Abstract
The highly polarized, typically very long, and nonmitotic nature of neurons present them with unique challenges in the maintenance of their homeostasis. This architectural complexity serves a rich and tightly controlled set of functions that enables their fast communication with neighboring cells and endows them with exquisite plasticity. The submembrane neuronal cytoskeleton occupies a pivotal position in orchestrating the structural patterning that determines local and long-range subcellular specialization, membrane dynamics, and a wide range of signaling events. At its center is the partnership between ankyrins and spectrins, which self-assemble with both remarkable long-range regularity and micro- and nanoscale specificity to precisely position and stabilize cell adhesion molecules, membrane transporters, ion channels, and other cytoskeletal proteins. To accomplish these generally conserved, but often functionally divergent and spatially diverse, roles these partners use a combinatorial program of a couple of dozens interacting family members, whose code is not fully unraveled. In a departure from their scaffolding roles, ankyrins and spectrins also enable the delivery of material to the plasma membrane by facilitating intracellular transport. Thus, it is unsurprising that deficits in ankyrins and spectrins underlie several neurodevelopmental, neurodegenerative, and psychiatric disorders. Here, I summarize key aspects of the biology of spectrins and ankyrins in the mammalian neuron and provide a snapshot of the latest advances in decoding their roles in the nervous system.
Keywords: ankyrin, axonal transport, cytoskeleton, plasma membrane, spectrin
1 |. INTRODUCTION
The canonical model of the cell’s submembranous skeleton depicts a spectrin network lining the inner lipid bilayer where it contributes to its shape and mechanical resilience. The spectrin skeleton attaches to the plasma membrane by direct association with membrane lipids and ankyrins, which are themselves coupled to the membrane via direct interaction with membrane-spanning proteins and membrane lipids (Bennett & Baines, 2001; Bennett & Lorenzo, 2013). The spectrin meshwork is formed by heterodimeric units of α- and β-spectrin assembled side-to-side in antiparallel fashion, which then form head-to-head tetramers that crosslink F-actin to form spectrin-actin arrays (Figure 1a,b). This model was first elucidated in erythrocytes, which exclusively express αI- and βI-spectrin, respectively, encoded by SPTA and SPTB, and ankyrin-R, encoded by ANK1 (Bennett & Stenbuck, 1979a). Subsequent findings of equivalent associations of actin, ankyrins, spectrins, and their partners in other cell types pointed at a ubiquitous mechanism that evolved as a conserved cytoskeleton strategy that serves as a functional template to the cell’s membrane (Burridge, Kelly, & Mangeat, 1982; Davies & Kloc, 1981; Davis & Bennett, 1982; Goodman, Zagon, & Kulikowski, 1981; Levine & Willard, 1981).
FIGURE 1.
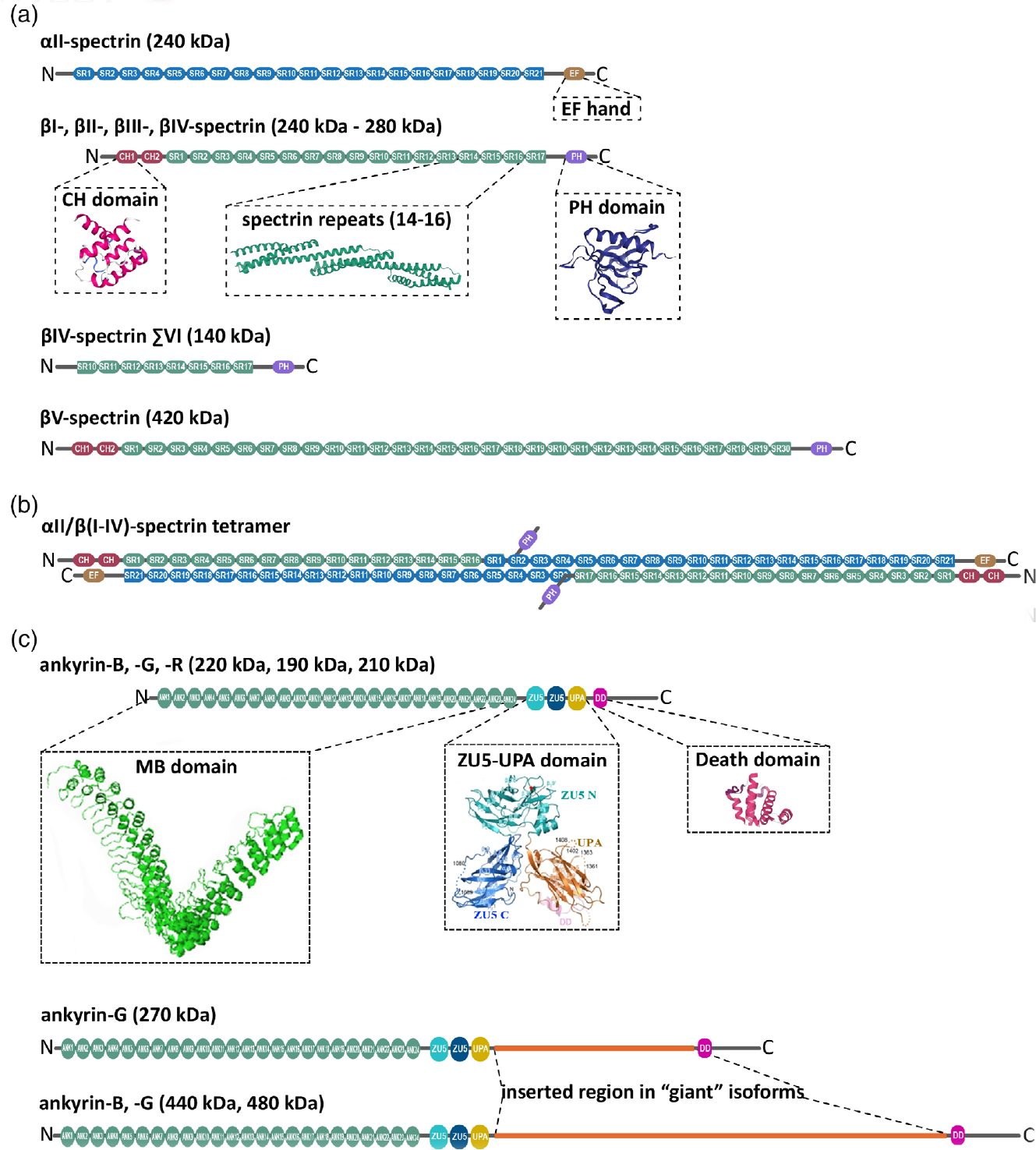
Structure domains of neuronal spectrins and ankyrins. (a) αII-spectrin spans 21 modular spectrin repeats (SR, blue), followed by a calcium-binding EF hand domain (light brown) close to the C-terminus. αII-spectrin also contains a Src-homology 3 (SH3) domain in SR9, and a calmodulin-binding loop in SR11. Neuronal spectrins also include canonical βI-, βII-, βIII-, and βIV-spectrins, which are comprised of 17 SR (green; ribbon diagram of the crystal structure of SR14–16 from Grum, MacDonald, & Mondragón, 1999), two in tandem calponin homology domains (CH, red; ribbon diagram of the crystal structure from Djinović-Carugo, Bañuelos, & Saraste, 1997) close to the N-terminus, and a pleckstrin homology domain (PH, purple; ribbon diagram of the crystal structure from Lemmon, Ferguson, & Abrams, 2002). βV-spectrin, the largest known mammalian spectrin, contains 13 additional SR. Alternatively spliced βIV-spectrin ΣVI, which lacks the PH domain and the first 9 SR, is a well-studied noncanonical, neuronal β-spectrin isoform. (b) αII-spectrin uses its N-terminus SR to bind the N-terminus SR of β-spectrins and associate as heterodimers. These heterodimers use antiparallel head-to-head association to form tetrameric units assembles with actin and networks with long-range regularity. (c) Canonical neuronal ankyrins contain a membrane-binding domain comprised of 24 ankyrin repeats (ANK) in the N-terminus (MBD, green; ribbon representation of the crystal structure from Michaely, Tomchick, Machius, & Anderson, 2002), followed by a supermodule that contains two ZU5 domains a UPA domain (teal, blue, orange; ribbon representation of crystal structure from Wang, Yu, Ye, Wei, & Zhang, 2012), which contains the ankyrin-binding site, a death domain (pink; ribbon representation of crystal structure from Wang, Simon, et al., 2019; Wang, Taki, et al., 2019) and a C-terminal unstructured regulatory domain (gray). The giant, neuron-specific ankyrin isoforms have an insertion of a single exon (orange) after the UPA domain
The ankyrin-spectrin partnership is likely to have first appeared ~580 million years ago in Cnidarians, the genomes of which contain a β-spectrin with a potential ankyrin-binding site and sequences resembling ankyrin that include a ZU5 domain with a putative spectrin-binding motif (Bennett & Lorenzo, 2013). Ankyrin and spectrin genes diversified through evolution to include a total of two α-spectrins (αI and αII), respectively, encoded by SPTA1 and SPTAN1, five β-spectrins (βI-V), correspondingly encoded by SPTB, SPTBN1, SPTBN2, SPTBN4, and SPTBN5, and three ankyrins (R, B, and G), encoded by ANK1, ANK2, and ANK3 in mammals. Alternative splicing events further expanded these protein families to include multiple isoforms with varying patterns of expressions across tissues and cell types. For example, while erythrocytes only express ankyrin-R and αI- and βI-spectrin, all three ankyrins and αII, βI, βII, and βIV spectrins are found in skeletal muscle and cardiomyocytes. In addition, muscle and cardiomyocyte ankyrins include multiple isoforms, some of which are uniquely enriched in these tissues (Cunha, Le Scouarnec, Schott, & Mohler, 2008; Hopitzan, Baines, Ludosky, Recouvreur, & Kordeli, 2005; Wu et al., 2015; Zhou et al., 1997). Neurons express the most expansive ankyrin-spectrin repertoire, which excludes erythrocyte-specific αI-spectrin, but includes two additional spectrins; βIII-spectrin (Ohara, Ohara, Yamakawa, Nakajima, & Nakayama, 1998; Sakaguchi et al., 1998; Stankewich, Tse, et al., 1998), which also shows high expression in the kidney (Sakaguchi et al., 1998), and βV-spectrin (Stabach & Morrow, 2000), which is primarily expressed in the cerebellum, photoreceptor cells (Papal et al., 2013), and auditory hair cells (Legendre, Safieddine, Küssel-Andermann, Petit, & El-Amraoui, 2008). Notably, neurons express “giant” ankyrin-B and ankyrin-G isoforms not found in other cell types, for which functional significance in now emerging (Jenkins et al., 2015; Tseng, Jenkins, Tanaka, Mooney, & Bennett, 2015; Yang, Walder-Christensen, Kim, et al., 2019).
The diversity of neuronal ankyrins and spectrins reflect the high level of functional specialization acquired by neurons. Consistent with the canonical spectrin role of imparting mechanical integrity to the lipid bilayer first described in erythrocytes (Agre, Orringer, & Bennett, 1982; Chasis, Agre, & Mohandas, 1988; reviewed in Eber & Lux, 2004), evidence suggests that spectrin networks may serve a similar function in neurons (Hammarlund, Jorgensen, & Bastiani, 2007). In an example of “function deduced from form”, this view has gained additional traction based on the recent discovery of actin-spectrin periodic structures lining neuronal processes (discussed below; Xu, Zhong, & Zhuang, 2013). However, it is the ankyrin-spectrin skeleton’s other canonical function in determining the spatial distribution and stabilization of transmembrane micro- and nanodomains that arguably serves a more critical role in neurons. First observed in erythrocytes where ankyrin-R stabilizes the anion exchanger 1 (AE1; encoded by SLC4A1) (Bennett & Stenbuck, 1979b; reviewed in Fowler, 2013), ankyrins and spectrins collaborate to properly localize, stabilize, and facilitate signal transduction of a wide range of membrane spanning protein complexes that include cell adhesion molecules (CAMs), ion channels, and membrane transporters (Bennett & Lorenzo, 2016). Consequently, ankyrins and spectrins play crucial roles in the nano- and micro-organization, development, and function of the axon initial segment (AIS), Nodes of Ranvier (NoR), axon branches, dendrites, and synaptic spines.
In a departure from their canonical roles as static plasma membrane organizers, members of the ankyrin and spectrin families associate with molecular motors and organelles to facilitate intracellular transport in neurons and other cell types (Lorenzo et al., 2010, 2014, 2019; Lorenzo & Bennett, 2017; Muresan et al., 2001; Qu et al., 2016; Takeda et al., 2000). For example, the 220 kDa ankyrin-B isoform couples the dynactin/dynein motor complex to multiple organelles to promote their retrograde axonal transport (Lorenzo et al., 2014). In contrast, the giant, neuron-specific 440 kDa ankyrin-B isoform binds the neural CAM L1 (L1CAM) to suppress axonal branching (Yang, Walder-Christensen, Kim, et al., 2019). Despite these divergent roles in neurons, ankyrin-B also fulfills the traditional, microdomain-stabilizing role in other cell types, such as cardiomyocytes, skeletal muscle, myocytes, and pancreatic beta cells (Ayalon, Davis, Scotland, & Bennett, 2008; Healy et al., 2010; Mohler, Davis, & Bennett, 2005). Therefore, 220 kDa ankyrin-B may multitask to exert a similar membrane protein-stabilizing function in neurons. This is indeed the case for some of the neuronal spectrins. For instance, in addition to crosslinking actin rings to form the periodic membrane structure (MPS) in axon and dendrites, a cytosolic pool of βII-spectrin independently facilitates bidirectional axonal transport of synaptic vesicles and other cargo (Lorenzo et al., 2019). Likewise, αII-spectrin and βIII-spectrin have been shown to both help assemble the MPS and to associate with motor protein complexes in neurons (Han, Zhou, Xia, & Zhuang, 2017; Holleran et al., 2001; Huang et al., 2017; Lorenzo et al., 2010; Takeda et al., 2000).
