Abstract
The use of immune checkpoint inhibitors (CPIs), such as pembrolizumab, for the treatment of cancer, is now prevalent. CPIs are associated with a significant side effect profile, termed immune-related adverse events (irAEs). Renal irAEs, such as interstitial nephritis, are rare, and CPI-related glomerulonephritis even rarer. This is a case report of a 72-year-old man with mesothelioma of the left lung, whose serum creatinine rose during pembrolizumab treatment. Renal biopsy revealed IgA nephropathy. Withdrawal of therapy for 2 months saw no improvement in renal function, and following recommencement, serum creatinine fluctuated at approximately 1.4 times original baseline. This report will highlight the renal irAEs to be the aware of when starting CPIs, and the importance of early renal biopsy in management.
Keywords: respiratory cancer, acute renal failure, unwanted effects / adverse reactions
Background
Immune checkpoint inhibitors (CPIs) are a relatively novel and rapidly expanding treatment option of a number of solid organ and haematological malignancies. Examples of CPIs include pembrolizumab and nivolumab, which are both anti-PD-1 agents. Their intended effect is to promote the T-cell-mediated antitumour response. However, overactivation of the immune system results in a unique category of side effects, termed immune-related adverse events (irAEs). Common irAEs include hepatitis, interstitial pneumonitis, colitis, thyroiditis and hypophysitis. Renal irAEs are relatively rare, and have only been reported in 2%–4.5% of patients receiving CPI treatment.1 In the majority of cases, CPI nephrotoxicity is associated with acute tubulointerstitial nephritis (ATIN); however, a number of glomerular pathologies (membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis and IgA nephropathy) have also been occasionally observed.1 Due to the rare nature of renal irAEs, the literature is limited, and reports of CPI-related IgA nephropathy are sparse. As a result, guidelines on the diagnosis and management of renal irAEs remain limited.
This report will present a case of IgA nephropathy, associated with elevated serum creatinine, observed in a 72-year-old man, diagnosed with mesothelioma, treated with pembrolizumab. Urinalysis showed no proteinuria or haematuria. Renal biopsy showed mild IgA nephropathy (M1, E0, S0, T0) Oxford score (see figures 1–4).
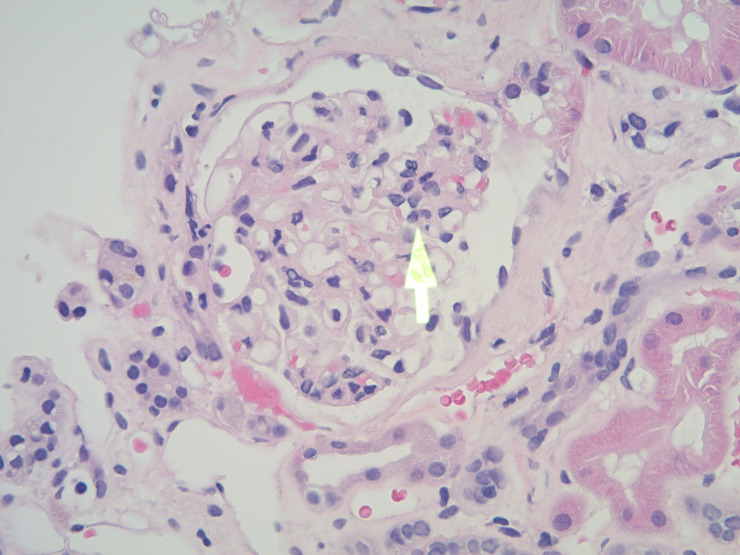
Figure 1.
H&E stain, magnification ×20 showing a glomerulus with peripheral segmental mesangial proliferation (arrow) (M1, Oxford classification), while the rest of the glomerular tuft appears normal.
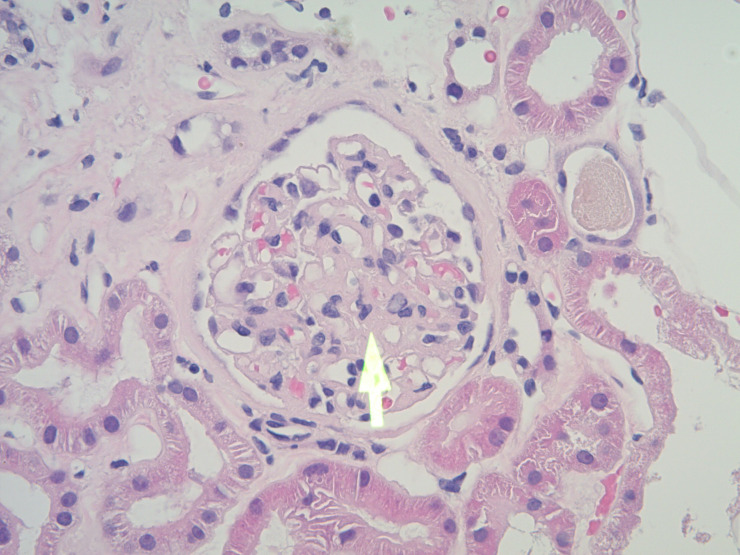
Figure 2.
