Abstract
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterised by inflammatory nodules, abscesses, sinus tract formation and scarring. There is a lack of evidence for the use of radical radiotherapy for patients with a diagnosis of HS. A 56-year-old woman with a long-standing diagnosis of HS presented with a cutaneous local recurrence of breast cancer. Radical radiotherapy was offered despite issues with previous prolonged postoperative wound healing associated with the underlying HS. A multidisciplinary evaluation was conducted with breast surgeons, dermatologists and radiation oncologists to assess the safety of delivery of radical radiotherapy. Five weeks post radiotherapy, the patient had no significant residual symptoms from her breast cancer treatment for her HS and no escalation of treatment was required for her HS. Factors contributing to safe delivery of radical radiotherapy include medical optimisation prior to and during treatment, radiation dose, radiation technique and vigilant post-treatment surveillance.
Keywords: Breast cancer, Radiotherapy, Dermatology
Background
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterised by recurrent painful inflammatory nodules, abscesses and draining sinus tracts, predominantly affecting skin-bearing apocrine glands.1 Most people develop HS in their early 20s although onset has been described in prepubertal individuals. The pathogenesis of the condition remains incompletely understood. Lymphocytic infiltration and hyperkeratosis of the pilosebaceous unit with subsequent dilatation and rupture lead to an acute inflammatory response. Following this, there is chronic inflammation with permanent architectural changes, including scarring and sinus tract formation. Patients with HS are more likely to have metabolic syndrome, obesity and type II diabetes mellitus. HS is recognised to have a profound impact on patients’ quality of life. Patients suffer from a significant mental health disease burden and have an increased risk of committing suicide.2 Documented reports of the prevalence of HS vary, but have been estimated at approximately 1%–4% within the UK population.3
Diagnosis of HS is based on clinical history and the presence of classic clinical features. HS may be broadly staged into three categories based on clinical severity, known as Hurley stage:4 stage I with abscess formation but no sinus tracts or scarring, stage II with recurrent abscesses, sinus tracts and scarring but affected areas are widely separated by normal skin, and stage III with diffuse involvement with multiple sinus tracts. Other important patient assessment measures include lesion count, pain scoring (such as the use of a visual analogue scale) and the Dermatology Life Quality Index.5 Treatment is directed at activity and extent of disease. Hurley stage I disease can often be effectively managed with topical antibiotics such as clindamycin, whereas more extensive stage II and III disease may require medical treatments, including long-term oral antibiotics, oral retinoids (such as acitretin), dapsone (a sulphonamide antibiotic),6 anti-tumor necrosis factor therapy (such as adalimumab or infliximab) or surgical interventions with excision and laying open of sinus tracts and fistulating disease as appropriate.7
Low-dose external beam radiotherapy has been shown to be effective in HS. A retrospective analysis of 231 patients receiving orthovoltage radiotherapy for HS with total doses ranging from 3 to 8 Gy found 38% had complete relief of symptoms and 40% had a clear improvement in symptoms.8 However, the use of radiotherapy is not widespread due to concerns regarding late skin toxicities, including secondary malignancy risk, and the advent of improved systemic and surgical treatment options.
There is lack of clinical evidence for the use of radical radiotherapy in patients with a diagnosis of HS. We present a case of successful adjuvant radiotherapy treatment of a locally advanced, recurrent breast cancer of the chest wall in a patient with long-standing Hurley stage III HS.
Case presentation
A 56-year-old mixed-race white/black Caribbean woman was diagnosed with a right-sided breast cancer in February 2015. This was a multifocal 48 mm and separate 12 mm grade 2 invasive ductal carcinoma, oestrogen and progesterone receptor positive, and human epidermal growth factor receptor 2 negative with evidence of lymphovascular invasion. She had a long-standing history of HS since puberty, affecting the axillae and groins. This had been well-controlled since initiation of dapsone in October 2018, escalated to the current dose of 150 mg in November 2018. Topically, she used Octenisan wash (Schulke) as a cleanser and Dalacin-T topical lotion (Pfizer) as needed. Previous treatments for HS had included minocycline, lymecycline and acitretin, all of which had provided only minimal benefit. Previous repeated 3-month courses of rifampicin and clindamycin had a good effect, and an excision of her left axillary HS in November 2018 resulted in an improvement in HS at that site.