Given the wide-ranging functionality of ankyrins and spectrins in scaffolding the neuron, transporting and delivering essential cargo along the axon, and enabling the proper and stable localization of membrane protein complexes in neuronal processes, it is not surprising that their deficiencies underlie several neurodevelopmental, neurogenerative, and psychiatric disorders (De Rubeis et al., 2014; Ikeda et al., 2006; Iossifov et al., 2014; Kloth et al., 2017; Saitsu et al., 2010; Wang et al., 2018; Wirgenes et al., 2014). However, despite considerable progress in decoding the molecular underpinnings of neuronal ankyrins and spectrins over four decades, our present understanding is far from complete. Likewise, their role in brain development, global and local connectivity, plasticity, and activity is only starting to emerge. Consequently, our understanding of pathogenic mechanisms of brain diseases and disorders involving spectrins (“spectrinopathies”) and ankyrins (“ankyrinopathies”) is still limited.
This review intends to provide a snapshot of our current understanding of the multifaceted roles of ankyrins and spectrins in neuronal and brain structure and function. Moreover, the discussion below aims to underscore the view that these classes of molecule, collaboratively or independently, within and across families, isotypes, and isoforms, can functionally coalesce, overlap, diverge, and specialize to provide a versatile molecular toolkit to orchestrate diverse cellular processes in neurons. Thus, arguably, as principal actors in normal brain function and disease, it is paramount to decipher how ankyrins and spectrins are integrated into diverse molecular pathways and physiological processes. First, I will briefly summarize the structural and biochemical logic that enables ankyrins and spectrins to form diverse molecular assemblies at the nano-, micro-, and macroscales in mammalian neurons. I will then illustrate how these molecular partnerships, in turn, play critical roles in the formation and function of specialized domains and various cellular processes. Where known, I will highlight connections between the specific molecular players and cellular processes and brain structure and function, and corresponding diseases. Throughout, I will call attention to gaps in our knowledge, along with thoughts and suggestions on how they may be addressed.
2 |. STRUCTURE AND NEURODIVERSITY OF SPECTRINS AND ANKYRINS
Mammalian neurons express the most diverse repertoire of ankyrins (B, G, and R) and spectrins (αII-spectrin and βI-V spectrins) of any cell type (Figure 1). Since the molecular structures of ankyrins and spectrins have been extensively reviewed elsewhere (Bennett & Lorenzo, 2013), here I will only briefly summarize their key features. Spectrins monomers are elongated and flexible molecules formed by multiple tandem copies of a triple-helical spectrin repeat domain containing between 99 and 114 residues and extending roughly 100 nm in length. High resolution crystal structures show that these homologous domains adopt left-handed, anti-parallel, three-helix coiled-coil topology, and are connected by short α-helical linkers (Grum et al., 1999; Figure 1a). αII-spectrin contains 21 spectrin repeats plus a partial repeat near the N-terminus that serves as site of noncovalent dimerization with the C-terminus of βI-IV spectrins (Figure 1a). α-β spectrin heterodimers assemble by antiparallel lateral association between complementary motifs in spectrin repeats 20 and 21 of αII-spectrin and repeats 1 and 2 of βI-IV spectrins (Speicher, Weglarz, & DeSilva, 1992; Figure 1b). αII-spectrin also contains a Src-homology 3 (SH3) domain in repeat 9, a calmodulin-binding loop in repeat 11, and a calcium-binding EF hand domain near the C-terminus. βI-IV spectrins, on the other hand, contain 16 spectrin repeats plus a partial repeat that facilitate tetramerization with the N-terminus of αII-spectrin (Ipsaro et al., 2010), two N-terminal tandem calponin homology (CH) domains, a C-terminus pleckstrin homology (PH) domain, and an ankyrin-binding site in repeats 14 and 15 (Figure 1a). βV-spectrin, a 417 kDa protein homologous of Drosophila spectrin β-heavy (βH), contains 29 spectrin repeats plus a partial repeat that binds αII-spectrin (Stabach & Morrow, 2000) and lacks the conserved ankyrin-binding region (Figure 1a).
αII/βI-V tetramers connect to F-actin filaments through direct binding of a highly conserved N-terminal actin-binding domain (ABD) in βI-V spectrins that includes two in-tandem CH domains. Actin-spectrin complexes are stabilized by accessory proteins such as adducin (Li & Bennett, 1996). This spectrin-actin assembly multimerizes in neurons to span a two dimensional (2D) network in the soma and the quasi one dimensional (1D) MPS in axons and dendrites (Han et al., 2017; Xu et al., 2013). Besides protein-mediated membrane attachment, the spectrin-actin skeleton associates with cell membranes by direct interaction of the PH domain of β-spectrins with phosphatidylinositol lipids (Das, Base, Dhulipala, & Dubreuil, 2006; Das, Base, Manna, Cho, & Dubreuil, 2008; He, Abdi, & Bennett, 2014; Lorenzo et al., 2019; Wang, Miller, Shaw, & Shaw, 1996), and via binding of membrane phosphatidylserine to structural elements within spectrin repeats of α- and β-spectrin polypeptides (An et al., 2004). The PH domain is absent in some isoforms of βI-spectrin (Riederer, Zagon, & Goodman, 1986) and βII-spectrin (Hayes et al., 2000). Similarly, the ΣVI isoform of βIV-spectrin lacks the N-terminal ABD and spectrin repeats 1–9 (Komada & Soriano, 2002; Figure 1a).
Canonical ankyrins (B, G, R) are monomers with an N-terminal membrane binding domain (MBD) comprised of 24 tandem ankyrin repeats (ANK) folded as an extended solenoid with a groove that interacts with unstructured peptides in binding partners (Michaely et al., 2002; Figure 1c). The MBD is followed by two ZU5 domains and a UPA domain folded into a tightly packed supermodule (ZU5N-ZU5C-UPA) (Wang et al., 2012; Figure 1c). The ZU5N-ZU5C-UPA tandem is highly conserved among ankyrins isoforms and across species, suggesting that it plays essential roles in ankyrin’s functions. The N-terminal ZU5 domain (ZU5N) contains the spectrin-binding site (Ipsaro & Mondragón, 2010). The C-terminal ZU5 domain (ZU5C) of ankyrin-B contains a pocket of positively charged residues required for binding to PtdIns(3)P lipids (Lorenzo et al., 2014). The PtdIns(3)P lipids binding site in the 220 kDa isoform of ankyrin-B is conserved in other family members (Wang et al., 2012) and in multiple species, suggesting that other ankyrins might also interact with lipids on membranes through a similar motif. The UPA domain of ankyrin-B contains a binding site for the dynactin subunit p62 (also known as dynactin-4) required for the association of ankyrin-B and the retrograde motor complex in neurons, skeletal muscle cells, embryonic fibroblasts, and adipocytes (Ayalon et al., 2011; Lorenzo et al., 2014; Lorenzo & Bennett, 2017; Qu et al., 2016). The ZU5N-ZU5C-UPA supermodule is followed by a death domain (DD). Interestingly, a similar ZU5N-ZU5C-UPA-DD tandem is present in p53-inducible death-domain-containing protein (PIDD), a scaffolding protein implicated in the regulation of apoptosis in response to DNA damage (Wang et al., 2012). The only known function for the DD of ankyrins to date is that is required to bind RabGAP1L to inactivate Rab22A and to efficiently recycle α5β1-integrin dimers to the leading edge of migrating fibroblasts, which promotes haptotaxis on a fibronectin gradient (Qu et al., 2016). The C-terminus portion of ankyrins consists of an unstructured regulatory domain, which engages in intramolecular interactions (Chen, Li, Wang, Wei, & Zhang, 2017; Mohler, Gramolini, & Bennett, 2002). As mentioned above, ANK2 and ANK3 genes include a giant exon, the peptide product of which are inserted between the UPA and death domains (Figure 1c). This large ankyrin transcripts are selectively expressed in neurons, where they encode for 440 kDa ankyrin-B, and the 270 and 480 kDa ankyrin-G isoforms.
3 |. SUBCELLULAR DISTRIBUTION AND NANO- AND MICRO-ASSEMBLY OF NEURONAL ANKYRINS AND SPECTRINS
The tandem ANK repeats in the MBD of ankyrins (Lee et al., 2006) and the triple-helical repeats of spectrins (Rief, Pascual, Saraste, & Gaub, 1999) exhibit tertiary-structure-based elasticity in single-molecule measurements by atomic force microscopy. Molecular spring-like behavior allows ankyrin and spectrin polypeptides to stretch and elastically recover when subjected to biomechanical challenges, such as the shear stress erythrocytes undergo in the circulation (Krieger et al., 2011) and the mechanical tension axons experience during growth, guidance, and fasciculation (Heidemann & Bray, 2015; Šmít, Fouquet, Pincet, Zapotocky, & Trembleau, 2017). These mechanical properties offer a rationale for the formation of ordered and periodic long-range cytoskeletal ankyrin-spectrin-actin assemblies in erythrocytes and neurons, the only cell types where these arrangements have been observed.
The red blood cell spectrin-actin meshwork is organized in a pseudohexagonal arrangement, where, on average, six spectrin tetramers bind short (~37 nm) actin protofilaments (Byers & Branton, 1985) capped on their fast-growing end by adducin (Gardner & Bennett, 1987; Kuhlman, Hughes, Bennett, & Fowler, 1996), and on the slow-growing end by tropomodulin (Ursitti & Fowler, 1994; Weber, Pennise, Babcock, & Fowler, 1994). Erythrocytic spectrin tetramers have been estimated to be 60–80 nm in length by electron microscopy (Byers & Branton, 1985) and three dimensional (3D)-Stochastic Optical Reconstruction Microscopy (STORM) (Pan, Yan, Li, & Xu, 2018), which corresponds to a “relaxed” conformation. A similar 2D polygonal lattice structure for βIII-spectrin has been visualized in the somatodendritic compartment in cultured rodent hippocampus neurons by 3D-STORM (Han et al., 2017) (Figure 2). Although αII-spectrin was not labeled in these experiments, the αII/βIII spectrin tetramer is the presumed structural unit in these 2D neuronal lattices. However, unlike in erythrocytes, the ~190 nm tetramer length corresponds to its fully extended conformation. The 2D spectrin lattice develops slowly in both the soma and in dendrites in vitro. The fraction of regions exhibiting the 2D pattern increases progressively in the soma from ~10% at day in vitro seven (DIV7) to over 40% at DIV28, and from 5 to 20% during the same time period in dendrites (Han et al., 2017). Interestingly, both treatment with actin-depolymerizing drugs and shRNA knockdown of βII-spectrin disrupt the 2D βIII-spectrin-actin lattice. Since βIII-spectrin expression is only slightly reduced upon βII-spectrin knockdown, this indicates that βII-spectrin is required for the organization of the 2D lattice structure in neurons (Han et al., 2017). However, it has not been shown whether βIII-spectrin is an integral component of the 2D lattice. Additionally, the functional role of the 2D lattice or of βIII-spectrin in the soma and dendritic shafts is not known. In accordance with spectrin skeleton’s canonical role, it is possible that one such function is to stabilize protein complexes in those regions, but those protein partners need to be identified.
FIGURE 2.
