Abstract
Background
Pulmonary resection is occasionally performed in postpneumonectomy patients with contralateral lung lesions, such as metachronous or metastatic lung cancer. Careful intraoperative respiratory management is essential in such patients. This study evaluated the respiratory management of postpneumonectomy patients who underwent contralateral pulmonary resection with selective bronchial blockade of the lobe or segment to be resected.
Methods
We retrospectively analyzed the surgical findings and safety of surgery in six patients who underwent contralateral pulmonary resection with selective bronchial blockade after pneumonectomy for non‐small cell lung cancer (NSCLC).
Results
The percutaneous oxygen saturation did not decrease in any of the patients during bronchial blockade under high oxygen concentration. The median blockade time was 57.5 minutes. The operative field was tolerable secured under conditions of partial lung collapse, and partial pulmonary resection was performed as planned. Postoperatively, one patient developed acute respiratory distress syndrome due to acute exacerbation of interstitial pneumonia; however, no patients died within one month postoperatively. Two patients underwent pulmonary resection in order to obtain adequate tissue specimens to evaluate the biomarkers of multiple lung metastases. On histopathology, one patient tested positive for anaplastic lymphoma kinase (ALK) and was subsequently administered an ALK inhibitor, which prolonged survival.
Conclusions
In all patients, intraoperative respiratory condition under partial lung collapse remained stable, and all partial pulmonary resections were safely performed. However, surgical indications should be carefully reviewed preoperatively in patients with interstitial pneumonia.
Key points
Significant findings of the study
Contralateral partial pulmonary resection was performed using selective bronchial blockade in postpneumonectomy patients.
Percutaneous oxygen saturation did not decrease during the bronchial blockade under high oxygen concentration, and the operative field was tolerable secured under conditions of partial lung collapse.
What this study adds
Oxygen concentration can be set to the minimum level, sufficient to maintain oxygenation, during contralateral partial pulmonary resection with selective bronchial blockade.
Keywords: Lung cancer, postpneumonectomy, pulmonary resection, selective bronchial blockade, video‐assisted thoracoscopic surgery
In this study, contralateral pulmonary resection was performed with selective bronchial blockade in postpneumonectomy patients, and we retrospectively analyzed the surgical findings and safety of the surgery.
The patients had stable intraoperative respiratory condition under partial lung collapse, and partial pulmonary resections were safe.
Introduction
The standard surgical treatment for primary lung cancer is lobectomy; however, 3%–7% of patients with lung cancer require pneumonectomy. 1 , 2 Reports show that the risk of tumor recurrence after complete resection of lung cancer is 2%–5% per year, 3 , 4 depending on the stage. Moreover, metachronous lung cancer occurs in 1%–5% of cases of lung cancer. 5 , 6 In postpneumonectomy patients, contralateral pulmonary resection may be required to manage new lung lesions, such as in cases of metachronous or metastatic lung cancer. Pulmonary resection can provide a chance to cure lung cancer, and it is suggested that contralateral pulmonary resection after pneumonectomy may be useful in some patients. 7 However, it requires multispecialty evaluation, with emphasis on the patient's performance status, cardiopulmonary function, and tumor stage and location. 8 Pneumonectomy has been reported to increase the total vascular resistance of the pulmonary circulation and may increase the right ventricular load 9 ; therefore, a history of pneumonectomy is considered a relative contraindication for further pulmonary resection. 2
Careful intraoperative respiratory management is essential during contralateral pulmonary resection in postpneumonectomy patients. During normal respiratory management, the operative field is suboptimal due to ventilation of the operative side 10 ; therefore, thoracotomy is performed in most patients. 11 In general, video‐assisted thoracoscopic surgery (VATS) causes lesser postoperative respiratory complications than thoracotomy. 12 VATS is performed by repeated inflation and deflation of the operative lung in postpneumonectomy patients. 10 However, the apnea time is limited to only a few minutes. Extracorporeal membrane oxygenation (ECMO) 13 and selective bronchial blockade of the lobe to be resected 14 , 15 have been reported as respiratory management strategies during contralateral pulmonary resection in postpneumonectomy patients. ECMO allows complete lung collapse on the operative side; however, administration of heparin is necessary to minimize the risk of thromboembolic events. 13 Furthermore, the use of extracorporeal circulation in patients with cancer increases the possibility of cancer progression because of cancer cell dissemination or involvement of the immune system. 16 On the other hand, selective bronchial blockade leads to partial lung collapse; however, the intraoperative respiratory management and safety of surgery under partial lung collapse remains to be established. To date, no study has reported which respiratory management strategy can be safely adopted for contralateral pulmonary resection in postpneumonectomy patients.