Other medical history included osteoarthritis of the knees, gout affecting the toes and depression. She was on citalopram 20 mg daily.
She underwent a mastectomy and axillary node clearance in March 2015 for her breast cancer, which found 1 of 16 lymph nodes positive for metastatic spread. She experienced issues with postoperative wound dehiscence, delayed healing and malodour for which she required oral lymecycline, potassium permanganate soaks and topical metronidazole gel to good effect. She declined adjuvant chemotherapy and adjuvant radiotherapy was not delivered due to a prolonged period of wound healing, persisting 8 months post surgery. She was commenced on the aromatase inhibitor anastrazole.
In July 2019, she underwent a biopsy of a suspicious mass superomedial to her mastectomy scar which confirmed breast cancer recurrence, and this was resected in August 2019, although with tumour cells present at the deep margin. A staging CT scan showed a suspicious lesion in the left ischium, and this was fluorodeoxyglucose-avid on a subsequent positron emission tomography/CT scan. The ischial lesion was treated with stereotactic ablative body radiotherapy in three fractions. Her endocrine therapy was switched from anastrazole to exemestane. She was deemed unsuitable for a deep inferior epigastric perforator artery reconstruction for her chest wall recurrence due a raised body mass index of 43 and ongoing electronic cigarette smoking.
Treatment
Due to a high risk of local recurrence, an adjuvant course of radical radiotherapy was recommended to the chest wall. This was delivered in February 2020 using a 3D conformal technique using tangential fields. The biological equivalent dose (BED) at 2 Gy per fraction for the standard dose of radical radiotherapy with 40.05 Gy in 15 fractions using an alpha-beta ratio for late tissue effects of 3 is 45.4 Gy. She was prescribed a dose of 50.4 Gy in 28 daily fractions over 5.5 weeks using tangential fields. A lower dose per fraction was used to allow better normal tissue recovery. Although the BED at 2 Gy per fraction is higher at 48.3 Gy, this was intentionally used due to the positive resection margin.
She was reviewed by her dermatology team prior to commencing radiotherapy. The non-healing wound on the chest wall was swabbed, which grew Staphylococcus aureus and Proteus mirabilis. A course of co-amoxiclav was prescribed. The patient was continued on dapsone 150 mg two times per day throughout radiotherapy. In case of a flare of her HS, it was recommended the patient start betamethasone valerate 0.1% with clioquinol 3% cream two times per day for up to 2 weeks.
Outcome and follow-up
Clinical photographs were taken before, during and post radiotherapy, and are shown in figures 1–6.
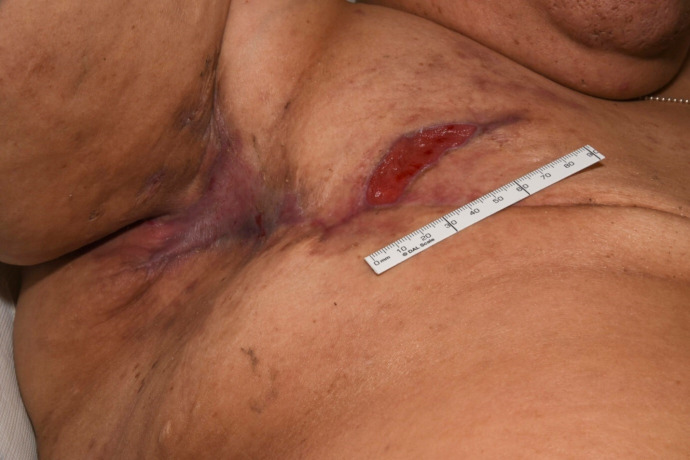
Figure 1.