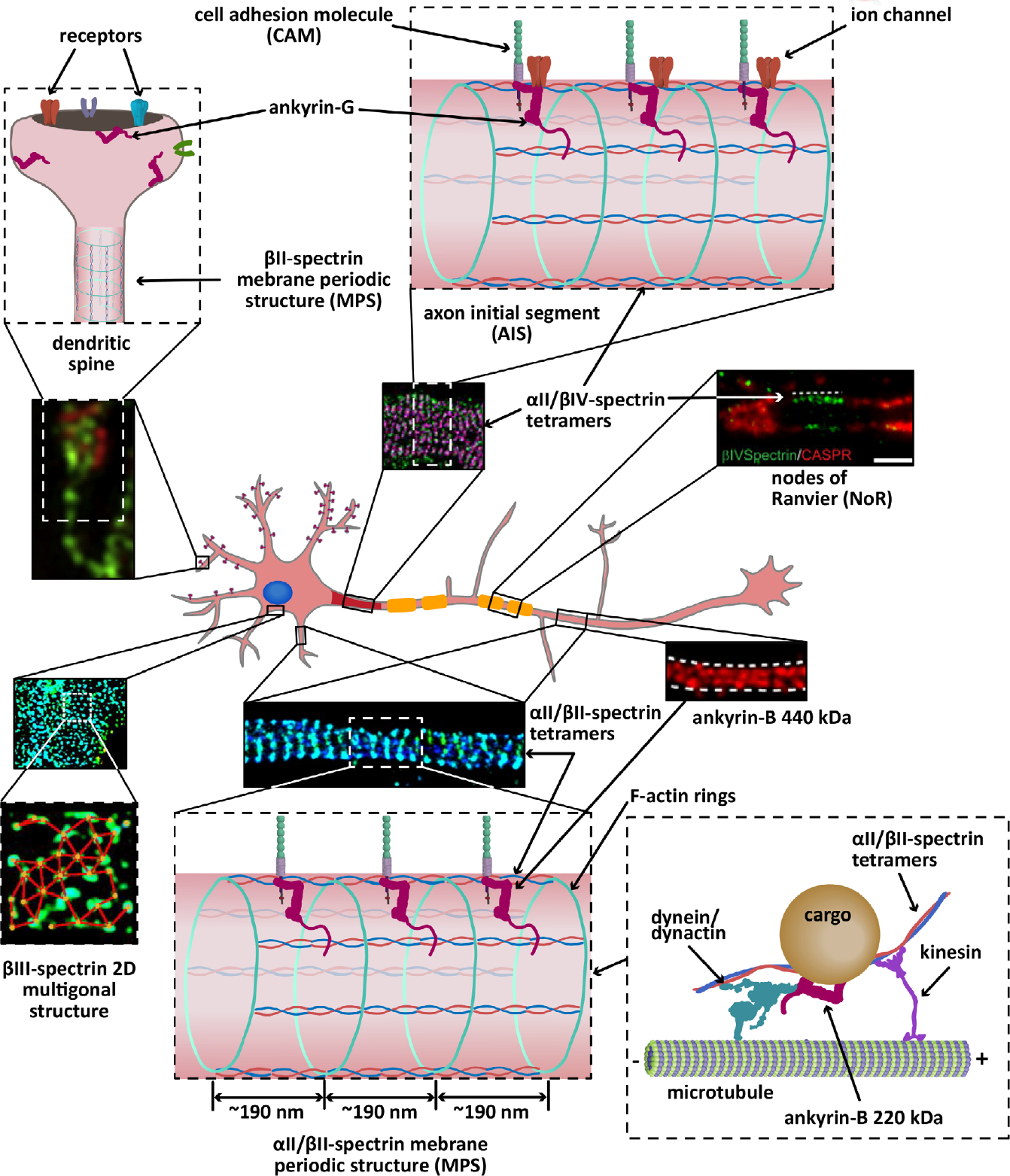
Cartoon representation of the localization and organization of ankyrins and spectrins in a hippocampal neuron. αII/βII-spectrin tetramers crosslink actin rings to line the axon (STORM image adapted from Zhong et al., 2014), parts of the shafts of dendrites, and a portion of spine necks (not shown), all with an average spacing regularity of ~190 nm. Both αII-spectrin and βII-spectrin also associate with microtubule motors to promote axonal transport of synaptic vesicles. The 440 kDa ankyrin-G isoform, which also shows long range periodicity (STED image adapted from Yang, Walder-Christensen, Kim, et al., 2019; Yang, Walder-Christensen, Lalani, et al., 2019), likely binds the periodic axonal βII-spectrin to stabilize cell adhesion molecules, such as L1CAM. Ankyrin-B 220 kDa (not shown) couples the retrograde transport machinery to organelles to facilitate axonal transport. Like βII-spectrin, βIII-spectrin forms periodic quasi-1D structures in dendritic processes (not shown), in addition to 2D polygonal lattices in the soma (STORM image adapted from Han et al., 2017, with permission). Similarly, αII/βIV-spectrin tetramers form periodic quasi-1D structures in the axon initial segment (AIS) (STORM image adapted from Huang, Zhang, Ho, et al., 2017) and nodes of Ranviers (NoR) (STED image adapted from D’Este, Kamin, Balzarotti, & Hell, 2017, with permission) in myelinated axons, where it binds ankyrin-G to stabilize cell adhesion molecules and ion channels. Ankyrin-R and βI-spectrin concentrate at the nodes to stabilize ion channels (not shown). βI-spectrin is also found in the soma, dendrites, and spines (not shown). Ankryin-G also localizes to dendritic spines (SIM image adapted from Smith et al., 2014, with permission), where it is required for the retention of AMPA receptors
3.1 |. Organization of ankyrins and spectrins: dendrites
Besides 2D lattices, βIII-spectrin and F-actin predominantly form a quasi 1D ladder-like MPS structure in dendrites (Han et al., 2017). Likewise, βII-spectrin, the most ubiquitous β-spectrin in neurons, forms an MPS lattice in dendrites and axons (Xu et al., 2013; Zhong et al., 2014; Figure 2). In all cases, the average spacing of the actin rings in the MPS remains constant at ~190 nm. The rate of formation of the dendritic 1D pattern for both βII-spectrin and βIII-spectrin in cultured mouse neurons is relatively slow, with only a small fraction of the 1D MPS structure appearing at DIV7 and progressively increasing to 60% at DIV28 (Han et al., 2017; Zhong et al., 2014). The frequency, regularity, and extension of the dendritic MPS are independent of their distance from the soma or of dendrite diameter. Loss of ankyrin-B results in an axo-dendritic redistribution of βII-spectrin (Zhong et al., 2014) without overall changes in its total brain expression (Lorenzo et al., 2014). βII-spectrin intensity in dendrites lacking ankyrin-B doubles compared to control neurons (Zhong et al., 2014). Interestingly, the periodicity of βII-spectrin in dendrites of DIV10 ankyrin-B knockout neurons becomes similar to that observed in axons, and differs from its more irregular pattern at this timepoint in dendrites of wild type neurons (Han et al., 2017; Zhong et al., 2014). This points to βII-spectrin local concentration as a determining factor in the formation of the MPS and to ankyrin-B playing a role in the polarized distribution of βII-spectrin between dendrites and axons, although the underlying mechanism is unknown.
βII-spectrin also forms an MPS in the dendritic spine neck of mature (DIV16–21) cultured neurons (Bär, Kobler, van Bommel, & Mikhaylova, 2016; He et al., 2016; Sidenstein et al., 2016) and likely in vivo (Bär et al., 2016; Figure 2). The periodic βII-spectrin pattern has been detected in around 25 or 50% of spines, a discrepancy that may be attributed to differences in culturing, staining, and imaging techniques (3D-STORM; He et al., 2016 vs. Stimulated emission depletion, Bär et al., 2016). These studies reveal that F-actin also organizes periodically at the spine neck, although, unlike βII-spectrin, it is present in virtually every spine independently of morphology and size (Bär et al., 2016). Notably, βII-spectrin periodicity pattern was present in some of the spine necks stemming from the shaft regions with nonperiodic βII-spectrin distribution.
Regardless of potential discrepancies in βII-spectrin staining, the presence of periodic actin rings without intercalating βII-spectrin tetramers in a fraction of spine necks suggest that other β-spectrins may selectively help assemble the dendritic MPS. βIII-spectrin is the most likely candidate, given its abundance in spines and enrichment at the neck of spines, as shown by confocal and platinum replica electron microscopy (Efimova et al., 2017). However, periodic βIII-spectrin arrangements in spines have not been reported. Nonperiodic βII-spectrin and βIII-spectrin signal can also be detected in spine heads (Bär et al., 2016; Han et al., 2017; He et al., 2016), but do not colocalize with postsynaptic density (PSD) markers. In contrast, EM immunogold labeling (Fifková & Morales, 1992; Sytnyk, Leshchyns’ka, Nikonenko, & Schachner, 2006) and confocal microscopy (Ursitti et al., 2001) images show βI-spectrin enrichment at PSD sites. Disruption of βI-spectrin binding to actin results in morphological and functional changes in dendritic spines (Nestor, Cai, Stone, Bloch, & Thompson, 2011). βI-spectrin is also found in the soma and throughout dendrites (Lambert & Bennett, 1993; Malchiodi-Albedi, Ceccarini, Winkelmann, Morrow, & Petrucci, 1993), but whether it forms periodic structures in neurons is not known. Interestingly, the absence of βI-spectrin colocalization with αII-spectrin in the head of spines suggests that it functions in its monomeric form in this subsynaptic domain (Ursitti et al., 2001). βIV-spectrin has also been suggested to be a component of dendritic spines based on the finding that an ankyrin-G mutation that abrogates binding to βIV-spectrin, also abolishes ankyrin-G localization in spines, and its function in regulating spine morphology (Smith et al., 2014). However, direct visualization of βIV-spectrin localization and distribution in spines has not been reported. On the other hand, structured illumination microscopy (SIM) images show nonperiodic nanoclusters of ankyrin-G in the head and neck of spines, the majority of which corresponds to its 190 kDa isoform, which promotes AMPA receptor retention in spines and spine plasticity (Smith et al., 2014; Figure 2).
3.2 |. Organization of ankyrins and spectrins: axons
Both major neuronal ankyrin-B isoforms are expressed throughout the axon and are required for axonal growth, guidance, and for establishing proper structural axonal connectivity (Lorenzo et al., 2014; Yang, Walder-Christensen, Kim, et al., 2019). Combined loss of 220 and 440 kDa ankyrin-B results in deficits in axonal growth in cultured neurons and absence of long-range axons in mouse brains (Lorenzo et al., 2014; Scotland, Zhou, Benveniste, & Bennett, 1998). In contrast, specific loss of 440 kDa ankyrin-B causes axonal hyperbranching in vitro and formation of ectopic axonal tracts in mice (Yang, Walder-Christensen, Kim, et al., 2019). 3D-STORM of axons labeled with a C-terminal pan-ankyrin-B antibody shows that axonal ankyrin-B proteins organize in a partially periodic pattern (Xu et al., 2013). This could be due to the additional presence of 220 kDa ankyrin-B on intracellular membranes (Lorenzo et al., 2014). This observation is supported by STED images of axons labeled with a 440 kDa ankyrin-B-specific antibody, which shows a ~190 nm periodicity, suggesting that 440 kDa ankyrin-B is a component of the MPS (Yang, Walder-Christensen, Kim, et al., 2019; Figure 2). Dual knockout of both ankyrin-B isoforms in neurons does not disrupt βII-spectrin-actin periodic assembly (Lorenzo et al., 2014), which suggests that recruitment of βII-spectrin to the axonal membrane lattice does not rely entirely, if at all, on its interaction with ankyrin-B.
Like ankyrin-B, βII-spectrin is also distributed through axons where it promotes axonal connectivity through biochemically distinct functions in axon growth and stability (Lorenzo et al., 2019). Targeted knockout of βII-spectrin in neuronal progenitors results in major loss of long-range axonal tracts, enlarged axonal diameter, as well as axonal degeneration (Lorenzo et al., 2019). βII-spectrin-deficient neurons have reduced axonal growth in vitro and exhibit loss of periodic axonal actin rings (Lorenzo et al., 2019). It is possible that the periodic membrane skeletal network confers stability to axons, and loss of this structure may contribute to the axonal degeneration in βII-spectrin-deficient brains.
3.2.1 |. Ankyrins and spectrins in axonal domains: axon initial segment
Ankyrin-G is widely recognized as the “master regulator” of the AIS because it orchestrates the recruitment and organization of all known AIS components (Jenkins & Bennett, 2001; Leterrier et al., 2017; reviewed in Leterrier, 2018; Rasband, 2010; Zhou et al., 1998). Both 270 and 480 kDa ankyrin-G isoforms preferentially localize to the AIS (Jenkins et al., 2015; Rasband, 2010). However, 480 kDa ankyrin-G plays the most prominent role in organizing the AIS, as demonstrated by the complete loss of known AIS components in 480 kDa-specific knockout neurons, and the full rescue of the correct length, position, and molecular organization of the AIS in total ankyrin-G null neurons by a 480 kDa ankyrin-G, but not a 270 kDa ankyrin-G plasmid (Jenkins et al., 2015).
βIV-spectrin, the β-spectrin most enriched at the AIS (Berghs et al., 2000; Lacas-Gervais et al., 2004), depends on ankyrin-G for its recruitment to this axonal domain (Jenkins et al., 2015; Komada & Soriano, 2002; Yang, Ogawa, Hedstrom, & Rasband, 2007), where both molecules are arranged with a ~190 nm periodicity (Leterrier et al., 2015; Zhong et al., 2014; Figure 2). The formation of a periodic βIV-spectrin-actin lattice at the AIS is preceded by an equivalent βII-spectrin-actin periodic structure, which is present in the proximal axon by DIV2, and subsequently expands toward the distal axon to virtually cover its full length by DIV10 (Zhong et al., 2014). Concomitantly with a reduction in both βII-spectrin signal and periodicity in the AIS by DIV8, ankyrin-G, and βIV-spectrin levels increase to become highly periodic by DIV12 (Zhong et al., 2014). Knockdown of βII-spectrin at DIV3, prior to the recruitment of βIV-spectrin to the AIS, but not at DIV7, affects βIV-spectrin periodicity (Zhong et al., 2014). These results suggest that βII-spectrin is important for the formation but not the maintenance of βIV-spectrin periodic assembly at the AIS (Zhong et al., 2014). Similarly, loss of βII-spectrin does not affect the morphology of the AIS in vivo and in cultured neurons (Lorenzo et al., 2019). These results differ from previous observations showing that knockdown of βII-spectrin affects the size of the AIS (Galiano et al., 2012). The source of this discrepancy is not clear, but it is possible that originates from experimental differences between the two studies. For example, normal AIS length was observed in DIV11 cortical neurons derived from mice constitutively lacking βII-spectrin (Lorenzo et al., 2019), while acute adenovirus-mediated shRNA knockdown of βII-spectrin in DIV7 rat hippocampal neurons resulted in longer AIS (Galiano et al., 2012). A similar disagreement was observed between histological characterizations of AIS length in the same βII-spectrin KO mice, possibly also due to experimental differences between the two studies. No apparent changes were observed in AIS length of postnatal cortical neuron day 6 (PND6) of βII-spectrin KO mice detected using an antibody specific for the AIS marker 480 kDa ankyrin-G (Lorenzo et al., 2019). In contrast, staining of hippocampal sections of βII-spectrin KO mice of unreported age with a βIV-spectrin-specific antibody, also an AIS marker, detected significant AIS fragmentation and increased area of immunoreactivity (Galiano et al., 2012). It is worth noting that loss of βII-spectrin, both in vivo and in vitro, results in over a 40% increase in βIV-spectrin-ΣVI (Lorenzo et al., 2019), which is critical for the maintenance of the AIS (discussed below; Yang, Walder-Christensen, Lalani, et al., 2019), and may explain some of the observed differences between the studies. Given the importance and multifaceted roles of βII-spectrin, it will important to further assess how it might impact AIS morphology, composition, and function.
