Abstract
Background
While prospective clinical studies on immunotherapy in epidermal growth factor receptor (EGFR) mutant non‐small‐cell lung cancer (NSCLC) with acquired resistance to EGFR tyrosine kinase inhibitors (TKIs) are ongoing, this study aimed to investigate the outcomes of immunotherapy combinations in such a population in a real‐world setting.
Methods
The clinical data of pretreated EGFR‐mutated NSCLC patients who acquired EGFR‐TKI resistance and received immunotherapy were retrospectively analyzed in this study. Progression‐free survival (PFS) was assessed using the Kaplan‐Meier log‐rank test, and univariate and multivariate analysis were performed.
Results
A total of 31 patients were analyzed in this study. A total of 25 (80.6%) patients received combination immunotherapy. In the univariate analysis, patients who received combination immunotherapy seemingly acquired longer PFS than those who received monotherapy, although there was no significant difference (3.42 months vs. 1.61; P = 0.078; hazard ratio (HR) 0.43, 95% CI: 0.16–1.13). Patients who received antiangiogenic drugs prior to immunotherapy acquired better PFS (3.42 months vs. 1.58; P = 0.027; HR 0.37, 95% CI: 0.15–0.93), while patients with liver metastasis had inferior PFS (2.04 months vs. 3.42; P = 0.031; HR 2.83, 95% CI: 1.05–7.60). Furthermore, multivariate analysis confirmed that the above three factors had independent prognostic value.
Conclusions
The study revealed that immunotherapy combinations are better choices than single‐agent regimens in previously treated and EGFR‐mutant NSCLC patients with progressive disease. In addition, antiangiogenic drugs administered before immunotherapy might be a favorable prognostic factor, while liver metastasis was associated with a short PFS in this setting. In future, more robust and prospective clinical trial results are expected to guide clinical practice.
Key points
Significant study findings
Immunotherapy‐based combination therapies are better choices than single‐agent regimens in heavily treated EGFR‐mutant NSCLC patients.
What this study adds
Patients without liver metastasis and with prior antiangiogenic drugs obtained more benefit from immunotherapy in this setting.
Keywords: Progression‐free survival (PFS), EGFR positive, immunotherapy, non‐small‐cell lung cancer (NSCLC), overall response rate (ORR)

Immunotherapy‐based combination therapies are better choices than single‐agent regimens in heavily treated EGFR‐mutant NSCLC patients. Patients without liver metastasis who have previously received antiangiogenic drugs obtain more benefit from immunotherapy in this setting.
Introduction
Non‐small‐cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer cases and is one of the most common causes of cancer‐associated deaths worldwide. 1 , 2 Epidermal growth factor receptor (EGFR) gene mutation is the most common genetic alteration driving NSCLC. 3 , 4 EGFR tyrosine kinase inhibitors (TKIs), including gefitinib, erlotinib, icotinib, afatinib, dacomitinib and osimertinib, have been confirmed to be effective and well tolerated as first‐line therapy. 5 , 6 , 7 , 8 , 9 , 10 However, acquired resistance inevitably occurs in advanced NSCLC patients, despite an initial dramatic response to EGFR‐TKIs. At present, chemotherapy is still the main treatment for patients who fail to respond to first‐line EGFR‐TKIs without acquired T790M mutation and acquired resistance to third‐line EGFR‐TKIs. 11
Immunotherapy has become a cornerstone of management in advanced NSCLC without driver gene mutations. 12 , 13 , 14 Nevertheless, it has not showed efficacy in patients with EGFR‐mutated tumors. Many patients with EGFR mutations who received anti‐programmed death 1 (PD‐1) antibodies or anti‐PD‐L1 antibodies did not achieve a favorable outcome in multiple clinical and retrospective studies. 13 , 15 , 16 , 17 , 18 , 19 The potential mechanisms underlying these poor outcomes are the uninflamed phenotype of the microenvironment and the low immunogenicity of EGFR‐mutant lung cancer. 20 , 21 , 22 Preclinical trials suggest that chemotherapy or antiangiogenic therapy plus immunotherapy has synergistic effects by reducing the percentage of tumor‐infiltrating regulatory T cells and mediating the tumor microenvironment. 23 , 24 , 25 Promising data from the IMpower150 study has inspired the combination of chemotherapy, bevacizumab and immune checkpoint inhibitors (ICIs) as a salvage option for this patient group. 26 , 27 Other clinical trials of combination immunotherapy, such as Keynote 789 and Checkmate 722, are ongoing. Therefore, the current data on the combination of chemotherapy or antiangiogenic drugs and ICIs in such patients are limited.