Patient’s right axilla and mastectomy scar 7 days prior to commencing radiotherapy. An ulcer is visible at the lateral aspect of the scar.
Figure 2.
Day 12 post commencing radiotherapy. Appearances are not significantly changed compared with prior to starting radiotherapy.
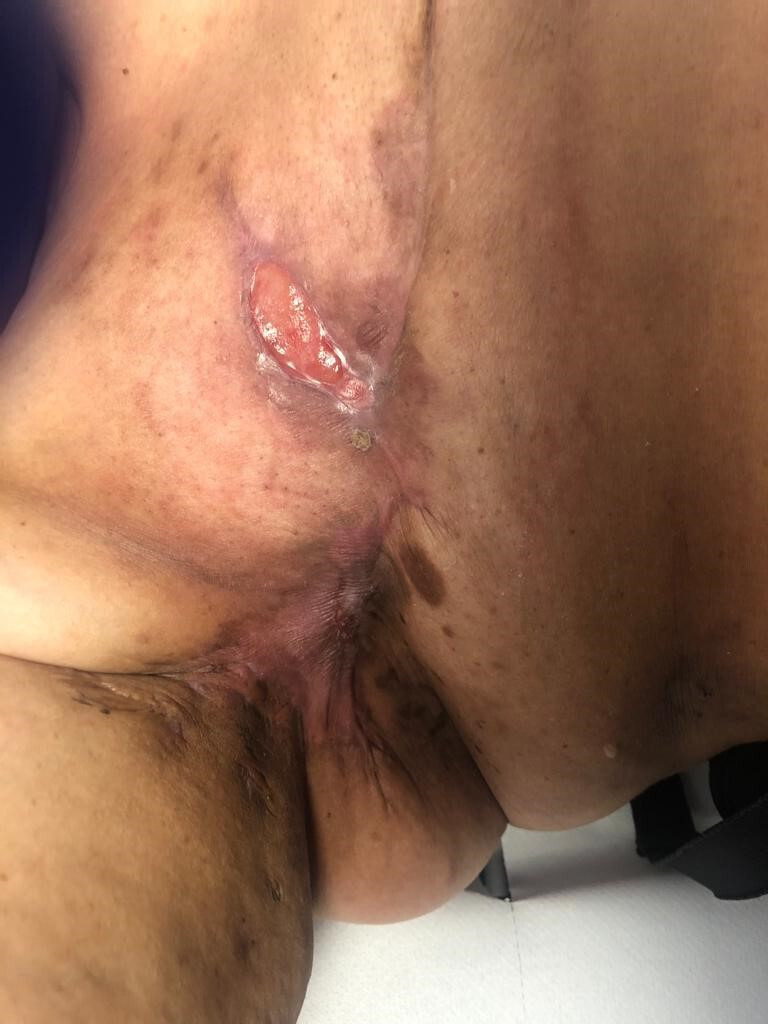
Figure 3.
Day 20 post commencing radiotherapy. Small, isolated areas of skin breakdown are noted cranial and caudal to the initially noted ulcer.