βIV-spectrin periodicity at the AIS is not affected by chemical disruption of the periodic actin rings (Leterrier et al., 2015). This contrasts with the interdependence of the periodic arrangement of βII-spectrin and actin, which individual disruptions cause their reciprocal disorganization (Zhong et al., 2014). The robustness of the periodic AIS βIV-spectrin lattice could be attributed to its binding to ankyrin-G, which may stabilize its localization in the absence of periodic actin (Leterrier et al., 2015). However, the DAR999AAA mutation in 480-kDa ankyrin-G, which eliminates the canonical binding of ankyrin-G to βII-spectrin (He et al., 2014; Kizhatil et al., 2007), has no effect on 480 kDa ankyrin-G or βIV-spectrin clustering at the AIS (Jenkins et al., 2015), suggesting that either the site for βIV-spectrin binding is not conserved in 480-kDa ankyrin-G and/or additional coupling mechanisms between these two partners exist (Jenkins et al., 2015). On the other hand, introduction of the Y1901A mutation (corresponding to the Y1874A mutation in βII-spectrin that disrupts binding to ankyrin-G) in βIV-spectrin-ΣI, the full-length βIV-spectrin splice variant, fails to restore AIS morphology and the clustering of 480-kDa ankyrin-G, and its AIS partners, in βIV-spectrin null neurons (Yang, Walder-Christensen, Lalani, et al., 2019). Surprisingly, deficits in AIS morphology and molecular composition of βIV-spectrin null neurons can be rescued in vitro with βIV-spectrin-ΣVI, the other βIV-spectrin isoform present in the AIS, which lacks the actin binding site plus the first nine spectrin repeats, but retains the ankyrin-G binding site and a PH domain (Yang, Walder-Christensen, Lalani, et al., 2019). This is consistent with the actin skeleton not being required for proper βIV-spectrin organization and function in the AIS, as mentioned above (Leterrier et al., 2015). Targeting of βIV-spectrin to the AIS can be further modified by conformational changes in 480 kDa ankyrin-G, which, in turn, are modulated by phosphorylation of specific residues (S1982A, S2619A, and S2417A) or by human neurodevelopmental mutations (T1861M, K2864N, and P2490L), all within the inserted region unique to this giant isoform (Jenkins et al., 2015; Yang, Walder-Christensen, Lalani, et al., 2019). 480 kDa ankyrin-G can expand 150 nm in its extended conformation in the mature AIS, as revealed by immunogold label platinum replica EM imaging (Jenkins et al., 2015; Jones, Korobova, & Svitkina, 2014), and inferred from proximity ligation signaling with antibodies against its N- and C-terminal ends (Yang, Walder-Christensen, Lalani, et al., 2019). Note that 3D-STORM imaging suggests an average radial conformation of ~32 nm of the inserted and C-terminus region of 480 kDa ankyrin-G, which might reflect a more relaxed conformation of the polypeptide, although it could also underestimate the true length of the protein depending on its orientation relative to the imaging direction (Leterrier et al., 2015). Nonetheless, the dangling unstructured C-terminal end of 480 kDa ankyrin-G and its N-terminal region may be far enough apart to prevent intramolecular interactions which could result in an inhibitory closed conformation and subsequent loss of binding and recruitment of βIV-spectrin to the AIS (Yang, Walder-Christensen, Lalani, et al., 2019).
αII-spectrin is also organized with a ~190 nm periodicity in the AIS, which is consistent with its incorporation into periodic αII-spectrin-βIV-spectrin tetramers (Huang, Zhang, Ho, et al., 2017). αII-spectrin associates with βIV-spectrin-ΣI and with βIV-spectrin-ΣVI, which lacks the canonical spectrin dimerization domain within the first two spectrin repeats. However, spectrin repeats 14 and 15, present in both βIV-spectrin isoforms can bind αII-spectrin and may provide a second binding region (Huang, Zhang, Ho, et al., 2017). STORM imaging of hippocampal neurons lacking αII-spectrin shows disruption of βIV-spectrin periodicity at the AIS, likely due to a significant reduction in βIV-spectrin-ΣI expression (Huang, Zhang, Ho, et al., 2017). This loss of βIV-spectrin periodicity in αII-spectrin knockout neurons happens despite a fourfold compensatory increase in βIV-spectrin-ΣVI, which has been shown to rescue normal features of the AIS (Yang, Walder-Christensen, Lalani, et al., 2019). Similarly, loss of αII-spectrin disrupts the periodicity of βII-spectrin in the distal axon (Huang, Zhang, Ho, et al., 2017), which could be due to the combined effects of the almost 80% reduction in expression of βII-spectrin in αII-spectrin null neurons, absence of the βII/αII-spectrin tetramer, and likely disruption of the actin rings.
3.2.2 |. Ankyrins and spectrins axonal domains: nodes of ranvier
NoR represent another specialized axonal domain where ankyrins and spectrins collaborate structurally and functionally (reviewed in Rasband & Peles, 2015). Although NoR are structurally, molecularly, and functionally similar to the AIS, their formation requires both intrinsic factors and myelinating glial cells (reviewed in Eshed-Eisenbach & Peles, 2013). The nodal gap is the region of voltage gated sodium channel (Nav) clustering and its boundaries and stabilization are determined by glial-axon interactions requiring specific CAMs and extracellular matrix (ECM) components (reviewed in Eshed-Eisenbach & Peles, 2013 and Rasband & Peles, 2015). Like in the AIS, ankyrin-G and βIV-spectrin are confined to the nodal gap, where they are arranged with a ~190 nm periodicity (D’Este et al., 2017; D’Este, Kamin, Gottfert, El-Hady, & Hell, 2015; Figure 2). Interestingly, while βIV-spectrin-ΣI is the predominant isoform at the NoR early in development, βIV-spectrin-ΣVI surpasses it in expression and continues to increase shortly after birth (Yoshimura, Stevens, Leterrier, Stankewich, & Rasband, 2016). It is not clear, however, how the switch in expression levels of these isoforms affect their skeletal ultrastructure at the nodes. Nav binding to ankyrin-G is necessary and sufficient for proper clustering at the nodes (Gasser et al., 2012). However, loss of ankyrin-G or βIV-spectrin does not affect Nav clustering in dorsal root ganglion (DRG) sensory neurons in vivo because their localization and stabilization is rescued by a compensatory mechanism whereby ankyrin-R and βI-spectrin concentrate at the nodes to stabilize these channels (Ho et al., 2014). However, whether ankyrin-R and/or βI-spectrin adopt a periodic structure at the nodal gap in ankyrin-G or βIV-spectrin deficient NoR has not been determined. In contrast to these findings, other reports have shown that loss of ankyrin-G progressively disrupts nodal organization in sciatic nerve (SN) and spinal cord (SC) neurons leading to axonal degeneration (Saifetiarova, Taylor, & Bhat, 2017). A potential reason for this discrepancy is that, unlike DRG neurons, both SN, SC neurons have an AIS, which structure, and function is affected by loss of ankyrin-G. Thus, it is possible that, if the ankyrin-R/βI-spectrin compensatory mechanism is also operating in these neurons, it may eventually be overriding by degenerating effects of loss of ankyrin-G in the AIS (Zhou et al., 1998).
βII-spectrin is a prominent constituent of the paranodal cytoskeleton, where it is also periodically organized (D’Este et al., 2017; Ogawa et al., 2006) and provides an alternative mechanism to maintain Nav channel clustering at the nodal gap in 186 kDa neurofascin-deficient axons (Amor et al., 2017). Paranodes serve as junctions where myelin attaches to the axons as well as physical barrier determinants of the adjacent juxtaparanodes, which extend beneath the myelin sheath, and are enriched with Kv1 potassium channels (reviewed in Poliak & Peles, 2003 and Rasband, 2004 and Salzer, 2003). Loss of βII-spectrin disrupts the paranode-juxtaparanode barrier and allows Kv1 channels to diffuse into paranodes and nodal gaps (Zhang, Susuki, Zollinger, Dupree, & Rasband, 2013). Similarly, αII-spectrin forms a periodic cytoskeleton at nodes and paranodes and is required for NoR assembly and maintenance (Huang, Zhang, Zollinger, Leterrier, & Rasband, 2017). Loss of αII-spectrin disrupts the periodicity of βIV-spectrin at the nodal gap and of βII-spectrin at paranodes, and leads to fewer intact nodes, in which Kv1.2 channels are no longer restricted to juxtaparanodes (Huang, Zhang, Zollinger, et al., 2017). Ankyrin-B also concentrates in the paranodal region, where it interacts with βII-spectrin (Ogawa et al., 2006). Ankyrin-B is expressed in both axons and myelinating glia, and it appears to need an intact paranode to concentrate in this domain (Chang et al., 2014; Ogawa et al., 2006). Interestingly, ankyrin-B has been shown to form a periodic structure in the paranode, which has been attributed to glial ankyrin-B, although it is not clear how its cellular identity was distinguished (D’Este et al., 2017). Ankyrin-B is also widely expressed in sensory axon terminals of the peripheral nervous systems in various mammalian species, where, like in myelinated CNS axons, concentrates in the paranodal regions (Engelhardt, Vorwald, Sobotzik, Bennett, & Schultz, 2013). However, unlike in CNS neurons, in these PNS neurons ankyrin-B, but not ankyrin-G, accumulates and shows a high degree of colocalization with NaV1 channels in the axon terminals, where it may have important roles supporting their mechanoreceptive and nociceptive properties (Engelhardt et al., 2013).
4 |. FUNCTIONAL ROLES OF THE SPECTRIN AND ANKYRIN ASSEMBLIES IN NEURONS
4.1 |. Structural and mechanical neurite stability
The functional significance of neuronal ankyrins and spectrins in the development and maintenance of neuronal processes is underscored by the striking morphological phenotypes of mice and humans with deficiencies in these proteins. For instance, conditional loss of αII-spectrin or βII-spectrin in mouse brains results in disruption of the MPS, axonal degeneration, and juvenile lethality (Galiano et al., 2012; Huang, Zhang, Zollinger, et al., 2017; Lorenzo et al., 2019). Loss of βII-spectrin or ankyrin-B leads to shorter axons in vivo and in vitro and deficits in long-range white matter connectivity in mice (Lorenzo et al., 2014, 2019). Mutations in αII-spectrin cause human early infantile epileptic encephalopathies (EIEE) with progressive brain atrophy (Saitsu et al., 2010; Syrbe et al., 2017; Tohyama et al., 2015), likely due to aberrant neurite development and defective GABAergic innervation of cortical pyramidal neurons (Wang et al., 2018). Similarly, human mutations in SPTBN2 that cause spinocerebellar ataxia 5 (SCA5) leads to cerebellar degeneration in mice (Armbrust et al., 2014) and patients (Ikeda et al., 2006), which is also observed upon loss of βIII-spectrin in mice (Gao et al., 2011; Stankewich et al., 2010).
The compromised structural integrity of the neuronal actin- and spectrin-based MPS may be a direct contributing factor to the cell-autonomous degeneration caused by ankyrin or spectrin deficiencies. This suspected role of the MPS in neurons has been substantiated by observations of increased membrane fragility in erythrocytes with molecular defects in the membrane skeleton (Agre et al., 1982; Chasis et al., 1988; reviewed in Eber & Lux, 2004), and of axonal breakage in Caenorhabditis elegans lacking β-spectrin that are exposed to torsion strain during motion (Hammarlund et al., 2007; Krieg, Dunn, & Goodman, 2014). However, even though neurons in mammalian systems experience some mechanical challenges under normal conditions (Heidemann & Bray, 2015; Šmít et al., 2017), whether these forces can cause damage in neurons with a disassembled MPS has not been shown. While this remains an open question, the role of the MPS in neuronal degeneration has started to emerge (Unsain et al., 2018; Wang, Simon, et al., 2019).