H&E stain, magnification ×20 showing another glomerulus with central mesangial proliferation (arrow).
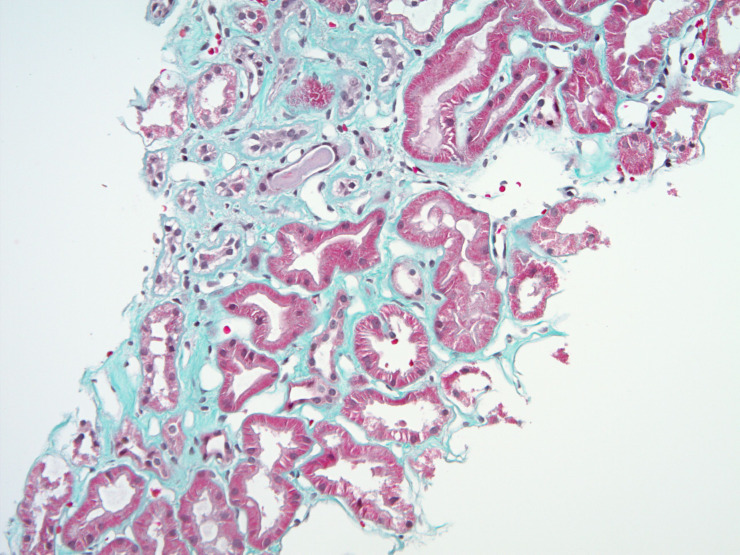
Figure 3.
Masson’s trichrome special stain, magnification ×10 displaying a focus of interstitial fibrosis. Fibrosis appears as green solid areas (T1, Oxford classification).
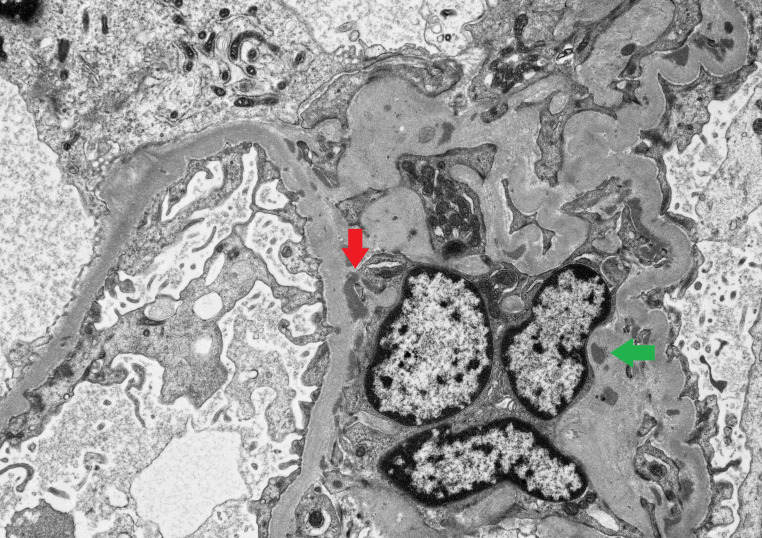
Figure 4.
Electron microscopy image showing, at the ultrastructural level, mesangial (green arrow) and paramesangial (red arrow) electron-dense, immune complex related deposits.
Case presentation
This 72-year-old man was diagnosed with T4N2M0 epitheiloid mesothelioma of the left lung in September 2016. He was originally treated with six cycles of first-line palliative cisplatin and pemetrexed from September 2016 to January 2017. Staging CT demonstrated only partial response. Subsequent monitoring showed disease progression, and the patient was commenced on second-line pembrolizumab therapy in January 2018, as part of a randomised controlled trial (pembrolizumab vs second-line chemotherapy).
Past medical history
Benign prostatic hypertrophy and irritable bowel syndrome.
