Abstract
Child acute malnutrition (AM) is an important cause of child mortality. Accurately estimating its burden requires cumulative incidence data from longitudinal studies, which are rarely available in low-income settings. In the absence of such data, the AM burden is approximated using prevalence estimates from cross-sectional surveys and the incidence correction factor 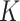 , obtained from the few available cohorts that measured AM. We estimated
, obtained from the few available cohorts that measured AM. We estimated 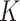 factors for severe acute malnutrition (SAM) and moderate acute malnutrition (MAM) from AM incidence and prevalence using representative cross-sectional baseline and longitudinal data from 2 cluster-randomized controlled trials (Innovative Approaches for the Prevention of Childhood Malnutrition—PROMIS) conducted between 2014 and 2017 in Burkina Faso and Mali. We compared K estimates using complete (weight-for-length z score, mid-upper arm circumference (MUAC), and edema) and partial (MUAC, edema) definitions of SAM and MAM.
factors for severe acute malnutrition (SAM) and moderate acute malnutrition (MAM) from AM incidence and prevalence using representative cross-sectional baseline and longitudinal data from 2 cluster-randomized controlled trials (Innovative Approaches for the Prevention of Childhood Malnutrition—PROMIS) conducted between 2014 and 2017 in Burkina Faso and Mali. We compared K estimates using complete (weight-for-length z score, mid-upper arm circumference (MUAC), and edema) and partial (MUAC, edema) definitions of SAM and MAM. 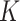 estimates for SAM were 9.4 and 5.7 in Burkina Faso and in Mali, respectively; K estimates for MAM were 4.7 in Burkina Faso and 5.1 in Mali. The MUAC and edema–based definition of AM did not lead to different
estimates for SAM were 9.4 and 5.7 in Burkina Faso and in Mali, respectively; K estimates for MAM were 4.7 in Burkina Faso and 5.1 in Mali. The MUAC and edema–based definition of AM did not lead to different 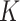 estimates. Our results suggest that
estimates. Our results suggest that 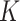 can be reliably estimated when only MUAC and edema-based data are available. Additional studies, however, are required to confirm this finding in different settings.
can be reliably estimated when only MUAC and edema-based data are available. Additional studies, however, are required to confirm this finding in different settings.
Keywords: burden of acute malnutrition, child acute malnutrition, cumulative incidence, incidence correction factor K, longitudinal data, moderate acute malnutrition, prevalence, severe acute malnutrition
Abbreviations
- AM
acute malnutrition
- SAM
severe acute malnutrition
- MAM
moderate acute malnutrition
- MUAC
mid-upper arm circumference
- PROMIS
Innovative Approaches for the Prevention of Childhood Malnutrition
- WLZ
weight-for-length z score
Acute malnutrition (AM) is a major public health challenge. Children with moderate acute malnutrition (MAM) are 3.4 times more likely to die compared with well-nourished children, and children with severe acute malnutrition (SAM) are 11.6 times more likely to die (1). Globally, 52 million children suffer from AM, a prevalence estimate based on cross-sectional surveys (2). AM, however, is not a chronic condition: Children with AM either recover or die, and recovered children can relapse to AM. Prevalence estimates are thus likely to underestimate the annual burden of AM, defined as the total number of AM cases that occur over the course of a year, and the associated number of child deaths. Reliable estimates of the AM burden help policy makers and nutrition program implementers predict the AM caseload and the necessary resources for AM treatment. The estimation of the AM burden, however, requires AM incidence data from longitudinal (i.e., cohort) data, which are rarely available in low-income settings. In the absence of incidence data, the AM burden or caseload for treatment programs is approximated by converting prevalence estimates from cross-sectional surveys to a cumulative AM incidence using the so-called incidence correction factor, 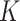 .
.
Only a handful of correction factors are available in the literature and many have limitations. Some are based on data from before the 2000s, when AM treatment coverage was much lower and the child disease burden was higher, both of which are likely to affect K-factor estimates; some are limited to SAM; some are based on data from treatment facilities, which are not representative of the general child population; some lack at least one of 3 diagnostic criteria for AM (mid-upper arm circumference (MUAC), weight-for-length z score (WLZ), and edema); some used treatment admission data instead of actual AM incidence; and some might have underestimated AM incidence by using cohort data with large intervals between follow-up measurements or data from preventive food-supplementation programs (3–7).