In this study, we retrospectively analyzed the surgical findings and safety of surgery in postpneumonectomy patients who underwent contralateral pulmonary resection with selective bronchial blockade, which to our knowledge, is the largest single‐center case series to be published to date.
Methods
Patient enrolment
Patients with a history of pneumonectomy for primary lung cancer, who underwent contralateral pulmonary resection with selective bronchial blockade at the Department of Thoracic Surgery of Yamaguchi Ube Medical Center between May 2013 and October 2017, were enrolled in this study. Six patients were considered eligible for inclusion (no patients were excluded). The patient characteristics are listed in Table 1. The cohort consisted of five men and one woman, with a median age of 62 years (range, 49–70 years). Three patients had previously undergone right pneumonectomy, while the other three patients had undergone left pneumonectomy. The site of the new lesion was the right upper lobe in one patient, right lower lobe in two patients, and left lower lobe in three patients. The median preoperative forced expiratory volume in the first second (FEV1.0) was 1.65 L (range: 1.22–2.11 L). Preoperative lung comorbidity included one case of interstitial pneumonia (case 1). None of the patients required home oxygen therapy before the surgery. Preoperatively, all patients had an Eastern Cooperative Oncology Group performance status score of 0, and transthoracic echocardiography revealed absence of right heart failure or pulmonary hypertension. A pulmonary resection was performed 12–128 months (median, 64 months) after initial pneumonectomy. Four patients (Cases 1–4) had a solitary pulmonary nodule of 2 cm or less, but no lymph node metastasis, and underwent pulmonary resection as radical treatment for suspected metastatic or metachronous lung cancer. In two patients (Cases 5 and 6), the lung cancer recurred as multiple lung metastases. Therefore, pulmonary resection was performed to obtain adequate tissue to evaluate biomarkers associated with recurrent lung cancer.
Table 1.
Clinical background of postpneumonectomy patients
| No. of case | Age | Sex | Pneumonectomy (right/left) | Past diagnosis | Interval after pneumonectomy (months) | Tumor site | Tumor size (mm) | Preoperative FEV1.0 (L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 61 | Male | Right | Ad | 12 | Left S6 | 20 | 1.41 |
| 2 | 63 | Female | Left | Atypical carcinoid | 45 | Right S2 | 20 | 1.60 |
| 3 | 59 | Male | Right | ACC | 128 | Left S6 | 17 | 1.22 |
| 4 | 69 | Male | Left | SqCC | 93 | Right S10 | 14 | 1.80 |
| 5 | 49 | Male | Left | AdSq | 83 | Right S8 | 5 | 2.11 |
| 6 | 70 | Male | Right | Ad | 39 | Left S10 | 32 | 1.69 |
ACC, adenoid cystic carcinoma; Ad, adenocarcinoma; AdSq, adenosquamous carcinoma; FEV1.0, forced expiratory volume in the first second; SqCC, squamous cell carcinoma.
Ethics, consent, and permissions
All included patients provided written informed consent for the publication of their medical data. The institutional review board of the Yamaguchi Ube Medical Center approved this study (IRB‐30‐2).
