Abstract
We report an unusual complication of COVID-19 infection in a 53-year-old Caucasian man. He presented with shortness of breath, fever and pleuritic chest pain. A CT pulmonary angiogram (CTPA) demonstrated acute bilateral pulmonary embolism and bilateral multifocal parenchymal ground glass change consistent with COVID-19 (SARS-CoV-2) infection. Right adrenal haemorrhage was suspected on the CTPA which was confirmed on triple-phase abdominal CT imaging. A short Synacthen test revealed normal adrenal function. He was treated initially with an intravenous heparin infusion, which was changed to apixaban with a planned outpatient review in 3 months’ time. He made an uncomplicated recovery and was discharged. Follow-up imaging nearly 5 months later showed near complete resolution of the right adrenal haemorrhage with no CT evidence of an underlying adrenal lesion.
Keywords: infections, adrenal disorders, radiology
Background
This case demonstrates a rare complication of COVID-19 infection. New and unusual sequelae of COVID-19 infection are being increasingly recognised, awareness of which are paramount when managing patients who are medically complex or unwell. Clinicians should be aware of the possibility of adrenal haemorrhage (AH) in patients with confirmed COVID-19 infection, particularly if a patient becomes haemodynamically unstable, develops multiorgan failure or signs of adrenal insufficiency to prevent a delay in diagnosis and allow the appropriate treatment to be instituted.
Case presentation
A previously well 53-year-old Caucasian man with a background of retinitis pigmentosa was admitted with pleuritic chest pain, shortness of breath and fever. He was an ex-smoker and was not taking any regular medication. Admission observations revealed: respiratory rate, 36/min; blood pressure (BP), 143/96; oxygen saturations, 97% self-ventilating in air; and heart rate, 99 beat/min. The white cell count was elevated (13.9×109/L, normal range 3.7–10.0 × 109/L) with a slightly elevated neutrophil count (10.3×109/L, normal range 3.7–10.0 × 109/L). The lymphocyte count and C reactive protein (CRP) level were normal. A COVID-19 viral PCR throat swab returned a negative result. He remained clinically well during his admission and was discharged 4 days later on oral amoxicillin.
The patient re-presented to the emergency department 8 days later with worsening shortness of breath and fever. Admission observations revealed: temperature, 37.6°C; heart rate 102 beats/min; BP 105/76; and oxygen saturations, 96% requiring 6 L/min of oxygen. An arterial blood gas revealed hypoxia and he was admitted for further management. His oxygen requirement increased on day 3 of his admission and a subsequent D-dimer result was elevated (3.9 µg/mL, normal <0.5 µg/mL). He was commenced empirically on therapeutic dose dalteparin (low molecular weight heparin) and a CT pulmonary angiogram (CTPA) was performed.
Investigations
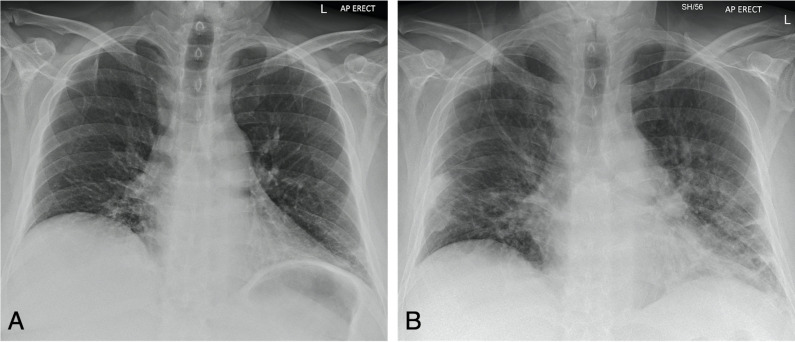
On his first admission, an ECG demonstrated normal sinus rhythm and there were no ST segment changes to indicate myocardial ischaemia or damage. The high-sensitivity cardiac troponin T was normal. A chest radiograph revealed low volume bilateral basal air space opacities (figure 1). On his second admission 12 days later, a repeat chest radiograph demonstrated progressive bilateral mid to lower zone air space opacities and atelectasis (figure 1) consistent with COVID-19 pneumonia. His acute phase reactants were elevated: CRP, 79 mg/L (normal range 0–10 mg/L), ferritin 2182 µg/L (normal range 41–400 µg/L), fibrinogen 7.75 g/L (normal range 1.9–4.1 g/L), platelets 504×109/L (normal range 150–450 x 109/L). His repeat COVID-19 PCR throat swab was positive.