The objective of this study was to calculate 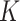 estimates for SAM and MAM that do not suffer from the methodological limitations listed above. Based on representative cross-sectional baseline and longitudinal data from 2 cluster-randomized controlled trials conducted in West Africa (Innovative Approaches for the Prevention of Childhood Malnutrition (PROMIS) Burkina Faso and PROMIS Mali (8, 9)), we calculated
estimates for SAM and MAM that do not suffer from the methodological limitations listed above. Based on representative cross-sectional baseline and longitudinal data from 2 cluster-randomized controlled trials conducted in West Africa (Innovative Approaches for the Prevention of Childhood Malnutrition (PROMIS) Burkina Faso and PROMIS Mali (8, 9)), we calculated 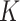 factors using both complete (based on WLZ, MUAC, and nutritional edema) and partial (based on MUAC and nutritional edema) AM case definitions. Because humanitarian programs with large AM caseloads often rely exclusively on MUAC and edema for diagnosis, we assessed whether using different AM definitions affects
factors using both complete (based on WLZ, MUAC, and nutritional edema) and partial (based on MUAC and nutritional edema) AM case definitions. Because humanitarian programs with large AM caseloads often rely exclusively on MUAC and edema for diagnosis, we assessed whether using different AM definitions affects 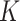 .
.
THE INCIDENCE CORRECTION FACTOR K
Using the assumptions in Miettinen (10), the AM incidence 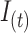 for a given period
for a given period 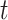 can be approximated using the AM prevalence
can be approximated using the AM prevalence 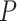 and the average duration of an AM episode
and the average duration of an AM episode 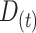 :
:
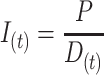 |
(1) |
The fraction 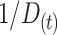 is referred to as the incidence correction factor,
is referred to as the incidence correction factor, 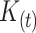 .
.
The burden of AM or cumulative AM incidence for a population of size 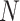 in period t is given by:
in period t is given by:
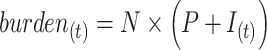 |
(2) |
By substituting 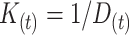 in equation 1, and subsequently substituting
in equation 1, and subsequently substituting 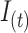 in equation 2, the burden of AM can be approximated as follows:
in equation 2, the burden of AM can be approximated as follows:
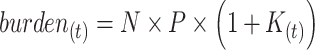 |
(3) |
DATA
The study setting of the PROMIS cohorts are described in detail elsewhere (8, 9). In brief, community health volunteers in Mali organized monthly village meetings with caregivers to screen their children aged 6–23 months for AM. AM cases were referred to the nearest health center for treatment. In Burkina Faso, AM screening and referral of children aged 1–23 months were performed by community health volunteers (as per national policy) at monthly well-baby consultations organized at health facilities. In both countries the national community-based management of acute malnutrition protocols for outpatient AM treatment were followed (11, 12). These involved weekly consultations for SAM at the health center, where children were given a weekly supply of ready-to-use therapeutic food. MAM children were expected every 2 weeks at the health center, where they received a 14-day supply of ready-to-use supplementary food.
In Burkina Faso, the study setting comprised all 32 rural health-center catchment areas of the Gourcy health district situated in the Nord region. In Mali, data were collected in 48 rural health-center catchment areas belonging to the Bla and San health districts situated in the Segou region. We conducted a cross-sectional baseline survey in children aged 0–17 months in Burkina Faso (November–December 2014) and aged 6–23 months in Mali (February–March 2015). Subsequently, an independent sample of children (2,450 children in Burkina Faso and 1,154 in Mali) was enrolled in the longitudinal study in both settings. At enrollment, children were aged 0 and 1.4 months in Burkina Faso and aged 6 and 6.9 months in Mali, and they did not suffer from AM. Children enrolled in the longitudinal studies were followed monthly for 18 months. For this analysis, we restricted the Burkina Faso sample to ages 7–17 months because MUAC is not used to screen for AM under the age of 6 months. In Mali, we used data only from the comparison study group because the PROMIS intervention had an impact on SAM and MAM incidence.