Pending the outcomes of prospective clinical studies and proven therapeutic strategies in this setting, attention must turn toward the potential applicability of currently licensed drugs and any available supporting evidence in real‐world clinical practice. Therefore, this study aimed to investigate the outcomes of immunotherapy in pretreated and advanced NSCLC patients with EGFR mutations in the real‐world setting.
Methods
Patient data collection
This retrospective study included pretreated and advanced EGFR mutant NSCLC patients who received immunotherapy with or without other regimens (chemotherapy or antiangiogenic drugs) from September 2018 to June 2020 at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital. Pretreated and advanced EGFR‐mutated NSCLC patients were defined as failure to respond to EGFR‐TKIs and/or other systemic regimens. Three patients were excluded because they received combination immunotherapy as their first‐line regimen before EGFR gene mutations were detected. One patient was excluded for an unknown regimen (Figure S1 in Appendix S1). This study was approved by the ethics committee of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital.
The following demographics, clinical characteristics and treatment information were collected from medical records: sex, age at the primary diagnosis, smoking status, EGFR mutations, EGFR T790M status after resistance to the first‐line EGFR‐TKIs, PD‐L1 status, Eastern Cooperative Oncology Group performance status (ECOG PS), tumor stage according to the eighth edition of the American Joint Committee on Cancer Tumor‐Node‐Metastasis (AJCC‐TNM) staging system, brain and liver metastasis at immunotherapy onset; local therapy including radiotherapy and surgical operation, chemotherapy usage, targeted therapy and antiangiogenic drug usage, and number of prior systemic regimens before immunotherapy.
In general, imaging examinations at baseline included computed tomography (CT) images of the chest and abdomen, brain magnetic resonance imaging (MRI) and whole bone scans. Treatment efficacy evaluation was undertaken by CT of the chest and abdomen every two or three cycles during treatment. Brain MRI was also performed if deemed necessary. The response to therapies was evaluated as: complete response (CR); partial response (PR); stable disease (SD); and progressive disease (PD) according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Progression‐free survival (PFS) was defined as the period from the initiation of immunotherapy to the date of disease progression. The overall response rate (ORR) to ICIs was calculated as the percentage of patients achieving CR and PR on the basis of RECIST 1.1. The disease control rate (DCR) was defined as the proportion of patients achieving CR, PR, or SD based on the RECIST 1.1.
Molecular diagnostics
EGFR gene mutations were detected before the initiation of first‐ or second‐line EGFR‐TKI therapy and confirmed by next‐generation sequencing (NGS) technology or polymerase chain reaction (PCR). Testing specimens originated from the lung (n = 13), lymph nodes (n = 5), brain metastases (n = 2), blood (n = 8), pleural fluid (n = 1) and uncertain origin (n = 2). The EGFR T790M status was detected when patients failed to respond to first‐ or second‐line EGFR‐TKIs. PD‐L1 expression was locally measured by immunohistochemistry.
Statistical analysis
Baseline characteristics in this study were described by applying descriptive statistics. All calculations included calculation of the means ± 95% confidence intervals (95% CIs). Univariate analysis was performed, and survival was assessed using the Kaplan‐Meier log‐rank test. Proportions were compared between groups using the chi‐squared test, and when pertinent, a two‐sided Fisher's test was conducted. Follow‐up visits continued until 17 August 2020. All statistical analyses were performed using SPSS version 20.0 (IBM Corp, Armonk, NY). Tests were two‐sided, and a P‐value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 31 patients were enrolled into this study (Table 1). All patients had adenocarcinoma, and received anti‐programmed death‐1 (anti‐PD‐1) inhibitors (Table S1). The median (range) age of patients at primary diagnosis was 53 (31–83) years. A total of 19 of 31 (61.3%) patients were female. The performance status (PS) ranged from 0 to 2, with 32.3% of patients having a poor performance status (PS = 2) prior to the initiation of immunotherapy, and 23 of 31 (74.2%) patients were never smokers. Of these patients, 23 patients (74.2%) were diagnosed with stage IVc disease, 18 (41.9%) patients had brain metastasis, and seven (22.6%) patients had liver metastasis. Genetic testing identified EGFR 19del in 16 (51.6%) patients, EGFR 21L858R in 12 (38.7%) patients and other EGFR mutations (S768I/G719X/unknown) in three (9.7%) patients. Tumors had a positive EGFR T790M status in eleven (35.5%) patients, and the PD‐L1 status was positive in seven (22.6%) patients.