Anesthetic management
Under general anesthesia, the patients were intubated with a 9 mm Mallinckrodt Lo‐Contour Reinforced Tracheal Tube (Covidien Japan Co., Ltd., Tokyo, Japan) with direct laryngoscopy guidance. A wire‐guided Arndt endobronchial blocker with a spherical‐shaped balloon (Cook Japan Co., Ltd., Tokyo, Japan) was used for segmental lung isolation (Fig 1). A wire‐guided endobronchial blocker (Arndt Blocker) was advanced through the blocker port of the Arndt Multiport Adapter, and a fiberoptic bronchoscope, which had been introduced through the fiberoptic port, was inserted through the wire loop. The bronchoscope was then advanced into the target bronchus, and the Arndt Blocker was placed into position. The bronchoscope was then removed, and the balloon of the Arndt Blocker was inflated. The ventilator was set to volume control ventilation mode. Selective bronchial blockade reduced the ventilated lung volume; therefore, the anesthesiologist reduced the ventilation volume and increased the ventilation frequency. Intraoperative monitoring included electrocardiography, percutaneous oxygen saturation (SpO2) measurement, arterial blood pressure monitoring, and end‐tidal carbon dioxide monitoring.
Figure 1.
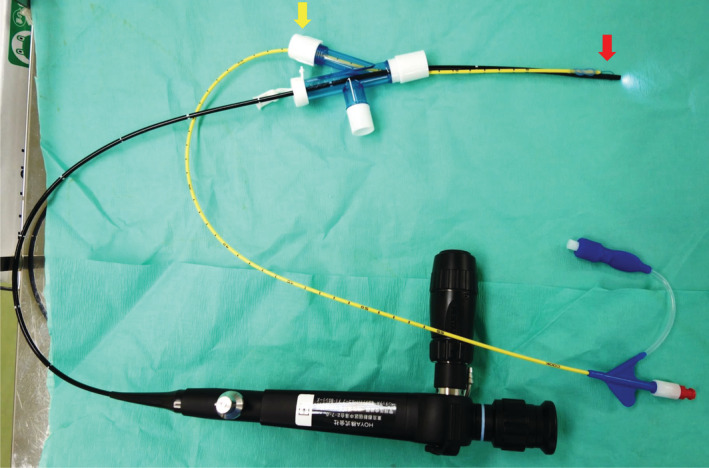
Selective bronchial blockade method. The wire‐guided endobronchial blocker is advanced through the blocker port (yellow arrow), and a bronchoscope is passed through the wire loop of the endobronchial blocker (red arrow). The bronchoscope is then inserted into the desired bronchus, and the endobronchial blocker is placed into position. The bronchoscope is removed, and the balloon of the blocker is inflated.
Outcomes
We retrospectively examined the surgical findings, safety of surgery, postoperative course, and prognosis.
Results
Surgical and postoperative information are listed in Table 2. In all six patients, single partial pulmonary resection with selective bronchial blockade was performed as originally planned. Three patients underwent thoracotomy and three underwent VATS. In two of the patients who underwent VATS, a 21‐G guiding‐marker system (Hakko Co., Ltd., Nagano, Japan) was percutaneously placed as a VATS marker near the target lung nodule under computed tomography (CT) guidance before the surgery. No patient developed complications associated with placement of the VATS marker. The bronchial blockade areas were the right upper lobe in one patient, right lower lobe in one patient, left lower lobe in two patients, right basal segment in one patient, and left basal segment in one patient. The median blockade time was 57.5 minutes (range, 30–111 minutes). The fraction of inspired oxygen (FiO2) was set to 1.0 during the bronchial blockade. However, FiO2 was set to 0.6–0.9 for one patient with interstitial pneumonia (Case 1). Almost 100% SpO2 was maintained intraoperatively in all patients during the bronchial blockade; interruption of blockade was not required to improve oxygenation. All patients were extubated immediately after surgery.
Table 2.