METHODS
SAM was defined as WLZ < −3 or MUAC < 115 mm or the presence of nutritional edema. MAM was defined as −3 ≤ WLZ < −2 or 115mm ≤ MUAC < 125 mm. WLZ was calculated using the World Health Organization growth standard (13). SAM cases were included in the incidence calculation if they were preceded by 1 or more SAM-free months. The calculation of MAM incidence was more complex: Children who were enrolled in a community-based management of acute malnutrition program and met the MAM criteria while recovering from SAM were not considered for the MAM incidence calculation. The rationale behind this approach is that SAM children enrolled in a community-based management of acute malnutrition program in both countries receive ready-to-use therapeutic foods until full recovery (i.e., even when their status has changed to MAM). The MAM incidence calculated from the longitudinal studies was thus limited to children who deteriorated from a well-nourished state to MAM and children who improved from SAM to MAM without receiving any SAM treatment, which allows us to estimate the specific burden for MAM treatment programs. For consistency, the same criteria were applied to define MAM in the cross-sectional data: MAM cases who received SAM treatment in the month preceding the survey were excluded.
Prior to analysis, we conducted multiple imputation of missing WLZ and MUAC values (approximately 15% for both outcomes) from the longitudinal studies using the 2-fold fully conditional specification algorithm. This imputation method takes the time dependence of the longitudinal measurements into account by using both covariate and outcome data from temporally adjacent values (14). We ran 50 iterations of the 2-fold fully conditional specification, which generated 50 imputed data sets. The imputed WLZ and MUAC values were used to create SAM and MAM indicators.
We calculated 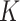 factors from estimates of I and P (equation 1), using bootstrapping to obtain valid 95% confidence intervals (15). The bootstrap strategy allows estimation of the uncertainty around the population value of the
factors from estimates of I and P (equation 1), using bootstrapping to obtain valid 95% confidence intervals (15). The bootstrap strategy allows estimation of the uncertainty around the population value of the 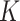 factor without the need to make a distributional assumption about
factor without the need to make a distributional assumption about 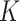 . The SAM and MAM incidence, defined as the total number of SAM or MAM cases divided by total person-years at risk, was thus estimated using 500 bootstrap samples for each of 50 imputed longitudinal data sets. Likewise, the SAM and MAM prevalence was estimated from 500 bootstrap samples of the cross-sectional baseline data. Estimates of I and P were combined to obtain 50 × 500 estimates of
. The SAM and MAM incidence, defined as the total number of SAM or MAM cases divided by total person-years at risk, was thus estimated using 500 bootstrap samples for each of 50 imputed longitudinal data sets. Likewise, the SAM and MAM prevalence was estimated from 500 bootstrap samples of the cross-sectional baseline data. Estimates of I and P were combined to obtain 50 × 500 estimates of 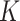 and 50 × 500 estimates of the AM burden (equation 2). Point estimates for I, P, K, and AM burden were obtained from the mean of their respective 50 × 500 estimates. The 95% confidence intervals were obtained from the percentiles following the MI Boot (pooled sample) method (15).
and 50 × 500 estimates of the AM burden (equation 2). Point estimates for I, P, K, and AM burden were obtained from the mean of their respective 50 × 500 estimates. The 95% confidence intervals were obtained from the percentiles following the MI Boot (pooled sample) method (15).
RESULTS
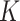 factors for SAM were 9.4 and 5.7 in Burkina Faso and in Mali, respectively. The
factors for SAM were 9.4 and 5.7 in Burkina Faso and in Mali, respectively. The 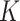 factors for MAM were 4.7 in Burkina Faso and 5.1 in Mali (Table 1). Given the similar SAM prevalence in both countries, the divergence in
factors for MAM were 4.7 in Burkina Faso and 5.1 in Mali (Table 1). Given the similar SAM prevalence in both countries, the divergence in 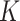 for SAM was driven by the higher SAM incidence in Burkina Faso compared with Mali. The difference in K is reflected in the annual burden of SAM as calculated using equation 3. It was larger in Burkina Faso (31 cases per 100 children per year) than in Mali (18 cases per 100 children per year); the annual estimated MAM burden was 77 and 71 cases per 100 children in Burkina Faso and Mali, respectively (Table 1).
for SAM was driven by the higher SAM incidence in Burkina Faso compared with Mali. The difference in K is reflected in the annual burden of SAM as calculated using equation 3. It was larger in Burkina Faso (31 cases per 100 children per year) than in Mali (18 cases per 100 children per year); the annual estimated MAM burden was 77 and 71 cases per 100 children in Burkina Faso and Mali, respectively (Table 1).
