Abstract
Electronic cigarettes (e-cigarettes) are the preferred smoking-cessation aid in the United States; however, there is little evidence regarding long-term effectiveness among those who use them. We used the Population Assessment of Tobacco and Health Study to compare long-term abstinence between matched US smokers who tried to quit with and without use of e-cigarettes as a cessation aid. We identified a nationally representative cohort of 2,535 adult US smokers in 2014–2015 (baseline assessment), who, in 2015–2016 (exposure assessment), reported a past-year attempt to quit and the cessation aids used, and reported smoking status in 2016–2017 (outcome assessment; self-reported ≥12 months continuous abstinence). We used propensity-score methods to match each e-cigarette user with similar nonusers. Among US smokers who used e-cigarettes to help quit, 12.9% (95% confidence interval (CI): 9.1%, 16.7%) successfully attained long-term abstinence. However, there was no difference compared with matched non–e-cigarette users (cigarette abstinence difference: 2%; 95% CI: −3%, 7%). Furthermore, fewer e-cigarette users were abstinent from nicotine products in the long term (nicotine abstinence difference: −4%; 95% CI: −7%, −1%); approximately two-thirds of e-cigarette users who successfully quit smoking continued to use e-cigarettes. These results suggest e-cigarettes may not be an effective cessation aid for adult smokers and, instead, may contribute to continuing nicotine dependence.
Keywords: e-cigarettes, long-term effectiveness, matching, nationally representative cohort, nicotine abstinence, propensity-score methods, smoking cessation
Abbreviations
- e-cigarette
electronic cigarette
- NRT
nicotine replacement therapy
- PATH
Population Assessment of Tobacco and Health
Electronic cigarette (e-cigarette) sales doubled in the United States between 2015 and 2017 (1). In the United Kingdom and the United States, e-cigarettes are now the most popular product type used to aid smoking cessation, ahead of US Food and Drug Administration–approved products including nicotine replacement therapy (NRT) such as a nicotine patch or nicotine gum, and prescription medications, including buproprion and varenicline. Although many herald e-cigarettes as a harm-reduction device (2–4), experts have noted potential public health risks, including the potential for increased smoking initiation among minors, and for increased nicotine addiction among dual users of cigarettes and e-cigarettes (5). In the United States, e-cigarettes can deliver high doses of nicotine, and there is evidence of substantial uptake among nonsmoking minors (6). Given these known risks, arguments for a net public health benefit rely on the effectiveness of e-cigarettes in helping adult smokers quit cigarette smoking for the long term (7, 8).
Several national reports have considered the evidence on whether e-cigarettes increase long-term smoking cessation (5, 9). The recent US Surgeon General’s report (10) concluded that evidence remains inadequate to infer that e-cigarettes increase smoking cessation. Only 4 randomized trials, all conducted outside of the United States, have directly tested whether e-cigarettes are efficacious for smoking cessation with a follow-up of at least 6 months. The most promising of these randomly assigned attendees of UK National Health Service stop-smoking services (n = 866) to either e-cigarettes or NRT and reported that use of e-cigarettes as a cessation aid increased successful quitting 1 year later (11). However, the importance of motivation was highlighted by a pragmatic trial conducted at 54 US businesses, which randomized 6,004 employees who smoked to provision of either free FDA approved cessation aids or to free e-cigarettes, as well as to 3 other arms. All arms received a brief communication intervention. As part of the primary analysis, the trial reported that provision of free e-cigarettes did not increase cessation as compared to provision of free FDA approved cessation aids (12). There also have been several reports from nationally representative longitudinal studies in which smokers self-selected to use e-cigarettes to help quit smoking. Use of e-cigarettes for quitting in the Adult Tobacco Cohort was associated with short-term but not long-term cigarette abstinence (13). There have been 5 reports using data from the US Population Assessment of Tobacco and Health (PATH) Study (14). Two analyses (15, 16) had biased results because they included smokers who did not make a quit attempt only in the comparison group (17). Authors of 1 of the analyses reported that use of e-cigarettes to quit was associated with increased short-term abstinence, measured at the same time e-cigarette use was assessed (18). In the other analysis (19), authors reported that substitution of e-cigarettes for cigarettes at wave 2 was not associated with sustained abstinence at wave 3, confirming an earlier report (20) that use of e-cigarettes after quitting was associated with increased relapse to smoking 1 year later. The authors of the latter 2 studies suggest nicotine abstinence after quitting cigarettes may be an important moderator of long-term abstinence from cigarette smoking.
In this article, we use more recent PATH data to address whether use of e-cigarettes to aid quitting contributed to increased successful smoking cessation in the US population (self-reported continuous abstinence of at least 12 months (21)). Many smokers use multiple cessation aids (22), thus, we focused on any e-cigarette use for quitting compared with no use. Furthermore, we include as a second comparison group those who used an approved pharmaceutical aid to quit but not an e-cigarette. The population of smokers who use e-cigarettes to quit is appreciably different from those who do not (18). Thus, we identified a priori 24 potential confounders and used propensity-score methods to match each e-cigarette user with up to 2 closely matched control respondents. We compared population-weighted abstinence rates in the matched samples. This approach estimates the causal effect of e-cigarette use explicitly among those who choose to use them as a cessation aid and is less dependent on modeling assumptions than regression-based approaches, which estimate average effects projected to the entire population (23). However, we report regression-based approaches as sensitivity analyses.