Surgical and postoperative data
| No. of cases | Approach | CT‐guided marking | Blockade area | Blockade time (minutes) | FiO2 | Histopathological diagnosis | Postoperative complication | Additional treatment | Prognosis | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Thoracotomy | No | Left lower lobe | 111 | 0.6–0.9 | Ad | Acute exacerbation of IP | None | Dead | 19 |
| 2 | Thoracotomy | No | Right upper lobe | 60 | 1.0 | NTM | None | None | Alive | 84 |
| 3 | Thoracotomy | No | Left lower lobe | 50 | 1.0 | ACC | None | None | Alive | 75 |
| 4 | VATS | Yes | Right basal segment | 30 | 1.0 | SqCC | None | Carbon‐ion radiotherapy | Alive | 75 |
| 5 | VATS | Yes | Right lower lobe | 57 | 1.0 | Ad | None | ALK inhibitor | Alive | 34 |
| 6 | VATS | No | Left basal segment | 58 | 1.0 | Ad | None | Chemotherapy, ICI | Dead | 18 |
ACC, adenoid cystic carcinoma; Ad, adenocarcinoma; ALK, anaplastic lymphoma kinase; CT, computed tomography; FiO2, fraction of inspired oxygen; ICI, immune checkpoint inhibitor; IP, interstitial pneumonia; NTM, nontuberculous mycobacteriosis; SqCC, squamous cell carcinoma; VATS, video‐assisted thoracoscopic surgery.
One patient (Case 1) developed acute exacerbation of interstitial pneumonia after surgery (Fig 2a–c). Respiratory distress in this patient was managed by high‐dose corticosteroid pulse therapy and blood purification therapy; however, supplemental oxygen was required at discharge. The postoperative course was uneventful in the other patients. The median postoperative hospital stay was 13 days (range, 3–53 days). In four patients, the histopathological findings were the same as those observed after the previous pneumonectomy. In Case 2, the lesion was diagnosed as a pulmonary carcinoid tumor by preoperative bronchoscopy with transbronchial biopsy, but subsequent postoperative histopathological examination established a diagnosis of nontuberculous mycobacteriosis.
Figure 2.
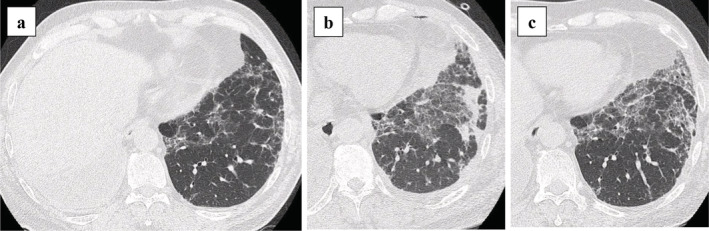
Computed tomography images of Case 1. (a) Interstitial pneumonia is observed in the left lower lobe before surgery. (b) Acute exacerbation of interstitial pneumonia developed on postoperative day 5. (c) After high‐dose corticosteroid pulse therapy and blood purification therapy, the ground‐glass shadow improved on postoperative day 38.
Among the two patients who underwent repeat biopsy of the lung metastatic lesions, the histopathology report of one patient (Case 5) was positive for echinoderm microtubule‐associated protein‐like 4 and anaplastic lymphoma kinase (EML4‐ALK). This patient received alectinib therapy, which resulted in long‐term survival. In Case 6, epidermal growth factor receptor (EGFR) exon 19 deletion mutation was detected in the previous pneumonectomy specimen, and the patient received afatinib therapy for multiple lung metastases. Because the lung metastases increased in size after temporary shrinkage, a repeat biopsy was performed to assess EGFR T790M mutation. However, the results for EGFR gene mutations were the same as those obtained earlier. The patient died 18 months after surgery, despite receiving chemotherapy and immune checkpoint inhibitor therapy. In five patients diagnosed with lung cancer (excluding Case 2), the overall median survival after surgery was 34 months (range, 18–75 months).
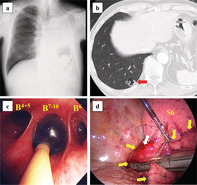
Case 4 involved a 69‐year‐old man who underwent pneumonectomy for left lung squamous cell carcinoma (Fig 3a). CT revealed a 1.4 cm lung nodule in the right 10th segment of the lower lobe (Fig 3b). A percutaneous VATS marker was placed near the lung nodule under CT guidance, and partial pulmonary resection was performed by VATS with right basal segmental blockade (Fig 3c,d). FiO2 was set to 1.0 during the bronchial blockade. SpO2 was maintained at 100%, and the operative field was tolerable secured under conditions of partial lung collapse. The partial pressure of arterial oxygen (PaO2) before, during, and after the bronchial blockade was 547, 410, and 542 mmHg, respectively. The partial pressure of arterial carbon dioxide (PaCO2) before, during, and after the bronchial blockade was 41.8 mmHg, 40.1 mmHg, and 51.6 mmHg, respectively. The postoperative course was uneventful, and the patient was discharged on postoperative day 12. The histopathological diagnosis was squamous cell carcinoma and the patient was diagnosed with metachronous primary lung cancer according to the definition proposed by Martini et al. 17 Carbon‐ion radiotherapy was performed for stump recurrence at 21 months after surgery; no recurrence was observed thereafter.