Table 1.
Estimated Incidence and Prevalence, 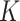 Factor, and Burden for Severe and Moderate Acute Malnutrition Among Children Aged 7–17 Months in Burkina Faso and Aged 7–23 Months in Mali, 2014–2017
Factor, and Burden for Severe and Moderate Acute Malnutrition Among Children Aged 7–17 Months in Burkina Faso and Aged 7–23 Months in Mali, 2014–2017
| SAM | MAM | |||||||
|---|---|---|---|---|---|---|---|---|
| Burkina Faso | Mali | Burkina Faso | Mali | |||||
| Definition | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| Based on WLZ, MUAC, and nutritional edema | ||||||||
| Incidencea | 28.1 | 25.8, 30.4 | 15.5 | 12.9, 18.2 | 63.8 | 59.3, 68.2 | 59.2 | 53.2, 65.8 |
| Prevalenceb | 3.1 | 2.3, 4.1 | 2.8 | 2.1, 3.5 | 13.5 | 11.9, 15.2 | 11.7 | 10.4, 13.2 |
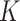
|
9.4 | 6.8, 12.4 | 5.7 | 4.3, 8.0 | 4.7 | 4.1, 5.3 | 5.1 | 4.3, 5.9 |
| Estimated AM burdenc | 31.2 | 28.8, 33.9 | 18.2 | 15.6, 21.1 | 77.4 | 72.6, 82.2 | 70.9 | 64.9, 77.7 |
| Based on MUAC and nutritional edema | ||||||||
| Incidencea | 17.0 | 15.1, 18.7 | 10.8 | 8.6, 12.7 | 35.7 | 33.2, 38.8 | 40.7 | 35.9, 45.9 |
| Prevalenceb | 1.3 | 0.8, 1.9 | 1.8 | 1.3, 2.5 | 9.1 | 7.6, 10.6 | 8.1 | 7.1, 9.3 |
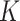
|
14.0 | 8.7, 23.7 | 6.0 | 4.2, 8.8 | 4.0 | 3.3, 4.8 | 5.0 | 4.2, 6.1 |
| Estimated AM burdenc | 18.3 | 16.3, 20.1 | 12.6 | 10.4, 14.8 | 44.8 | 41.7, 48.1 | 48.8 | 43.9, 54.1 |
Abbreviations: AM, acute malnutrition; CI, confidence interval; MAM, moderate acute malnutrition; MUAC, mid-upper arm circumference; SAM, severe acute malnutrition; WLZ, weight-for-length z score.
a Per 100 children per year.
b Per 100 children.
c Cases per 100 children per year.
Omitting WLZ from the case definition of SAM and MAM resulted in lower prevalence and incidence estimates. A higher 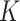 -factor point estimate for SAM and a slightly lower
-factor point estimate for SAM and a slightly lower 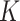 -factor point estimate for MAM were found in Burkina Faso, but their 95% confidence intervals overlap.
-factor point estimate for MAM were found in Burkina Faso, but their 95% confidence intervals overlap.
DISCUSSION
Using unique cross-sectional and longitudinal data from Burkina Faso and Mali, we estimated incidence correction or 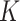 factors for SAM and MAM that do not suffer from the methodological limitations of previous estimates. Point estimates for SAM were different between countries (9.4 in Burkina Faso vs. 5.7 in Mali), although confidence intervals were wide. For MAM, we obtained similar
factors for SAM and MAM that do not suffer from the methodological limitations of previous estimates. Point estimates for SAM were different between countries (9.4 in Burkina Faso vs. 5.7 in Mali), although confidence intervals were wide. For MAM, we obtained similar 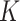 factors in both countries (4.7 for Burkina Faso and 5.1 for Mali).
factors in both countries (4.7 for Burkina Faso and 5.1 for Mali).