METHODS
Data source and sample
Data were from the restricted public use file of the PATH Study (24). The surveys are conducted at approximately annual intervals (waves) with stratified oversampling for adult (aged 18 to 24 years) tobacco users, and Black adults. Response rates were as follows: initial household screener survey, 54%; in-depth adult interview at wave 1, 74.0%; annual follow-up, 83.1%, 78.4%, and 73.5% for waves 2, 3, and 4, respectively. Surveys included informed consent and the study is overseen by the Westat Institutional Review Board. Our sample was identified from 10,722 cigarette smokers at wave 2 (2014–2015, baseline assessment), of whom 2,852 reported a past-year quit attempt at wave 3 (2015–2016, exposure assessment), with 2,535 completing the wave 4 outcome assessment in 2017–2018. The data collection schema is provided in Web Figure 1 (available at https://academic.oup.com/aje).
Measures
Tobacco and nicotine use.
During each interview, after viewing an image of each tobacco product, participants were asked whether they had ever used that product and whether they currently used it every day or some days. Noncurrent users were asked “In the past 30 days, have you smoked/used (product), even one or two puffs” and “In the past 12 months, have you smoked/used (product), even one or two puffs.” Ever-smokers were asked whether they had used the following NRT products in the past 12 months: a nicotine patch, gum, inhaler, nasal spray, lozenge, or pill. Our 2 outcome variables (≥12 months’ abstinence from 1) cigarettes and 2) all nicotine products) were identified from these questions on the wave 4 survey. Nicotine use includes any use of cigarettes, e-cigarettes, NRT, cigars (traditional, cigarillo, and filtered), pipes, hookah, snus, or other smokeless products.
Use of e-cigarettes and pharmaceutical aids to quit smoking.
Each survey asked smokers whether they had made a quit attempt within the past 12 months and which of the following products was used for their most recent quit attempt: e-cigarettes, NRT, varenicline (Chantix; Pfizer, Groton, Connecticut), or buproprion (Wellbutrin or Zyban; GlaxoSmithKline, London, UK). The primary exposure was reported use of e-cigarettes to quit at wave 3 (e-cigarette group, n = 427); comparison groups were those who did not use e-cigarettes to quit smoking (no–e-cigarette group, n = 2,108) and those who reported use of a pharmaceutical cessation aid at wave 3 (varenicline, buproprion, or NRT) but not e-cigarettes (n = 465).
Study covariates.
Web Appendix 1 presents survey questions for 24 potential confounders, which we identified a priori. These include sociodemographic variables, cigarette-smoking history, duration of previous quit attempt reported prior to baseline, timing of most recent quit attempt from survey (assessed at wave 3); self-efficacy about quitting; interest in quitting cigarettes; exposure to smoking; perceived harm of cigarettes and e-cigarettes; daily e-cigarette use reported at current or prior surveys (“ever” daily use); nicotine dependence level (average agreement with a series of 15 statements on emotional and physical response to nicotine products, scaled from 0 to 100) (25); and health-related covariates. All were assessed at wave 2, with the exception of timing of most recent quit attempt from the wave 3 survey, used to control potential recall bias associated with type of aid used (26). Univariate distributions by cessation aid category are listed in Web Table 1.
Statistical analyses
Estimates were weighted using the wave 1 through wave 4 longitudinal survey weights, which were adjusted for the sampling design, survey nonresponse, and longitudinal drop out (27). Weighted percentages and Wilson confidence intervals for proportions were calculated. For confidence intervals and P values, the replicate survey weights were used with balanced repeated replication with Fay adjustment (ρ = 0.3) (28) in R, version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria), except for the propensity-score matched analyses, where bootstrap percentile confidence intervals were used.
For propensity-score matching, within each bootstrap sample for each participant we calculated a propensity score by estimating the probability of membership in the e-cigarette–use group using logistic regression. To obtain complete data for the 24 covariates, we used simple imputation (R package “mice”). To identify the optimal set of covariates among the 24 variables, we used a 10-fold cross-validated LASSO (29) procedure for each logistic regression model, with a tuning parameter selected from among the sequence 0–0.1 with step of 0.005 (R package “glmnet”), conducted without survey weights. We repeated this propensity-score estimate for each bootstrap-resampled data set. Using the propensity score, we matched up to 2 controls for each case (nearest-neighbor matches using R package “Matchit”) within the a priori caliper distance of 0.1 (if possible) or 0.2 (maximum allowed) (30). We chose the caliper that provided the lowest standardized difference averaged across all covariates after matching. Cases that did not have a match were omitted from the sample.
For each matched bootstrap sample, we used logistic regression with survey weights (R package “survey”) to estimate the average risk difference between the 2 matched groups, for each outcome. The model included an indicator of the matched pair (or triple), the overall propensity score, and, to adjust for any remaining covariate imbalance, any covariate with median standardized difference between the 2 study groups larger than 0.10 (31, 32). We report the bootstrap mean estimate of risk difference and adjusted 95% bootstrap quantile confidence intervals, with Bonferroni adjustment (33) to account for the 2 abstinence outcomes studied. To identify a sufficient bootstrap sample size, we required a jackknife quality estimate (34) to be less than 0.1, resulting in 1,500 bootstrap samples for the comparison of e-cigarette use versus no e-cigarette use.