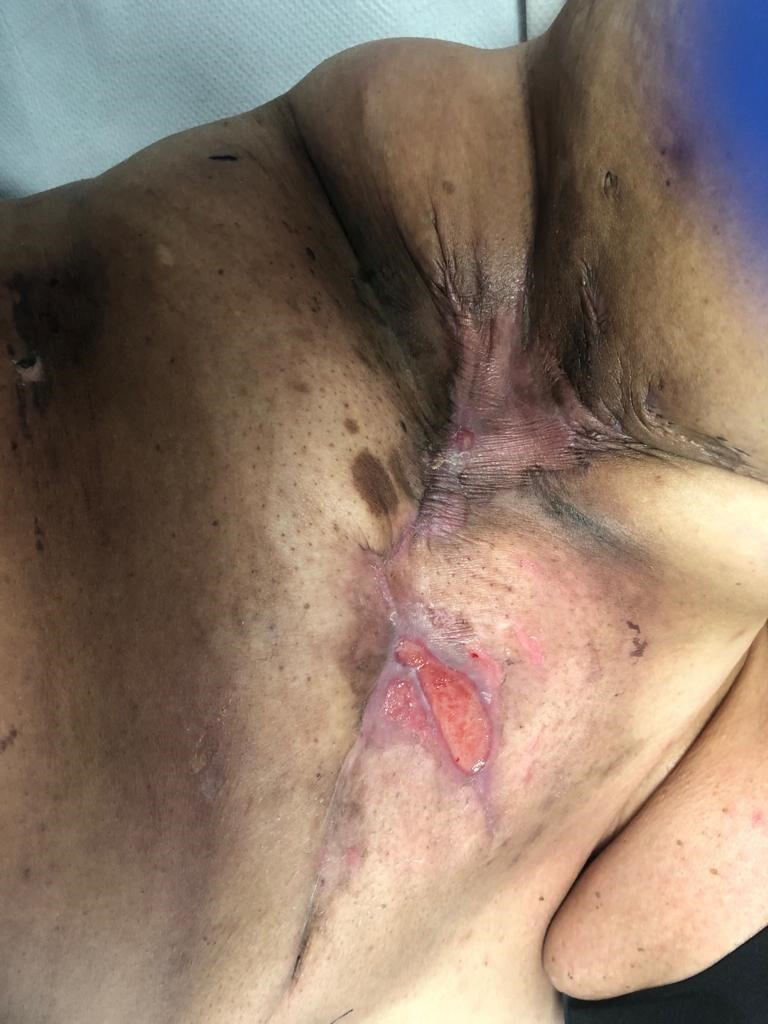
Figure 4.
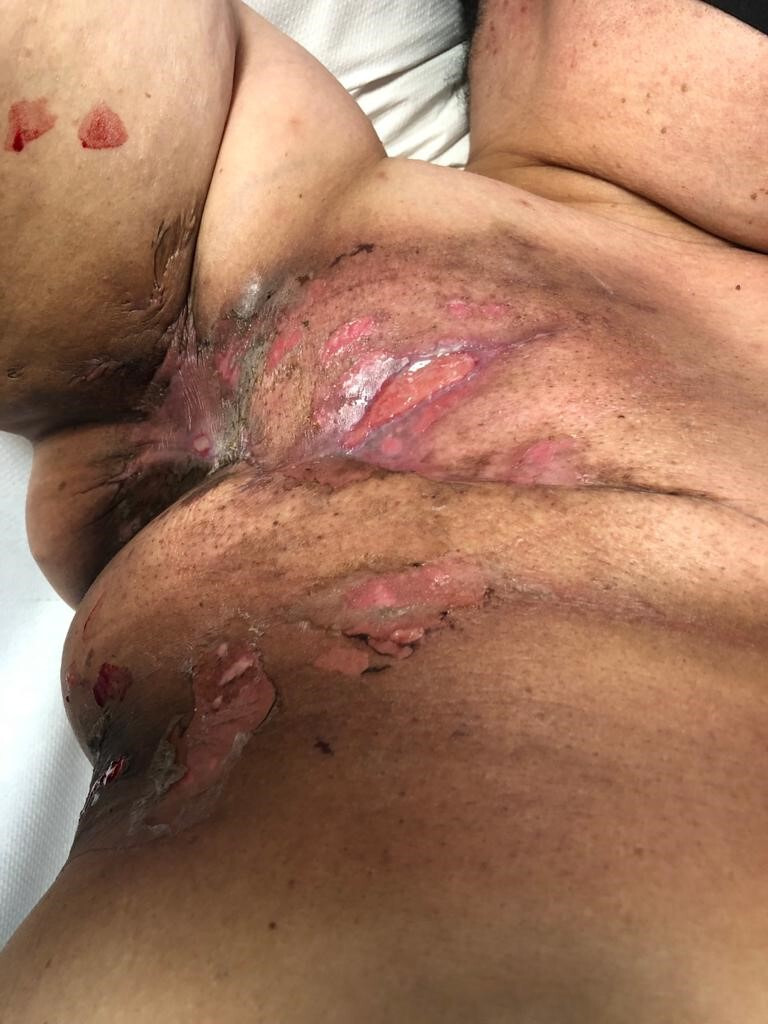
Day 28 post commencing radiotherapy. Further small, sloughy areas of skin breakdown are noted, both cranial and caudal to the mastectomy scar.
