Abstract
Background
Pulmonary sarcomatoid carcinoma (PSC) is rare with a poor outcome and is resistant to conventional cytotoxic chemotherapy. The efficacy and safety of durvalumab and tremelimumab for treating recurrent or metastatic PSCs were assessed by a nonrandomized, open‐label, phase II study.
Methods
A total of 18 patients with recurrent or metastatic PSC received 1500 mg of durvalumab and 75 mg of tremelimumab every four weeks, followed by 750 mg of durvalumab every two weeks until the disease progressed, or an unacceptable toxicity level was reached. The primary endpoint was the objective response rate (ORR). The secondary endpoints were progression‐free survival (PFS), overall survival (OS), and toxicity. Genomic profiling of PSC by next‐generation sequencing (NGS) and determination of peripheral blood lymphocyte subsets using flow cytometry were performed for exploratory analysis.
Results
A total of 15 out of 18 patients were evaluated for the analysis of the primary endpoint. At the data cutoff point, the ORR of 26.7% (95% confidence interval [CI]: 7.8–55.1) was achieved with the median follow‐up duration of 12.0 months (range, 8.4–16.1). Median PFS and OS were 5.9 months (95% CI: 1.1–11.9) and 15.4 months (95% CI: 11.1‐not reached), respectively. Treatment‐related adverse events (AEs) of any grade were reported in 16 patients; the most common AEs were pruritus (n = 5), pneumonitis (n = 4), and rash (n = 4). Treatment was discontinued in two patients due to AEs of grade ≥ 3.
Conclusions
Durvalumab and tremelimumab demonstrated clinical benefit with a prolonged survival and manageable toxicity profile in patients with recurrent or metastatic PSC.
Keywords: Durvalumab, immunotherapy, non‐small cell lung cancer, pulmonary sarcomatoid carcinoma, tremelimumab
This first prospective phase II trial for recurrent or metastatic pulmonary sarcomatoid carcinomas demonstrated that durvalumab and tremelimumab was effective with a confirmed ORR of 26.7%. The combination of durvalumab and tremelimumab was well‐tolerated with favorable toxicity profiles.
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare type of lung malignancy with poorly differentiated characteristics, accounting for less than 1% of all lung cancers. 1 , 2 It occurs mainly in elderly men and is associated with a heavy smoking history. 3 , 4 , 5 Its clinical course is aggressive and a poor prognosis is observed, even in the early phases of the disease. 6 These tumors are also highly resistant to conventional cytotoxic chemotherapy. 7 It has been previously reported that the response rate of conventional cytotoxic chemotherapy used for non–small cell lung cancer (NSCLC) was 0%–16.5% in PSC and the median overall survival (OS) was only 5–6.3 months. 7 , 8 Thus, novel therapeutic strategies are desperately needed.
Immunotherapy has risen to the forefront as the most rapidly evolving treatment strategy in oncology. Immune checkpoint inhibitors (ICIs) have shown notable success in several clinical trials involving multiple tumor types including NSCLC. 9 , 10 , 11 Durvalumab (MEDI4736) is a selective human immunoglobulin (Ig) G1 monoclonal antibody (mAb) against programmed death ligand 1 (PD‐L1). Tremelimumab is a selective human IgG2 mAb binding to cytotoxic T‐lymphocyte–associated antigen‐4 (CTLA‐4). An early phase study reported the clinical efficacy of durvalumab plus tremelimumab treatment with its safety profile manageable regardless of PD‐L1 expression level. 12
Given that PSC shows high positivity rate of PD‐L1 up to 90.2% and exhibits strong immune‐cell infiltration, 13 , 14 , 15 it is reasonable to apply ICIs in PSC. However, ICIs have not been explored prospectively in PSC. Here, we performed a phase II study (NCT03022500) to investigate the efficacy and safety of the combined treatment of durvalumab plus tremelimumab in patients with recurrent or metastatic PSC.