Sensitivity analyses
Sensitivity analyses included incorporating matching as random effects instead of fixed effects, and 1:1 rather than 1:2 propensity-score matching without additional covariate adjustment. We also used weighted multivariable logistic regression on the full sample; covariates included were age, sex, ethnicity, race, education, income, nicotine dependence, relative perceived harm of e-cigarettes, and previous daily e-cigarettes use, with simple imputation. Finally, we tested whether the results were robust to omission of adjustment for multiple comparisons.
As a post hoc exploratory sensitivity analyses, we used logistic regression to test whether the association of e-cigarette use with long-term cigarette abstinence and nicotine abstinence differed by baseline smoking status, nicotine dependence, age, sex, education level, and race/ethnicity. Statistical inference was based on 95% confidence intervals for interaction terms (uncorrected for multiple comparisons), and a stratified analysis was conducted when the boundary of the confidence interval was close to 1.
RESULTS
Population rates of cigarette and nicotine abstinence at wave 4 follow-up
Among this representative sample of US smokers who reported a past-year quit attempt in 2015–2016 (wave 3), 17.4% used e-cigarettes to help quit smoking. Those who used e-cigarettes were younger, more nicotine dependent, more likely to be non-Hispanic White, and had higher income and level of education (Table 1).
Table 1.
Sample Characteristics of Smokersa in 2014–2015 Reporting a Past-Year Quit Attempt in 2015–2016, According to Use or No Use, of e-Cigarettes to Aid Quitting, Population Assessment of Tobacco and Health Study
| Sociodemographic Factors | Used e-Cigarettes on Quit Attempt b (n = 427) | Did Not Use e-Cigarettes on Quit Attempt b (n = 2,108) | |||||
|---|---|---|---|---|---|---|---|
| No. | Weighted % | 95% CI | No. | Weighted % | 95% CI | P Value | |
| Age, years | <0.001 | ||||||
| 18–34 | 218 | 46.8 | 41.1, 52.5 | 922 | 38.3 | 35.6, 41.0 | |
| 35–50 | 127 | 32.0 | 26.7, 37.3 | 546 | 28.5 | 26.0, 31.0 | |
| ≥50 | 82 | 21.2 | 16.9, 25.5 | 640 | 33.2 | 30.5, 35.9 | |
| Sex | 0.500 | ||||||
| Male | 202 | 50.7 | 44.8, 56.6 | 1,012 | 53.0 | 50.5, 55.5 | |
| Female | 225 | 49.3 | 43.4, 55.2 | 1,095 | 47.0 | 44.5, 49.5 | |
| Education | 0.006 | ||||||
| Less than high school | 89 | 19.4 | 14.9, 23.9 | 593 | 26.8 | 24.6, 29.0 | |
| High school graduate | 90 | 23.1 | 17.2, 29.0 | 502 | 27.5 | 25.0, 30.0 | |
| Some college or higher | 230 | 55.2 | 48.9, 61.5 | 944 | 43.7 | 41.2, 46.2 | |
| Ethnicity | <0.001 | ||||||
| Hispanic | 37 | 6.9 | 4.7, 9.1 | 334 | 15.1 | 13.1, 17.1 | |
| Non-Hispanic | 390 | 93.1 | 90.9, 95.3 | 1732 | 82.9 | 80.7, 85.1 | |
| Race | <0.001 | ||||||
| White | 354 | 85.8 | 82.5, 89.1 | 1,400 | 69.1 | 66.6, 71.6 | |
| Black | 26 | 5.5 | 3.3, 7.7 | 433 | 19.5 | 17.5, 21.5 | |
| Other | 43 | 8.0 | 5.1, 10.9 | 223 | 9.0 | 7.6, 10.4 | |
| Income (annually, US$) | <0.001 | ||||||
| <35,000 | 220 | 47.5 | 42.2, 52.8 | 1,341 | 59.8 | 56.9, 62.7 | |
| ≥35,000 | 190 | 48.0 | 42.7, 53.3 | 633 | 34.0 | 31.3, 36.7 | |
| Smoking-related diseases | 0.178 | ||||||
| Marked | 201 | 47.0 | 41.7, 52.3 | 1,069 | 51.1 | 48.4, 53.8 | |
| Not marked | 226 | 53.0 | 47.7, 58.3 | 1,039 | 48.9 | 46.2, 51.6 | |
| Nicotine dependence scale score | 0.009 | ||||||
| 0–33.3 | 89 | 22.4 | 17.1, 27.7 | 571 | 28.5 | 25.6, 31.4 | |
| 33.4–66.7 | 172 | 38.6 | 33.9, 43.3 | 839 | 39.4 | 36.9, 41.9 | |
| 66.8–100 | 165 | 38.7 | 33.4, 44.0 | 648 | 29.6 | 27.4, 31.8 | |
| Relative perceived harm of e-cigarettes | <0.001 | ||||||
| Less harmful | 262 | 61.3 | 56.4, 66.2 | 726 | 34.7 | 32.3, 37.1 | |
| More harmful | 158 | 36.9 | 31.8, 42.0 | 1,306 | 61.4 | 58.9, 63.9 | |
| e-Cigarette use before W2 | 0.001 | ||||||
| Never | 44 | 10.9 | 7.4, 14.4 | 949 | 48.1 | 45.9, 50.3 | |
| Ever | 383 | 89.1 | 85.6, 92.6 | 1,154 | 51.7 | 49.5, 53.9 | |
| e-Cigarette use before W2 | <0.001 | ||||||
| Daily use at W1 or W2 | 106 | 24.5 | 20.0, 29.0 | 96 | 4.3 | 3.3, 5.3 | |
| Not daily use at W1 or W2 | 321 | 75.5 | 71.0, 80.0 | 2012 | 95.7 | 94.7, 96.7 | |
Abbreviations: W1, wave 1 of the study; W2, wave 2 of the study.