Table 1.
Baseline characteristics of advanced NSCLC patients with EGFR mutations
| Character | N (%) |
|---|---|
| Age, year | |
| Median (range) | 53 (31–83) |
| Gender | |
| Female | 19 (61.3%) |
| Male | 12 (38.7%) |
| ECOG PS | |
| 0 | 4 (12.9%) |
| 1 | 17 (54.8%) |
| 2 | 10 (32.3%) |
| Smoking status | |
| Never | 23 (74.2%) |
| Ever | 8 (25.8%) |
| Pathology | |
| Adenocarcinoma | 31 (100%) |
| Tumor stage | |
| IVA | 5 (16.1%) |
| IVB | 3 (9.7%) |
| IVC | 23 (74.2%) |
| Metastatic sites | |
| Number (median, range) | 3 (1–5) |
| Lymph nodes | 18 (58.1%) |
| Brain | 18 (41.9%) |
| Lung | 17 (54.8%) |
| Bone | 16 (51.6%) |
| Liver | 7 (22.6%) |
| Pleura | 11 (35.5%) |
| Adrenal glands | 4 (12.9%) |
| EGFR gene mutation | |
| EGFR 19del | 16 (51.6%) |
| EGFR 21L858R | 12 (38.7%) |
| Other | 3 (9.7%) |
| EGFR T790M status | |
| Positive | 11 (35.5%) |
| Negative | 15 (48.4%) |
| Not detected | 5 (16.1%) |
| PD‐L1 status | |
| ≥50% | 5 (16.1%) |
| 1–50% | 2 (6.5%) |
| Negative/unknown | 24 (77.4%) |
The line number of systemic regimens ranged from 1 to 9, with a median line number of four, prior to the initiation of ICIs. All patients received targeted therapy. More than half of patients received chemotherapy (74.2%, 23/31) or antiangiogenic drugs (74.2%, 23/31) before ICIs. A total of 11 (35.5%) patients received radiotherapy or surgery before ICIs. Six patients (19.4%) were treated with immunotherapy as a single agent. Approximately 25 of 31 (80.6%) patients received combination immunotherapy with other agents: osimertinib (1, 3.2%); chemotherapy (9, 29.0%); antiangiogenic drugs (9, 29.0%); chemotherapy and antiangiogenic drugs (6, 19.4%) (Table 2).
Table 2.
Immunotherapy therapy and clinical outcomes of advanced NSCLC patients with EGFR mutations
| Character | N (%) |
|---|---|
| Number of prior systemic regimens | |
| Median (range) | 4 (1–9) |
| Prior systemic therapy | |
| Targeted therapy | 31 (100%) |
| Chemotherapy | |
| No | 8 (25.8%) |
| Yes | 23 (74.2%) |
| Antiangiogenic therapy | |
| No | 8 (25.8%) |
| Yes | 23 (74.2%) |
| Local therapy | |
| No | 20 (64.2%) |
| Yes | 11 (35.5%) |
| Immunotherapy regimens | |
| Combination therapy | 25 (80.6%) |
| ICIs plus osimertinib | 1 (3.2%) |
| ICIs plus chemotherapy | 9 (29.0%) |
| ICIs plus antiangiogenic drugs | 9 (29.0%) |
| ICIs plus chemotherapy and antiangiogenic drugs | 6 (19.4%) |
| Monotherapy | 6 (19.4%) |
Univariate and multivariate analysis
The median follow‐up time was 11.24 (95% CI: 8.69–13.78) months. The median PFS (mPFS) was 3.25 (95% CI: 1.65–4.86) months, and the median overall‐survival (OS) was not reached. In the univariate analysis, patients who received combination immunotherapy therapy seemingly acquired longer PFS than those who received immunotherapy as a single agent, although there was no significant difference in terms of PFS (3.42 vs. 1.61 months; P = 0.078; hazard ratio (HR) 0.43, 95% CI: 0.16–1.13) (Fig 1a). Patients who received antiangiogenic drugs prior to immunotherapy acquired better PFS than those did not (3.42 vs. 1.58 months; P = 0.027; HR 0.37, 95% CI: 0.15–0.93) (Fig 1b). However, patients with liver metastasis obtained inferior PFS (2.04 vs. 3.42 months; P = 0.031; HR 2.83, 95% CI: 1.05–7.60) (Fig 1c). In addition, multivariate analysis confirmed that immune‐based combination regimens, prior antiangiogenic drugs and liver metastasis were independent prognostic factors of PFS in this setting (Table 3).
