Abstract
Background:
Traumatic hemorrhagic contusions are associated with iodine leak; however, quantification of leakage and its importance to outcome is unclear.
Purpose:
To identify iodine-based dual-energy CT variables that correlate with in-hospital mortality and short-term outcomes for contusions at hospital discharge.
Materials and Methods:
In this retrospective study, consecutive patients with contusions from May 2016 through January 2017 were analyzed. Two radiologists evaluated CT variables from unenhanced admission head CT and follow-up head dual-energy CT scans obtained after contrast material–enhanced whole-body CT. The outcomes evaluated were in-hospital mortality, Rancho Los Amigos scale (RLAS) score, and disability rating scale (DRS) score. Logistic regression and linear regression were used to develop prediction models for categorical and continuous outcomes, respectively.
Results:
The study included 65 patients (median age, 48 years; interquartile range, 25–65.5 years); 50 were men. Dual-energy CT variables that correlated with mortality, RLAS score, and DRS score were iodine concentration, pseudohematoma volume, iodine quantity in pseudohematoma, and iodine quantity in contusion. The single-energy CT variable that correlated with mortality, RLAS score, and DRS score was hematoma volume at follow-up CT. Multiple logistic regression analysis after inclusion of clinical variables identified two predictors that enabled determination of mortality: postresuscitation Glasgow coma scale (P-GCS) (adjusted odds ratio, 0.42; 95% confidence interval [CI]: 0.2, 0.86; P = 0.01) and iodine quantity in pseudohematoma (adjusted odds ratio, 1.4 per milligram; 95% CI: 1.02 per milligram, 1.9 per milligram; P = 0.03), with a mean area under the receiver operating characteristic curve of 0.96 ± 0.05 (standard error). For RLAS, the predictors were P-GCS (mean coefficient, 0.32 ± 0.06; P < .001) and iodine quantity in contusion (mean coefficient, −0.04 per milligram ± 0.02; P = 0.01). Predictors for DRS were P-GCS (mean coefficient, −1.15 ± 0.27; P < .001), age (mean coefficient, 0.13 per year ± 0.04; P = .002), and iodine quantity in contusion (mean coefficient, 0.19 per milligram ± 0.07; P = .02).
Conclusion:
Iodine-based dual-energy CT variables correlate with in-hospital mortality and short-term outcomes for contusions at hospital discharge.
Summary
Quantitative iodine-based parameters derived from dual-energy CT are used to measure both the volume of penumbra that manifests as a nonhemorrhagic component of contusion and the magnitude of capillary disruption in the epicenter and penumbra while also enabling prediction of in-hospital mortality and short-term outcome.
Traumatic brain injury (TBI) is a disabling injury that leads to physical and behavioral impairments (1–3). Pathoanatomic description of injury type at admission and follow-up head CT (at 2–6 hours) carries prognostic importance (4–6). There are four pathoanatomic types of TBI: hemorrhagic contusions; subarachnoid hemorrhage; hematomas, including epidural and subdural hematomas; and diffuse axonal injury (DAI) (5). One of the most severe types of TBI is contusion (7,8). Contusions are often complicated by a secondary mechanosensitive molecular cascade that eventually results in capillary dysfunction and fragmentation in the penumbra of the contusion, leading to hemorrhagic progression of contusion (HPC) (7–9). Reports exist on the identification of an iodine leak in contusions that depend on qualitative assessment after CT angiography of the brain (10–13). These single-energy (SE) CT studies show a correlation between threshold levels of Hounsfield unit elevation, which are used to confirm leak and HPC, worsening Glasgow coma scale, and in-hospital mortality. Although use of CT angiography to evaluate TBI is not an accepted standard of care, contrast-enhanced whole-body (WB) CT is widely used in the work-up of patients with polytrauma (14). The contrast material bolus administered during WB CT leaks into and is retained by the epicenter and penumbra of the contusion due to capillary fragmentation and dysfunction (8).
Dual-energy (DE) CT is used to quantify the amount of iodine leak in cerebral infarctions, subdural spaces, and spontaneous hemorrhages (15–17). Similarly, it is possible to quantify the amount of iodine leak in contusions and the volume of enhancing penumbra, which is not possible with single-energy (SE) CT (8). Quantification of the epicenter volume that manifests as a hemorrhagic component and the penumbra that manifests as a nonhemorrhagic component of the contusion may serve as radiologic markers. These markers may be used to assess the entire volume of tissue damage in contusions. The ability to quantify the amount of iodine leak through the damaged endothelium from primary and secondary injuries may help measure the magnitude of capillary fragmentation in the epicenter and penumbra. Thus, iodine-based DE CT variables have the potential to enable measurement of not only the volume of the entire contusion but also the severity of capillary fragmentation. Exploration of the feasibility and identification of the potential strengths of quantitative iodine-based DE CT variables over the previously validated SE CT predictor variables may have an important role in prognostication, injury classification, and pathophysiology characterization.
The purpose of this study was to use both unenhanced admission and follow-up head dual-energy CT scans obtained after whole-body CT to identify iodine-based dual-energy CT variables that correlate with in-hospital mortality and short-term outcome at hospital discharge.