Figure 5.
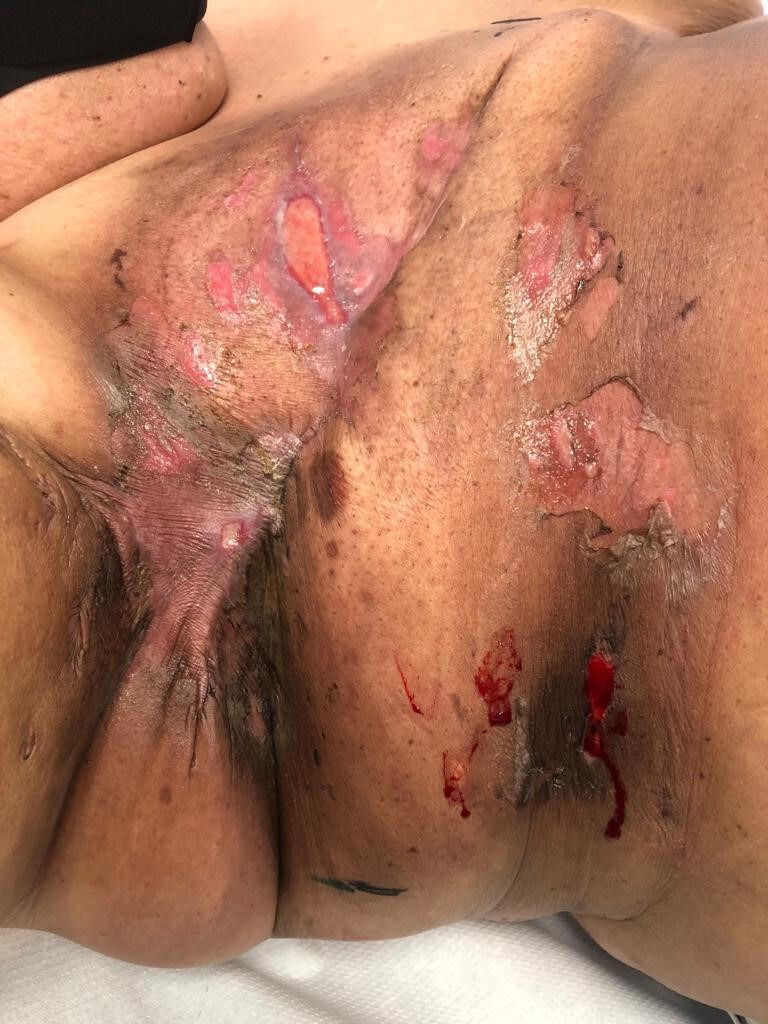
Day 7 post completion of radiotherapy. No significant change in skin health noted compared with day 28 into radiotherapy treatment.