Figure 1.

The Kaplan‐Meier curve for progression‐free survival (PFS) in terms of (a) immunotherapy regimens ( ) single and (
) single and ( ) combination; (b) prior antiangiogenic drugs therapy; (
) combination; (b) prior antiangiogenic drugs therapy; ( ) No and (
) No and ( ) Yes and (c) liver metastasis (
) Yes and (c) liver metastasis ( ) Absent and (
) Absent and ( ) Present.
) Present.
Table 3.
Univariate and multivariate analysis
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Covariates | Comparisons | Median PFS in months (95% CI) | P‐value | P‐value | HR (95% CI) |
| Gender | Male vs. female | 1.70 (0.00–4.94) vs. 3.25 (1.65–4.86) | 0.820 | ||
| ECOG PS | 1 vs. 2 | 3.25 (0.00–6.61) vs. 1.81 (0.22–3.40) | 0.225 | ||
| Smoking status | Never vs. ever | 2.69 (0.99–4.40) vs. NR | 0.168 | ||
| Tumor stage | IVB vs. IVA | 1.38 (0.91–1.85) vs. 2.69 (1.42–3.96) | 0.452 | ||
| IVC vs. IVA | 3.42 (0.92–5.92) vs. 2.69 (1.42–3.96) | 0.857 | |||
| Brain metastasis | Present vs. absent | 2.69 (1.00–4.39) vs. 4.63 (0.45–8.82) | 0.397 | ||
| Liver metastasis | Present vs. absent | 2.04 (0.49–3.59) vs. 3.42 (0.88–5.96) | 0.031 | 0.041 | 2.96 (1.05–8.34) |
| EGFR gene mutation | EGFR L858R vs. EGFR 19del | 5.52 (0.00–11.24) vs. 2.04 (1.32–2.76) | 0.284 | ||
| EGFR T790M status | Positive vs. negative | 2.69 (1.20–4.19) vs. 3.42 (0.98–5.85) | 0.893 | ||
| PD‐L1 status | 1%–50% vs. ≥50% | NA vs. 6.14 (2.19–4.63) | 0.771 | ||
| Negative/unknown vs. ≥ 50% | 2.69 (0.76–4.63) vs. 6.14 (2.19–10.10) | 0.348 | |||
| Local therapy | Yes vs. no | 1.58 (1.33–1.83) vs. 2.10 (0.00–4.47) | 0.762 | ||
| Prior chemotherapy | Yes vs. no | 3.25 (1.22–5.28) vs. 2.10 (1.93–2.27) | 0.736 | ||
| Prior antiangiogenic drugs therapy | Yes vs. no | 3.42 (0.97–5.87) vs. 1.58 (0.80–2.35) | 0.027 | 0.023 | 0.27 (0.09–0.83) |
| Immunotherapy regimens | Combination therapy vs. monotherapy | 3.42 (1.94–4.90) vs. 1.61 (1.33–1.89) | 0.078 | 0.044 | 0.346 (0.12–0.97) |
Immunotherapy regimens and clinical outcomes
The swimmer plot of different therapeutic strategies in this setting is shown in Fig 2. No patients achieved partial response in the monotherapy cohort, and the DCR was 2/6 in this group. Among the combination groups, the ORR was 36.0% and the DCR was 64.0%. There was no significant difference between ICI monotherapy and immune‐based combination therapy in terms of ORR (P = 0.15) (Fig 3a,b). In the ICIs plus chemotherapy cohort, the ORR and the DCR was 5/9 and 6/9, respectively. In the ICIs plus antiangiogenic drugs group, the ORR and the DCR was 2/9 and 6/9, respectively. The ORR was 2/6 and the DCR was 4/6 in patients receiving ICIs plus chemotherapy and antiangiogenic drugs. No significant difference was analyzed among three different combination strategies in terms of ORR (ICIs plus antiangiogenic drugs vs. ICIs plus chemotherapy and antiangiogenic inhibitors, P = 1.00; ICIs plus chemotherapy vs. ICIs plus chemotherapy and antiangiogenic inhibitors, P = 0.61; Fig 3c,d).