a Weighted US population estimates.
b e-Cigarette use status for most recent quit attempt, among all smokers reporting a quit attempt at wave 3.
Among US smokers who used e-cigarettes to quit, 12.9% (95% confidence interval (CI): 9.1%, 16.7%) achieved at least 12 months’ abstinence from cigarettes at wave 4, compared with 11.3% (95% CI: 9.6, 13.0) among US smokers who did not use e-cigarettes to quit (Table 2). Among US smokers who used e-cigarettes to quit, the population-weighted estimate of at least 12 months of nicotine abstinence at wave 4 was 2.8% (95% CI: 0.9%, 4.8%), compared with 8.1% (95% CI: 6.5%, 9.7%) among those who did not use e-cigarettes to quit. Table 2 lists population abstinence rates among these smokers by baseline consumption level (daily or nondaily).
Table 2.
Long-Term Abstinence at Follow-Upa, b Among US Smokers Who Made a Quit Attempt in 2015–2016, According to Use or No Use, of e-Cigarettes to Aid Quitting, Population Assessment of Tobacco and Health Study
| Cigarette Smoking Status (W2) and e-Cigarettes Used to Aid Quit Attempt? c | Cigarette Abstinence (W4) | Nicotine d Abstinence (W4) | ||||
|---|---|---|---|---|---|---|
| No. | Weighted % Abstinent | 95% CI | No. | Weighted % Abstinent | 95% CI | |
| All current cigarette smokers | ||||||
| Yes | 427 | 12.9 | 9.1, 16.7 | 427 | 2.8 | 0.9, 4.8 |
| No | 2,108 | 11.3 | 9.6, 13.0 | 2,108 | 8.1 | 6.5, 9.7 |
| Daily cigarette smokers | ||||||
| Yes | 290 | 13.7 | 8.8, 18.7 | 290 | 3.4 | 0.8, 6.1 |
| No | 1,455 | 9.5 | 7.7, 11.3 | 1,455 | 7.3 | 5.7, 9.0 |
| Nondaily cigarette smokers | ||||||
| Yes | 137 | 11.1 | 5.7, 16.5 | 137 | 1.5 | −0.6, 3.7 |
| No | 653 | 15.1 | 11.7, 18.4 | 653 | 9.6 | 6.6, 12.6 |
Abbreviations: W2, wave 2 of the study; W4, wave 4 of the study.
a Abstinence of ≥12 months, reported at wave 4.
b Weighted US population estimates.
c e-Cigarette use status for most recent quit attempt, among all smokers reporting a quit attempt at wave 3.
d Nicotine use includes any of cigarettes, e-cigarettes, and nicotine replacement therapy.
Propensity score–matched samples
We assessed appropriateness of the propensity-score match by comparing kernel density estimates of the propensity score (i.e., the estimated probability of using e-cigarettes to quit on the index quit attempt). Comparing smokers who used e-cigarettes to quit and smokers who did not, the 2 density estimates were very different prior to matching (Web Figure 2A). In particular, there were few respondents with propensities greater than 0.6 in the no–e-cigarette population, indicating that some population subgroups are unlikely to use e-cigarettes. Matching, although restricted to respondents with propensity score less than 0.8, resulted in good overlap of the density estimates (Web Figure 2B). Matching used all the 427 available e-cigarette users, with a median sample size of 386 for the matched sample. For each matching variable, we also plotted the standardized absolute mean difference between study groups across the 1,500 bootstrap re-samples, for the full sample and the matched samples (Web Figure 3). The matched samples had a small between-group difference across all covariates with the exception of prior daily e-cigarette use (Web Table 2). This variable was controlled for in the logistic regression comparing abstinence rates between the matched samples.
Figures assessing the quality of the match between the e-cigarette group and the matched US Food and Drug Administration–approved pharmaceutical aid group are presented in Web Figure 4. The propensity scores were always positive, indicating that some respondents in each group were at least somewhat likely to belong to the other group. However, the fewer available respondents in the comparison group resulted in fewer successful matches: all 427 e-cigarette users were included in at least 1 matched sample but the median matched sample size was 244. We again used 1,500 bootstrap samples and the matching was improved in the between-group balance for all covariates. However, a residual difference remained for age, prior daily e-cigarette use, relative perceived harm of e-cigarettes, and smoking-related diseases, which we controlled for in the logistic regression.