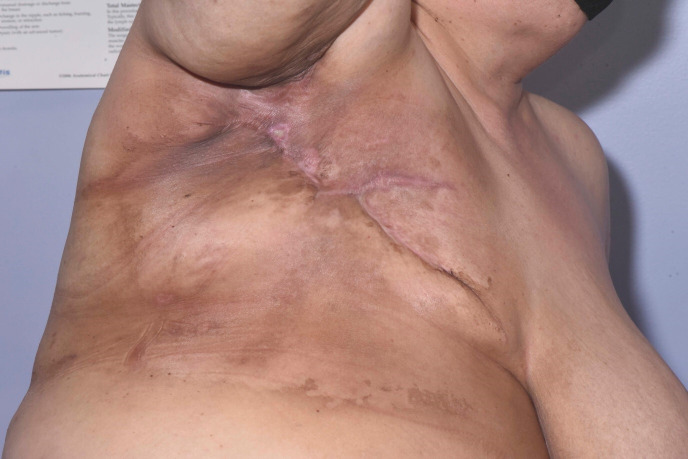
Figure 6.
Day 25 post radiotherapy. Resolution of the previously noted small ulcers around the mastectomy scar. No residual areas of skin breakdown are noted.
As seen in the clinical photographs, although patchy Radiation Therapy Oncology Group grade 2b-3 areas of moist desquamation did develop, the patient’s HS did not flare during or after the treatment course. Betamethasone valerate 0.1% with clioquinol 3% cream was not required. The skin healed completely 5 weeks post completion of radiotherapy. The patient will remain under regular review by her surgical, radiation oncology and dermatology teams.
Discussion
This case presentation reports a case of a patient with active HS who received a radical course of radiotherapy to the right chest wall without a clinically significant flare of her HS. The interaction between radiotherapy and HS is complex and multifaceted. An understanding of the mechanisms underpinning HS and the effect of both low-dose and high-dose radiotherapy is required to explain the interplay between the two.
The pathogenesis of HS is complex and the understanding of its underlying mechanisms is evolving. Sabat et al describe the pathogenesis in two stages:7 an initial pathogenetic event with perivascular and perifollicular immune cell infiltration, subsequent follicular plugging and stasis, and proliferation of bacteria. This leads to a secondary pro-inflammatory cytokine release, leading to chronic disease. CD4(+) T cells produce interleukin (IL)-17 and the IL-17 pathway may be important in the pathogenesis of HS.9 Circulation of these inflammatory cytokines leads to systemic inflammation and an increased risk of other syndromes, including metabolic syndrome and inflammatory bowel disease.10 11
Low-dose radiotherapy is a treatment option for HS, though rarely utilised. Radiotherapy for benign conditions is not common in the UK, and there is significant variation in practice across different centres. A multicentre questionnaire survey to analyse the use of radiotherapy for non-neoplastic conditions was undertaken in 2012.12 This showed only one of the responding 25 radiotherapy departments reported the use of the radiotherapy for HS. This is in contrast to the current practice in Germany where radiation is regularly used for hyperproliferative disorders such as HS.13 The effectiveness of radiotherapy in treating HS is likely due to a combination of both an anti-proliferative and an anti-inflammatory effect.14 15 Quantifying the risk of radiation-induced malignancies following low doses of radiotherapy is difficult for a number of reasons, including the small number of patients treated, variations in prescribed radiation regimens and variations in anatomical sites of treated benign lesions.