Materials and Methods
Study Design
This retrospective study was compliant with the Health Insurance Portability and Accountability Act, and permission was obtained from our institutional review board. Written informed consent was waived. Consecutive patients referred to a level I trauma center between May 2016 and January 2017 were eligible for inclusion. The inclusion criteria were as follows: (a) history of blunt trauma with acquisition of head CT scans as a part of WB CT performed within 6 hours after traumatic impact, with confirmed diagnosis of one or more hemorrhagic contusions; (b) acquisition of follow-up head DE CT scans within 10 hours after admission CT to evaluate the progression of TBI; and (c) age of at least 18 years. Patients were excluded if (a) they underwent decompressive craniectomy to evacuate extraparenchymal hematomas before follow-up; (b) they had either nonfocal extraparenchymal hematoma extending over more than one lobe with a width of more than 2 mm or holohemispheric subdural hematoma, irrespective of the width, to select patients with contusions as the main type of injury; or (c) they had subcortical white matter punctate hemorrhages concomitant with DAI. The protocol used for work-up of patients with polytrauma at the study institute is shown in Appendix E1 (online).
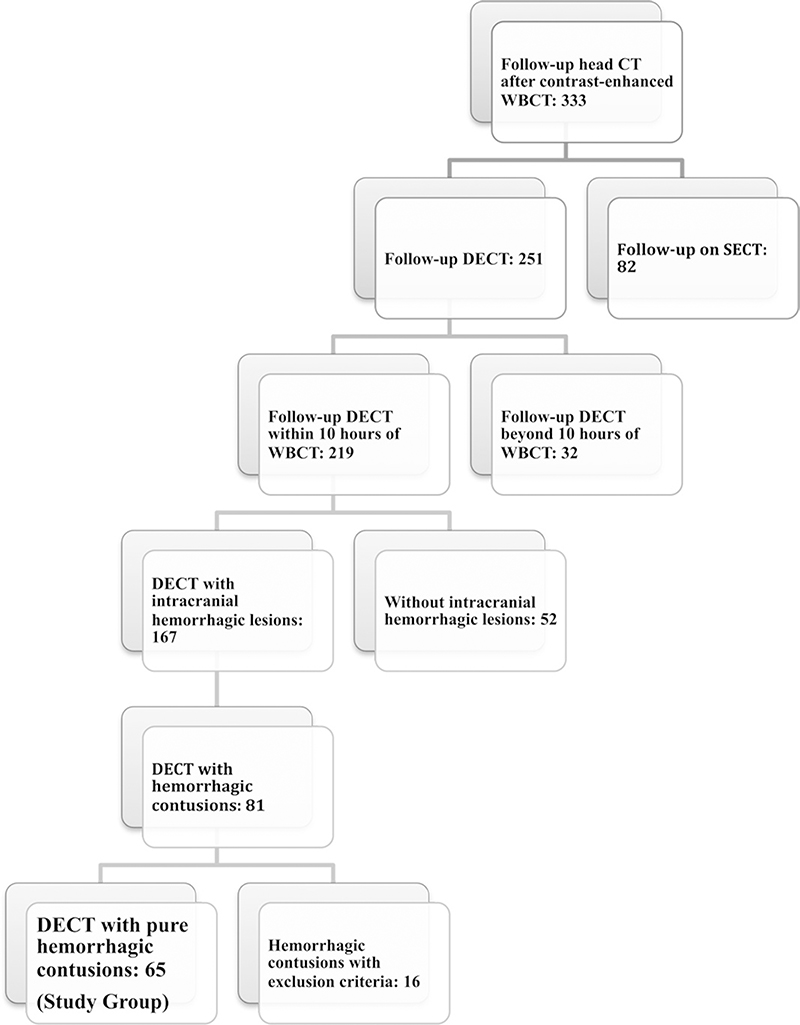
Reviewer 1 (U.B., a trauma radiologist with 10 years of experience) evaluated the initial and follow-up studies to select all the patients with pure contusions (n = 65) who would comprise the study group. A patient selection flowchart is shown in Figure 1.
Figure 1:
Patient selection flowchart. DECT = dual-energy CT, SECT = single-energy CT, WBCT = whole-body CT.
Clinical information, in-hospital mortality, and outcome measures at hospital discharge (Rancho Los Amigos scale [RLAS] and disability rating scale [DRS]) were obtained from comprehensive occupational and physical therapy discharge summaries. Medical students (M.R., S.T.) obtained clinical data under the supervision of a neurocritical care attending physician (G.P., 6 years of clinical experience). In intubated patients, a verbal score for postresuscitation Glasgow coma scale (P-GCS) was derived (18).
Image Analysis and Definitions
Imaging details are provided in Appendix E1 (online). Two full-time trauma radiologists who were blinded to clinical information reviewed the imaging studies (reviewer 1 and reviewer 2 [K.S., 25 years of experience]). A total of three image sets were used for volume measurements in each patient (ie, 120-kV DE CT or SE CT images were acquired with 120 kVp [from admission CT] and 120-kV and 190-keV image sets were acquired from follow-up DE CT scans). Reviewer 1 annotated all the contusions that were meant for volume measurements, measured the volumes using semiautomated three-dimensional segmentation on a thin-client server (Philips Intellispace Portal; Philips Healthcare, Best, the Netherlands), and evaluated admission CT scans for Marshall CT scores (19).
To measure iodine concentration in the contusion, the modified brain hemorrhage application on the postprocessing workstation (syngo.via, version VB10B; Siemens Healthcare, Forchheim, Germany) was used (8). Reviewers 1 and 2 independently measured the iodine concentrations on follow-up DE CT scans by drawing a region of interest around each contusion. For every contusion, three regions of interest were drawn; these were in the axial, coronal, and sagittal sections at the level of the maximum diameter. The average of the three regions of interest was obtained. The mean values obtained by the two reviewers were used for analysis.
Dependent Variables
Dependent variables included in-hospital mortality, RLAS, and DRS (20,21). Details about the variables are provided in Appendix E1 (online).
Independent Variables
Independent variables included 21 predictors derived from CT, clinical, and laboratory data (Table 1).