Comparisons of abstinence rates between matched samples
There was no evidence for a difference in the proportion of persons who achieved long-term abstinence from cigarettes between those who used e-cigarettes to help quit smoking and the matched sample of those who did not use e-cigarettes as a cessation aid (risk difference: 0.02, 95% CI: −0.03, 0.07) (Figure 1A). However, e-cigarettes users were less likely to be nicotine abstinent in the long term at follow up (risk difference: –0.04, 95% CI: −0.07, −0.01).
Figure 1.
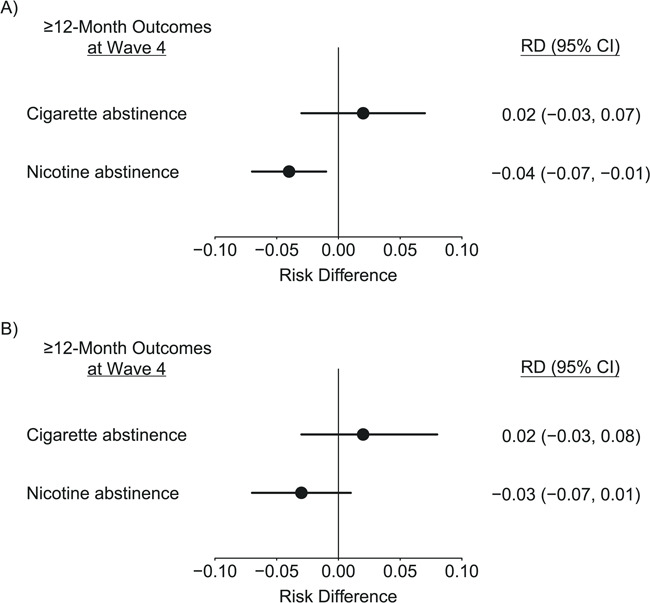
Differences in long-term abstinence rates from smoking cigarettes and from use of any nicotine-containing product, comparing the type of aid used for smoking cessation, 2014–2108, Population Assessment of Tobacco and Health (PATH) Study. A) e-Cigarettes used for cessation versus no e-cigarettes used for cessation. B) e-Cigarettes used for cessation versus pharmacotherapy but no e-cigarettes used for cessation. Weighted differences in rates of ≥12 months’ abstinence between e-cigarette users and a matched sample of non–e-cigarette users, matched on 26 smoking-related characteristics and further adjusted by logistic regression. Bars represent Bonferroni adjusted 95% bootstrap confidence intervals (CI). Samples were drawn from 2,852 adult respondents to the PATH Study who reported smoking at wave 2 (2014–2015), reported a quit attempt and cessation aids used at wave 3 (2015–2016), and reported abstinence outcomes at wave 4 (2017–2018). RD, risk difference.
Comparing e-cigarette users with the matched sample of those who used pharmaceutical aids (but not e-cigarettes) to quit (Figure 1B), there was no difference in the proportion who achieved either abstinence outcome (cigarette abstinence: risk difference: 0.02, 95% CI: −0.03, 0.08; nicotine abstinence: risk difference: –0.03, 95% CI: −0.07, 0.01).
Sensitivity analyses were consistent with these results (Web Figure 5, Web Appendices 2 and 3, Web Table 3). Exploratory analyses of interaction terms between e-cigarette use and baseline smoking status, nicotine dependence, age, sex, education level, and race/ethnicity revealed that all confidence intervals included 1, unadjusted for multiple comparisons (Web Appendix 4, Web Table 4). However, the interaction terms for the association of e-cigarettes with daily or nondaily smoking status, and with educational level appeared to be worth future exploration, and stratified analyses for these variables are presented in Web Appendix 4 and Web Table 4.
US abstinence rates by product among those who successfully quit cigarettes
Table 3 lists population abstinence rates from various nicotine-containing products among all those who were long-term abstinent from cigarettes at wave 4. Among those who successfully used e-cigarettes to quit cigarette smoking, only approximately one-third were also long-term abstinent from e-cigarettes at follow-up. Among those who successfully used approved pharmacotherapy to quit smoking, approximately 70% were abstinent from NRT. Among the larger group who successfully quit smoking without use of e-cigarettes (who may have used no aid or approved pharmacotherapy), greater than 90% were long-term abstinent from each of NRT and e-cigarettes at follow-up. Importantly, in each comparison group of cigarette-abstinent smokers, 7%–17% were still using some form of combusted tobacco at follow-up. Overall, among US smokers in 2014–2015 who reported using e-cigarettes to quit in the following year, 8.4% (95% CI: 5.4%, 11.4%) had quit smoking and appeared to have substituted e-cigarettes for their cigarettes by 2016–2017.
Table 3.