Figure 1.
Anteroposterior (AP) erect chest radiographs of a 53-year-old man. (A) Initial radiograph performed for sudden onset right chest pain and shortness of breath demonstrates low volume bibasal lower zone air space opacities, more so at the left costophrenic angle. (B) Performed 12 days after (A) for a persistent history of shortness of breath and fever, demonstrates progressive changes with bilateral mid and lower zone air space opacities with atelectasis consistent with COVID-19 infection.
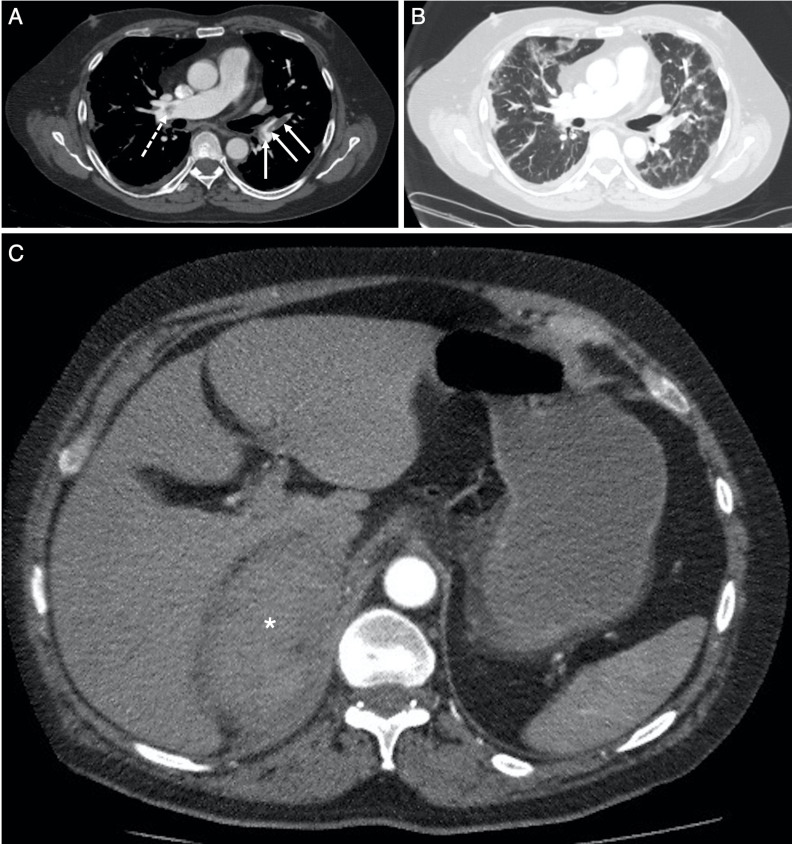
The CTPA performed following readmission demonstrated acute bilateral pulmonary emboli (PE) and bilateral multifocal parenchymal ground glass change consistent with COVID-19 infection (figure 2). The imaging also identified an approximate 12×6.5 cm heterogeneous soft tissue density in the right suprarenal region with thickening of the right anterior perirenal fascia suspicious for right AH (figure 2). A triple phase abdomen and pelvis CT was performed and confirmed a large, stable density, right AH: there was no active contrast extravasation (figure 3). Serum cortisol was appropriately elevated at 608 nmol/L (normal range 85–460 nmol/L) and thus considered normal. There were no clinical signs of adrenal insufficiency.
Figure 2.
Selected axial slices from the CT pulmonary angiogram obtained 5 days following the chest radiograph in figure 1B, performed for persistent oxygen requirement and a raised D-dimer result. (A) Through the level of the pulmonary trunk and right main pulmonary artery on soft-tissue window (W:450, L:50), demonstrates acute pulmonary emboli in right main pulmonary artery proximal to the trifurcation (dashed arrow) with further emboli at the origin, and within, the left lower lobe segmental pulmonary arteries (solid arrows). There was no saddle embolus, right heart strain or pericardial effusion. (B) Through the same level as (A) on lung window (W:1500, L:−50), demonstrates bilateral multifocal ground glass opacification consistent with COVID-19 infection. (C) More inferiorly in the upper abdomen, there is a soft-tissue density mass (average Hounsfield Unit, 55) in the right suprarenal region (*): the right adrenal gland is not identified, and appearances are suspicious for right adrenal haemorrhage.