Table 1:
Demographic, Clinical, and CT Characteristics for the Patient Studies
| Characteristic | Summary Statistic (n = 65) |
|---|---|
| Age (y)* | 48 (25–65.5) |
| Sex | |
| Male | 50 (76.9) |
| Female | 15 (23.1) |
| P-GCS* | 11 (7–14) |
| Motor score* | 5 (4.25–6) |
| Systolic blood pressure (mm Hg)* | 151 (133–170) |
| Diastolic blood pressure (mm Hg)* | 87 (79–96) |
| Prothrombin time (sec)* | 14.3 (13.6–15.7) |
| aPTT (sec)* | 28 (26–30) |
| Fibrinogen level (mg/dL)†‡ | 295.2 6 97.3 |
| Platelet count (103/μL)* | 210 (172–250) |
| Marshall CT score | |
| DI II | 57 (87.7) |
| DI III | 7 (10.8) |
| DI 1 V | 1 (1.5) |
| Multiple contusions | 28 (43.1) |
| Hemorrhagic progression | 44 (69.8) |
| Fraction of hemorrhagic progression* | 1.33 (0.12–3.8) |
| Iodine concentration (mg/mL)* | 0.43 (0.23–0.53) |
| Hematoma volume on admission CT scan (cm3)* | 1.52 (0.4–5.1) |
| Hematoma volume on follow-up CT scan (cm3)* | 3.2 (1–9) |
| Total pseudohematoma volume from all contusions (cm3)* | 1.02 (0.2–3.1) |
| Fractional pseudohematoma volume to true hematoma* | 0.3 (0.1–0.57) |
| Iodine quantity in all pseudohematomas (mg)* | 0.34 (0.08–1.12) |
| Iodine quantity in all contusions (mg)* | 1.33 (0.46–6) |
Note.—Unless otherwise indicated, data are number of patients, and data in parentheses are percentages. aPTT = activated partial thromboplastin time, DI = diffuse injury, P-GCS = postresuscitation Glasgow coma scale.
Data are median, and data in parentheses are the interquartile range.
Data are mean ± standard deviation.
To convert to SI units (micromoles per liter), multiply by 0.0294.
Initial and follow-up hematoma volumes.—
Initial hematoma volumes were measured on 120-kV or SE CT images (defined as the variable A) (Fig 2a, Fig E1a [online]). Volume measurements on follow-up DE CT images were obtained on both 120-kV images (Fig 2b, Fig E1b [online]) and 190-keV images (Fig 2c, Fig E1c [online]).
Figure 2:
Images in a 25-year-old man with traumatic brain injury sustained after a motorcycle crash. (a) Axial unenhanced dual-energy 120-kV CT image of the head at admission shows hemorrhagic contusion (arrowhead) in the right parietal lobe. (b) Axial unenhanced dual-energy 120-kV CT image of the head at 4-hour follow-up shows hemorrhagic progression of the contusion (arrow) with increased volume of the existing hematoma. (c) Virtual high monochromatic axial image (190-kV) from the unenhanced follow-up dual-energy CT scan shows smaller hemorrhagic contusion volume (arrow) when compared with a and b due to negligible attenuation contribution from leaked iodinated contrast material. (d) Iodine overlay axial image from the unenhanced follow-up dual-energy CT scan shows contrast staining of the penumbra of the contusions (black arrowheads), and helps demarcate from the epicenter (white arrowhead).
Pseudohematoma and fraction of pseudohematoma.—
Pseudohematoma is defined as enhancing penumbra caused by iodine leak on follow-up 120-kV images (8). A detailed explanation is given in Appendix E1 (online). Pseudohematoma volume was calculated by subtracting volume on 190-keV images from volume on 120-kV images. Fraction of pseudohematoma was calculated with the equation (B – C)/C, where B is volume on 120-kV images and C is volume on 190-keV images.
Iodine concentration.—
Average iodine concentration (in milligrams per milliliter) was measured with the modified brain hemorrhage application (Fig 2d, Fig E1d [online]).
Iodine quantity in pseudohematoma and contusion.—
Iodine quantity in pseudohematoma (Ip) was calculated as follows: Ip = ([B – C] × Ic), where Ic is iodine concentration. Iodine quantity in total contusion (Itc) was calculated as follows: Itc = (B × Ic).
HPC and fraction of HPC.—
HPC is designated as enlargement of the existing hemorrhagic contusion volumes (30% volume increase) or appearance of a new lesion (8). Fraction of HPC was calculated with the equation HPC = (C– A)/(A).
Fractional rate of iodine washout, adjusted iodine concentration and adjusted iodine-based variables. —
These are discussed in Appendix E1 (online).
Statistical Analysis
Data from continuous variables are summarized as mean ± standard deviation for normally distributed variables and as median and interquartile range for nonnormally distributed variables. Categorical variables are summarized as counts and percentages. The association between each predictor of interest and in-hospital mortality was examined by using the χ2 or Fisher exact test for categorical predictors and simple logistic regression for continuous predictors. The effect of each predictor of interest on in-hospital mortality without adjusting for other predictors and the unadjusted odds ratio (OR) and corresponding 95% confidence intervals (CIs) were calculated. For continuous outcome variables (ie, RLAS and DRS), simple linear regression was performed first. For both categorical and continuous outcomes, predictors with P < .1 at unadjusted analysis were incorporated into the model selection process. The final model for each outcome was developed by using the backward elimination method.