Methods
Study design and patients
This was an open‐label and multicenter single‐arm phase II study in patients with recurrent or metastatic PSC. The histology of PSC was classified as sarcomatoid carcinoma, spindle cell carcinoma, giant cell carcinoma, pleomorphic carcinoma, carcinosarcoma, and pulmonary blastoma on the basis of the World Health Organization classification. 16 Patients with recurrent or metastatic PSC were eligible if the following criteria were met: ≥ 20 years of age; patients had not received any, or prior lines of chemotherapy or targeted therapies; had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; and had a minimum of one measureable lesion using the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1. Patients with brain metastases were eligible if they had no symptoms or were neurologically stable without administration of steroids after surgery or radiotherapy. Patients were not eligible if they had a history of carcinomatous meningitis, had an active autoimmune disease for the last two years, were receiving systemic steroid therapy or other form of immunosuppressive treatment, or had been on anti‐PD‐1, –PD‐L1, or –CTLA‐4 mAb treatments. This study received the approval of the Institutional Review Board of each participating center and was performed in compliance with the protocol, good clinical practice guidelines, and the Declaration of Helsinki. Informed consents were acquired from patients prior to study entry.
Treatments and assessments
Patients received 1500 mg of durvalumab and 75 mg of tremelimumab; both were administered intravenously every four weeks for up to four cycles, followed by 750 mg of durvalumab every two weeks. Treatment was continued until investigator‐assessed and confirmed progression, intolerable toxicities, withdrawal of consent, or a total of 18 cycles had been reached.
The modified RECIST version 1.1 17 was used to evaluate tumor response every eight weeks. Once radiological progression was initially identified, the study treatment was continued on patients who were clinically stable until the confirmation of progression which was a minimum of four weeks later. Patients administered at least one cycle of study treatment were eligible for safety evaluation. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Immunohistochemistry of PD‐L1
Immunohistochemistry analysis of PD‐L1 was done on pretreatment formalin‐fixed paraffin‐embedded tumor tissues using the SP263 assay (Ventana Medical Systems, Tucson, AZ, United States) or the 22C3 pharmDx assay (Dako, Carpinteria, CA, United States) according to the manufacturer's recommendations. The expression of PD‐L1 was evaluated by the percentage of tumor cells with membranous staining of PD‐L1 18 and assessed by pathologists in a blinded fashion.
Fluorescence‐activated cell sorting (FACS)
Freshly collected whole blood was labeled at room temperature for 15 minutes with the following fluorescence‐conjugated mAbs: BD Multitest CD3 FITC/CD8 PE/CD45 PerCP/CD4 APC and BD Multitest CD3 FITC/CD16 + CD56 PE/CD45 PerCP/CD19 APC (BD Biosciences, San Jose, CA, United States). To remove red blood cells, FACS lysis solution (BD Biosciences, San Jose, CA, United States) was added and incubated at room temperature for 15 minutes. A FACSCalibur flow cytometer and CellQuest software (BD Biosciences, San Jose, CA, United States) was used to acquire and analyze the data.
Next‐generation sequencing
Formalin‐fixed paraffin‐embedded (FFPE) tumor tissues from 12 patients were deeply sequenced with a next‐generation sequencing (NGS) panel developed by Seoul National University Hospital (SNUH) FIRST Lung Cancer Panel (LCP), which comprised 64 lung cancer‐related target genes. 19
Statistical analysis
Overall response rate (ORR) by investigator review was the primary endpoint. All patients receiving any study treatment were eligible to be evaluated for a response. A null hypothesis of 5% ORR and alternative hypothesis of 30% ORR were established. To prove the hypothesis, Simon optimal 2‐stage design was used with a one‐sided type I error of 5% and power of 80%. If one or more responses occurred in the first five patients in the first stage, then the study would proceed to the second stage. At the end of the study, it was considered positive if at least three responses were observed out of 18 patients. OS, progression‐free survival (PFS), safety, and biomarker analysis were secondary endpoints. PFS and OS were estimated using the Kaplan‐Meier method.