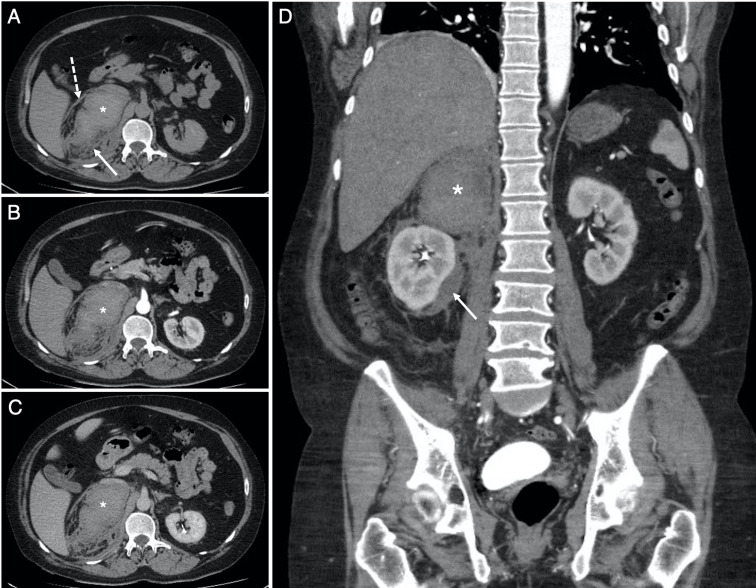
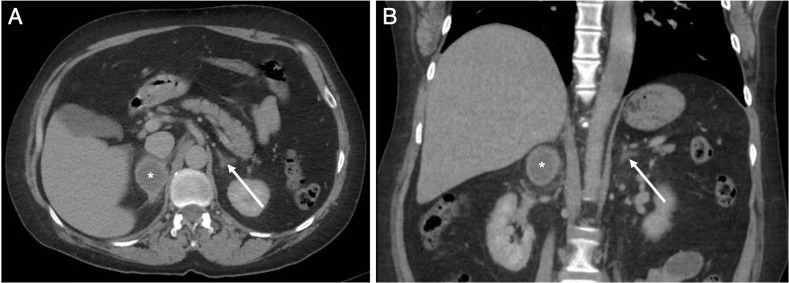
Figure 3.
Selected soft-tissue window (W:450, L:50) axial slices from the (A) non-contrast, (B) arterial and (C) venous phases of the abdomen and pelvis CT examination through the level of the coeliac trunk, with (D) venous phase coronal reconstruction, obtained the same day as the CTPA in figure 2. Images (A–C) confirm the recent right adrenal haemorrhage (*). The average density (HU, 55) is stable across all phases with no active extravasation of contrast. The haemorrhage extends to the right perirenal space (solid arrows in A and D) and tracks down into the right paracolic gutter, with thickening of the right anterior perirenal (Gerota) fascia (dashed arrow in A).
Differential diagnosis
The working diagnosis on the patient’s second attendance was COVID-19 related acute respiratory distress syndrome complicated by acute PE and unilateral AH. The AH was not clinically suspected but was identified incidentally on CTPA and confirmed on further abdominal CT imaging. Other differential diagnoses included an underlying adrenal tumour (not supported by the history or serology, and there was no previous cross-sectional imaging available with which to compare) and disseminated intravascular coagulation (DIC) secondary to sepsis which was not supported by the clotting panel results. Following senior medical review and discussion with the haematology team, it was felt extremely unlikely that four doses of therapeutic dalteparin would result in iatrogenic AH.
Treatment
The patient was treated with intravenous fluids and antibiotics alongside titrated oxygen therapy for his COVID-19 pneumonia. Given that the CTPA demonstrated both acute PE and AH, the haematology team advised treatment with intravenous heparin infusion due to its prompt reversibility.
The vascular radiology team felt that the AH was likely to be venous in nature and could be managed conservatively without the need for radiological intervention. The endocrinology team advised checking for signs of adrenal insufficiency, undertaking a short Synacthen test, and measuring adrenocorticotropic hormone (ACTH) levels in 6 weeks’ time. The patient remained haemodynamically stable on intravenous heparin for 5 days following which he was successfully switched to apixaban, a direct factor Xa inhibitor.