Correlation analysis was used to examine linear associations among pairs of predictors. For two predictors with an absolute value of a correlation coefficient of 0.8 or higher, one of them was deleted from the backward model selection process, and a sensitivity analysis was conducted by removing the other predictor from the model selection process. The final prediction model for each outcome of interest was selected based on Akaike information criterion. In addition, variance inflation factor was used to verify that there was no multicollinearity issue in the final model. To examine the degree of overfitting of the final prediction model, 10-fold cross-validation was performed. Overall root-mean-square error and general trend in the coefficients within each fold were evaluated.
For the prediction model for in-hospital mortality, receiver operating characteristic analysis was performed to assess the overall predictive ability of the model by using the area under the receiver operating characteristic curve (AUC) and corresponding standard error. All analyses were performed by using commercially available statistical software (JMP 12; SAS Institute, Cary, NC; STATA/SE, version 15, Stata, College Station, Texas).
Results
Baseline clinical characteristics of the cohort (n = 65) are shown in Table 1 and Table E1 (online). The median time from traumatic impact to WB CT was 1 hour 30 minutes (interquartile range, 1.12–2 hours), and median time to follow-up head DE CT was 6 hours (interquartile range, 4–7.25 hours). All contusions showed iodine leak, with a concentration ranging from 0.1 to 1.0 mg/mL (median, 0.43 mg/mL; interquartile range, 0.23–0.53 mg/mL]). In 28 patients with multiple contusions, there was a strong correlation between the iodine concentration in the largest contusion and the mean concentration in smaller contusions (r = 0.94; 95% CI: 0.9, 0.96). The correlation between the two reviewers’ measurements of iodine concentration was strong (r = 0.92; 95% CI: 0.87, 0.95). Median RLAS and DRS were 4 (interquartile range, 3–6) and 5 (interquartile range, 3–8), respectively. The mean length of hospital stay was 13 days ± 9 (standard deviation).
In-Hospital Mortality
Six patients died of TBI during acute hospitalization. Without adjusting for other predictors, the DE CT variables that correlated with mortality were higher iodine concentration (OR, 362; 95% CI: 5.46, 24 075; P = .006), pseudohematoma volume (OR, 1.21; 95% CI: 1.05, 1.4; P = .006), iodine quantity in pseudohematoma (OR, 1.23; 95% CI: 1.04, 1.45; P = .01), and iodine quantity in contusion (OR,1.1; 95% CI: 1.02, 1.18; P = .01). A threshold iodine concentration of 0.8 mg of iodine per milliliter and iodine quantity in contusion of 3.9 mg yielded optimal sensitivity and specificity, with individual mean AUCs of 0.78 ± 0.11 and 0.84 ± 0.09, respectively. An SE CT variable that correlated was hematoma volume at follow-up CT. Clinical variables that correlated were P-GCS, motor score, activated partial thromboplastin time, and platelet count. Complete details are provided in Table 2. Correlation between predictors is shown in Figure E2 (online). For the prediction model, redundant and correlated predictors (r > 0.8) were dropped from the full logistic regression model (Table E2 [online]) to prevent the problem with unstable parameter estimates of regression. Iodine quantity in pseudohematoma and the volume of pseudohematoma showed strong linear correlation (r > 0.95) with iodine quantity in contusion; hence, these factors can be interchangeable in the regression models. Among these correlated predictors, iodine quantity in contusions was used in the full model with the knowledge that it incorporates the maximum information related to iodine leak and hematoma volume. However, iodine quantity in pseudohematoma yielded lower Akaike information criterion for predicting mortality; hence, it was replaced in the final model. The final model yielded two predictors with significance (Table 2): P-GCS and iodine quantity in pseudohematoma. On average, a one-unit (milligrams) increase in iodine quantity in pseudohematoma increased the odds of mortality by 40% (adjusted OR, 1.4; 95% CI:1.02, 1.9; P = .03). The two predictors resulted in a coefficient of determination for multivariate analysis (or R2) of 0.57 (P < .001), with overall root-mean-square error of 0.217 on 10-fold cross-validation. Receiver operating characteristic analysis of the regression model in predicting mortality yielded a mean AUC of 0.96 ± 0.05 (Fig 3). In the subset of patients with moderate and severe TBI, the model yielded a mean AUC of 0.94 ± 0.07 (Fig E3 [online]).
Table 2:
Unadjusted and Adjusted Associations between Predictor Variables and In-Hospital Mortality
| Independent Variable | Unadjusted Odds Ratio | P Value | Adjusted Odds Ratio | P Value |
|---|---|---|---|---|
| P-GCS | 0.53 (0.33, 0.86) | .01* | 0.42 (0.2, 0.86) | .01* |
| Iodine quantity in all pseudohematomas (mg) | 1.23 (1.04, 1.45) | .02* | 1.4 (1.02, 1.9) | .03* |
| Age (y) | 1 (0.96, 1.04) | .82 | … | … |
| Sex (men vs women) | NA | .19† | … | … |
| Motor score | 0.43 (0.24, 0.74) | .002* | … | … |
| Systolic blood pressure (mm Hg) | 0.98 (0.95, 1) | .13 | … | … |
| Diastolic blood pressure (mm Hg) | 0.96 (0.91, 1.01) | .14 | … | … |
| Prothrombin time (sec) | 1.13 (0.97, 1.32) | .12 | … | … |
| aPTT (sec) | 1.23 (1.03, 1.46) | .02* | … | … |
| Fibrinogen level (mg/dL)‡ | 0.99 (0.98, 1) | .2 | … | … |
| Platelet count (103/μL) | 0.98 (0.96, 0.99) | .01* | … | … |
| Marshall CT score | ||||
| DI II | 1 | … | … | … |
| DI III and 1 V | 4.42 (0.6, 29.4) | .15† | … | … |
| Multiple contusions (%) | 2.92 (0.49, 17.2) | .21† | … | … |
| Hemorrhagic progression | NA | .1† | … | … |
| Fraction of hemorrhagic progression | 0.95 (0.78, 1.16) | .62 | … | … |
| Iodine concentration (mg/mL) | 362 (5.46, 24 075) | .006* | … | … |
| Hematoma volume on admission CT scan (cm3) | 1.07 (0.99, 1.17) | .09 | … | … |
| Hematoma volume on follow-up CT scan (cm3) | 1.07 (1, 1.13) | .03* | … | … |
| Total pseudohematoma from all contusions (cm3) | 1.21 (1.05, 1.4) | .006* | … | … |
| Fractional pseudohematoma to true hematoma | 5.66 (0.7, 46.3) | .11 | … | … |
| Iodine quantity in all contusions (mg) | 1.1 (1.02, 1.18) | .01* | … | … |
Note.—Data in parentheses are 95% confidence intervals. aPTT = activated partial thromboplastin time, DI = diffuse injury, NA = not available, as no deaths were reported in one of the groups, P-GCS = postresuscitation Glasgow coma scale.