Figure 2.
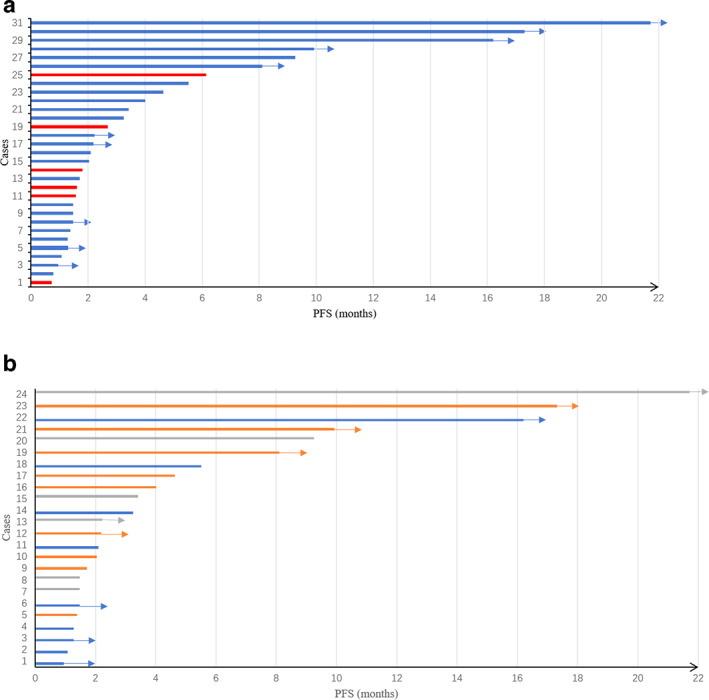
The swimmer plot of progression‐free survival (PFS) from the initiation of immunotherapy according to (a) the entire cohort; ( ) Combination therapy and (
) Combination therapy and ( ) monotherapy and (b) different combination regimens (
) monotherapy and (b) different combination regimens ( ) ICIs plus chemotherapy and antiangiogenic drugs, (
) ICIs plus chemotherapy and antiangiogenic drugs, ( ) ICIs plus chemotherapy, and (
) ICIs plus chemotherapy, and ( ) ICIs plus antiangiogenic drugs.
) ICIs plus antiangiogenic drugs.
Figure 3.
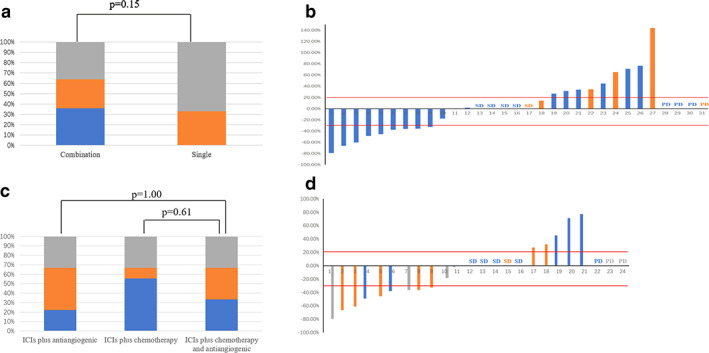
The best response of enrolled patients received immunotherapy. (a) The overall response rate (ORR) was not significant different between immunotherapy as single regimens and combination regimens ( ) PR, (
) PR, ( ) SD, and (
) SD, and ( ) PD. (b) The best response rate of all enrolled patients with immunotherapy is illustrated (
) PD. (b) The best response rate of all enrolled patients with immunotherapy is illustrated ( ) combination therapy, and (
) combination therapy, and ( ) monotherapy. (c) The ORR was not statistically different among three different combination immunotherapy regimens. (d) The best response rate of all enrolled patients with combination immunotherapy is illustrated (
) monotherapy. (c) The ORR was not statistically different among three different combination immunotherapy regimens. (d) The best response rate of all enrolled patients with combination immunotherapy is illustrated ( ) ICIs plus antiangiogenic, (
) ICIs plus antiangiogenic, ( ) ICIs plus chemotherapy, and (
) ICIs plus chemotherapy, and ( ) ICIs plus chemotherapy and antiangiogenic drugs.