Baseline serum creatinine prior to commencement of pembrolizumab was 102 umol/L.
Of note, the patient developed adrenal insufficiency secondary to pembrolizumab treatment, requiring oral steroid replacement. This diagnosis, and commencement of steroids, preceded his renal deterioration by almost 2 months, and the two pathologies were felt to be unrelated.
Drug history
Ranitidine, hydrocortisone, fludrocortisone, solifenacin, and quinine. No known drug allergies.
Deteriorating renal function following commencement of pembrolizumab
The patient was commenced on three-weekly cycles of pembrolizumab in January 2018 with renal function at baseline. In May, 5 months into treatment, serum creatinine rose to 138 umol/L. This was monitored closely; however, renal function did not recover despite adequate oral rehydration. In June, with a serum creatinine of 131 umol/L, the decision was made to hold pembrolizumab. Clinically, the patient had an unremarkable examination with no evidence of fluid overload. Urine output was within normal limits for weight. Urinalysis showed no evidence of protein or blood. Blood pressure was stable at 138/76 mm Hg. In July, with an entirely negative glomerulonephritis screen, serum creatinine climbed to 147 umol/L. As per the clinical trial, and local trust guidelines, a renal opinion was sought, and their decision was to proceed to early renal biopsy while continuing to hold pembrolizumab.
Investigations
A renal biopsy was performed in July 2018. It showed no evidence of ATIN or acute tubular necrosis (ATN). Instead, mild IgA nephropathy (M1, E0, S0, T0) Oxford score (see figures 1–4) was observed.
At the time of biopsy (1 month off pembrolizumab), serum creatinine was 138 umol/L, urea 11.7 mmol/L, potassium 5.2 mmol/L, bicarbonate 25 mmol/L, haemoglobin 139 g/L and serum albumin 44 g/L. Blood pressure remained stable, and urinalysis remained clear.
Outcome and follow-up
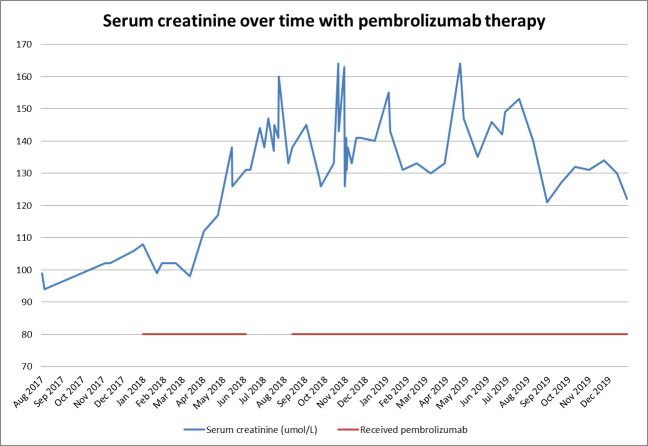
Pembrolizumab was suspended for 2 months from June 2018 to August 2018; however, this did not result in renal recovery (see figure 5). Following the diagnosis of IgA nephropathy, the decision was made to recommence pembrolizumab, and monitor renal function. Over the following year, the patient’s serum creatinine fluctuated between 121 umol/L and 164 umol/L while on pembrolizumab therapy. A staging scan in November 2019 showed stable disease. At no point did renal function recover to original baseline (see figure 5).
Figure 5.
Serum creatinine over time shown in blue. Pembrolizumab therapy timeline shown in red.
Literature review
CPI nephrotoxicity is a rare but known phenomenon; however, there are only a handful of cases reported of CPI-related IgA nephropathy. A literature review of CPI nephrotoxicity shows the majority of cases demonstrate ATIN on renal biopsy. Cases of ATN, minimal change disease and other forms of glomerulonephritis have also been occasionally described.