P value indicates a significant difference.
P value was calculated with the Fisher exact test.
To convert to SI units (micromoles per liter), multiply by 0.0294.
Figure 3:
Receiver operating characteristic curve for the final prediction model that consists of postresuscitation Glasgow coma scale and iodine quantity in pseudohematomas yielded a mean area under the curve of 0.96 ± 0.05 (standard error) in predicting in-hospital mortality. The yellow line represents a 45° angle tangent to the receiver operating characteristic curve, and the point of intersection marks a good cutoff point under the assumption that false-negative and false-positive findings have similar costs. Prediction formula: Mortality = 3.23 + −0.87 × P-GCS + 0.33 × iodine quantity in pseudohematoma.
Short-term Outcome Measures
The DE CT variables that correlated with RLAS and DRS were iodine concentration (mean coefficient, −0.04 ± 0.01 [P = .002] and 0.006 ± 0.003 [P = .03], respectively), pseudohematoma volume (mean coefficient, −0.87 ± 0.3 [P = .005] and 0.21 ± 0.07 [P = .004], respectively), iodine quantity in pseudohematoma (mean coefficient, −0.92 ± 0.38 [P = .02] and 0.26 ± 0.09 [P = .004], respectively), and iodine quantity in contusion (mean coefficient, −1.88 ± 0.66 [P = .006] and 0.49 ± 0.15 [P = .002], respectively). SE CT variables that correlated with RLAS and DRS were hematoma volume at both admission CT and follow-up CT, while Marshall CT score correlated only with RLAS. P-GCS and motor score were the clinical parameters that correlated with both RLAS and DRS, while activated partial thromboplastin time and age correlated with DRS only. Complete details are provided in Table 3. For RLAS and DRS regression, pseudohematoma volume and iodine quantity in pseudohematoma were dropped from the full regression model because the variance inflation factor was greater than five (Table E3 [online]). Iodine quantity in contusion yielded the lowest Akaike information criterion among the correlated variables for the model predicting RLAS and DRS. For RLAS, the significant predictors (P-GCS and iodine quantity in contusions) resulted in an adjusted R2 value of 0.39 (P < .001) (Table 3), with overall RMSE of 1.68 at cross-validation. For DRS, the predictors were P-GCS, age, and iodine quantity in contusion, with an adjusted R2 value of 0.36 (P < .001) (Table 4) and overall RMSE of 7.92. On average, a one-unit (milligrams) increase in iodine quantity in contusions decreased RLAS by 0.04 (mean coefficient, −0.04 ± 0.02; P = .01) and increased DRS by 0.19 (mean coefficient, 0.19 ± 0.07; P = .02).
Table 3:
Unadjusted and Adjusted Associations between Predictor Variables and Rancho Los Amigos Scale
| Independent Variable | Unadjusted Coefficient | P Value | Adjusted Coefficient | P Value |
|---|---|---|---|---|
| P-GCS | 0.94 ± 0.17 | <.001* | 0.32 ± 0.06 | <.001* |
| Iodine quantity in all contusions (mg) | −1.88 ± 0.66 | .006* | −0.044 ± 0.018 | .01* |
| Age (y) | 0.07 ± 1.3 | .95 | … | … |
| Sex (men vs women) | 0.04 ± 0.14 | .75 | … | … |
| Motor score | 0.27 ± 0.07 | <.001* | … | … |
| Systolic blood pressure (mm Hg) | −0.63 ± 1.83 | .7 | … | … |
| Diastolic blood pressure (mm Hg) | −0.16 ± 1.05 | .87 | … | … |
| Prothrombin time (sec) | −0.23 ± 0.22 | .29 | … | … |
| aPTT (sec) | −0.5 ± 0.27 | .07 | … | … |
| Fibrinogen level (mg/dL)† | 7.36 ± 0.002 | .2 | … | … |
| Platelet count (103/μL) | 0.003 ± 4.65 | .99 | … | … |
| Marshall CT score | … | … | ||
| DI II | 1 | … | … | … |
| DI III and 1 V | 0.5 ± 0.26 | .05* | … | … |
| Multiple contusions (%) | 0.14 ± 0.12 | .24 | … | … |
| Hemorrhagic progression | 0.17 ± 0.14 | .22 | … | … |
| Fraction of hemorrhagic progression | 0.19 ± 0.48 | .68 | … | … |
| Iodine concentration (mg/mL)‡ | −0.04 ± 0.012 | .002* | … | … |
| Hematoma volume on admission CT scan (cm3) | −0.82 ± 0.39 | .04* | … | … |
| Hematoma volume on follow-up CT scan (cm3) | −1.49 ± 0.63 | .02* | … | … |
| Total pseudohematoma from all contusions (cm3) | −0.87 ± 0.3 | .005* | … | … |
| Fractional pseudohematoma to true hematoma | −0.04 ± 0.02 | .05* | … | … |
| Iodine quantity in all pseudohematomas (mg) | −0.92 ± 0.38 | .02* | … | … |
Note.—Unless otherwise indicated, data are mean ± standard error. aPTT = activated partial thromboplastin time, DI = diffuse injury, P-GCS– postresuscitation Glasgow coma scale.