) ICIs plus chemotherapy and antiangiogenic drugs.
Intracranial response to ICIs
There were 18 patients diagnosed with brain metastasis prior to the initiation of ICIs. Three and 15 patients received single agent and combination therapies, respectively. The best intracranial response to ICIs was SD. The DCR in terms of intracranial response was in two of three patients who had received immunotherapy as a single agent and 60% (9/15) in the combination group, respectively.
Safety
Four patients (12.9%) experienced grade 1 to grade 2 immune‐related adverse events, including one patient receiving single‐agent therapy who had a grade 1 rash, one patient who received ICI combination therapy and antiangiogenic drugs who had grade 2 hypothyroidism, and two patients receiving combination immunotherapy with chemotherapy who had a grade 2 rash.
Discussion
This retrospective study provided evidence on the efficacy of immunotherapies in advanced EGFR‐mutated NSCLC patients who failed to respond to EGFR‐TKIs and multiple conventional systemic therapies in a real‐world setting. Immunotherapy‐based combination therapy was a favorable prognostic factor, and furthermore, in addition to the results of previous studies, this study showed the PFS benefit of anti‐PD‐1
inhibitors when
combined with other agents.
ICI monotherapy has been reported to have a reduced efficacy in heavily treated EGFR mutant NSCLC patients who have failed to respond to EGFR‐TKIs. However, the efficacy of immunotherapy combination therapy has been under debate until now. Previous studies have demonstrated that the PFS of ICI monotherapy in patients ranged from 1.6 to 1.9 months in this population, 19 , 28 which is similar to the finding in our monotherapy cohort. Furthermore, using ICI monotherapy in patients with EGFR mutant NSCLC who failed to respond to EGFR‐TKIs and without acquired T790M mutation is not recommended in the clinical practice guidelines of the European Society for Medical Oncology (ESMO). 29 However, rare data on ICI‐based combination therapies has been reported and the guidelines regarding combined immunotherapy in this setting remains unclear. Previous mainstream systematic regimen in this setting was conventional chemotherapy. In the AURA3 study, the median PFS and ORR of the platinum plus pemetrexed group was reported to be 4.4 months and 31%, respectively. 30 Although there were more heavily‐treated patients in our study, the median PFS of immunotherapy‐based combination therapy in our study was comparable to the efficacy of platinum‐based chemotherapy in the AURA3 study. The ABCP (atezolizumab plus platinum‐based chemotherapy and bevacizumab) arm in the IMpower150 study is the first and only reported example of a survival benefit for ICI‐based combination therapy in EGFR‐mutant NSCLC patients who failed to respond to prior treatment with TKIs, achieving an ORR of 72% and a median OS of 19.4 months. 27 The ORR of 33.3% (2/6) in a four‐drug combination regimen in our study was obviously lower than that of previously reported data. The difference might be explained by the limited population size and relatively heavily treated patients in this study. The synergistic activities of immunotherapy with chemotherapy or antiangiogenic agents have been confirmed in preclinical studies, 31 and relevant prospective clinical trials; for example Keynote 789 (NCT03515837), and Checkmate 722 (NCT02864251), are presently under investigation. In the meantime, several immunotherapy‐based combination regimens illustrated in our study have been utilized in the real‐world. Qiu et al. recently reported a median PFS of 5.4 months in EGFR‐mutant patients who received ICIs plus antiangiogenic drugs; however they did not provide a specific description of this setting. 32 This study, which complements existing studies, highlighted that immunotherapy, especially anti‐PD‐1, combined with different agents benefited pretreated EGFR‐mutated NSCLC patients in a real‐world setting.