The effects of radical doses of radiation to the skin are well-documented. The skin contains several radiosensitive components, including stem cells and basal keratinocytes. Radiotherapy causes the generation of free radicals which result in acute inflammation and disruption of the ability of skin cells to replicate. This, combined with skin necrosis, leads to radiation dermatitis.16 Late effects have been demonstrated to be due to increased signalling of transforming growth factor-beta1 (TGF-β1) that may lead to increased levels of extracellular matrix production during wound healing that result in complications such as radiation-induced fibrosis.17
The effects of high-dose radical radiotherapy on HS are sparsely documented. A literature review revealed a case of de novo HS occurring within the radiation treatment field several weeks following completion of 25 fractions of radical radiotherapy for a uterine adenocarcinoma.18 A further case report presented a case of a patient with a known diagnosis of HS but having had no treatment for 12 years undergoing breast radiotherapy and developing a Hurley stage II flare on completion.19 The mechanism through which high-dose radiotherapy may cause a flare of HS has been hypothesised to include the increase in the number of local T-helper (TH) 17-derived cytokines by way of disruption to the lymphatic network and compression of neuromediators running along the peripheral nerves caused by dermal fibrosis.20
Radiation dermatitis, both the acute reaction within 90 days and the chronic reaction thereafter, are recognised complications of breast radiotherapy. Pre-existing skin conditions are likely to deter radiation oncologists from offering potentially life-prolonging radiotherapy in the fear that an exacerbation will significantly reduce quality of life, a significant concern in patients who have an incurable condition such as metastatic cancer. There is a biological basis for the exacerbation of conditions such as HS following high-dose radiotherapy in the form of lymphatic and neurological deregulation leading to a heightened immune response, mainly by TH-17-mediated cytokines.20 This would be in keeping with the two published case reports of HS following radical radiotherapy, where the development of HS occurred within the radiation field.18 19
We hypothesise that the reason this patient did not experience a flare of her HS, despite high-dose radiation, is the initial stability achieved in her HS disease control. At a histological level, skin affected by acute radiation dermatitis is often found to have an increase of immune-mediated cytokines, chemokines response and various ILs.21 Immunomodulatory treatment of HS prior to commencing radiation may act against this response and prevent a flare of HS. We further hypothesise that specific treatment with dapsone may ameliorate the acute inflammatory response of radical radiotherapy by virtue of its multiple anti-inflammatory and immunomodulatory properties.22 Further studies are required for clarity on dapsone’s complex mechanisms of actions to further support this link.
The role of fractionation in mitigating the risk of side effects is uncertain. While hypofractionation theoretically carries a greater risk of acute toxicity, large trials of hypofractionation in the radical treatment of the breast with radiotherapy have conversely found lower rates of acute toxicity compared with standard fractionation.23 24 This suggests modern techniques of 3D conformal hypofractionated regimens should also result in acceptable toxicities in patients with pre-existing skin conditions, provided they are medically optimised.
Caution must be maintained following radical radiotherapy to ensure that the HS continues to be stable through the chronic skin response. As discussed previously, the immune response implicated in chronic radiation dermatitis appears to be mediated via TGF-β1, resulting in radiation-induced fibrosis, and can occur years after initial radiotherapy treatment.17 This separate immune mechanism has unknown effects on HS and physicians need to be vigilant against the possibility of a late flare. However, this case report does demonstrate that well-controlled HS can withstand the acute immune flare caused by a radical high-dose course of radiotherapy. Informed patient choice and multidisciplinary management is advisable in ensuring optimal management. The multidisciplinary team consisting of surgeons, dermatologists and radiation oncologists among others is of paramount importance in not only ensuring stability of disease prior to undergoing treatment but also in informing patients of potential risks to ensure informed consent, and for monitoring against the potential for late flares.
Learning points.
Hidradenitis supprativa (HS) is more common than most physicians realise.
Radiotherapy should not be contraindicated in cases of chronic skin conditions such as HS.
A multidisciplinary approach involving surgeons, radiation oncologists and dermatologists can provide safe treatment, even if delivered directly at the affected skin areas.
It is important to be vigilant with follow-up to assess for long-term side effects.
Footnotes
Contributors: HR, FJF, ER and GA all made significant contributions to the writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Sellheyer K, Krahl D. "Hidradenitis suppurativa" is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol 2005;44:535–40. 10.1111/j.1365-4632.2004.02536.x [DOI] [PubMed] [Google Scholar]
- 2.Patel KR, Lee HH, Rastogi S, et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol 2020;83:737–44. 10.1016/j.jaad.2019.11.068 [DOI] [PubMed] [Google Scholar]
- 3.Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-Based clinical practice research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018;178:917–24. 10.1111/bjd.16101 [DOI] [PubMed] [Google Scholar]
- 4.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian hidradenitis suppurativa foundations. J Am Acad Dermatol 2019;81:76–90. 10.1016/j.jaad.2019.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–6. 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 6.Medicines.org.uk Dapsone Tablets BP 50mg - Summary of Product Characteristics (SmPC) - (emc) [Internet], 2020. Available: https://www.medicines.org.uk/emc/medicine/23938#PHARMACOLOGICAL_PROPS [Accessed 16 Jun 2020].