P value indicates a significant difference.
To convert to SI units (micromoles per liter), multiply by 0.0294.
To convert to SI units (nanomoles per liter), multiply by 7.880.
Table 4:
Unadjusted and Adjusted Associations between Predictor Variables and Disability Rating Scale
| Independent Variable | Unadjusted Coefficient | P Value | Adjusted Coefficient | P Value |
|---|---|---|---|---|
| P-GCS | −0.1 ± 0.04 | .001* | −1.15 ± 0.27 | <.001* |
| Iodine quantity in all contusions (mg) | 0.49 ± 0.15 | .002* | 0.19 ± 0.07 | .02* |
| Age (y) | 0.64 ± 0.3 | .04* | 0.13 ± 0.04 | .002* |
| Sex (men vs women) | −0.013 ± 0.03 | .7 | … | … |
| Motor score | −0.06 ± 0.01 | .001* | … | … |
| Systolic blood pressure (mm Hg) | 0.09 ± 0.44 | .84 | … | … |
| Diastolic blood pressure (mm Hg) | −0.16 ± 0.25 | .5 | … | … |
| Prothrombin time (sec) | 0.1 ± 0.05 | .06 | … | … |
| aPTT (sec) | 0.17 ± 0.06 | .009* | … | … |
| Fibrinogen level (mg/dL)† | 0.54 ± 1.4 | .7 | … | … |
| Platelet count (103/μL) | −1.11 ± 1.1 | .3 | … | … |
| Marshall CT Score | .06 | |||
| DI II | 1 | … | … | … |
| DI III and 1 V | −0.07 ± 0.03 | … | … | … |
| Multiple contusions (%) | −0.02 ± 0.03 | .43 | … | … |
| Hemorrhagic progression | −0.03 ± 0.03 | .4 | … | … |
| Fraction of hemorrhagic progression | −0.04 ± 0.11 | .7 | … | … |
| Iodine concentration (mg/mL)‡ | 0.006 ± 0.003 | .03* | … | … |
| Hematoma volume on admission CT scan (cm3) | 0.26 ± 0.09 | .006* | … | … |
| Hematoma volume on follow-up CT scan (cm3) | 0.4 ± 0.14 | .008* | … | … |
| Total pseudohematoma from all contusions (cm3) | 0.21 ± 0.07 | .004* | … | … |
| Fractional pseudohematoma to true hematoma | 0.01 ± 0.004 | .04* | … | … |
| Iodine quantity in all pseudohematomas (mg) | 0.26 ± 0.09 | .004* | … | … |
Note.—Unless otherwise indicated, data are mean ± standard error. aPTT = activated partial thromboplastin time, DI = diffuse injury, P-GCS– postresuscitation Glasgow coma scale.
P value indicates a significant difference.
To convert to SI units (micromoles per liter), multiply by 0.0294.
To convert to SI units (nanomoles per liter), multiply by 7.880.
Iodine Washout Rates
A second follow-up DE CT scan was available in 37 patients. In these patients, there was a slow and gradual decrease in iodine concentration, with a median decrease of 2.22% (interquartile range, 0%–9.6%). There was no correlation between iodine concentration and washout rate (Spearman ρ = 0.25, P = 0.13). Serial iodine concentrations measured in patients with more than two follow-up studies (n = 22) demonstrated complete washout as soon as 56 hours after contrast material injection, and iodine was retained for as long as 147 hours after injection. Analysis performed with adjusted iodine-based variables to the median time for follow-up CT of 6 hours resulted in a model with identical predictors for RLAS and DRS, while iodine quantity in pseudohematomas was replaced by iodine quantity in contusions in the prediction model for mortality.
Discussion
Brain contusions may result in capillary dysfunction and fragmentation in the penumbra of the contusion, leading to hemorrhagic progression of the contusion. In this study, we evaluated patients with a traumatic contusion by using dual-energy (DE) CT. We used iodine quantification to identify DE CT iodine-based variables (ie, volume of pseudohematoma, iodine quantity in pseudohematoma, and iodine quantity in contusion) that correlated with in-hospital mortality and short-term outcome. Multivariable analysis showed that iodine quantity in patients with pseudohematoma was the strongest imaging predictor of mortality (adjusted odds ratio = 1.4 per milligram; 95% confidence interval: 1.02 per milligram, 1.9 per milligram; P = .03). Iodine quantity in contusion was the strongest imaging predictor of RLAS (mean coefficient, −0.04 per milligram ± 0.02; P = .01) and DRS (mean coefficient, 0.19 per milligram ± 0.07; P = 0.02). In this patient population, all cerebral contusions were associated with an iodine leak, whereas 41%–67% of contusions were associated with leaks reported at SE CT (10–13).