Figure 3.
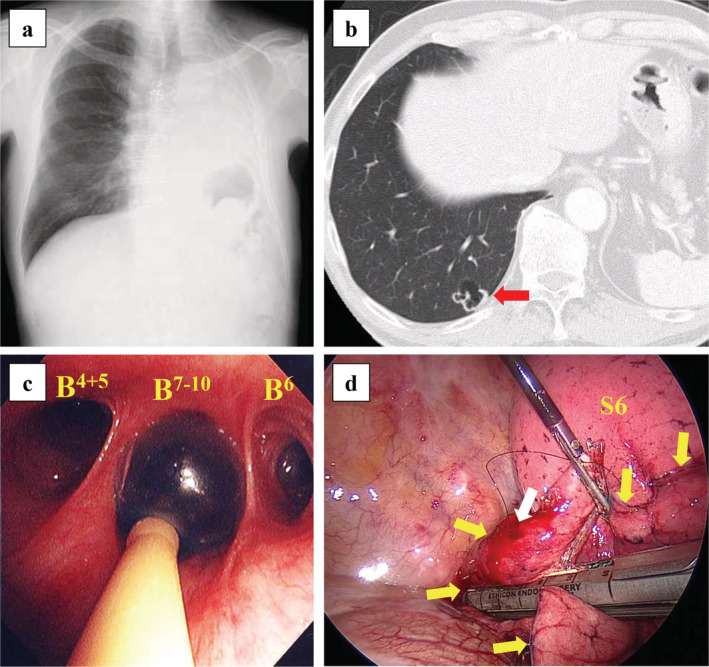
Clinical findings of Case 4. (a) Chest radiography after left pneumonectomy. (b) Computed tomography image shows a 1.4 cm sized lung tumor in the right 10th segment (red arrow). (c) In respiratory management, the endobronchial blocker is positioned in the right basal segmental bronchus. (d) Surgical findings show that partial pulmonary resection is performed under partial lung collapse by video‐assisted thoracoscopic surgery (VATS). The white arrow indicates a VATS marker. The yellow arrows indicate the nonventilated lung of the right lower lobe, which is the right basal segment. S6, right sixth segment.
Discussion
Herein, we report a case series of postpneumonectomy patients who underwent pulmonary resection using selective bronchial blockade. Their respiratory condition remained stable intraoperatively, and no patient required interruption of bronchial blockade to improve oxygenation. The operative field was secured under conditions of partial lung collapse, and partial pulmonary resection was safe in all patients.
In this study, pulmonary resection was performed using VATS in three patients. VATS has been shown to cause less damage to the inspiratory muscles than thoracotomy, which consequently leads to better postoperative respiratory function. 18 Therefore, VATS is useful for patients with limited lung function, such as those who have undergone pneumonectomy. As it is difficult to locate pulmonary nodules during VATS under conditions of partial lung collapse, placement of a VATS marker is useful. However, CT‐guided percutaneous marking has been reported to cause iatrogenic pneumothorax in 18.6%–23.0% of cases. 19 , 20 Pneumothorax can be life‐threatening for postpneumonectomy patients. 10 It is advisable to prepare for thoracic drainage when placing a percutaneous VATS marker. Recent studies have reported the usefulness of bronchoscopic marking. 21 , 22 Unlike percutaneous VATS marking, bronchoscopic marking does not damage the visceral pleura. Although bronchoscopic marking was not used in this study, the procedure is significantly superior to CT‐guided percutaneous marking because of the lower incidence of iatrogenic pneumothorax.