Trophic deprivation (TD) of DGR neurons, which induces axonal degeneration, results in an early and significant disruption of the MPS prior to axonal fragmentation (Unsain et al., 2018; Wang, Simon, et al., 2019). This early MPS disassembly after TD is independent of protein loss and of apoptotic pathway activation, as demonstrated in sensory axons that either overexpressed the apoptotic protein Bcl-xL, or that lacked the apopotic factor Bax (Wang, Simon, et al., 2019). Similarly, pharmacological stabilization of F-actin prevents both MPS loss and axonal fragmentation upon TD treatment (Unsain et al., 2018; Wang, Simon, et al., 2019). Conversely, loss of actin polymerization, which disrupts the MPS (Xu et al., 2013), also induces axonal degeneration (Wang, Simon, et al., 2019). It is believed that MPS stabilization and disruption, respectively, suppresses and activates the prodegenerative anterograde signaling molecules MAPKKK dual leucine zipper kinase (DLK) and c-Jun (Wang, Simon, et al., 2019). The structural reorganization of the MPS may act as a signal in TD-induced axon degeneration. This proposed new role is supported by the surprising finding that genetic knockdown or knockout of βII-spectrin suppresses retrograde signaling and protects axons from TD-induced degeneration (Wang, Simon, et al., 2019). An plastic MPS could modulate retrograde signaling during TD-induced axon degeneration through two plausible and nonmutually exclusive mechanisms, which are dependent on the integrity of the MPS and/or the normal abundance of its components. One possibility is that the MPS acts as a regular supra-structure that correctly docks and distributes the molecules involved in the degenerative pathway in a latent state along the axon (see discussion below) (Wang, Simon, et al., 2019). Conceivably, when the MPS does not form, as in the case of genetic knockout of βII-spectrin (Lorenzo et al., 2019; Xu et al., 2013), mislocalization of prodegenerative signaling molecules impairs the retrograde degeneration cascade. Alternatively, or in parallel, absence of βII-spectrin, which results in bidirectional deficits in axonal transport (Lorenzo et al., 2019), may obstruct the retrograde flow of degradative signaling molecules. However, competing transport defects of other cargo caused by loss of βII-spectrin, which are independent from the formation of the MPS, do eventually result in degeneration of cortical axons (Lorenzo et al., 2019), despite the protection against TD-induced degeneration of sensory axons resulting from the loss of βII-spectrin (Wang, Simon, et al., 2019).
4.2 |. Recruitment and stabilization of neuronal membrane partners
The core competency of the ankyrin-spectrin partnership is its ability to stabilize binding partners, including various CAMs, ion channels, and membrane receptors at specialized plasma membrane sites (reviewed in Bennett & Lorenzo, 2013 and Bennett & Lorenzo, 2016). A typical scheme for ankyrin recruitment and stabilization of a specific partner to a specialized membrane is the anchoring of that complex to an αII/β-spectrin scaffold, in which the β-spectrin family member may be determined by preferential binding to that particular ankyrin, and/or its segregation to that specialized domain through independent mechanisms. Since ankyrins possess multivalent binding abilities throughout their functional domains, the same or a neighboring ankyrin molecule can bind additional partners. Given the repertoire of ankyrins and spectrins, and their ability for long-range spatial organization, this strategy allows them to position diverse molecular nanoclusters of various dimensions, proximity, and regularity along the neuronal membrane to facilitate local and global physiological demands.
4.2.1 |. Ankyrin binding partners
CAMs and ion channels
As described above, 480 kDa ankyrin-G orchestrates the organization of the AIS and NoR, where it binds the 186 kDa isoform of the CAM neurofascin (Jenkins & Bennett, 2001; Jenkins et al., 2015; reviewed in Leterrier, 2018; Leterrier et al., 2017; reviewed in Rasband, 2010; Zhou et al., 1998). Neurofascin binds ankyrin-G’s MBD through the conserved amino acid sequence FIGQY in its cytosolic domain, which is modulated by tyrosine (Y) phosphorylation (Garver, Ren, Tuvia, & Bennett, 1997; Tuvia, Garver, & Bennett, 1997). Ankyrin-G’s MBD has two partially overlapping neurofascin-interacting motifs that allow a single ankyrin-G to simultaneously bind two neurofascin molecules (Michaely & Bennett, 1995; Wang et al., 2014). Similarly, ankyrin-G also uses its MBD to bind voltage-gated sodium channels of the Nav1 family by recognizing a nine amino acid motif in the cytosolic loop that links the transmembrane domains II and III in these channels (Garrido et al., 2003; Lemaillet, Walker, & Lambert, 2003). The AIS and NoR generally express the Nav1 family members Nav1.2 and Nav1.6, which expression are developmentally controlled (Kaplan et al., 2001), but may also include other Nav1 members, depending on the neuron type (Wang, Ou, & Wang, 2017). Ankyrin-G also binds potassium channels Kv7.2 (KCNQ2) and Kv7.3 (KCNQ3) (expressed as either KCNQ2/3 heteromers or KCNQ2 homomers) through a conserved 10 amino acid stretch in these channels, which has a striking similarity with the ankyrin-G-binding sequence in Nav1 channels (Pan et al., 2006).
The association of ankyrin-G with Nav1 and KCNQ2/3 is further modulated through phosphorylation of the channels by protein kinase CK2, which significantly augments their binding affinity (Bréchet et al., 2008; Hien et al., 2014; Xu & Cooper, 2015). Ankyrin-G shares the binding region for Nav1 and KCNQ2/3 located in the N-terminus half of the MBD, although different residues in the MDB determine the specific binding and the strength of their relative affinities to each channel, which in turn controls channel ratio and relative activity, and might modulate AIS excitability (Wang et al., 2014). Structural, biochemical, and cellular studies have shown that ankyrins may use an autoinhibitory mechanism, whereby unstructured portions of these molecules located in the C-terminus or linker regions interact with sites within the MBD in a quasi-independent and combinatorial manner (Chen et al., 2017; Galiano et al., 2012; He, Tseng, & Bennett, 2013). This mechanism offers a rationale for the phosphorylation-induced conformational change of ankyrin-G that may lead to autoinhibition in the AIS (Yang, Walder-Christensen, Lalani, et al., 2019). A common feature of the interacting sites across all these different partners of ankyrin-G is that they correspond to unstructured regions within these partnering molecules, which makes it sterically and energetically favorable to have their binding regions interact with ankyrin-G along its MBD solenoid (Wang et al., 2014). Unsurprisingly, given their close association with ankyrin-G, neurofascin, Nav1, and Kv7 channels all show periodic arrangements in the AIS or NoR (D’Este et al., 2017; Leterrier et al., 2015; Xu et al., 2013). Likewise, mutations in these proteins have been linked to converging or overlapping pathologies, such as epilepsy, ASD, and bipolar disorder (Kloth et al., 2017; Singh et al., 1998; Spratt et al., 2019; Wirgenes et al., 2014).
The CAM family member L1CAM also associates with ankyrins through the conserved FIGQY sequence in the cytosolic tail (Davis & Bennett, 1994). A few hundred pathogenic mutations in the L1CAM gene have been described in patients with a broad spectrum of neurological abnormalities, including aphasia, shuffling gait, adducted thumbs, hydrocephalus, agenesis of the corpus callosum, SPG1 (X-linked hereditary spastic paraplegia type 1), and mental retardation, collectively referred to as CRASH syndrome (Schafer et al., 2010; Vos et al., 2010;). L1CAM is expressed along the length of the axon and L1CAM-deficient mice show defects in axon guidance, corpus callosum dysgenesis, and other brain malformations (Dahme et al., 1997; Demyanenko, Tsai, & Maness, 1999; Rathjen & Schachner, 1984). Ankyrin-B as candidate partner of L1CAM was first reported based on evidence of functional coupling between the two proteins demonstrated by their co-distribution in premyelinated axons in the cerebral cortex, the reduction in L1CAM but not in NCAM expression in brains of ankyrin-B knockout mice, and some similarities in brain defects between ankyrin-B and L1CAM knockout mice (Scotland et al., 1998). Additional characterization of the ankyrin-B knockout mouse revealed that loss of 220 kDa ankyrin-B contributes to the axonal phenotype by impairing axonal transport, a function that likely does not involve L1CAM (Lorenzo et al., 2014). Consistent with that observation is the finding that 440 kDa ankyrin-B colocalizes with L1CAM in a proximity ligation assay (PLA), and that signal is markedly diminished in brain tissue along the corpus callosum of 440 kDa ankyrin-B-specific knockout mice (Yang, Walder-Christensen, Kim, et al., 2019). Brains of knock-in L1CAM Y1229H mice (Buhusi, Schlatter, Demyanenko, Thresher, & Maness, 2008), which lack ankyrin-binding activity, shows a similar reduction in PLA signal (Yang, Walder-Christensen, Kim, et al., 2019). Since both 220 and 440 kDa ankyrin-B isoforms shared the known L1CAM binding region in the MBD, the structural basis for the preferential association of L1CAM and 440 kDa ankyrin-B is not clear, but it may involve difference in autoinhibition mechanisms caused by the insertion of the giant exon in 440 kDa ankyrin-B (Yang, Walder-Christensen, Kim, et al., 2019). Both complete loss and 50% reduction in 440 kDa ankyrin-B, as well as expression of L1CAM Y1229H cause axon hyperbranching in vitro, which constitutes more evidence of functional partnering by these proteins (Yang, Walder-Christensen, Kim, et al., 2019).
Interestingly, neurons differentiated from human embryonic stem cells carrying conditional L1CAM loss-of-function mutations or lacking L1CAM showed shorter axons, decreased axonal branching and AIS area, and mild dendritic arborization defects (Patzke, Acuna, Giam, Wernig, & Sudhof, 2016). L1CAM-deficient human neurons showed 20–30% reduction in total ankyrin-B, including lower 220 kDa ankyrin-B levels, which may be partially responsible for the shorter axons (Lorenzo et al., 2014). However, this slight reduction in ankyrin-B did not positively affect axonal branching, as recently shown (Yang, Walder-Christensen, Kim, et al., 2019). These differing results suggests that there is either a minimum threshold for loss of 440 kDa ankyrin-B expression to have an impact in branch development, and/or L1CAM may also promote axonal branching through ankyrin-B-independent mechanisms. Loss of L1CAM in human neurons also resulted in 20–50% reduction in ankyrin-G expression, which partially explains the AIS defects and modest reductions in sodium currents (Patzke et al., 2016). The functional interaction between L1CAM and ankyrins is further demonstrated by the inability of L1CAM human mutations (R1166X and S1224L) within the ankyrin-binding region to rescue several of the phenotype of L1CAM deficient human neurons (Patzke et al., 2016). However, unlike for ankyrin-B, it is not clear whether the functional partnership between L1CAM and ankyrin-G results from their direct interaction. A recent report describing the requirement of L1CAM in neocortical pyramidal neurons (PyNs) to facilitate innervation of their AIS by chandelier cells (ChCs) and to establish axo-axonic synapse provides additional evidence for a functional, and potentially structural, L1CAM-ankyrin-G complex (Tai, Gallo, Wang, Yu, & Van Aelst, 2019). In this study, the L1CAM Y1229H mutation in the ankyrin-binding region did not rescue loss of ChC/PyN AIS innervation in L1CAM knockout PyNs, and knockout of βIV-spectrin phenocopied the L1CAM null phenotype (Tai et al., 2019). Since the Y1229H mutation also abrogates L1CAM association with 440 kDa ankyrin-B, and ankyrin-B is also present in the AIS, it would be interesting to test if ankyrin-B is also involved in the mechanism of L1CAM stabilization at the AIS of PyNs.
Microtubules and microtubule-associated proteins
Both ankyrin-G and ankyrin-B associate with microtubules in neurons (Davis & Bennett, 1984; Fréal et al., 2016; Leterrier et al., 2011; Sobotzik et al., 2009; Yang, Walder-Christensen, Kim, et al., 2019). EM micrographs of the AIS from Purkinje neurons devoid of all ankyrin-G isoforms showed absence of cytoplasmic microtubule bundles (Sobotzik et al., 2009). These authors speculated that 480 kDa ankyrin-G, which contains an elongated tail domain, was a likely candidate to structurally interact with and stabilize microtubules at the AIS (Sobotzik et al., 2009). Subsequent immunogold labeling from platinum replica EM of cultured hippocampal neurons lacking 480 and 270 kDa ankyrin-G isoforms showed similar loss of AIS microtubule bundles (Jenkins et al., 2015). Ankyrin-G has also been suggested to facilitate the organization of microtubules at the AIS via interactions with microtubule end-binding (EB) proteins (Fréal et al., 2016; Leterrier et al., 2011). Pulldown assays from transfected COS-7 cells demonstrated association of EB1 and EB3 with 270 kDa ankyrin-G and co-localization of these EB proteins with ankyrin-G at the AIS (Leterrier et al., 2011). It has been shown that 480 kDa ankyrin-G selectively associates with EB proteins through 10 Ser-x-Ile-Pro (SxIP) motifs present along the giant inserted region (Fréal et al., 2016). These authors discarded a potential interaction of 270 kDa ankyrin-G with EBs based on their lack of colocalization and cotransport in transfected COS-7 cells, in contrast to previous findings (Leterrier et al., 2011). This discrepancy is likely due to the high overexpression of EBs used in the recruitment experiments (Leterrier et al., 2011), which could induce an interaction with the two SxIP motifs present in 270 kDa ankyrin-G. The interaction of 480 kDa ankyrin-G and EB proteins at the AIS is reciprocally required for their mutual localization and stability in the proximal axon, proper microtubule organization, and AIS formation (Fréal et al., 2016). A recent report demonstrated that microtubule-bound TRIM46 is required for the recruitment of microtubules to the AIS plasma membrane by 480 kDa ankyrin-G and to drive retrograde transport of neurofascin-186 to the membrane in the AIS (Fréal et al., 2019). Collectively, these studies provide strong evidence for a role of giant ankyrin-G in organizing microtubules in the AIS.