- 7.Sabat R, Jemec GBE, Matusiak Łukasz, et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020;6:18. 10.1038/s41572-020-0149-1 [DOI] [PubMed] [Google Scholar]
- 8.Fröhlich D, Baaske D, Glatzel M. [Radiotherapy of hidradenitis suppurativa--still valid today?]. Strahlenther Onkol 2000;176:286–9. [PubMed] [Google Scholar]
- 9.Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol 2015;173:1431–9. 10.1111/bjd.14075 [DOI] [PubMed] [Google Scholar]
- 10.Shalom G, Freud T, Harman-Boehm I, et al. Hidradenitis suppurativa and metabolic syndrome: a comparative cross-sectional study of 3207 patients. Br J Dermatol 2015;173:464–70. 10.1111/bjd.13777 [DOI] [PubMed] [Google Scholar]
- 11.Deckers IE, Benhadou F, Koldijk MJ, et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol 2017;76:49–53. 10.1016/j.jaad.2016.08.031 [DOI] [PubMed] [Google Scholar]
- 12.Taylor RE, Hatfield P, McKeown SR, et al. Radiotherapy for benign disease: current evidence, benefits and risks. Clin Oncol 2015;27:433–5. 10.1016/j.clon.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 13.Kriz J, Seegenschmiedt HM, Bartels A, et al. Updated strategies in the treatment of benign diseases-a patterns of care study of the German Cooperative group on benign diseases. Adv Radiat Oncol 2018;3:240–4. 10.1016/j.adro.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodemann HP, Blaese MA. Responses of normal cells to ionizing radiation. Semin Radiat Oncol 2007;17:81–8. 10.1016/j.semradonc.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 15.Arenas M, Sabater S, Hernández V, et al. Anti-Inflammatory effects of low-dose radiotherapy. Strahlentherapie und Onkologie 2012;188:975–81. 10.1007/s00066-012-0170-8 [DOI] [PubMed] [Google Scholar]
- 16.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol 2006;54:28–46. 10.1016/j.jaad.2005.08.054 [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Lefaix J-L, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 2000;47:277–90. 10.1016/S0360-3016(00)00435-1 [DOI] [PubMed] [Google Scholar]
- 18.Haber R, Gottlieb J, Zagdanski A-M, et al. Radiation-Induced hidradenitis suppurativa: a case report. JAAD Case Rep 2017;3:182–4. 10.1016/j.jdcr.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher M, Larsen L. A case of radiation-induced localized exacerbation of hidradenitis suppurativa. JAAD Case Rep 2016;2:44–6. 10.1016/j.jdcr.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vita V, Ruocco E. Hidradenitis suppurativa after radiotherapy for uterine adenocarcinoma: a typical example of an isoradiotopic response. JAAD Case Rep 2017;3:570–1. 10.1016/j.jdcr.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller K, Meineke V. Radiation-Induced alterations in cytokine production by skin cells. Exp Hematol 2007;35:96–104. 10.1016/j.exphem.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 22.Debol SM, Herron MJ, Nelson RD. Anti-Inflammatory action of dapsone: inhibition of neutrophil adherence is associated with inhibition of chemoattractant-induced signal transduction. J Leukoc Biol 1997;62:827–36. 10.1002/jlb.62.6.827 [DOI] [PubMed] [Google Scholar]
- 23.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs Hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol 2015;1:931. 10.1001/jamaoncol.2015.2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagsi R, Griffith KA, Boike TP, et al. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule. JAMA Oncology 2015;1:918 10.1001/jamaoncol.2015.2590 [DOI] [PubMed] [Google Scholar]