With regard to the criteria for preoperative evaluation of contralateral pulmonary resection in postpneumonectomy patients, previous reports recommend a preoperative performance status score of 0, with a preoperative FEV1.0 of 0.8–1 L or more. 23 , 24 All of the patients in this study met these criteria. Pulmonary resection is recommended for postpneumonectomy patients with tumors that are 2 cm or less in diameter but without lymph node metastasis. 2 , 8 Single partial resection has been reported to have a better prognosis than more extensive surgeries, such as multiple partial resections, segmentectomy, and lobectomy. 2 , 23 , 24 , 25 In general, complications after pulmonary resection occur in 24%–41% of patients, and the postoperative mortality rate with partial pulmonary resection is <1%. 26 In postpneumonectomy patients, postoperative complications occur in 12.5%–41% of patients, and the one‐month postoperative mortality rate is 0%–11.1%. 2 , 10 , 11 , 24 , 27 In this study, one patient (Case 1) developed acute respiratory distress syndrome due to acute exacerbation of interstitial pneumonia. However, it is possible that the high oxygen concentration administered intraoperatively caused the exacerbation. Therefore, in patients with interstitial pneumonia, we recommend setting the oxygen concentration to the minimum level that maintains oxygenation. Moreover, in such patients, the indications for surgery should be carefully reviewed.
Stereotactic body radiotherapy (SBRT) is an alternative to surgery for patients who cannot tolerate pulmonary resection. In postpneumonectomy patients, the three‐year survival rate after pulmonary resection has been reported to be 46%–61%, 2 , 24 , 27 which is comparable to the three‐year survival rate of 36%–63% after SBRT. 28 , 29 Biopsies should also be performed before SBRT to obtain a pathological diagnosis of the lung lesions. However, iatrogenic pneumothorax has been reported to occur in 13%–25% of the patients who undergo percutaneous lung biopsy 11 , 30 and 1%–6% of those who undergo bronchial lung biopsy. 31 Therefore, SBRT is often performed only on the basis of imaging diagnosis, without pathological diagnosis. 32 , 33 Because biopsy and surgery may show different histological tumor types, as observed in Case 2, the imaging and pathological diagnoses are often different. With regard to the prognosis reported for SBRT, previous studies might have included patients with benign disease; therefore, the prognosis could be overestimated. Additionally, radiation pneumonitis can be life‐threatening for postpneumonectomy patients. 28 The risk of radiation pneumonitis due to unnecessary radiation therapy must be avoided; therefore, pathological examination is essential before SBRT.
Recently, the treatment strategy reported for recurrent lung cancer has been based on molecular biomarkers, such as EGFR mutation, EML4‐ALK fusion gene, and PD‐L1 expression, etc. 34 , 35 In Cases 5 and 6, pulmonary resection was performed as a repeat biopsy to evaluate the biomarkers of lung metastases. In Case 5, ALK‐targeted therapy resulted in long‐term survival. The prognosis after contralateral pulmonary resection for metastatic lung cancer in postpneumonectomy patients is reported to be poor. 11 , 24 However, the development of personalized targeted therapies can improve long‐term survival; and accordingly, a repeat biopsy using this procedure is worth considering.
In conclusion, intraoperative respiratory condition of postpneumonectomy patients who underwent selective bronchial blockade remained stable. Partial pulmonary resections were safe under partial lung collapse, and there were no one‐month postoperative mortalities. However, surgical indications should be carefully reviewed in patients with interstitial pneumonia. Pulmonary resection can be curative in patients with early stage lung cancer. Moreover, evaluation of biomarkers is essential in patients with recurrent lung cancer. Therefore, it is anticipated that this surgical procedure should be increasingly utilized in the future.
Disclosure
None of the authors have any potential conflicts of interest relevant to this report.
Acknowledgment
We thank Editage (http://www.editage.jp) for English language editing.
No funding was received for this case series.