Results
Patient characteristics
A total of 18 patients with a diagnosis of PSC were enrolled between May 2017 and February 2018. Baseline characteristics are described in Table 1. The median age of 12 men and six women was 61.5 years (range, 44–78) and nearly 60% of patients had a smoking history. The PD‐L1 expression was evaluated in 14 patients. Nine of 14 patients (64.3%) included had ≥50% PD‐L1 expression. A total of 12 patients (67%) had received prior cytotoxic chemotherapy for PSC; nine (50%) of 18 patients had received only one line of prior cytotoxic chemotherapy; and two patients (11.1%) had received two lines of prior cytotoxic chemotherapy.
Table 1.
Baseline characteristics of the patients (N = 18)
| Characteristics | No. of patients (%) | |
|---|---|---|
| Median age, years (range) | 62 (44–78) | |
| Sex | Male | 12 (66.7) |
| Female | 6 (33.3) | |
| ECOG performance status | 0 | 9 (50.0) |
| 1 | 9 (50.0) | |
| PD‐L1 expression † |
High (≥ 50%) Low (< 50%) |
9 (64.3) 5 (35.6) |
| Brain metastases |
Yes No |
3 (16.7) 15 (83.3) |
| Smoking status | Current smoker | 1 (5.6) |
| Former smoker | 10 (55.6) | |
| Never smoker | 7 (38.9) | |
| Type of prior therapy ‡ | Surgery | 10 (55.6) |
| Radiotherapy | 4 (22.2) | |
| Chemotherapy | 12 (66.7) | |
| Lines of prior chemotherapy § | 0 | 7 (38.9) |
| 1 | 8 (44.4) | |
| 2 | 3 (17.7) | |
PD‐L1 data were not available for four patients due to lack of tumor tissues.
Includes adjuvant treatment.
Only given in advanced diseases.
ECOG, Eastern Cooperative Oncology Group.
Response and survival outcome
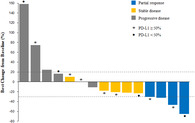
All 18 patients who had received at least one cycle of study treatment were eligible for the efficacy and safety analyses. Three patients did not undergo tumor response assessment. While partial responses were achieved in four patients, no complete response occurred. Thus the primary endpoint of the study was met. The ORR was 26.7% (95% confidence interval [CI]: 7.8–55.1) with the disease control rate of 60.0% (95% CI: 32.3–83.7) (Table 2). The amount of tumor shrinkage of the target lesion from baseline for individual patients is shown in Fig 1a,b. Tumor response was not significantly correlated with the PD‐L1 expression. The median duration of response was 10.3 months (95% CI: 7.8–10.3). The responses were durable and two patients still showed ongoing responses at the data analysis point (Fig 1c).
Table 2.
Efficacy results (investigator‐assessed) in the total population
| Efficacy | No. of patients (%) |
|---|---|
| Best overall response | |
|
Complete response Partial response |
0 (0.0) 4 (26.7) |
|
Stable disease Progressive disease |
5 (33.3) 6 (40.0) |
| Not evaluable | 3 (16.7) |
| Median PFS, months (95% CI) | 5.9 (1.1–11.9) |
| Median OS, months (95% CI) | 15.4 (11.1–not reached) |
OS, overall survival; PFS, progression‐free survival.
Figure 1.