Long-Term Abstinencea, b (≥12 Months) From e-Cigarettes, Nicotine Replacement Therapy, and Other Tobacco Productsc Among US Smokers Who Were ≥12 Months’ Cigarette Abstinent at Follow-up in 2016–2017, Population Assessment of Tobacco and Health Study
Products Abstained from for  12-Months at W4 12-Months at W4
|
e-Cigarettes Used To Quit d (n = 49) | e-Cigarettes Not Used To Quit d (n = 227) | Pharmaceutical Aid e Used To Quit d (n = 45) | |||
|---|---|---|---|---|---|---|
| Weighted % Abstinent | 95% CI | Weighted % Abstinent | 95% CI | Weighted % Abstinent | 95% CI | |
| e-Cigarettes | 31.7 | 16.4, 47.0 | 93.0 | 89.0, 96.9 | 96.1 | 89.7, 102.4 |
| NRT | 94.5 | 85.3, 103.8 | 91.9 | 87.4, 96.3 | 71.0 | 55.7, 86.4 |
| Other tobacco products | 82.2 | 70.0, 94.5 | 82.9 | 77.3, 88.5 | 93.1 | 85.2, 101.1 |
| Combustiblef | 83.0 | 70.7, 95.2 | 86.1 | 80.5, 91.6 | 93.1 | 85.2, 101.1 |
| Smokelessg | 93.3 | 84.0, 102.6 | 95.6 | 92.6, 98.6 | 97.2 | 91.5, 102.9 |
Abbreviations: W4, wave 4 of the study; NRT, nicotine replacement therapy.
a Abstinence of ≥12 months, reported at wave 4.
b Weighted US population estimates.
c Other tobacco products include cigars (traditional, cigarillo, and filtered), pipes, hookah, snus, or other smokeless products.
d e-Cigarette use and pharmaceutical-aid status for most recent quit attempt, among all smokers reporting a quit attempt at wave 3.
e Pharmaceutical aids include varenicline and buproprion.
f Combustible products include cigars, pipes, and hookahs.
g Smokeless products include snus, moist snuff, dip, and spit and chewing tobacco.
DISCUSSION
We used the PATH survey to prospectively compare long-term cessation outcomes between a nationally representative sample of US smokers who tried to quit smoking with the help of e-cigarettes in 2016–2017 and a matched sample of US smokers who also tried to quit but without using e-cigarettes. We found that e-cigarette users did not have higher rates of long-term abstinence from cigarette smoking but did have lower rates of abstinence from nicotine than their matched peers. This difference appeared to be largely due to high rates of continuing use of e-cigarettes among those who quit smoking cigarettes. Two-thirds of those who successfully used e-cigarettes to attain long-term abstinence from cigarettes were still using e-cigarettes during the follow-up year. It would be important to assess eventual relapse rates among these groups (35). We also compared abstinence rates among those who used e-cigarettes to quit and a matched sample of those who used US Food and Drug Administration–approved pharmaceutical cessation aids. Estimated effects were very similar, but confidence intervals were wider, likely due to the smaller matched sample sizes.
The low rates of nicotine abstinence found in our study are worth noting. We included in this measure e-cigarettes, other tobacco products, and NRT products. Long-term nicotine abstinence was well under 5% for US smokers who used e-cigarettes to quit, and less than 10% for those who did not. Our matched analysis attributes 4 percentage points of this difference to the use of e-cigarettes. Of particular concern is the high rate of continued smoking of other forms of tobacco among those who successfully quit cigarettes, ranging from 17% of those who successfully used e-cigarettes to quit to 7% among successful pharmaceutical aid users.
Smokers who used e-cigarettes to try to quit smoking were younger, more educated and affluent, had higher nicotine dependence levels, and were more likely to report mental health symptoms than smokers who tried to quit without e-cigarettes. We used propensity-score methods to match each e-cigarette user with up to 2 similar smokers who did not use e-cigarettes, and we compared the difference in abstinence rates for the matched samples. This procedure allowed us to estimate the average causal effect of e-cigarettes among the population of people who use them (36). Alternatively, regression-based modeling can estimate average causal effects over the whole population, although at the risk of extrapolation to smokers who are unlikely to ever use e-cigarettes. Indeed, there were few non–e-cigarette users with a propensity score greater than 50%, whereas approximately 20% of e-cigarette users had a propensity score greater than 50%, indicating that such model-based extrapolation is needed to estimate a population-averaged effect. However, we used these types of model-based methods in our sensitivity analyses and obtained qualitatively similar results.
At the population level, we estimated that approximately 13% of US smokers who made a quit attempt using e-cigarettes achieved long-term smoking cessation success, as did approximately 11% of US smokers who tried to quit without use of e-cigarettes, similar to the propensity-score matched estimate of a difference of 2 percentage points in cessation rates. The 95% confidence interval for the matched difference in cessation rates was from −3 percentage points to 7 percentage points. These cessation rates observed in PATH are similar to those seen in other population studies. For example, the 2008 clinical practice guidelines for smoking cessation estimated that approximately 13% of US smokers who tried to quit smoking attained 6–12 months abstinence.
In our study, as in other population studies, daily smokers were less likely to quit successfully than nondaily smokers. Interestingly, the unadjusted observed association of e-cigarette use for cessation differed in direction between daily and nondaily smokers. In exploratory post hoc analyses, we used adjusted multivariable logistic regression to investigate interactions between the association of e-cigarette use and daily vs nondaily smoking, as well as with age, education, sex, and race ethnicity. All confidence intervals for these interaction terms included 1; however, estimated interactions for education and daily versus nondaily smoking appeared to be worth future investigation and are reported in Web Appendix 4 and Web Table 4.