Our study also found that patients with liver metastasis had an inferior PFS. The underlying mechanism might be explained by the highly immunosuppressive liver microenvironment. 33 , 34 In addition, several studies have previously reported shorter OS in advanced NSCLC patients with liver metastasis than those without it, while receiving ICI as a single agent. 27 , 35 , 36 , 37 , 38 Although many prospective clinical studies enrolled some patients with liver metastasis, few studies have referred to the efficacy of immunotherapy combination regimens in advanced lung cancer patients with liver metastasis. The subgroup analysis in the IMpower150 study demonstrated that improved median OS with ABCP regimens was observed in patients with liver metastasis. 27 Considering the poor prognosis, patients with liver metastasis might need more aggressive and combined therapy to control their disease. This study revealed that patients with antiangiogenic drugs prior to immunotherapy obtained PFS benefit. Although few studies have previously reported this phenomenon, it has been speculated that tumor vascular normalization after use of antiangiogenic agents might potentially enhance the efficacy of subsequent treatments. 39
Some limitations in this study should be considered. First, heterogenous treatment options in this study were due to its retrospective nature and limited sample size, and therefore the results should be interpreted with caution. Second, the median overall survival was not reached, as we were unable to conclude whether combined immunotherapy would lead to long‐term survival. In addition, some details regarding the detection kit used to evaluate PD‐L1 expression were unavailable. We failed to detect the EGFR T790M gene beyond first‐ or second‐line EGFR‐TKI resistance in a small number of patients due to unavailable or inadequate tissue biopsy specimens.
In conclusion, the findings of our study indicated that ICI‐based combination therapies might be more beneficial than ICI monotherapy in pretreated and EGFR‐mutant NSCLC patients with progressive disease. This study provided more evidence on anti‐PD‐1 inhibitors in combination with different regimens in this setting. In addition, prior antiangiogenic drugs before immunotherapy might be a favorable prognostic factor, while liver metastasis was associated with a short PFS in such patients. More robust clinical trial results should be performed in order to guide clinical practice in the future.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Appendix S1: Supporting information.
References
- 1. Li S, Li L, Zhu Y et al Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: A comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer 2014; 110 (11): 2812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136 (5): E359–86. [DOI] [PubMed] [Google Scholar]
- 3. She J, Yang P, Hong Q, Bai C. Lung cancer in China: Challenges and interventions. Chest 2013; 143 (4): 1117–26. [DOI] [PubMed] [Google Scholar]
- 4. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non‐small‐cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015; 5 (9): 2892. [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y‐L, Cheng Y, Zhou X et al Dacomitinib versus gefitinib as first‐line treatment for patients with EGFR‐mutation‐positive non‐small‐cell lung cancer (ARCHER 1050): A randomised, open‐label, phase 3 trial. Lancet Oncol 2017; 18 (11): 1454–66. [DOI] [PubMed] [Google Scholar]
- 6. Mok TS, Wu Y‐L, Thongprasert S et al Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361 (10): 947–57. [DOI] [PubMed] [Google Scholar]
- 7. Wu Y‐L, Zhou C, Liam C‐K et al First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: Analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol 2015; 26 (9): 1883–9. [DOI] [PubMed] [Google Scholar]
- 8. Yang JC‐H, Wu Y‐L, Schuler M et al Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16 (2): 141–51. [DOI] [PubMed] [Google Scholar]
- 9. Soria J‐C, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non–small‐cell lung cancer. N Engl J Med 2018; 378 (2): 113–25. [DOI] [PubMed] [Google Scholar]
- 10. Shi Y, Zhang L, Liu X et al Icotinib versus gefitinib in previously treated advanced non‐small‐cell lung cancer (ICOGEN): A randomised, double‐blind phase 3 non‐inferiority trial. Lancet Oncol 2013; 14 (10): 953–61. [DOI] [PubMed] [Google Scholar]
- 11. Ramalingam SS, Vansteenkiste J, Planchard D et al Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med 2019; 382 (1): 41–50. [DOI] [PubMed] [Google Scholar]
- 12. Mok TS, Wu Y‐L, Kudaba I et al Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): A randomised, open‐label, controlled, phase 3 trial. Lancet 2019; 393 (10183): 1819–30. [DOI] [PubMed] [Google Scholar]
- 13. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med 2015; 373 (17): 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brahmer JR, Rodríguez‐Abreu D, Robinson AG et al Health‐related quality‐of‐life results for pembrolizumab versus chemotherapy in advanced, PD‐L1‐positive NSCLC (KEYNOTE‐024): A multicentre, international, randomised, open‐label phase 3 trial. Lancet Oncol 2017; 18 (12): 1600–9. [DOI] [PubMed] [Google Scholar]