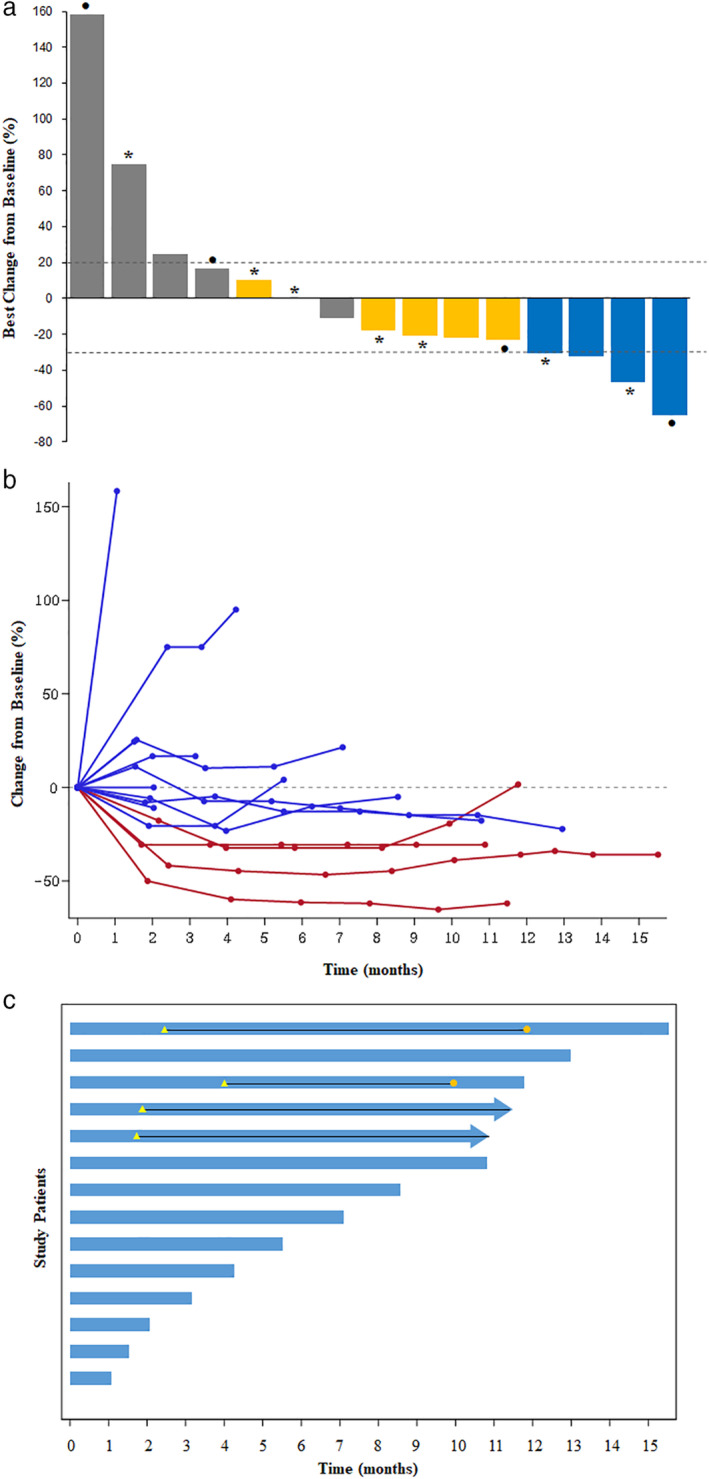
Response to durvalumab plus tremelimumab and treatment duration. (a) Waterfall plot of maximum percent change in tumor size from baseline as assessed by the investigators. (b) Spider plot of change in tumor size from baseline over time. (c) Swimmer's plot of time from start of treatment to time of last treatment.  , Partial response;
, Partial response;  , Stable disease;
, Stable disease;  , Progressive disease;
, Progressive disease;  , PD‐L1 ≥ 50%;
, PD‐L1 ≥ 50%;  , PD‐L1 < 50%;
, PD‐L1 < 50%;  , PR;
, PR;  , SD/PD;
, SD/PD;  , Response start;
, Response start;  , Response end;
, Response end;  , Partial Response.
, Partial Response.
At the data cutoff of 27 February 2019, the median follow‐up duration was 12.0 months (95% CI: 8.4–16.1). Median PFS and OS were 5.9 months (95% CI: 1.1–11.9) and 15.4 months (95% CI: 11.1‐not reached), respectively (Table 2). The Kaplan‐Meier curves of PFS and OS of the evaluated patients are shown in Fig 2a,b, respectively.