In 2019, at the Anderson Cancer Center, University of Texas, Mamlouk et al1 retrospectively reviewed all cancer patients from 2008 to 2018 treated with a CPI, and subsequently underwent renal biopsy. Sixteen cases were identified. Fourteen of 16 cases showed evidence of ATIN alongside multiple glomerular pathologies, including pauci-immune glomerulonephritis, membranous nephropathy, C3 glomerulonephritis, IgA nephropathy and amyloid A amyloidosis. A combination of CPI discontinuation and immunosuppression helped the majority of patients achieve partial to complete renal recovery. In 2019, Izzedine et al2 conducted a monocentric case study looking at all pembrolizumab-related nephrotoxicity cases at a local centre in France from 2015 to 2017. Twelve of 676 patients treated with pembrolizumab suffered renal toxicity (acute kidney injury or proteinuria). Four patients had ATIN, five had ATN alone, one had minimal change disease and ATN, and one had minimal change disease alone. Again, treatment involved withdrawing pembrolizumab and immunosuppression; however, one patient developed severe renal failure requiring dialysis. In 2018, Abudayyeh et al3 retrospectively reviewed all renal biopsies performed at a local centre between 2008 and 2017. Ten cases of CPI nephrotoxicity were identified. Five patients had ATIN as well as varying forms of glomerulonephritis. The glomerular pathologies observed included membranous nephropathy, IgA nephropathy, pauci-immune glomerulonephritis and amyloid A amyloidosis. In 2016, Cortazar et al4 reviewed 13 patients with CPI nephrotoxicity. Twelve patients had evidence of ATIN on renal biopsy.
To date, two case reports have been written describing CPI-related IgA nephropathy. In 2018, Kishi et al5 reported a case of a 72-year-old man with postoperative recurrence of lung squamous cell carcinoma, treated with nivolumab. The patient developed acute kidney injury associated with proteinuria. Renal biopsy showed IgA nephropathy. Nivolumab withdrawal resulted in partial renal recovery. In 2019, Oki et al6 reported a case of a 75-year-old woman with non-small cell lung carcinoma who developed proteinuria and microscopic haematuria while on pembrolizumab. Renal biopsy showed ATIN and IgA nephropathy. The patient’s proteinuria and haematuria slowly improved after discontinuation of therapy, and renal function remained stable.
Discussion
Renal irAEs are relatively rare. CPI nephrotoxicity is most commonly associated with ATIN, and CPI-related glomerulonephritis, including IgA nephropathy, is extremely rare.
Although there have been reported cases of CPI-related IgA nephropathy, it is still unclear if the aetiology of our patient’s IgA nephropathy was related to pembrolizumab. It is possible that the patient had IgA nephropathy prior to commencing pembrolizumab, and this could have been an incidental renal biopsy finding. The patient’s raised serum creatinine could also be unrelated to his IgA nephropathy, especially given his urine was always clear of blood and protein. It is of note, the patient was having regular contrast scans to monitor disease, and the renal physicians offered a differential diagnosis of contrast nephropathy as a cause of renal impairment. This, of course, does not explain the presence of IgA nephropathy, and the renal biopsy showed no features suggestive of contrast nephropathy.
Finally, elevated serum creatinine, while receiving pembrolizumab, has been observed in 11%–35% of cases.7 The aetiology of this remains unclear. It is important to note that withdrawal of therapy for 2 months saw no improvement in renal function, and following recommencement, serum creatinine fluctuated at approximately 1.4 times original baseline (see figure 5). Fortunately, the patient was able to continue CPI therapy with regular renal monitoring without further deterioration in function.
Learning points.
Renal immune-related adverse events are rare, especially in the form of glomerulonephritis.
In patients presenting with acute kidney injury, proteinuria or haematuria, initial management involves a full renal workup and renal biopsy.
Discontinuation of checkpoint inhibitor (CPI) therapy and commencement of immunosuppression (if appropriate) can help to arrest or even reverse CPI-related renal injury in many cases.
Footnotes
Contributors: RW: main author of the paper and responsible for design, data collection, literature review, analysis and discussion. TD: involved in the patient’s care, editing and approval of final draft. AT: provided renal biopsy pathology images and descriptions, editing and approval of final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mamlouk O, Selamet U, Machado S, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer 2019;7:2. 10.1186/s40425-018-0478-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izzedine H, Mathian A, Champiat S, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J 2019;12:81–8. 10.1093/ckj/sfy100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abudayyeh A, Abdelrahim M, Selamet U, et al. Checkpoint inhibitor induced glomerulonephritis. JCO 2018;36:e15083 10.1200/JCO.2018.36.15_suppl.e15083 [DOI] [Google Scholar]
- 4.Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishi S, Minato M, Saijo A, et al. Iga nephropathy after nivolumab therapy for postoperative recurrence of lung squamous cell carcinoma. Intern Med 2018;57:1259–63. 10.2169/internalmedicine.9814-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oki R, Hirakawa Y, Kimura H, et al. Renal effects after pembrolizumab treatment for non-small cell lung carcinoma. Intern Med 2020;59:977–81. 10.2169/internalmedicine.3928-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UpToDate UpToDate [Online], 2020. Available: https://www.uptodate.com