Outcome and follow-up
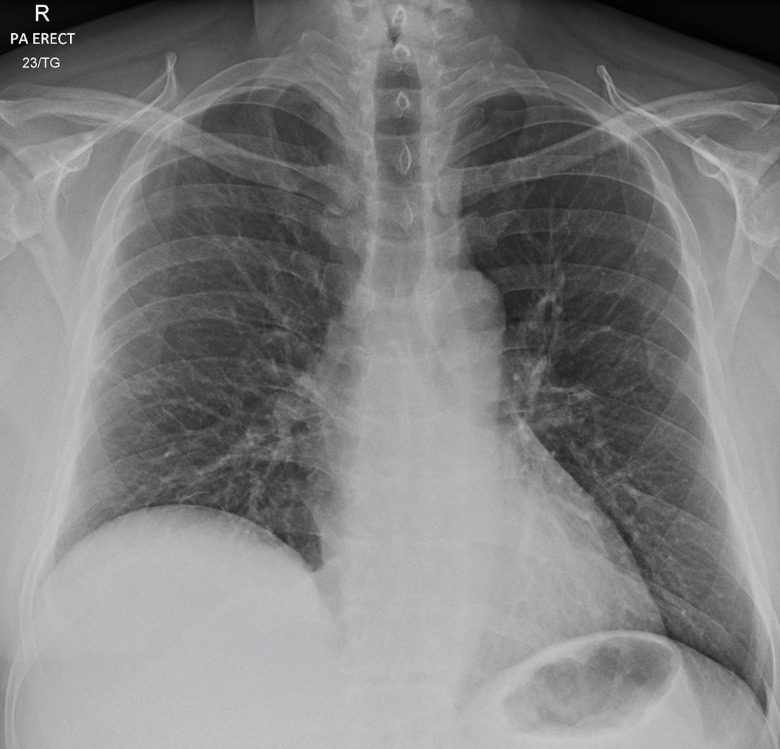
The patient made a full recovery. Forty-eight hours after discontinuing the intravenous heparin, he was discharged home with endocrinology outpatient follow-up. During his admission, there was no clinical or serological evidence of adrenal insufficiency and he remained haemodynamically stable. A short Synacthen test was undertaken 6 weeks after discharge, which demonstrated a normal response to ACTH (table 1). A follow-up chest radiograph demonstrated near complete resolution of the bilateral airspace opacities (figure 4). Repeat CT imaging performed nearly 5 months later revealed a marked improvement of the appearances of the right adrenal gland with subtotal resorption of the previously identified large AH (figure 5). There was no CT evidence of an underlying right adrenal lesion and the left adrenal gland was normal.
Table 1.
Assessment of serum adrenal function 6 weeks post discharge
| Serological test | Result | Normal range | |
| Cortisol (nmol/L) | Pretest | 400 | 85–460 |
| 30 min | 812 | ||
| 60 min | 920 | ||
| ACTH (ng/L) | 36 | 7.2–63.3 | |
ACTH, adrenocorticotropic hormone.
Figure 4.
Follow-up posteroanterior (PA) erect chest radiograph obtained nearly 7 weeks after figure 1A demonstrates significant improvement in appearances with near complete resolution of the bilateral airspace opacities seen in figure 1B.
Figure 5.
Selected (A) axial and (B) coronal slices from a portal venous phase CT of the adrenal glands on soft-tissue window (W:450, L:50) obtained approximately 5 months after the CTPA (figure 2) and CT abdomen and pelvis (figure 3). The images demonstrate marked improvement in the appearances of the right adrenal gland (*) with subtotal resorption of the previous identified large adrenal haemorrhage (figures 2 and 3). There is no CT evidence of an underlying adrenal lesion and the left adrenal gland (arrow) is normal.
Discussion
AH represents a rare and underestimated cause of acute clinical decompensation, multiorgan failure and death.1 It is often under-recognised due to its non-specific presentation; however, early identification can be life-saving.2 In autopsy series, the incidence of asymptomatic AH ranges from 0.14% to 1.10%.3 4
AH is a very rare but important complication of COVID-19 infection. Bilateral AH as a complication of COVID-19 infection has been described recently in a patient with antiphospholipid syndrome who presented with abdominal pain, fever, nausea, vomiting and dyspnoea. This patient developed clinical and biochemical signs of adrenal insufficiency and serological testing revealed a coagulopathy. Imaging confirmed a small filling defect in the left renal vein consistent with a non-obstructing thrombus.5 A further recently published case report describes non-haemorrhagic adrenal infarction in a patient with COVID-19 infection who presented with abdominal pain and hyponatraemia.6 To our knowledge, we report the first case of a clinically occult unilateral AH to present in a patient with confirmed COVID-19 infection in the absence of abdominal symptoms or clinical signs of adrenal insufficiency.
