%20pneumonia&source=search_result&selectedTisel=1~47&usage_type=default&display_rank=1 [Accessed 27 Oct 2020].
- 9.King JTE. Hypersensitivity pneumonitis (extrinsic allergic alveolitis): Clinical manifestations and diagnosis [Internet], 2020. Available: https://www.uptodate.com/contents/hypersensitivity-pneumonitis-extrinsic-allergic-alveolitis-clinical-manifestations-and-diagnosis?search=acute%20hypersensitivity%20pneumonitis&source=search_result&selecteselect=1~150&usage_type=default&display_rank=1 [Accessed 27 Oct 2020].
- 10.Betancourt SL, Martinez-Jimenez S, Rossi SE, et al. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol 2010;194:103–9. 10.2214/AJR.09.3040 [DOI] [PubMed] [Google Scholar]
- 11.King JTE. Cryptogenic organizing pneumonia [Internet], 2020. Available: https://www.uptodate.com/contents/cryptogenic-organizing-pneumonia?search=cryptogenic%20organizing%20pneumonia&source=search_result&selecselected=1~107&usage_type=default&display_rank=1 [Accessed 27 Oct 2020].
- 12.King JTE. The diffuse alveolar hemorrhage syndromes [Internet], 2020. Available: https://www.uptodate.com/contents/the-diffuse-alveolar-hemorrhage-syndromes?search=diffuse%20alveolar%20hemorrhage&source=search_result&selectedTitle=1~131&usage_type=default&display_rank=1 [Accessed 27 Oct 2020].
- 13.Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med 2010;42:28–35. 10.5152/eajm.2010.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castañer E, Alguersuari A, Gallardo X, et al. When to suspect pulmonary vasculitis: radiologic and clinical clues. Radiographics 2010;30:33–53. 10.1148/rg.301095103 [DOI] [PubMed] [Google Scholar]
- 15.Baer AN. Diagnosis and classification of Sjögren’s syndrome [Internet], 2018. Available: https://www.uptodate.com/contents/diagnosis-and-classification-of-sjogrens-syndrome [Accessed 16 Dec 2019].
- 16.Hollingsworth H. E-cigarette or vaping product use associated lung injury (EVALI) [Internet], 2020. Available: https://www.uptodate.com/contents/e-cigarette-or-vaping-product-use-associated-lung-injury-evali?search=evali&source=search_result&selectedTitle=1~10&usage_type=default&display_rank=1#H862560329[Accessed 27 Oct 2020].
- 17.Civiletto CW, Hutchinson J. Electronic vaping delivery of cannabis and nicotine [Internet]: StatPearls, 2020. Available: https://www.ncbi.nlm.nih.gov/books/NBK545160/ [PubMed]
- 18.Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol 2014;46:65–81. 10.1007/s12016-013-8374-y [DOI] [PubMed] [Google Scholar]
- 19.Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013;10:239–47. 10.1513/AnnalsATS.201212-127FR [DOI] [PubMed] [Google Scholar]
- 20.DynaMed Ipswich (MA): EBSCO information services. 1995 -. record No. T1568994420333, Vaping, 2019. Available: https://www.dynamed.com/topics/dmp~AN~T1568994420333 [Accessed 1 Dec 2019].
- 21.Salzman GA, Alqawasma M, Asad H. Vaping associated lung injury (EVALI): an explosive United States epidemic. Mo Med 2019;116:492–6. [PMC free article] [PubMed] [Google Scholar]