Our finding that e-cigarette use to quit smoking did not increase cigarette abstinence to 12 months or longer is similar to results from the Adult Tobacco Use Cohort (133), in which researchers found a cessation benefit for e-cigarettes at 6 months but not at 12 or 18 months. Using an earlier PATH Study cohort (18), we reported that using an e-cigarette to quit was associated with short-term abstinence (≥30 days); here, abstinence was reported contemporaneously with the report of use of e-cigarettes to quit. Thus, it is possible that e-cigarettes help short-term quitting but not sustained abstinence rates. These results are also consistent with those of a recent study using the PATH waves 1–3 database (19), in which e-cigarette use among older smokers was associated with abstinence at wave 2 but relapse by wave 3.
Our results on substitution of e-cigarettes for cigarettes are qualitatively similar to the randomized trial of attendees to UK National Health Service stop-smoking services, in which 80% of successful quitters in the e-cigarette arm continued to use e-cigarettes at 1 year, compared with persistent use of NRT by only 9% of successful quitters in the NRT arm (11). However, we did not replicate this trial’s findings of a sustained cessation benefit from use of an e-cigarette to quit. The difference in our results may be related to the intensiveness of the UK intervention or to the lower level of nicotine in UK e-cigarettes. The motivation level of participants might also account for these differences: only 43% of those screened for the UK trial were randomly assigned to the study, whereas the PATH Study estimates are representative of the US population. Similar differences in conclusions between randomized trials and observational studies have been reported regarding use of NRT to quit (18, 37). Our findings, however, are consistent with the lack of efficacy of e-cigarettes in the recent pragmatic randomized trial of provision of e-cigarettes to help cessation among employees at US workplaces who smoke (12). It is possible that participants in the pragmatic trial more closely match the general population of US smokers who want to quit.
Strengths of this study include that data were drawn from a large representative sample of the US population who report tobacco use annually, that we used a prospectively assessed measure of 12-month abstinence, and we aimed to assess the causal effect of e-cigarettes for cessation as they are used in the US population. Results were robust to a variety of sensitivity analyses, and our propensity-score approach is relatively robust to modeling assumptions (23). However, a limitation of all observational studies is the possibility of unmeasured confounding, such as differences in motivation level to quit smoking, in quitting history, or in self-efficacy to successfully quit smoking. The survey measures used are self-reported and, as such, may have measurement error. Although biomarkers of tobacco use are collected in the PATH Study, these were not available to validate the outcome at the time of writing. However, in an analysis of earlier PATH data, self-reported tobacco use was strongly associated with biomarker data (38). In this study, the e-cigarette devices used were those that were generally available in 2015–2016 and the results may not generalize to the modifications in available products since that time.
In conclusion, we compared long-term abstinence rates between a nationally representative cohort of US smokers who tried to quit smoking using e-cigarettes as a cessation aid, and a matched sample of smokers who tried to quit without using e-cigarettes. We found no evidence that e-cigarettes helped these smokers to successfully quit smoking. We estimated that approximately 8% of all adult US smokers who used an e-cigarette to quit cigarettes in 2015–2016 were able to successfully substitute e-cigarettes for cigarette smoking. However, our propensity score–matched results suggest these smokers would have been equally successful in quitting smoking without the use of e-cigarettes. Furthermore, our results suggest, these respondents were more likely to remain dependent on nicotine, largely due to continuing use of e-cigarettes.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Biostatistics, Department of Family Medicine and Public Health, University of California, San Diego (UCSD), San Diego, California (Ruifeng Chen, Karen Messer); Moores Cancer Center, UCSD, San Diego, California (John P. Pierce, Eric C. Leas, Sheila Kealey, David R. Strong, Dennis R. Trinidad, Tarik Benmarhnia, Karen Messer); Department of Family Medicine and Public Health, UCSD, San Diego, California (John P. Pierce, Martha M. White, David R. Strong, Dennis R. Trinidad); and Scripps Institution of Oceanography, UCSD, San Diego, California (Tarik Benmarhnia).
This work was funded by National Institutes of Health grant 1 RO1CA234539. The data were analyzed as part of a restricted-use file made available by the National Addiction & HIC Data Archive Program hosted by the Inter-university Consortium for Political and Social Research at University of Michigan. J.P.P. was supported by grants from National Cancer Institute during the conduct of the study; M.M.W. was supported by grants from National Institute of Health during the conduct of the study; and D.R.S. was supported by the National Cancer Institute (grant RO1CA234539) during the conduct of the study. D.R.T was funded on the Tobacco-Related Disease Program of the University of California, Office of the President (grant 28IR - 0066).
Conflict of interest: none declared.