AH can be categorised as either traumatic or non-traumatic and can be unilateral or bilateral. AH is believed to be initiated by thrombosis of the adrenal veins.7 During stress, a surge of circulating catecholamines and ACTH leads to vasoconstriction of the draining adrenal venules8 which, alongside increased platelet aggregation as a result of the high levels of catecholamines, predisposes to adrenal vein thrombosis.9 Subsequently, venous stasis and increased adrenal venous pressure leads to rupture of the thin walled adrenal venules.10 Furthermore, the presence of ACE2 receptor has been shown to be used by SARS-CoV-2 to enter endothelial cells isolated from the vessels of multiple organs, including the lungs and adrenal glands, which may cause direct endothelial damage.11 Moreover, distinct structural changes of the adrenal gland have been reported during the course of COVID-19 infection which may result in both direct adrenal lesions due to generalised infection and of the involvement of CD8+ T lymphocytes.12
Classically, infection-driven AH is associated with meningococcal septicaemia, also known as Waterhouse-Friderichsen syndrome. However, an adrenal histopathology series of 65 patients with fatal bacterial infections showed a broad collection of pathogenic bacteria (both gram-positive and negative) including Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella spp, and Legionella spp, 60% of which of which resulted in AH.13
Although adrenal insufficiency is rare after unilateral AH,14 it is important to assess the patient for haemodynamic instability and, to exclude adrenal insufficiency and hormonally active adrenal tumours. Hypotension, hyponatraemia, hypoglycaemic and hyperkalaemia may all indicate adrenal insufficiency. Serum cortisol and short Synacthen tests aid the diagnosis. Literature on the management of AH is limited but in the published case reports, most patients are initially treated conservatively.
Given that our patient had no medical history of a pre-existing coagulopathy or bleeding diathesis, we conclude that the right AH was most likely secondary to COVID-19 infection. While it is known that AH may occur secondary to the use of dalteparin in relation to heparin induced thrombocytopenia or coagulopathy,15 16 neither of these were present in our patient. In the absence of previous cross-sectional imaging, a pre-existing right adrenal lesion could not be excluded at the time of the initial imaging (figure 3) given the size of the AH. As such, follow-up cross-sectional imaging is necessary to exclude an underlying adrenal lesion17: in our patient, there was no CT evidence of an underlying adrenal lesion on follow-up imaging (figure 5).
The true prevalence of AH in patients coinfected with COVID-19 has not been established. To our knowledge, this is the first case to demonstrate AH in a patient with COVID-19 infection with no abdominal symptoms or clinical signs of adrenal insufficiency. As such, the incidence of AH is likely to be higher than currently reported. Clinicians should be aware of the possibility of AH in the event that a patient becomes unstable or, develops multiorgan failure or signs of adrenal insufficiency to prevent a delay in diagnosis and allow the appropriate treatment to be instituted. However, clinicians should also be aware that AH might be detected as an incidental finding on imaging performed for other indications in the presence of SARS-CoV-2 infection.
Patient’s perspective.
Having COVID-19 has changed my life. I used to worry about paying my bills and now I just enjoy life to the full. Family and friends gave me the strength to fight. I am so grateful to all the staff in Barnsley Hospital. I wouldn’t be here without them. I just want people to learn from my case.
Learning points.
Adrenal haemorrhage (AH) is a rare complication of COVID-19 infection, the incidence of which has not yet been established.
AH represents a rare and underestimated cause of acute decompensation, multiorgan failure and death.
Non-traumatic AH can occur as a result of underlying stressors, including sepsis.
Assessment of haemodynamic stability, exclusion of hormonally active adrenal tumours and adrenal insufficiency is required.
Adrenal insufficiency is rare after unilateral AH.
Acknowledgments
The authors are grateful to Kanwar Gill, Consultant Radiologist at Barnsley Hospital NHS Foundation Trust, for providing the follow-up radiological report (Figure 5). They would also like to thank the Medical Education department at Barnsley NHS Foundation Trust for funding the publication of this case report.