- 15. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389 (10066): 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herbst RS, Baas P, Kim D‐W et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387 (10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 17. Gainor JF, Shaw AT, Sequist LV et al EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non–small cell lung cancer: A retrospective analysis. Clin Cancer Res 2016; 22 (18): 4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee CK, Man J, Lord S et al Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: A systematic review and meta‐analysis. JAMA Oncol 2018; 4 (2): 210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hastings K, Yu H, Wei W et al EGFR mutation subtypes and response to immune checkpoint blockade treatment in non‐small‐cell lung cancer. Ann Oncol 2019; 30 (8): 1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong Z‐Y, Zhang J‐T, Liu S‐Y et al EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD‐1 blockade in non‐small cell lung cancer. Onco Targets Ther 2017; 6 (11): e1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature 2017; 541 (7637): 321–30. [DOI] [PubMed] [Google Scholar]
- 22. Jia Y, Li X, Jiang T et al EGFR‐targeted therapy alters the tumor microenvironment in EGFR‐driven lung tumors: Implications for combination therapies. Int J Cancer 2019; 145 (5): 1432–44. [DOI] [PubMed] [Google Scholar]
- 23. Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: The right path to achieve cancer cure? Ann Oncol 2015; 26 (9): 1813–23. [DOI] [PubMed] [Google Scholar]
- 24. Zhang P, Ma Y, Lv C et al Upregulation of programmed cell death ligand 1 promotes resistance response in non‐small‐cell lung cancer patients treated with neo‐adjuvant chemotherapy. Cancer Sci 2016; 107 (11): 1563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manegold C, Dingemans A‐MC, Gray JE et al The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 2017; 12 (2): 194–207. [DOI] [PubMed] [Google Scholar]
- 26. Socinski MA, Jotte RM, Cappuzzo F et al Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378 (24): 2288–01. [DOI] [PubMed] [Google Scholar]
- 27. Reck M, Mok TS, Nishio M et al Atezolizumab plus bevacizumab and chemotherapy in non‐small‐cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open‐label phase 3 trial. Lancet Respir Med 2019; 7 (5): 387–401. [DOI] [PubMed] [Google Scholar]
- 28. Yamada T, Hirai S, Katayama Y et al Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR‐mutated non‐small cell lung cancer. Cancer Med 2019; 8 (4): 1521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Y‐L, Planchard D, Lu S et al Pan‐Asian adapted clinical practice guidelines for the management of patients with metastatic non‐small‐cell lung cancer: A CSCO–ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol 2019; 30 (2): 171–210. [DOI] [PubMed] [Google Scholar]
- 30. Mok TS, Wu Y‐L, Ahn M‐J et al Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med 2016; 376 (7): 629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y, Yuan J, Righi E et al Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci 2012; 109 (43): 17561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiu L, Zhao X, Shi W et al Real‐world treatment efficacy of anti‐programmed death‐1 combined with anti‐angiogenesis therapy in non‐small cell lung cancer patients. Medicine 2020; 99 (24): e20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tagliamonte M, Petrizzo A, Tornesello ML, Ciliberto G, Buonaguro FM, Buonaguro L. Combinatorial immunotherapy strategies for hepatocellular carcinoma. Curr Opin Immunol 2016; 39: 103–13. [DOI] [PubMed] [Google Scholar]
- 34. Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM. Challenges in cancer vaccine development for hepatocellular carcinoma. J Hepatol 2013; 59 (4): 897–903. [DOI] [PubMed] [Google Scholar]
- 35. Sridhar S, Paz‐Ares L, Liu H et al Prognostic significance of liver metastasis in durvalumab‐treated lung cancer patients. Clin Lung Cancer 2019; 20 (6): e601–8. [DOI] [PubMed] [Google Scholar]
- 36. Tumeh PC, Hellmann MD, Hamid O et al Liver metastasis and treatment outcome with anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5 (5): 417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vokes EE, Ready N, Felip E et al Nivolumab versus docetaxel in previously treated advanced non‐small‐cell lung cancer (CheckMate 017 and CheckMate 057): 3‐year update and outcomes in patients with liver metastases. Ann Oncol 2018; 29 (4): 959–65. [DOI] [PubMed] [Google Scholar]
- 38. Funazo T, Nomizo T, Kim YH. Liver metastasis is associated with poor progression‐free survival in patients with non–small cell lung cancer treated with nivolumab. J Thorac Oncol 2017; 12 (9): e140–1. [DOI] [PubMed] [Google Scholar]
- 39. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol 2018; 15 (5): 325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information.