Figure 2.
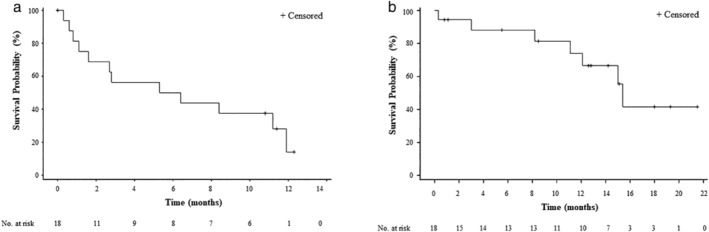
Kaplan‐Meier curve for progression‐free survival (a) and overall survival (b).
Safety
AEs of any grade, regardless of attribution, were reported in 16 patients with 84 AEs and one serious AE. The worst grades of treatment‐related AEs (TEAEs) per patient are shown in Table 3. The most frequently occurring TEAEs of any grade were pruritus (n = 4), rash (n = 4), pneumonitis (n = 3), and amylase elevation (n = 3). TEAEs of grade 3 or higher occurred in two patients. One patient had grade 4 of lipase and grade 3 of amylase elevation, respectively. This led to discontinuation of durvalumab and tremelimumab treatment. The other patient experienced grade 3 pneumonitis and grade 3 pruritus, which also led to study treatment discontinuation. Overall, grade 3 and 4 AEs were managed using the standard guidelines and the patients fully recovered. Seven deaths were reported during the study. However, none were related to the treatment, and occurred as a result of disease progression.
Table 3.
Treatment‐related adverse events (N = 18)
| Adverse event | Any grade | Grade ≥ 3 |
|---|---|---|
| No. of patients (%) | ||
| Anemia | 1 (5.6) | 0 |
| Arthralgia | 1 (5.6) | 0 |
| Nausea | 1 (5.6) | 0 |
| Diarrhea | 1 (5.6) | 0 |
| Amylase increased | 3 (16.7) | 1 (5.6) |
| Lipase increased | 2 (11.1) | 1 (5.6) |
| AST increased | 1 (5.6) | 0 |
| Hyperthyroidism | 1 (5.6) | 0 |
| Hypothyroidism | 1 (5.6) | 0 |
| Dyspnea | 1 (5.6) | 0 |
| Pneumonitis | 3 (16.7) | 1 (5.6) |
| Pruritus | 4 (22.2) | 1 (5.6) |
| Rash | 4 (22.2) | 0 |
Genomic profiling
At the data analysis point, pretreatment tumor tissues (archival or prior to first dosing) were obtained from 12 patients that were eligible for exploring the potential molecular biomarkers via NGS. The mutational analysis results are summarized in Fig 3. The most commonly occurring genetic mutations occurred in the genetic loci such as TP53, KRAS, and PIK3CA in eight, three, and three patients, respectively. The MET exon 14 skipping mutation and BRAF K601E mutation were also observed in a single case each. However, no single cases of EGFR mutations or ALK translocation were detected in this study. Notably, this mutational analysis also identified a single case of JAK2 A597fs mutation known to affect the downstream signaling of the interferon gamma receptor pathway, which interrupts PD‐L1 expression and leads to primary resistance to ICIs. The patient with the JAK2 inactivating mutation showed no tumor response with very rapid disease progression.
Figure 3.