The predictive strength of iodine quantity in a contusion in determining RLAS and DRS can be explained not only by its ability to account for the volume of epicenter and penumbra of the contusion but also by the magnitude of capillary dysfunction and fragmentation in both the epicenter and the penumbra. Functional outcome in contusions is largely determined by the volume of irreversible tissue damage from both the primary injury and the secondary injury (5). Animal models have demonstrated the concept of progressive microvascular failure associated with contusions, where the peak kinetic energy delivered to the epicenter resulted in immediate capillary fragmentation. The energy deposited in the penumbra is not enough to fracture capillaries, but it is enough to activate mechanosensitive molecular processes. This upregulates sulfonylurea receptor 1, which has been implicated in delayed microvascular dysfunction and fragmentation (7–9). SE CT variables cannot enable assessment of the volume of the penumbra and the magnitude of microvascular dysfunction, proving inferior to DE CT variables as predictors of in-hospital mortality and short-term outcome. Given the independent prediction ability, the specific iodine-based DE CT variables may represent a neuroimaging marker that can fill a gap in the realm of current imaging tools to measure the extent of secondary injury and treatment response that targets limiting secondary injury.
Consistent with previous reports, P-GCS was the strongest clinical predictor of in-hospital mortality and outcomes in these patients (22,23). Similarly, age was another known prognostic predictor, with older age associated with poorer performance at DRS (23–25). Our study used the most commonly used CT classification scheme (Marshall CT score), as well as contusion volumes at admission and follow-up CT that influence outcomes and compared them with the quantitative iodine-based DE CT variables (26–28). The Marshall CT score was originally derived and validated in cohorts containing patients with moderate or severe TBI and injuries of pathoanatomic heterogeneity (26). Therefore, our results showing a lack of correlation between Marshall CT score and mortality and DRS are likely due to our inclusion of patients with a specific injury type (contusions) and of all injury severities (P-GCS) in our cohort.
We excluded large extraparenchymal hematomas and DAI lesions detected at CT, as those pathoanatomic injuries do not share a common physiologic mechanism of primary and secondary damage with contusions (5,6). However, it should be noted that contusions coexist with DAI. A recent study demonstrated both focal lesions (contusions, epidural or subdural hematomas, etc) and DAI in half of the patients with moderate or severe TBI; however, the study did not mention the incidence based on the specific type of focal lesion (29). Although we excluded DAIs that were seen on CT scans, a large majority of patients with coexistent DAI would have been included in our cohort in the absence of concurrent MRI scans. Hence, our study did not account for the confounding effects of DAI on outcomes.
CT perfusion and contrast-enhanced MRI are current imaging techniques used to detect microvascular disruption. However, these techniques are not widely accepted for use in patients with TBI due to additional cost, time, and potential for additional radiation (30). Thus, this emerging technique with its ability to measure the volume of penumbra and the degree of capillary disruption from secondary injury can be easily automated for hematoma segmentation and iodine quantification to streamline patients for treatment approach of limiting the secondary injury (17).
Our study had limitations, including its retrospective and single-center design, which introduced selection and institutional biases. Additionally, this cohort comprised patients with a contusion as the major form of injury. Thus, this data cannot be generalized to all patients with TBI, especially those with major extraparenchymal bleeds. Coexistence of DAI, especially in patients with moderate or severe TBI, is another factor that has not been accounted for and might have confounded the outcomes. Finally, the small cohort used in this preliminary investigation limits its statistical power. Thus, the current study should be considered exploratory.
Quantitative iodine-based parameters derived from dual-energy CT have the ability to measure the volume of penumbra that manifests as a nonhemorrhagic component of contusion but also the magnitude of capillary disruption in the epicenter and penumbra while also predicting in-hospital mortality and short-term outcome by using unenhanced admission and follow-up head dual-energy CT scans obtained after whole-body CT.
Supplementary Material
Key Points.
Quantification of iodine leak derived from short-term unenhanced follow-up dual-energy head CT scans obtained after contrast-enhanced whole-body CT scans at admission was associated with in-hospital mortality and short-term outcome for traumatic hemorrhagic contusions at hospital discharge (disability rating scale and Rancho Los Amigos scale).
On average, a one-unit (milligrams) increase in iodine quantity in pseudohematoma increases the odds of in-hospital mortality by 40% (adjusted odds ratio, 1.4; P = .03).
A one-unit (milligrams) increase in iodine quantity in contusions was associated with lower Rancho Los Amigos scale score by 0.04 per milligram (P = .01) and greater disability rating scale score by 0.19 per milligram (P = .02).
Acknowledgments
Disclosures of Conflicts of Interest: U .K.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Siemens; is a conference speaker for Siemens. Other relationships: disclosed no relevant relationships. K.S. disclosed no relevant relationships. M.R. disclosed no relevant relationships. S.T. disclosed no relevant relationships. B.A. disclosed no relevant relationships. Y.G.P. disclosed no relevant relationships. G.S. disclosed no relevant relationships. D.D. disclosed no relevant relationships. J.M.S. disclosed no relevant relationships. T.P. disclosed no relevant relationships. G.L. disclosed no relevant relationships. Y.L. disclosed no relevant relationships. T.R.F. disclosed no relevant relationships.
Abbreviations
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- DAI
diffuse axonal injury
- DE
dual energy
- DRS
disability rating scale
- HPC
hemorrhagic progression of contusion
- OR
odds ratio
- P-GCS
postresuscitation Glasgow coma scale
- RLAS
Rancho Los Amigos scale
- SE
single energy
- TBI
traumatic brain injury
- WB
whole body
Contributor Information
Uttam K. Bodanapally, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Kathirkamanathan Shanmuganathan, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Meghna Ramaswamy, University of Maryland School of Medicine, Baltimore, Md.