References
- 1. Rosen JE, Hancock JG, Kim AW, Detterbeck FC, Boffa DJ. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg 2014; 98: 1953–60. [DOI] [PubMed] [Google Scholar]
- 2. Ayub A, Rehmani SS, Al‐Ayoubi AM, Raad W, Flores RM, Bhora FY. Pulmonary resection for second lung cancer after pneumonectomy: A population‐based study. Ann Thorac Surg 2017; 104: 1131–7. [DOI] [PubMed] [Google Scholar]
- 3. Pairolero PC, Williams DE, Bergstralh EJ, Piehler JM, Bernatz PE, Payne WS. Post‐surgical stage I bronchogenic carcinoma: Morbid implications of recurrent disease. Ann Thorac Surg 1984; 38: 331–8. [DOI] [PubMed] [Google Scholar]
- 4. Fleisher AG, McElvaney G, Robinson CLN. Multiple primary bronchogenic carcinomas: Treatment and follow‐up. Ann Thorac Surg 1991; 51: 48–51. [DOI] [PubMed] [Google Scholar]
- 5. Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 1998; 90: 1335–45. [DOI] [PubMed] [Google Scholar]
- 6. Deschamps C, Pairolero PC, Trastek VF, Payne WS. Multiple primary lung cancers. Results of surgical treatment. J Thorac Cardiovasc Surg 1990; 99: 769–77. [PubMed] [Google Scholar]
- 7. Toufektzian L, Patris V, Potaris K, Konstantinou M. Is it safe and worthwhile to perform pulmonary resection after contralateral pneumonectomy? Interact Cardiovasc Thorac Surg 2015; 20: 265–9. [DOI] [PubMed] [Google Scholar]
- 8. Asai M, Scott WJ. Lung resection in the postpneumonectomy patient. Thorac Surg Clin 2018; 28: 19–25. [DOI] [PubMed] [Google Scholar]
- 9. Okada M, Ota T, Okada M, Matsuda H, Okada K, Ishii N. Right ventricular dysfunction after major pulmonary resection. J Thorac Cardiovasc Surg 1994; 108: 503–11. [PubMed] [Google Scholar]
- 10. Zampieri D, Marulli G, Comacchio GM, Schiavon M, Zuin A, Rea F. Thoracoscopic wedge resection in single‐lung patients. J Thorac Dis 2018; 10: 861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grodzki T, Alchimowicz J, Kozak A et al Additional pulmonary resections after pneumonectomy: Actual long‐term survival and functional results. Eur J Cardiothorac Surg 2008; 34: 493–8. [DOI] [PubMed] [Google Scholar]
- 12. Flores RM, Park BJ, Dycoco J et al Lobectomy by video‐assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009; 138: 11–8. [DOI] [PubMed] [Google Scholar]
- 13. Heward E, Hayes T, Evison M, Booton R, Barnard J, Shah R. Extracoporeal membrane oxygenation assisted segmentectomy for metachronous lung cancer after pneumonectomy. Ann Thorac Surg 2016; 102: e187–9. [DOI] [PubMed] [Google Scholar]
- 14. Nakanishi R, Hirai A, Muranaka K, Shinohara K. Successful video‐assisted thoracic surgery lobectomy in a single‐lung patient. Surg Laparosc Endosc Percutan Tech 2007; 17: 562–4. [DOI] [PubMed] [Google Scholar]
- 15. Walsh K, Park B, Amar D. Segmental lung isolation in a postpneumonectomy patient undergoing contralateral lung resection. J Cardiothorac Vasc Anesth 2017; 31: 1048–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. La Francesca S, Frazier OH, Radovancevic B, De Caro LF, Reul GJ, Cooley DA. Concomitant cardiac and pulmonary operations for lung cancer. Tex Heart Inst J 1995; 22: 296–300. [PMC free article] [PubMed] [Google Scholar]
- 17. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70: 606–12. [PubMed] [Google Scholar]
- 18. Donahoe LL, de Valence M, Atenafu EG et al High risk for thoracotomy but not thoracoscopic lobectomy. Ann Thorac Surg 2017; 103: 1730–5. [DOI] [PubMed] [Google Scholar]
- 19. Chen YR, Yeow KM, Lee JY et al CT‐guided hook wire localization of subpleural lung lesions for video‐assisted thoracoscopic surgery (VATS). J Formos Med Assoc 2007; 106: 911–8. [DOI] [PubMed] [Google Scholar]