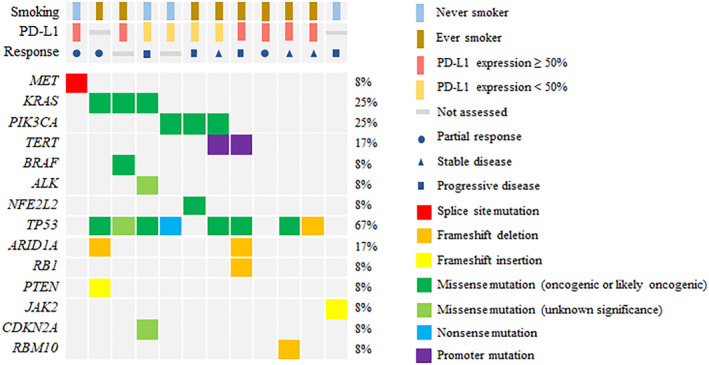
Integrated clinical and genomic profiling data (N = 12). Each column represents one patient.
Circulating lymphocyte subsets
Whole blood from 14 patients with and without clinical benefit was analyzed to compare the composition of circulating lymphocyte subsets. Clinical benefit was defined as partial response or stable disease of more than six months. Patients with clinical benefit (n = 7) had higher proportions of circulating CD8+ T cells (28.4% vs. 19.0%, P = 0.051) and lower CD4+ to CD8+ T‐cell ratio (1.6 vs. 2.6, P = 0.116) in pretreatment blood than the ones without clinical benefit (n = 7) (Fig S1).
Discussion
In this study, durvalumab and tremelimumab demonstrated antitumor activity in recurrent or metastatic PSC and met the primary endpoint of the prespecified ORR. To the best of our knowledge, this is the first prospective trial of PSC and the first positive trial of PSC. Although a retrospective study of 39 patients with PSC demonstrating an ORR of 38.5% with ICI alone has been previously reported, 20 ICI combinations have not been explored in PSC. The combined treatment of durvalumab and tremelimumab was effective with an ORR of 26.7%. The median PFS and OS of the durvalumab and tremelimumab regimen in previous studies were reported to be much longer than those of cytotoxic chemotherapy in this clinical setting. 8 , 21 Furthermore, the median duration of response was 10.3 months with the durable response of two patients still having an ongoing response at the data cutoff point. This is in contrast with the relatively short duration of response observed with cytotoxic chemotherapies. Thus, the clinical benefit achieved with this regimen is greater than that of conventional chemoagents obtained in patients with PSC.
The safety profile of durvalumab and tremelimumab reported in this study was as expected based on the known data about AEs reported from ICIs with no new safety signals identified. All AEs were manageable and consistent with those previously reported in other tumor types. 12 , 22 , 23 The discontinuation rate due to AEs was low with only two patients reported during the study.
High immune‐cell infiltration has been described in PSC, which indicates the existence of strong antitumor immune responses. 15 However, this malignancy has a poor outcome compared with other histological subtypes. Enhanced PD‐L1 level might explain this conflict. In agreement with this, our data demonstrated that 64% of patients expressed a high level of PD‐L1 (≥50% positivity). This approximately corresponds to the range of 53% to 90.2% in PSC as previously reported in other studies. 13 , 14 , 15 The therapeutic resistance of PSC might be also explained by other immune inhibitory mechanisms including inhibitory immune cells (regulatory T cells and myeloid‐derived suppressor cells), cytokines, and expression of other immune checkpoint molecules. Further study is needed to delineate the underlying mechanisms of therapeutic resistance in this type of malignancy.
In contrast to common subtypes of lung cancer extensively studied for the presence of targetable genetic alterations, little data is available on PSC. A total of 12 cases of PSC in our study were tested for genetic‐alterations in 64 genes. Of these, 11 out of 12 cases had at least one genetic alteration. Consistent with a previous study, 24 the majority of cases (eight cases, 66.7%) had TP53 mutations and three cases (25%) had KRAS and PIK3CA mutations. One patient harbored a MET exon 14 skipping mutation and another patient with a KRAS hotspot mutation harbored a concurrent non‐V600E BRAF mutation, as reported previously. 25 , 26 Therefore, our NGS analysis for these patients suggests that PSC has a similar genotype to that of high‐grade adenocarcinoma found in smokers. Of note, A JAK2 inactivating mutation was identified in the tumor of a patient who did not respond to durvalumab and showed progressive disease. When the JAK2 is inactivated, PD‐L1 expression is inhibited through interruption of downstream signaling of the interferon gamma receptor pathway. 27 , 28 This implies that JAK2 inactivation might be associated with primary resistance to anti‐PD‐1/PD‐L1 inhibitors.