How do our estimates compare with the best available estimates using cohort data in the literature? A study using data from Niger, Mali, and Burkina Faso found a pooled SAM 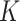 factor of 4.8 (ranging from 2.5 for Mali to 13.3 for Burkina Faso) (5). The study, however, had some limitations that might have biased the estimation of
factor of 4.8 (ranging from 2.5 for Mali to 13.3 for Burkina Faso) (5). The study, however, had some limitations that might have biased the estimation of 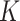 . In Mali and Niger, the SAM incidence used to calculate
. In Mali and Niger, the SAM incidence used to calculate 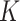 was likely underestimated. In Mali, follow-up measurements were done quarterly which led to fewer incident cases detected, and in Niger, the cohort data used included data from children that were given preventive food supplements (250–820 kcal/day), which likely reduced SAM incidence. In Burkina Faso, the SAM incidence was estimated from admission data to a SAM treatment program instead of from cohort data from a representative sample. Another study in Niger that used 8 months of monthly follow-up data derived
was likely underestimated. In Mali, follow-up measurements were done quarterly which led to fewer incident cases detected, and in Niger, the cohort data used included data from children that were given preventive food supplements (250–820 kcal/day), which likely reduced SAM incidence. In Burkina Faso, the SAM incidence was estimated from admission data to a SAM treatment program instead of from cohort data from a representative sample. Another study in Niger that used 8 months of monthly follow-up data derived 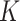 factors from the estimated episode durations. For SAM a
factors from the estimated episode durations. For SAM a 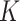 factor of 8 was found, and for MAM estimates ranged from 3.1 to 4.8 (16). Our estimates thus appear in line with the few available estimates in the literature. More studies, however, are needed to assess to what extent the
factor of 8 was found, and for MAM estimates ranged from 3.1 to 4.8 (16). Our estimates thus appear in line with the few available estimates in the literature. More studies, however, are needed to assess to what extent the 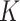 factors are site-specific.
factors are site-specific.
We found no evidence that excluding WLZ from the definition of AM led to a different 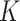 for SAM or MAM, a finding that is consistent with previous work that estimated
for SAM or MAM, a finding that is consistent with previous work that estimated 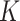 for SAM (5). The lack of impact on K of changing the SAM and MAM case definition can be explained by a similar decrease in both incidence
for SAM (5). The lack of impact on K of changing the SAM and MAM case definition can be explained by a similar decrease in both incidence 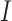 and prevalence
and prevalence 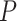 , while
, while 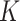 was calculated by the fraction
was calculated by the fraction 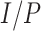 (equation 1). Our findings suggest that when using the MUAC and edema–only definitions of SAM or MAM,
(equation 1). Our findings suggest that when using the MUAC and edema–only definitions of SAM or MAM, 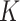 can still be reliably estimated. Additional studies are required to assess whether this finding holds in different settings. Even though it appears that K is independent of the definition of AM, the annual burden of SAM and MAM per 100 children is highly sensitive to the AM prevalence estimate used (equation 3, Table 1). Thus, program planners should use prevalence estimates that use the same AM definition as the one that is used for AM screening in the program.
can still be reliably estimated. Additional studies are required to assess whether this finding holds in different settings. Even though it appears that K is independent of the definition of AM, the annual burden of SAM and MAM per 100 children is highly sensitive to the AM prevalence estimate used (equation 3, Table 1). Thus, program planners should use prevalence estimates that use the same AM definition as the one that is used for AM screening in the program.
This study has a number of strengths and limitations. An important strength is the use of representative monthly follow-up data, in contrast to previous studies, which used 3- to 6-month follow-up frequencies (5, 7). The less frequent follow-ups might have led to longer episode length estimates given that the shortest observable episode length is defined by the length of the measurement interval. Another strength of this study is the use of multiple imputation for longitudinal data, a method that is superior to the often used “last observation carried forward” approach, which inflates episode length when data are missing between adjacent AM episodes. The main limitation of this study concerns the external validity of its findings. First, we used prevalence estimates that were derived from the cross-sectional surveys and thus might not have been representative for the whole year. Second, our estimates were derived from 3 districts representing limited and geographically contiguous areas of Mali and Burkina Faso. Third, enumerators conducted monthly visits and were instructed to refer MAM and SAM cases for treatment for ethical reasons. This could have led to more referrals and higher treatment coverage and, consequently, to shorter episode durations. AM treatment coverage for this cohort of children, however, was between 8% and 22% in Burkina Faso and Mali, respectively (8, 9), suggesting that the impact of this nutritional surveillance on treatment coverage, and possibly episode length, was limited.