Footnotes
Contributors: NS: involved in direct patient care; cowrote and edited the manuscript text; obtained informed signed patient consent. CTB: involved in direct patient care; cowrote and edited the manuscript text. MP: reported the imaging; cowrote and edited the manuscript text; extracted, reformatted and labelled all figures; wrote the figure legends. EU: involved in direct patient care; cowrote and edited the manuscript text.
Funding: Medical Education department, Barnsley NHS Foundation Trust.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Charalampakis V, Stamatiou D, de Bree E, et al. Spontaneous adrenal hemorrhage. report of two cases and review of pathogenesis, diagnosis and management. J Surg Case Rep 2018;2018:rjy129. 10.1093/jscr/rjy129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo clinic. Mayo Clin Proc 2001;76:161–8. 10.1016/S0025-6196(11)63123-6 [DOI] [PubMed] [Google Scholar]
- 3.Moore MA, Biggs PJ. Unilateral adrenal hemorrhage: an unusual presentation. South Med J 1985;78:989–92. 10.1097/00007611-198508000-00025 [DOI] [PubMed] [Google Scholar]
- 4.Cardwell MS. Spontaneous adrenal hemorrhage in pregnancy. A case report. J Reprod Med 1988;33:233–5. [PubMed] [Google Scholar]
- 5.Frankel M, Feldman I, Levine M, et al. Bilateral adrenal hemorrhage in coronavirus disease 2019 patient: a case report. J Clin Endocrinol Metab 2020;105. 10.1210/clinem/dgaa487. [Epub ahead of print: 01 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar R, Guruparan T, Siddiqi S, et al. A case of adrenal infarction in a patient with COVID 19 infection. BJR Case Rep 2020;6:20200075. 10.1259/bjrcr.20200075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajasekhar A, Zumberg M. Venous thromboses at unusual sites. 4 edn, 2019: 323–4. [Google Scholar]
- 8.Wharton J, Cohen D. Delayed unilateral adrenal hemorrhage complicating pneumococcal septic shock. Del Med J 2015;87:310–2. [PubMed] [Google Scholar]
- 9.Kovacs KA, Lam YM, Pater JL. Bilateral massive adrenal hemorrhage. assessment of putative risk factors by the case-control method. Medicine 2001;80:45–53. 10.1097/00005792-200101000-00005 [DOI] [PubMed] [Google Scholar]
- 10.Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med 1989;110:227–35. 10.7326/0003-4819-110-3-227 [DOI] [PubMed] [Google Scholar]
- 11.Li J, Gao J, Xu Y-ping, Y-ping X, et al. [Expression of severe acute respiratory syndrome coronavirus receptors, ACE2 and CD209L in different organ derived microvascular endothelial cells]. Zhonghua Yi Xue Za Zhi 2007;87:833–7. [PubMed] [Google Scholar]
- 12.Zinserling VA, Semenova NY, Markov AG, et al. Inflammatory cell infiltration of adrenals in COVID-19. Horm Metab Res 2020;52:639–41. 10.1055/a-1191-8094 [DOI] [PubMed] [Google Scholar]
- 13.Guarner J, Paddock CD, Bartlett J, et al. Adrenal gland hemorrhage in patients with fatal bacterial infections. Mod Pathol 2008;21:1113–20. 10.1038/modpathol.2008.98 [DOI] [PubMed] [Google Scholar]
- 14.Marti JL, Millet J, Sosa JA, et al. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg 2012;36:75–82. 10.1007/s00268-011-1338-6 [DOI] [PubMed] [Google Scholar]
- 15.Rosenberger LH, Smith PW, Sawyer RG, et al. Bilateral adrenal hemorrhage: the unrecognized cause of hemodynamic collapse associated with heparin-induced thrombocytopenia. Crit Care Med 2011;39:833–8. 10.1097/CCM.0b013e318206d0eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houlden RL, Janmohamed A. Bilateral adrenal hemorrhage with adrenal insufficiency after dalteparin use post hip ATHROPLASTIES. AACE Clin Case Rep 2020;6:e141–3. 10.4158/ACCR-2019-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali A, Singh G, Balasubramanian SP. Acute non-traumatic adrenal haemorrhage-management, pathology and clinical outcomes. Gland Surg 2018;7:428–32. 10.21037/gs.2018.07.04 [DOI] [PMC free article] [PubMed] [Google Scholar]