PD‐L1 expression and circulating lymphocyte sets were analyzed to find biomarkers for treatment prediction, but there was no statistically significant finding. This could be due to an insufficient number of patients being analyzed. Appropriate selection of patients with validated biomarkers remains the key to success.
This study has several limitations. First, this trial enrolled a heterogeneous patient population with different types of previous therapies and a range of previous lines of chemotherapy. Second, as this was not a randomized trial, direct comparisons of durvalumab and tremelimumab with conventional chemotherapy or anti‐PD‐1/PD‐L1 inhibitor monotherapy were not possible. Third, sample size was relatively small due to the rare incidence of PSC. However, this study was the first prospective trial of PSC and met the prespecified ORR primary endpoint.
In conclusion, durvalumab and tremelimumab combination therapy has demonstrated promising efficacy with a favorable toxicity profile in the treatment of PSC. Given this antitumor activity in this patient population, further randomized studies with durvalumab plus tremelimumab should be considered for patients with PSC.
Disclosure
Bhumsuk Keam received research funding from MSD (Merck Sharp & Dohme Corp) MSD and Ono Pharmaceutical Co., Ltd. and served as an advisor for AstraZeneca, MSD, and Genexine outside of the current work. Dong‐Wan Kim received grants from AstraZeneca/MedImmune, outside the submitted work. Tae Min Kim received research grants from AstraZeneca ‐KHIDI, outside the submitted work. All remaining authors have declared no conflicts of interest.
Supporting information
Figure S1. Comparison of pretreatment lymphocyte subsets in blood between patients with clinical benefit (n = 7) and those without clinical benefit (n = 7). Clinical benefit was defined as partial response or stable disease of more than six months. Error bars represent standard deviation of the mean value.
Acknowledgments
AstraZeneca supported us with the drug supply and funding. This study was supported by a grant from the Korea Health Technology R&D Project “"Strategic Center of Cell and Bio Therapy for Heart, Diabetes, & Cancer”" through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant no: HI17C2085). The research was supported by the Korean Cancer Study Group (KCSG) and KCSG data center. KCSG Translational Research Committee funding was independent of study performance and interpretation. We thank the participating patients and their families, all study coinvestigators, and research coordinators. We also thank HERINGS for support with statistical analyses and Seonah Ha, PhD, who assisted in medical writing.
References
- 1. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J 2001; 18 (6): 1059–68. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med 2010; 134 (11): 1645–58. [DOI] [PubMed] [Google Scholar]
- 3. Mochizuki T, Ishii G, Nagai K et al Pleomorphic carcinoma of the lung: Clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008; 32 (11): 1727–35. [DOI] [PubMed] [Google Scholar]
- 4. Rossi G, Cavazza A, Sturm N et al Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: A clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003; 27 (3): 311–24. [DOI] [PubMed] [Google Scholar]
- 5. Ung M, Rouquette I, Filleron T et al Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin Lung Cancer 2016; 17 (5): 391–7. [DOI] [PubMed] [Google Scholar]
- 6. Yendamuri S, Caty L, Pine M et al Outcomes of sarcomatoid carcinoma of the lung: A surveillance, epidemiology, and end results database analysis. Surgery 2012; 152 (3): 397–402. [DOI] [PubMed] [Google Scholar]
- 7. Bae HM, Min HS, Lee SH et al Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer 2007; 58 (1): 112–5. [DOI] [PubMed] [Google Scholar]
- 8. Lee J, Jung HA, Kim Y et al Efficacy of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in patients with advanced pulmonary pleomorphic carcinoma. Lung Cancer 2018; 122: 160–4. [DOI] [PubMed] [Google Scholar]