- 20. Park JB, Lee SA, Lee WS et al Computed tomography‐guided percutaneous hook wire localization of pulmonary nodular lesions before video‐assisted thoracoscopic surgery: Highlighting technical aspects. Ann Thorac Med 2019; 14: 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anayama T, Hirohashi K, Miyazaki R et al Near‐infrared dye marking for thoracoscopic resection of small‐sized pulmonary nodules: Comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anayama T, Hirohashi K, Okada H et al Simultaneous cone beam computed tomography‐guided bronchoscopic marking and video‐assisted thoracoscopic wedge resection in a hybrid operating room. Thorac Cancer 2019; 10: 579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Massard G, Wihlm JM, Morand G. Surgical management for metachronous bronchogenic cancer occurring after pneumonectomy. J Thorac Cardiovasc Surg 1995; 109: 597–600. [DOI] [PubMed] [Google Scholar]
- 24. Donington JS, Miller DL, Rowland CC et al Subsequent pulmonary resection for bronchogenic carcinoma after pneumonectomy. Ann Thorac Surg 2002; 74: 154–9. [DOI] [PubMed] [Google Scholar]
- 25. Levasseur P, Regnard JF, Icard P, Dartevelle P. Cancer surgery on a single residual lung. Eur J Cardiothorac Surg 1992; 6: 639–40. [DOI] [PubMed] [Google Scholar]
- 26. Bommart S, Berthet JP, Durand G et al Imaging of postoperative complications following surgery for lung cancer. Diagn Interv Imaging 2017; 98: 11–20. [DOI] [PubMed] [Google Scholar]
- 27. Terzi A, Lonardoni A, Scanagatta P, Pergher S, Bonadiman C, Calabrò F. Lung resection for bronchogenic carcinoma after pneumonectomy: A safe and worthwhile procedure. Eur J Cardiothorac Surg 2004; 25: 456–9. [DOI] [PubMed] [Google Scholar]
- 28. Thompson R, Giuliani M, Yap ML et al Stereotactic body radiotherapy in patients with previous pneumonectomy: Safety and efficacy. J Thorac Oncol 2014; 9: 843–7. [DOI] [PubMed] [Google Scholar]
- 29. Senthi S, Haasbeek CJ, Lagerwaard FJ et al Radiotherapy for a second primary lung cancer arising post‐pneumonectomy: Planning considerations and clinical outcomes. J Thorac Dis 2013; 5: 116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cronin CG, Sharma A, Digumarthy SR et al Percutaneous lung biopsy after pneumonectomy: Factors for improving success in the care of patients at high risk. AJR Am J Roentgenol 2011; 196: 929–34. [DOI] [PubMed] [Google Scholar]
- 31. Izbicki G, Shitrit D, Yarmolovsky A et al Is routine chest radiography after transbronchial biopsy necessary: A prospective study of 350 cases. Chest 2006; 129: 1561–4. [DOI] [PubMed] [Google Scholar]
- 32. Testolin A, Favretto MS, Cora S, Cavedon C. Stereotactic body radiation therapy for a new lung cancer arising after pneumonectomy: Dosimetric evaluation and pulmonary toxicity. Br J Radiol 2015; 88: 20150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giaj Levra N, Filippi AR, Guarneri A et al Efficacy and safety of stereotactic ablative radiotherapy in patients with previous pneumonectomy. Tumori 2015; 101: 148–53. [DOI] [PubMed] [Google Scholar]
- 34. Mok TS. Personalized medicine in lung cancer: What we need to know. Nat Rev Clin Oncol 2011; 8: 661–8. [DOI] [PubMed] [Google Scholar]
- 35. Aoki MN, Amarante MK, de Oliveira CEC, Watanabe MAE. Biomarkers in non‐small cell lung cancer: Perspectives of individualized targeted therapy. Anticancer Agents Med Chem 2018; 18: 2070–7. [DOI] [PubMed] [Google Scholar]