Ankyrin-G also interacts with neuronal microtubule-associated proteins (MAPs), including the nuclear distribution protein nudE-like 1 (NDEL1; Kuijpers et al., 2016) and the GABAA receptor-associated protein (GABARAP; Tseng et al., 2015). NDEL1 complexes with 270 kDa ankyrin-G, likely through binding of its C-terminus region to the inserted portion in 270 kDa ankyrin-G, also shared by 480 kDa ankyrin-G. Ankyrin-G is necessary for NDEL1 localization to the AIS, where it promotes the polarized distribution of dendritic cargo by reversing the direction of transport of somatodendritic vesicles at the AIS (discussed below; Kuijpers et al., 2016). A recent report showed that NDEL1’s C-terminal coiled-coil region (CT-CC) binds a similar ~70-amino acids stretch within the inserted region of both ankyrin-B and ankyrin-G with 2:1 stoichiometry (Ye et al., 2020). The crystal structure of the ankyrin-B-NDEL1’s CT-CC complex revealed a stable 5-helix bundle dominated by hydrophobic interactions spread across six distinct interaction layers (Ye et al., 2020). However, while blocking of the NDEL1-binding region in ankyrin-G impaired NDEL1 accumulation at the AIS (Ye et al., 2020), the significance of the ankyrin-B-NDEL1 interaction remains unknown. Similarly, 480 kDa ankyrin-G interacts with GABARAP, a MAP member of the ubiquitin-like LC3 family at extrasynaptic somatodendritic plasma membranes of pyramidal neurons (Tseng et al., 2015). 480 kDa ankyrin-G binds GABARAP through a tryptophan residue (W1989) within a LC3-interacting (LIR) motif in the inserted region (Alemu et al., 2012; Nelson et al., 2018; Tseng et al., 2015). 480 kDa ankyrin-G localization at extrasynaptic GABAergic microdomains is promoted by protein-protein interactions via the inserted region and by its direct insertion into the plasma membrane through C70-dependent palmitoylation, which are both required for stabilizing GABAA receptor synapses (Tseng et al., 2015). Coupling of ankyrin-G to GABARAP stabilizes somatodendritic GABAergic synapses by opposing GABAA receptor internalization through a clathrin-mediated mechanism (Tseng et al., 2015). Interestingly, ankyrin-G does not require its usual neuronal partner βIV-spectrin to form these extrasynaptic microdomains. Instead, ankyrin-G associates with βII-spectrin, as it does in other cell types (reviewed in Bennett & Lorenzo, 2013), but whether this association is necessary to form and stabilize GABAergic extrasynaptic domains has not been tested.
Ankyrin-B co-sediments with microtubules polymerized from brain tubulin (Davis & Bennett, 1984). The P2580fs mutation in mouse 440 kDa ankyrin-B, which mimics the autism-linked R2608fs frameshift mutation in the giant inserted exon of human ANK2, produces a truncated ankyrin-B protein of around 290 kDa missing the C-terminus regulatory domain and a portion of the inserted region (Yang, Walder-Christensen, Kim, et al., 2019). Neurons carrying this mutated ankyrin-B peptide show displacement of microtubule bundles away from the plasma membrane, as observed in EM cross-sections of callosal neurons from mutant mice (Yang, Walder-Christensen, Kim, et al., 2019). P2580fs ankyrin-B mutant neurons also exhibit increased microtubule dynamics, with more EB3 comets entering the shafts of nascent axonal branches P2580fs (Yang, Walder-Christensen, Kim, et al., 2019). Interestingly, loss of 440 kDa ankyrin-B interaction with L1CAM due to expression of the Y1229H mutation in L1CAM phenocopies these microtubule defects (Yang, Walder-Christensen, Kim, et al., 2019). These observations imply a potential interaction between 440 kDa ankyrin-B and microtubules and offer a rationale for the axon hyperbranching observed in Y1229H L1CAM and 440 kDa ankyrin-B deficient neurons. However, confirmation of a direct ankyrin-B-microtubule interaction in native cellular environments and elucidation of its functional significance awaits further study.
4.2.2 |. Spectrin binding partners
It has been proposed that βII-spectrin associates with CAM close homolog of L1 (CHL1) based on their partial colocalization along the axon, the growth cone, and the soma of cultured hippocampal neurons, their co-immunoprecipitation from brain homogenates, and on the ability of purified βII-spectrin to capture the intracellular domain of recombinant CHL1 in an in vitro assay (Tian et al., 2012). Ligand-induced clustering of CHL1 promotes the dissociation of the CHL1-βII-spectrin complex at the plasma membrane as well as lipid-raft dependent internalization of CHL1, which is required for neurite outgrowth (Tian et al., 2012). Although shRNA knockdown of βII-spectrin promotes CHL1 internalization (Tian et al., 2012), constitutive loss of βII-spectrin affects axonal extension, which results from independent mechanisms that negatively regulate axonal growth (Lorenzo et al., 2019).
Neural cell adhesion molecule (NCAM) associates with spectrin in neurons (Leshchyns’ka, Sytnyk, Morrow, & Schachner, 2003; Pollerberg, Burridge, Krebs, Goodman, & Schachner, 1987; Puchkov, Leshchyns’ka, Nikonenko, Schachner, & Sytnyk, 2011; Sytnyk et al., 2006). 180 and 140 kDa NCAM isoforms colocalize with βI-spectrin in neurites of cultured hippocampal neurons and co-precipitate with βI-spectrin from brain homogenates (Pollerberg et al., 1987). Association of βI-spectrin and NCAM’s intracellular domain requires spectrin repeats 2 and 3 (Pollerberg et al., 1987). βI-spectrin recruits protein kinase PKCβ2 to the NCAM complex to trigger NCAM-mediated neurite outgrowth in cultured neurons (Pollerberg et al., 1987). Both 180 kDa NCAM and βI-spectrin levels are increased at axo-dendritic contacts in cultured neurons, where βI-spectrin accumulation depends on NCAM (Leshchyns’ka et al., 2003). NCAM-rich axon-dendritic contact regions also cluster other PSD components, such as CaMKIIα, PSD95, and Shank. In particular, CaMKIIα and the NMDA receptor subunits NR1 and NR2B co-immunoprecipitate with NCAM (Sytnyk et al., 2006). Binding of βI-spectrin to NMDA receptors had also been previously reported (Wechsler & Teichberg, 1998). Multiple experimental results suggest that a βI-spectrin-NCAM complex participates in the assembly of the PSD. First, levels of βI-spectrin are reduced in both PDS fractions and immunogold EM staining of PSDs from NCAM knockout mouse brains. Second, blocking the NCAM-βI-spectrin interaction with a spectrin fragment containing the NCAM binding region reduced the accumulation of PSD95, NR1, and CaMKIIα at the PSD of cultured neurons (Leshchyns’ka et al., 2003). Last, loss of NCAM results in an increased of perforated PSDs in a βI-spectrin-dependent manner (Puchkov et al., 2011). A recent report shows that NCAM significantly increases its association with the βII-spectrin MPS upon exogenous addition of an NCAM antibody specific to its extracellular domain (Zhou, Han, Xia, & Zhuang, 2019). With it, fibroblast growth factor receptor 1 (FGFR1) and other effectors also came into close proximity to both NCAM and βII-spectrin and resulted in the transactivation of extracellular signal-regulated kinase (ERK) signaling (Zhou et al., 2019). These authors observed a similar effect upon activation of the cannabinoid type 1 receptor (CB1; Zhou et al., 2019). Knockdown of βII-spectrin disrupted those signaling cascades, which constitutes another demonstration of the spectrin skeleton facilitating the spatial and temporal association of functionally related molecules.
βIII-spectrin binds the glutamate transporter EAAT4, which is highly expressed in extrasynaptic sites of cerebellar Purkinje neurons (Jackson et al., 2001). βIII-spectrin stabilizes EAAT4 at the plasma membrane based on observations of decreased EAAT4 membrane lateral mobility in HEK293 cells co-expressing βIII-spectrin (Ikeda et al., 2006) and reduced surface EAAT4 levels in Purkinje neurons of βIII-spectrin knockout mice (Perkins et al., 2010; Stankewich et al., 2010). Furthermore, EAAT4 cellular distribution is affected in postmortem cerebellum from SCA5 patients expressing a 13 amino acid in-frame deletion in βIII-spectrin (“American mutation”; Ikeda et al., 2006). Consistent with that observation, SCA5 mutant βIII-spectrin did not rescue normal EAAT4 localization in HEK293 cells (Ikeda et al., 2006). βIII-spectrin also associates with meta-botropic glutamate receptor 1α (mGluR1α) and expression of the SCA5 American mutation in a mouse model leads to mGluR1α mislocalization and disfunction at Purkinje cell dendritic spines, which likely result from a deficient anchoring of mGluR1α at the membrane (Armbrust et al., 2014). βIII-spectrin, as well as other spectrins and ankyrins associate with organelles, molecular motors, and intracellular proteins involved in neuronal transport, which I discuss in 4.3.
4.3 |. Spectrin and ankyrins in neuronal intracellular transport
The involvement of ankyrins and spectrins in neuronal intracellular transport is relatively less studied, but arguably as relevant as their canonical roles in membrane protein organization discussed above. Therefore, their capacity to act in parallel as transport facilitators has critical functional and physiological consequences (Lorenzo et al., 2010, 2014, 2019). Spectrins’ participation in axonal transport was first recognized when “fodrin,” the preceding name of βII-spectrin, was observed comigrating with organelles following metabolic pulse labeling of axonal cargo in the optic nerve (Cheney, Hirokawa, Levine, & Willard, 1983). Likewise, αII-spectrin was found associated with the kinesin motor KIF3 and axonal organelles and also noticed to travel with similar velocities as KIF3 in rat optic nerve (Takeda et al., 2000). βIII-spectrin has been shown to be in a complex with the dynactin subunit actin-related protein 1 (Arp1), a critical adaptor of the retrograde motor dynein (Holleran et al., 2001). βIII-spectrin directly associates with Arp1 through at least one binding site that overlaps the actin-binding motif conserved across spectrins (Holleran et al., 2001). However, βIII-spectrin’s affinity for Arp1 is considerably higher than for F-actin, which likely allows the partitioning of βIII-spectrin between both motor-bound and membrane skeleton-associated pools, given that F-actin concentration is much higher than that of Arp1 (Holleran et al., 2001). In support of a general role of neuronal β-spectrins in promoting axonal transport via association with dynactin, pulldown experiments from mouse brain lysates show that βII-spectrin is in a complex with p150Glued (Lorenzo et al., 2019). Interestingly, recent cryo-EM structures of dynactin indicate that p150Glued is sterically less hindered than the Arp1 subunit (reviewed in Reck-Peterson, Redwine, Vale, & Carter, 2018, Urnavicius et al., 2015). Similarly, βV-spectrin binds the dynactin subunit dynamitin (p50) in photoreceptor cells (Papal et al., 2013). Like αII-spectrin (Takeda et al., 2000), βII-spectrin also associates with anterograde motors, including KIF3A, KIF5B, and KIF1A, although it is not clear whether their interaction is direct or through intermediary protein(s) (Lorenzo et al., 2019). Similarly, βV-spectrin associates with KIF3A (Papal et al., 2013).
The current proposed role for spectrins in neuronal transport is that they serve as accessory proteins that facilitate the coupling of microtubule motors and organelles. This model is supported by findings that spectrins simultaneously associate with motors and various intracellular cargo (Lorenzo et al., 2019; Muresan et al., 2001; Papal et al., 2013; Takeda et al., 2000). For example, βII-spectrin interacts with synaptic vesicles and lysosomes in mouse brain lysates, and its constitutive loss reduces the axonal bidirectional motility of these organelles in cultured neurons (Lorenzo et al., 2019). Reconstituted motility assays using squid axon’s cytoplasmic material and liposomes coated with acidic phospholipids showed that a dominant-negative construct containing the PH domain of βII-spectrin or βIII-spectrin affected organelle and liposome retrograde motility, suggesting that β-spectrins’ association with membrane lipids is required for dynein/dynactin-driven axonal transport (Muresan et al., 2001). βII-spectrin’s PH domain interacts with PI(4,5)P, PI(3,4)P2, and PI(3,4,5)P3 lipids through a highly conserved binding pocket (Hyvönen et al., 1995). Transfection of the K2207Q βII-spectrin PH mutant, which eliminates phosphoinositide binding (He et al., 2014) fails to restore both axonal transport dynamics and axon length in βII-spectrin null neurons (Lorenzo et al., 2019). These results indicate that βII-spectrin binding to PIP lipids, which likely enables its coupling to organelles, is required for axonal transport and growth.
Given the dual function of βII-spectrin in assembling the axonal MPS and facilitating axonal transport, a critical question is whether transport deficits in βII-spectrin null neurons may be the indirect result of deficient axon outgrowth and/or degeneration resulting from disruption of the spectrin skeleton, or other affected functions. EM sections of callosal axons from PND25 βII-spectrin KO mouse brains show signs of early degeneration (Lorenzo et al., 2019). However, bidirectional synaptic vesicle transport was already impaired in DIV2 axons lacking βII-spectrin, which indicates that deficits in transport result from an inherent deficiency in βII-spectrin (Lorenzo et al., 2019). Surprisingly, the presence of the axonal-spectrin periodic structure is not a prerequisite for the enablement of normal axonal transport. Loss of βII-spectrin and the MPS does not affect microtubule stability, as suggested by the normal dynamics of axonal EB3 comets (Lorenzo et al., 2019). In addition, there is no difference in the transport dynamics of synaptic vesicles at DIV2 between the axon proximal region, where the MPS is already detectable, and the distal region, where the MPS has not yet formed (Lorenzo et al., 2019; Zhong et al., 2014). Together, these results indicate that βII-spectrin independently assembles the neuronal actin periodic structure and promotes axonal organelle transport.