REFERENCES
- 1. Huang JD, Duan ZS, Kwok JL, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2019;28(2):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abrams DB, Glasser AM, Pearson JL, et al. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu Rev Public Health. 2018;39:193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levy DT, Borland R, Lindblom EN, et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob Control. 2018;27(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green LW, Fielding JE, Brownson RC. The debate about electronic cigarettes: harm minimization or the precautionary principle. Annu Rev Public Health. 2018;39(1):189–191. [DOI] [PubMed] [Google Scholar]
- 5. National Academies of Sciences Engineering and Medicine Public Health Consequences of e-Cigarettes. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 6. Miech R, Johnston L, O'Malley PM, et al. Trends in adolescent vaping, 2017-2019. N Engl J Med. 2019;381(15):1490–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fairchild AL, Bayer R, Lee JS. The e-cigarette debate: what counts as evidence? Am J Public Health. 2019;109(7):1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warner KE, Mendez D. E-cigarettes: comparing the possible risks of increasing smoking initiation with the potential benefits of increasing smoking cessation. Nicotine Tob Res. 2019;21(1):41–47. [DOI] [PubMed] [Google Scholar]
- 9. McNeill A, Brose LS, Calder R, et al. Evidence Review of e-Cigarettes and Heated Tobacco Products 2018. A Report Commissioned by Public Health England. London: Public Health England; 2018. [Google Scholar]
- 10. US Department of Health and Human Services Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–637. [DOI] [PubMed] [Google Scholar]
- 12. Halpern SD, Harhay MO, Saulsgiver K, et al. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. New Engl J Med. 2018;378(24):2302–2310. [DOI] [PubMed] [Google Scholar]
- 13. Sweet L, Brasky TM, Cooper S, et al. Quitting behaviors among dual cigarette and e-cigarette users and cigarette smokers enrolled in the tobacco user adult cohort. Nicotine Tob Res. 2019;21(3):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berry KM, Reynolds LM, Collins JM, et al. E-cigarette initiation and associated changes in smoking cessation and reduction: the population assessment of tobacco and health study, 2013-2015. Tob Control. 2019;28(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalkhoran S, Chang Y, Rigotti NA. Electronic cigarette use and cigarette abstinence over 2 years among U.S. smokers in the population assessment of tobacco and health study. Nicotine Tob Res. 2020;22(5):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pierce JP, Messer K, Leas EC, et al. A source of bias in studies of e-cigarettes and smoking cessation. Nicotine Tob Res. 2020;22(5):861–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benmarhnia T, Pierce JP, Leas E, et al. Can e-cigarettes and pharmaceutical aids increase smoking cessation and reduce cigarette consumption? Findings from a nationally representative cohort of American smokers. Am J Epidemiol. 2018;187(11):2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watkins SL, Thrul J, Max W, et al. Real-world effectiveness of smoking cessation strategies for young and older adults: findings from a nationally representative cohort. Nicotine Tob Res. 2020;22(9):1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dai H, Leventhal AM. Association of electronic cigarette vaping and subsequent smoking relapse among former smokers. Drug Alcohol Depend. 2019;199:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilpin EA, Pierce JP, Farkas AJ. Duration of smoking abstinence and success in quitting. J Natl Cancer Inst. 1997;89(8):572–576. [DOI] [PubMed] [Google Scholar]
- 22. Kasza KA, Cummings KM, Carpenter MJ, et al. Use of stop-smoking medications in the United States before and after the introduction of varenicline. Addiction. 2015;110(2):346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse, US Department of Health and Human Services Food and Drug Administration, Center for Tobacco Products Population Assessment of Tobacco and Health (PATH) Study (United States) Restricted-Use Files. Ann Arbor, MI: Inter-university Consortium for Political and Social Research (distributor); 2019. 10.3886/ICPSR36231.v18; Accessed April 8, 2019. [DOI]
- 25. Strong DR, Pearson J, Ehlke S, et al. Indicators of dependence for different types of tobacco product users: descriptive findings from wave 1 (2013-2014) of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend. 2017;178:257–266. [DOI] [PubMed] [Google Scholar]
- 26. Borland R, Partos TR, Cummings KM. Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine Tob Res. 2012;14(12):1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Addiction & HIV Data Archive Program Population Assessment of Tobacco and Health (PATH) Study Series. https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/606. Accessed June 22, 2020.
- 28. Judkins DR. Fay’s method for variance estimation. J Off Stat. 1990;6(3):223. [Google Scholar]
- 29. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol. 1996;58(1):267–288. [Google Scholar]
- 30. Wang Y, Cai H, Li C, et al. Optimal caliper width for propensity score matching of three treatment groups: a Monte Carlo study. PLoS One. 2013;8(12):e81045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pimentel SD, Kelz RR, Silber JH, et al. Large, sparse optimal matching with refined covariate balance in an observational study of the health outcomes produced by new surgeons. J Am Stat Assoc. 2015;110(510):515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ho D, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15. [Google Scholar]
- 33. Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56(293):52–64. [Google Scholar]
- 34. Efron BS, Stein C. The jackknife estimate of variance. Ann Stat. 1981;9(3):586–596. [Google Scholar]
- 35. Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA. 2019;322(18):1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(13):41–55. [Google Scholar]
- 37. Leas EC, Pierce JP, Benmarhnia T, et al. Effectiveness of pharmaceutical smoking cessation aids in a nationally representative cohort of American smokers. J Natl Cancer Inst. 2018;110(6):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rostron BL, Corey CG, Chang JT, et al. Associations of cigarettes smoked per day with biomarkers of exposure among U.S. adult cigarette smokers in the Population Assessment of Tobacco and Health (PATH) Study wave 1 (2013-2014). Cancer Epidemiol Biomarkers Prev. 2019;28(9):1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