In conclusion, estimates of the burden of acute malnutrition are often presented in terms of AM prevalence (2). Our analyses show that using prevalence underestimates the true number of cases affected by AM every year. The SAM prevalence of 3.1 per 100 children in Burkina Faso and 2.8 per 100 children in Mali, translates into a yearly estimated SAM burden of 31 and 18 cases per 100 children, respectively. Similarly, the MAM prevalence of 13.5 and 11.7 in Burkina Faso and Mali, respectively, corresponds to a yearly MAM burden of 77 (Burkina Faso) and 71 (Mali) cases per 100 children. In this study, prevalence estimates thus underestimated the annual burden of SAM by a factor of 7–10 and that of MAM by a factor of 6, which highlights the need for incidence data to accurately quantify the AM burden as well as its attributable child mortality.
ACKNOWLEDGMENTS
Author affiliations: Poverty, Health and Nutrition Division, International Food Policy Research Institute, Washington, DC (Francisco M. Barba, Lieven Huybregts, and Jef L. Leroy).
This research was funded by the Children’s Investment Fund Foundation and Innocent Foundation through the No Wasted Lives Framework. The PROMIS project was funded by Global Affairs Canada (https://www.international.gc.ca/) (grant 52308/5252/0200) and by the CGIAR Agriculture for Nutrition and Health program led by the International Food Policy Research Institute.
We acknowledge the Council for Research and Technical Advice on Acute Malnutrition for providing useful comments to the initial study proposal. We thank Dr. Marie Ruel for thoughtful comments. The PROMIS intervention was implemented by Helen Keller International with the support from the Gourcy, Bla, and San health districts.
Conflict of interest: none declared.
REFERENCES
- 1. Olofin I, McDonald CM, Ezzati M, et al. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One. 2013;8(5):e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. [DOI] [PubMed] [Google Scholar]
- 3. Dale NM, Myatt M, Prudhon C, et al. Using cross-sectional surveys to estimate the number of severely malnourished children needing to be enrolled in specific treatment programmes. Public Health Nutr. 2017;20(8):1362–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bulti A, Briend A, Dale NM, et al. Improving estimates of the burden of severe acute malnutrition and predictions of caseload for programs treating severe acute malnutrition: experiences from Nigeria. Arch Public Heal. 2017;75(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isanaka S, Boundy EO'N, Grais RF, et al. Improving estimates of numbers of children with severe acute malnutrition using cohort and survey data. Am J Epidemiol. 2016;184(12):861–869. [DOI] [PubMed] [Google Scholar]
- 6. Deconinck H, Pesonen A, Hallarou M, et al. Challenges of estimating the annual caseload of severe acute malnutrition: the case of Niger. PLoS One. 2016;11(9):e0162534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garenne M, Willie D, Maire B, et al. Incidence and duration of severe wasting in two African populations. Public Health Nutr. 2009;12(11):1974–1982. [DOI] [PubMed] [Google Scholar]
- 8. Becquey E, Huybregts L, Zongrone A, et al. Impact on child acute malnutrition of integrating a preventive nutrition package into facility-based screening for acute malnutrition during well-baby consultation: a cluster-randomized controlled trial in Burkina Faso. PLoS Med. 2019;16(8):e1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huybregts L, Le Port A, Becquey E, et al. Impact on child acute malnutrition of integrating small-quantity lipid-based nutrient supplements into community-level screening for acute malnutrition: a cluster-randomized controlled trial in Mali. PLoS Med. 2019;16(8):e1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miettinen O. Estimability and estimation in case referent studies. Am J Epidemiol. 1976;103(2):226–235. [DOI] [PubMed] [Google Scholar]
- 11. Ministère de la Santé Protocole de prise en charge integrée de la malnutrition aiguë au Mali. Bamako, Mali: Republique du Mali; 2011. [Google Scholar]
- 12. Ministère de la Santé Protocole national prise en charge integrée de la malnutrition aigue (PCIMA) Burkina Faso. Ouagadougou, Burkina Faso: Burkina Faso; 2014. [Google Scholar]
- 13. WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 14. Welch CA, Petersen I, Bartlett JW, et al. Evaluation of two-fold fully conditional specification multiple imputation for longitudinal electronic health record data. Stat Med. 2014;33(21):3725–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37(14):2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Isanaka S, Grais RF, Briend A, et al. Estimates of the duration of untreated acute malnutrition in children from Niger. Am J Epidemiol. 2011;173(8):932–940. [DOI] [PubMed] [Google Scholar]