Loss of ankyrin-B causes very similar deficits in axonal growth and long-range axonal connectivity to βII-spectrin deficiency both in vivo and in vitro (Lorenzo et al., 2014; Scotland et al., 1998). While loss of ankyrin-B does not disrupt the MPS, it markedly impairs the retrograde axonal transport of multiple organelles (Lorenzo et al., 2014). Specifically, the 220-kDa ankyrin-B isoform promotes organelle dynamics via dual interactions with membrane PI3P phosphoinositide lipids and the p62/dynactin4 subunit of the dynein/dynactin motor complex. Neither the 220 kDa ankyrin-B DD1320AA mutant, unable to associate with the dynactin complex, nor the R1194E or K1195E mutants, which abrogates binding to PI3P lipids, rescue the axonal transport and growth deficits (Lorenzo et al., 2014). On the other hand, the βII-spectrin Y1874A mutant, which is unable to bind ankyrin-B, rescues both synaptic vesicle transport and axonal length deficits in neurons lacking βII-spectrin (Lorenzo et al., 2019). This result, together with the strong effects of loss of βII-spectrin on anterograde cargo motion, which are not observed in ankyrin-B-deficient neurons, suggest that βII-spectrin promotes axonal transport independently of ankyrin-B. While individual knockout of either ankyrin-B or βII-spectrin in mouse brains causes postnatal lethality, their combined loss results in early embryonic death. Additionally, mice with total loss of one of these two genes and 50% reduction in the other showed earlier lethality and more severe morphological deficits and motor impairments than either of the single gene knockout or the double heterozygous mice (Lorenzo et al., 2019). Moreover, simultaneous loss of ankyrin-B and βII-spectrin in cultured hippocampal neurons almost entirely stalled synaptic vesicle transport. That dual loss of these transport facilitators causes a more severe impairment of transport dynamics than their individual deficits supports the conclusion that ankyrin-B and βII-spectrin promote axonal transport of synaptic cargo through independent pathways.
Ankyrin-G has also been implicated in transport at the AIS (Barry et al., 2014; Garza et al., 2018; Kuijpers et al., 2016). Ankyrin-G associates with the anterograde motor KIF5 via interaction between the first sets of ankyrin repeats and a 70-residue region in the KIF5B tail (Barry et al., 2014). The ankyrin-G-KIF5 complex facilitates the anterograde transport of Nav1.2 in the proximal axon. Ankyrin-G also interacts with neuronal microtubule-associated protein NDEL1 through its giant inserted region, and it is necessary for NDEL1 localization to the AIS (Kuijpers et al., 2016). NDEL1 transiently activates dynein on cargos that enter the proximal axon to selectively sort their direction between somatodendritic and axonal trajectories. Thus, depletion of ankyrin-G or the NDEL1 partner LIS1, also a dynein regulator, results in aberrant entry of dendritic cargo into the axon. As mentioned above, ankyrin-G binds EBs and stabilizes microtubules in the AIS (Fréal et al., 2016; Garza et al., 2018; Leterrier et al., 2011; Sobotzik et al., 2009). ANK3 heterozygous neurons show enhanced EB3 and microtubule dynamics at the AIS concomitant with an increase in GSK3 and a decrease in CRMP2 activities, which indicate dysregulation of a microtubule stabilization pathway. The association of ankyrin-G with regulators of both microtubule dynamics and dynein activation offers indirect evidence for ankyrin-G role in cargo transport. However, the specific consequences of axonal cargo motility or microtubule disruption caused by ankyrin-G deficits have not been determined. Given the multifaceted roles of ankyrin-G in axons and dendrites, it will be important to further clarify its role in organelle transport.
5 |. PERSPECTIVES
Our understanding of how ankyrins and spectrins drive neuronal biology has advanced remarkably since they were first observed four decades ago to be ubiquitously and abundantly distributed in the mammalian brain. The discovery and study of the multiple members of both protein families that followed uncovered several of their shared, synergistic, and specialized functions, and elevated their place as critical and multifaceted players in neuronal biology. Nonetheless, this same diversity and pleiotropy foretells that there is still much we do not know about this molecular partnership and makes it almost certain that new and exciting discoveries will come to light in the next few years.
New improvements in superresolution (Culley, Tosheva, Matos Pereira, & Henriques, 2018; Wang, Taki, et al., 2019) and correlative microscopies (Vassilopoulos, Gibaud, Jimenez, Caillol, & Leterrier, 2019) should soon enable us to capture the dynamic itineraries of ankyrins and spectrins at nanoscale resolution, which will in turn help us more precisely pinpoint their roles in, and individual commitments to, known and novel pathways. This will represent a significant step in deciphering the code behind their modes of functional partitioning and long-range and local organization. Intrinsic to achieving this goal is the need to advance our understanding of the regulatory programs that determine alternative splicing and posttranslational modifications, both of which we know virtually nothing about in ankyrins and spectrins, together with how they may modulate cell-specific and local functions. Although remarkable progress has been made in uncovering the roles of ankyrins and spectrins as principal actors in the AIS, we do not fully understand how they integrate into the developing model of AIS plasticity and its relevance to disease (Sohn et al., 2019). Technical advances, such as AIS-targeted proteomics (Hamdan et al., 2020), should enable us to gain deeper insights into the organization and function of AIS. As we continue to learn more about axonal functions of these molecules, it is important that we prioritize research on their understudied dendritic roles. Their roles in other nervous system cells, such as glia and peripheral neurons, where they are unquestionably key molecules, represent a much less explored frontier in need of attention. Thus, integrating all this knowledge will be critical to advance our understanding of how ankyrins and spectrins effect plasticity, connectivity, and maintenance in the nervous system.
The continued and growing interest in the neurobiology of ankyrins and spectrins over the last few years has been partly fueled by genetic discoveries that connects them to several brain disorders, which, in turn, have been facilitated by several large-scale genomics efforts across the world. As these studies grow in number, power, and sophistication, it is inevitable that over the next few years we will see the spectrum of ankyrinopathies and spectrinopathies, and the number of patients affected by them, grow appreciably. With them, the urgency to gain deeper mechanistic insights that will translate into diagnostic and therapeutic strategies will continue to mount. Due to the inaccessibility of the human brain for molecular in vivo studies, animal models of these diseases will remain a valuable tool. Fortunately, the utility of these models continues to be enhanced by our growing ability to more finely manipulate and measure gene effects at the brain region, circuit, and cell type levels. In parallel, more comprehensive cellular studies using neurons and glial cells differentiated from patient-derived iPSCs will help us characterize the pathogenic underpinnings of genetic variants. Additionally, the exciting inroads being made in the design of brain organoids holds promise as powerful systems to more faithfully model the pathobiology of these diseases across scales in a not distant future. Last, because several individual ankyrinopathies and spectrinopathies of the nervous system can span a spectrum of comorbid disorders, they should be considered as model pathologies in the study of the genetic and functional convergence of brain diseases.
ACKNOWLEDGMENTS
The author regrets that the work of several contributors to the themes discussed above may have been omitted due to space limitations. The author thanks Dr. Keith Burridge for kindly reading the manuscript and providing valuable comments. D.N.L. was supported by the University of North Carolina at Chapel Hill School of Medicine as a Simmons Scholar, by the National Ataxia Foundation, and by the US National Institutes of Health (NIH) grant R01NS110810.
Footnotes
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Agre P, Orringer EP, & Bennett V (1982). Deficient red-cell spectrin in severe, recessively inherited spherocytosis. The New England Journal of Medicine, 306, 1155–1161. 10.1056/nejm198205133061906 [DOI] [PubMed] [Google Scholar]
- Alemu EA, Lamark T, Torgersen KM, Birgisdottir AB, Larsen KB, Jain A, et al. (2012). ATG8 family proteins act as scaffolds for assembly of the ULK complex: Sequence requirements for LC3-interacting region (LIR) motifs. Journal of Biological Chemistry, 287, 39275–39290. 10.1074/jbc.M112.378109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor V, Zhang C, Vainshtein A, Zhang A, Zollinger DR, Eshed-Eisenbach Y, et al. (2017). The paranodal cytoskeleton clusters Na(+) channels at nodes of Ranvier. eLife, 6, e21392 10.7554/eLife.21392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Guo X, Sum H, Morrow J, Gratzer W, & Mohandas N (2004). Phosphatidylserine binding sites in erythroid spectrin: Location and implications for membrane stability. Biochemistry, 43, 310–315. [DOI] [PubMed] [Google Scholar]
- Armbrust KR, Wang X, Hathorn TJ, Cramer SW, Chen G, Zu T, et al. (2014). Mutant β-III spectrin causes mGluR1α mislocalization and functional deficits in a mouse model of spinocerebellar ataxia type 5. The Journal of Neuroscience, 34, 9891–9904. 10.1523/JNEUROSCI.0876-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon G, Davis JQ, Scotland P, & Bennett V (2008). An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell, 135, 1189–1200. 10.1016/j.cell.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Ayalon G, Hostettler JD, Hoffman J, Kizhatil K, Davis JQ, & Bennett V (2011). Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. The Journal of Biological Chemistry, 286, 7370–7378. 10.1074/jbc.M110.187831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär J, Kobler O, van Bommel B, & Mikhaylova M (2016). Periodic F-Actin structures shape the neck of dendritic spines. Scientific Reports, 6, 37136 10.1038/srep37136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J, Gu Y, Jukkola P, O’Neill B, Gu H, Mohler PJ, et al. (2014). Ankyrin-G directly binds to kinesin-1 to transport voltage-gated Na+ channels into axons. Developmental Cell, 28(2), 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, & Baines AJ (2001). Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiological Reviews, 81, 1353–1392. [DOI] [PubMed] [Google Scholar]
- Bennett V, & Lorenzo DN (2013). Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Current Topics in Membranes, 72, 1–37. 10.1016/B978-0-12-417027-8.00001-5 [DOI] [PubMed] [Google Scholar]
- Bennett V, & Lorenzo DN (2016). An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Current Topics in Membranes, 77, 143–184. 10.1016/bs.ctm.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Bennett V, & Stenbuck PJ (1979a). Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. Journal of Biological Chemistry, 254, 2533e–2541e. [PubMed] [Google Scholar]
- Bennett V, & Stenbuck PJ (1979b). The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature, 280, 468e473. [DOI] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R Jr., Maksimova E, Stabach P, Hermel JM, et al. (2000). betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. The Journal of Cell Biology, 151, 985–1002. 10.1083/jcb.151.5.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréchet A, Fache M-P, Brachet A, Ferracci G, Baude A, Irondelle M, et al. (2008). Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. The Journal of Cell Biology, 183, 1101–1114. 10.1083/jcb.200805169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi M, Schlatter MC, Demyanenko GP, Thresher R, & Maness PF (2008). L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. The Journal of Neuroscience, 28, 177–188. 10.1523/jneurosci.3573-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Kelly T, & Mangeat P (1982). Nonerythrocyte spectrins: Actin-membrane attachment proteins occurring in many cell types. The Journal of Cell Biology, 95, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers TJ, & Branton D (1985). Visualization of the protein associations in the erythrocyte membrane skeleton. Proceedings of the National Academy of Sciences, 82, 6153–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KJ, Zollinger DR, Susuki K, Sherman DL, Makara MA, Brophy PJ, et al. (2014). Glial ankyrins facilitate paranodal axoglial junction assembly. Nature Neuroscience, 17(12), 1673–1681. 10.1038/nn.3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis JA, Agre P, & Mohandas N (1988). Decreased membrane mechanical stability and in vivo loss of surface area reflect spectrin deficiencies in hereditary spherocytosis. The Journal of Clinical Investigation, 82, 617–623. 10.1172/jci113640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li J, Wang C, Wei Z, & Zhang M (2017). Autoinhibition of ankyrin-B/G membrane target bindings by intrinsically disordered segments from the tail regions. eLife, 6, e29150 10.7554/eLife.29150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney R, Hirokawa N, Levine J, & Willard M (1983). Intracellular movement of fodrin. Cell Motility, 3, 649–655. [DOI] [PubMed] [Google Scholar]
- Culley S, Tosheva KL, Matos Pereira P, & Henriques R (2018). SRRF: Universal live-cell super-resolution microscopy. The International Journal of Biochemistry & Cell Biology, 101, 74–79. 10.1016/j.biocel.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha SR, Le Scouarnec S, Schott JJ, & Mohler PJ (2008). Exon organization and novel alternative splicing of the human ANK2 gene: Implications for cardiac function and human cardiac disease. Journal of Molecular and Cellular Cardiology, 45, 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, & Mantei N (1997). Disruption of the mouse L1 gene leads to malformations of the nervous system. Nature Genetics, 17, 346–349. 10.1038/ng1197-346 [DOI] [PubMed] [Google Scholar]