Solomiya Tsymbalyuk, University of Maryland School of Medicine, Baltimore, Md.
Bizhan Aarabi, Department of Neurosurgery, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Gunjan Y. Parikh, Department of Neurology, R. Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Gary Schwartzbauer, Department of Neurosurgery, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
David Dreizin, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
J. Marc Simard, Department of Neurosurgery, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Thomas Ptak, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Guang Li, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Yuanyuan Liang, Department of Epidemiology and Public Health, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
Thorsten R. Fleiter, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21(5): 375–378. [DOI] [PubMed] [Google Scholar]
- 2.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil 2008;23(2):123–131. [DOI] [PubMed] [Google Scholar]
- 3.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil 1999;14(6):602–615. [DOI] [PubMed] [Google Scholar]
- 4.Lobato RD, Gomez PA, Alday R, et al. Sequential computerized tomography changes and related final outcome in severe head injury patients. Acta Neurochir (Wien) 1997;139(5):385–391. [DOI] [PubMed] [Google Scholar]
- 5.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma 2008;25(7):719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gennarelli TA, Spielman GM, Langfitt TW, et al. Influence of the type of intracranial lesion on outcome from severe head injury. J Neurosurg 1982a;56(1): 26–32. [DOI] [PubMed] [Google Scholar]
- 7.Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma 2012;29(1): 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodanapally UK, Shanmuganathan K, Issa G, et al. Dual-energy CT in hemorrhagic progression of cerebral contusion: overestimation of hematoma volumes on standard 120-kV images and rectification with virtual high-energy monochromatic images after contrast-enhanced whole-body imaging. AJNR Am J Neuroradiol 2018;39(4):658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med 2006;12(4):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosa M Jr, da Rocha AJ, Maia ACM Jr, Saade N, Veiga JCE, Romero JM. Contusion contrast extravasation depicted on multidetector computed tomography angiography predicts growth and mortality in traumatic brain contusion. J Neurotrauma 2016;33(11):1015–1022. [DOI] [PubMed] [Google Scholar]
- 11.Letourneau-Guillon L, Huynh T, Jakobovic R, Milwid R, Symons SP, Aviv RI. Traumatic intracranial hematomas: prognostic value of contrast extravasation. AJNR Am J Neuroradiol 2013;34(4):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang APH, Lee CW, Hsieh HJ, et al. Early parenchymal contrast extravasation predicts subsequent hemorrhage progression, clinical deterioration, and need for surgery in patients with traumatic cerebral contusion. J Trauma 2011;71(6):1593–1599. [DOI] [PubMed] [Google Scholar]
- 13.Orito K, Hirohata M, Nakamura Y, et al. Predictive value of leakage signs for pure brain contusional hematoma expansion. J Neurotrauma 2018;35(5):760–766. [DOI] [PubMed] [Google Scholar]
- 14.Huber-Wagner S, Lefering R, Qvick LM, et al. Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet 2009;373(9673):1455–1461. [DOI] [PubMed] [Google Scholar]
- 15.Bodanapally UK, Dreizin D, Issa G, Archer-Arroyo KL, Sudini K, Fleiter TR. Dual-energy CT in enhancing subdural effusions that masquerade as subdural hematomas: diagnosis with virtual high-monochromatic (190-keV) images. AJNR Am J Neuroradiol 2017;38(10):1946–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonatti M, Lombardo F, Zamboni GA, et al. Iodine extravasation quantification on dual-energy CT of the brain performed after mechanical thrombectomy for acute ischemic stroke can predict hemorrhagic complications. AJNR Am J Neuroradiol 2018;39(3):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan CO, Lam S, Kuppens D, et al. Spot and Diffuse Signs: Quantitative Markers of Intracranial Hematoma Expansion at Dual-Energy CT. Radiology 2019;290(1): 179–186. [DOI] [PubMed] [Google Scholar]
- 18.Meredith W, Rutledge R, Fakhry SM, Emery S, Kromhout-Schiro S. The conundrum of the Glasgow Coma Scale in intubated patients: a linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma 1998;44(5):839–844; discussion 844–845. [DOI] [PubMed] [Google Scholar]
- 19.Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 1992;9(Suppl 1):S287–S292. [PubMed] [Google Scholar]
- 20.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil 1982;63(3): 118–123. [PubMed] [Google Scholar]
- 21.Wright J The Disability Rating Scale. The Center for Outcome Measurement in Brain Injury. http://www.tbims.org/combi/drs. Published 2000. [Google Scholar]
- 22.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007;24(2):329–337. [DOI] [PubMed] [Google Scholar]
- 23.Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AI. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol 2010;9(5):543–554. [DOI] [PubMed] [Google Scholar]
- 24.Mushkudiani NA, Engel DC, Steyerberg EW, et al. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007;24(2):259–269. [DOI] [PubMed] [Google Scholar]
- 25.Tokutomi T, Miyagi T, Ogawa T, et al. Age-associated increases in poor outcomes after traumatic brain injury: a report from the Japan Neurotrauma Data Bank. J Neurotrauma 2008;25(12):1407–1414. [DOI] [PubMed] [Google Scholar]
- 26.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008;5(8):e165; discussion e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005;57(6):1173–1182; discussion 1173–1182. [DOI] [PubMed] [Google Scholar]
- 28.Nelson DW, Nyström H, MacCallum RM, et al. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J Neurotrauma 2010;27(1):51–64. [DOI] [PubMed] [Google Scholar]
- 29.Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg 2010;113(3):556–563. [DOI] [PubMed] [Google Scholar]
- 30.Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT; American College of Radiology Head Injury Institute. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol 2015;36(2):E1–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.