- 9. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373 (2): 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387 (10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 11. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375 (19): 1823–33. [DOI] [PubMed] [Google Scholar]
- 12. Antonia S, Goldberg SB, Balmanoukian A et al Safety and antitumour activity of durvalumab plus tremelimumab in non‐small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol 2016; 17 (3): 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim S, Kim MY, Koh J et al Programmed death‐1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015; 51 (17): 2698–707. [DOI] [PubMed] [Google Scholar]
- 14. Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand‐1 (PD‐L1). J Thorac Oncol 2013; 8 (6): 803–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vieira T, Antoine M, Hamard C et al Sarcomatoid lung carcinomas show high levels of programmed death ligand‐1 (PD‐L1) and strong immune‐cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016; 98: 51–8. [DOI] [PubMed] [Google Scholar]
- 16. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10 (9): 1243–60. [DOI] [PubMed] [Google Scholar]
- 17. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366 (26): 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hendry S, Byrne DJ, Wright GM et al Comparison of four PD‐L1 immunohistochemical assays in lung cancer. J Thorac Oncol 2018; 13 (3): 367–76. [DOI] [PubMed] [Google Scholar]
- 19. Im SW, Chae J, Jang SS et al A newly developed capture‐based sequencing panel for genomic assay of lung cancer. Genes Genomics 2020; 42: 751–9. [DOI] [PubMed] [Google Scholar]
- 20. Domblides C, Monnet I, Mazieres J et al Efficacy of immune checkpoint inhibitors in lung sarcomatoid carcinoma: Data from a French multicentric cohort. Ann Oncol 2018; 29 (suppl_8): viii400–41. 10.1093/annonc/mdy1288. [DOI] [PubMed] [Google Scholar]
- 21. Vieira T, Girard N, Ung M et al Efficacy of first‐line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013; 8 (12): 1574–7. [DOI] [PubMed] [Google Scholar]
- 22. Bondarenko I, Juan‐Vidal O, Pajkos G et al Preliminary efficacy of durvalumab plus tremelimumab in platinum‐refractory/resistant ED‐SCLC from arm a of the phase II BALTIC study. Ann Oncol 2018; 29 (Suppl 8): viii596–602. 10.1093/annonc/mdy1298. [DOI] [Google Scholar]
- 23. Siu LL, Even C, Mesia R et al Safety and efficacy of durvalumab with or without tremelimumab in patients with PD‐L1‐low/negative recurrent or metastatic HNSCC: The phase 2 CONDOR randomized clinical trial. JAMA Oncol 2019; 5 (2): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terra SB, Jang JS, Bi L et al Molecular characterization of pulmonary sarcomatoid carcinoma: Analysis of 33 cases. Mod Pathol 2016; 29 (8): 824–31. [DOI] [PubMed] [Google Scholar]
- 25. Kwon D, Koh J, Kim S et al MET exon 14 skipping mutation in triple‐negative pulmonary adenocarcinomas and pleomorphic carcinomas: An analysis of intratumoral MET status heterogeneity and clinicopathological characteristics. Lung Cancer 2017; 106: 131–7. [DOI] [PubMed] [Google Scholar]
- 26. Tissot C, Couraud S, Tanguy R, Bringuier PP, Girard N, Souquet PJ. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016; 91: 23–8. [DOI] [PubMed] [Google Scholar]
- 27. Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 1998; 93 (3): 397–409. [DOI] [PubMed] [Google Scholar]
- 28. Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in aging, inflammation, hematopoiesis and malignant transformation. Cell 2019; 8 (8): 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of pretreatment lymphocyte subsets in blood between patients with clinical benefit (n = 7) and those without clinical benefit (n = 7). Clinical benefit was defined as partial response or stable disease of more than six months. Error bars represent standard deviation of the mean value.