- Das A, Base C, Dhulipala S, & Dubreuil RR (2006). Spectrin functions upstream of ankyrin in a spectrin cytoskeleton assembly pathway. The Journal of Cell Biology, 175, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Base C, Manna D, Cho W, & Dubreuil RR (2008). Unexpected complexity in the mechanisms that target assembly of the spectrin cytoskeleton. The Journal of Biological Chemistry, 283, 12643–12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PLA, & Kloc CB (1981). Calmodulin-binding proteins: A high molecular weight calmodulin-binding protein from bovine brain. Biochemistry International, 3, 203–212. [Google Scholar]
- Davis J, & Bennett V (1982). Microtubule-associated protein 2, a microtubule-associated protein from brain, is immunologically related to the alpha subunit of erythrocyte spectrin. Journal of Biological Chemistry, 257, 5816–5820. [PubMed] [Google Scholar]
- Davis JQ, & Bennett V (1984). Brain ankyrin. A membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. The Journal of Biological Chemistry, 259, 13550–13559. [PubMed] [Google Scholar]
- Davis JQ, & Bennett V (1994). Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. Journal of Biological Chemistry, 269, 27163–27166. [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. (2014). Synaptic, transcriptional and chromatin genes disrupted in autism. Nature, 515, 209–215. 10.1038/nature13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, & Maness PF (1999). Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. The Journal of Neuroscience, 19, 4907–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Este E, Kamin D, Balzarotti F, & Hell SW (2017). Ultrastructural anatomy of nodes of Ranvier in the peripheral nervous system as revealed by STED microscopy. Proceedings of the National Academy of Sciences of the United States of America, 114, E191–E199. 10.1073/pnas.1619553114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Este E, Kamin D, Gottfert F, El-Hady A, & Hell SW (2015). STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Reports, 10, 1246–1251. 10.1016/j.celrep.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Djinović-Carugo K, Bañuelos S, & Saraste M (1997). Crystal structure of a calponin homology domain. Nature Structural Biology, 4, 175–179. 10.1038/nsb0397-175 [DOI] [PubMed] [Google Scholar]
- Eber S, & Lux SE (2004). Hereditary spherocytosis—Defects in proteins that connect the membrane skeleton to the lipid bilayer. Seminars in Hematology, 41, 118–141. [DOI] [PubMed] [Google Scholar]
- Efimova N, Korobova F, Stankewich MC, Moberly AH, Stolz DB, Wang J, et al. (2017). βIII spectrin is necessary for formation of the constricted neck of dendritic spines and regulation of synaptic activity in neurons. The Journal of Neuroscience, 37, 6442–6459. 10.1523/JNEUROSCI.3520-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt M, Vorwald S, Sobotzik JM, Bennett V, & Schultz C (2013). Ankyrin-B structurally defines terminal microdomains of peripheral somatosensory axons. Brain Structure and Function, 218(4), 1005–1016. 10.1007/s00429-012-0443-0 [DOI] [PubMed] [Google Scholar]
- Eshed-Eisenbach Y, & Peles E (2013). The making of a node: A co-production of neurons and glia. Current Opinion in Neurobiology, 23, 1049–1056. 10.1016/j.conb.2013.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifková E, & Morales M (1992). Actin matrix of dendritic spines, synaptic plasticity, and long-term potentiation. International Review of Cytology, 139, 267–307. [DOI] [PubMed] [Google Scholar]
- Fowler VM (2013). The human erythrocyte plasma membrane: A Rosetta Stone for decoding membrane-cytoskeleton structure. Current Topics in Membranes, 72, 39–88. [DOI] [PubMed] [Google Scholar]
- Fréal A, Fassier C, Le Bras B, Bullier E, De Gois S, Hazan J, et al. (2016). Cooperative interactions between 480 kDa Ankyrin-G and EB proteins assemble the axon initial segment. The Journal of Neuroscience, 36, 4421–4433. 10.1523/JNEUROSCI.3219-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréal A, Rai D, Tas RP, Pan X, Katrukha EA, van de Willige D, … Hoogenraad CC (2019). Feedback-driven assembly of the axon initial segment. Neuron, 104, 305–321. 10.1016/j.neuron.2019.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiano MR, Jha S, Ho TS-Y, Zhang C, Ogawa Y, Chang K-J, et al. (2012). A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell, 149, 1125–1139. 10.1016/j.cell.2012.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Perkins EM, Clarkson YL, Tobia S, Lyndon AR, Jackson M, & Rothstein JD (2011). β-III spectrin is critical for development of purkinje cell dendritic tree and spine morphogenesis. The Journal of Neuroscience, 31, 16581–16590. 10.1523/JNEUROSCI.3332-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K, & Bennett V (1987). Modulation of spectrin-actin assembly by erythrocyte adducin. Nature, 328, 359–362. 10.1038/328359a0 [DOI] [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, et al. (2003). A targeting motif involved in sodium channel clustering at the axonal initial segment. Science, 300, 2091–2094. 10.1126/science.1085167 [DOI] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, & Bennett V (1997). Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. The Journal of Cell Biology, 137, 703–714. 10.1083/jcb.137.3.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Qi X, Gjeluci K, Leussis MP, Basu H, Reis SA, et al. (2018). Disruption of the psychiatric risk gene Ankyrin 3 enhances microtubule dynamics through GSK3/CRMP2 signaling. Translational Psychiatry, 8(1), 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A, Ho TS-Y, Cheng X, Chang K-J, Waxman SG, Rasband MN, & Dib-Hajj SD (2012). An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. The Journal of Neuroscience, 32, 7232–7243. 10.1523/JNEUROSCI.5434-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SR, Zagon IS, & Kulikowski RR (1981). Identification of a spectrin-like protein in nonerythroid cells. Proceedings of the National Academy of Sciences of the United States of America, 78, 7570–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grum VL, MacDonald RI, & Mondragón A (1999). Structures of two repeats of spectrin suggest models of flexibility. Cell, 98, 523–535. 10.1016/s0092-8674(00)81980-7 [DOI] [PubMed] [Google Scholar]
- Hamdan H, Lim BC, Torii T, Joshi A, Konning M, Smith C, et al. (2020). Mapping axon initial segment structure and function by multiplexed proximity biotinylation. Nature Communications, 11(1), 100 10.1038/s41467-019-13658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Jorgensen EM, & Bastiani MJ (2007). Axons break in animals lacking beta-spectrin. The Journal of Cell Biology, 176, 269–275. 10.1083/jcb.200611117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Zhou R, Xia C, & Zhuang X (2017). Structural organization of the actin-spectrin-based membrane skeleton in dendrites and soma of neurons. Proceedings of the National Academy of Sciences of the United States of America, 114, E6678–E6685. 10.1073/pnas.1705043114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NV, Scott C, Heerkens E, Ohanian V, Maggs AM, Pinder JC, et al. (2000). Identification of a novel C-terminal variant of beta II spectrin: Two isoforms of beta II spectrin have distinct intracellular locations and activities. Journal of Cell Science, J113, 2023–2034. [DOI] [PubMed] [Google Scholar]
- He J, Zhou R, Wu Z, Carrasco MA, Kurshan PT, Farley JE, et al. (2016). Prevalent presence of periodic actin-spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. Proceedings of the National Academy of Sciences of the United States of America, 113, 6029–6034. 10.1073/pnas.1605707113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Abdi KM, & Bennett V (2014). Ankyrin-G palmitoylation and bIIspectrin binding to phosphoinositide lipids drive lateral membrane assembly. Journal of Cell Biology, 206, 273e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Tseng WC, & Bennett V (2013). A single divergent exon inhibits ankyrin-B association with the plasma membrane. Journal of Biological Chemistry, 288, 14769–14779. 10.1074/jbc.M113.465328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy JA, Nilsson KR, Hohmeier HE, Berglund J, Davis J, Hoffman J, et al. (2010). Cholinergic augmentation of insulin release requires ankyrin-B. Science Signaling, 3, ra19 10.1126/scisignal.2000771 [DOI] [PubMed] [Google Scholar]
- Heidemann SR, & Bray D (2015). Tension-driven axon assembly: A possible mechanism. Frontiers in Cellular Neuroscience, 9, 316 10.3389/fncel.2015.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien YE, Montersino A, Castets F, Leterrier C, Filhol O, Vacher H, & Dargent B (2014). CK2 accumulation at the axon initial segment depends on sodium channel Nav1. FEBS Letters, 588, 3403–3408. 10.1016/j.febslet.2014.07.032 [DOI] [PubMed] [Google Scholar]
- Ho TS-Y, Zollinger DR, Chang K-J, Xu M, Cooper EC, Stankewich MC, et al. (2014). A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nature Neuroscience, 17, 1664–1672. 10.1038/nn.3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, & Holzbaur EL (2001). Beta III spectrin binds to the Arp1 subunit of dynactin. Journal of Biological Chemistry, 276, 36598–36605. [DOI] [PubMed] [Google Scholar]
- Hopitzan AA, Baines AJ, Ludosky MA, Recouvreur M, & Kordeli E (2005). Ankyrin-G in skeletal muscle: Tissue-specific alternative splicing contributes to the complexity of the sarcolemmal cytoskeleton. Experimental Cell Research, S309, 86–98. [DOI] [PubMed] [Google Scholar]
- Huang CY, Zhang C, Ho TS, Oses-Prieto J, Burlingame AL, Lalonde J, et al. (2017). αII spectrin forms a periodic cytoskeleton at the axon initial segment and is required for nervous system function. The Journal of Neuroscience, 37, 11311–11322. 10.1523/JNEUROSCI.2112-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY-M, Zhang C, Zollinger DR, Leterrier C, & Rasband MN (2017). An αII spectrin-based cytoskeleton protects large-diameter myelinated axons from degeneration. The Journal of Neuroscience, 37, 11323–11334. 10.1523/JNEUROSCI.2113-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvönen M, Macias MJ, Nilges M, Oschkinat H, Saraste M, & Wilmanns M (1995). Structure of the binding site for inositol phosphates in a PH domain. EMBO Journal, 14, 4676–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, et al. (2006). Spectrin mutations cause spinocerebellar ataxia type 5. Nature Genetics, 38, 184–190. 10.1038/ng1728 [DOI] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature, 515, 216–221. 10.1038/nature13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsaro JJ, Harper SL, Messick TE, Marmorstein R, Mondragón A, & Speicher DW (2010). Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex. Blood, 115, 4843–4852. 10.1182/blood-2010-01-261396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsaro JJ, & Mondragón A (2010). Structural basis for spectrin recognition by ankyrin. Blood, 115(20), 4093–4101. 10.1182/blood-2009-11-255604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, et al. (2001). Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature, 410(6824), 89–93. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Kim N, Jones SL, Tseng WC, Svitkina TM, Yin HH, et al. (2015). Giant ankyrin-G: A critical innovation in vertebrate evolution of fast and integrated neuronal signaling. Proceedings of the National Academy of Sciences of The United States of America, 112, 957e–964e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, & Bennett V (2001). Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. The Journal of Cell Biology, 155, 739–746. 10.1083/jcb.200109026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Korobova F, & Svitkina T (2014). Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. The Journal of Cell Biology, 205, 67–81. 10.1083/jcb.201401045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, & Barres BA (2001). Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron, 30, 105–119. [DOI] [PubMed] [Google Scholar]
- Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, & Bennett V (2007). Ankyrin-G and beta2-spectrin collaborate in bio-genesis of lateral membrane of human bronchial epithelial cells. Journal of Biological Chemistry, 282, 2029–2037. 10.1074/jbc.M608921200 [DOI] [PubMed] [Google Scholar]
- Kloth K, Denecke J, Hempel M, Johannsen J, Strom TM, Kubisch C, & Lessel D (2017). First de novo ANK3 nonsense mutation in a boy with intellectual disability, speech impairment and autistic features. European Journal of Medical Genetics, 60, 494–498. 10.1016/j.ejmg.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Komada M, & Soriano P (2002). [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. The Journal of Cell Biology, 156, 337–348. 10.1083/jcb.200110003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Dunn AR, & Goodman MB (2014). Mechanical control of the sense of touch by β-spectrin. Nature Cell Biology, 16(3), 224–233. 10.1038/ncb2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger CC, An X, Tang HY, Mohandas N, Speicher DW, & Discher DE (2011). Cysteine shotgun-mass spectrometry (CS-MS) reveals dynamic sequence of protein structure changes within mutant and stressed cells. Proceedings of the National Academy of Sciences of the United States of America, 108, 8269–8274. 10.1073/pnas.1018887108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman PA, Hughes CA, Bennett V, & Fowler VM (1996). A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. The Journal of Biological Chemistry, 271, 7986–7991. 10.1074/jbc.271.14.7986 [DOI] [PubMed] [Google Scholar]
- Kuijpers M, van de Willige D, Freal A, Chazeau A, Franker MA, Hofenk J, et al. (2016). Dynein regulator NDEL1 controls polarized cargo transport at the axon initial segment. Neuron, 89, 461–471. 10.1016/j.neuron.2016.01.022 [DOI] [PubMed] [Google Scholar]
- Lacas-Gervais S, Guo J, Strenzke N, Scarfone E, Kolpe M, Jahkel M, et al. (2004). BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. The Journal of Cell Biology, 166, 983–990. 10.1083/jcb.200408007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, & Bennett V (1993). Postmitotic expression of ankyrinR and beta R-spectrin in discrete neuronal populations of the rat brain. Journal of Neuroscience, 13, 3725–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, & Marszalek PE (2006). Nanospring behaviour of ankyrin repeats. Nature, 440, 246–249. 10.1038/nature04437 [DOI] [PubMed] [Google Scholar]
- Legendre K, Safieddine S, Küssel-Andermann P, Petit C, & El-Amraoui A (2008). alphaII-betaV spectrin bridges the plasma membrane and cortical lattice in the lateral wall of the auditory outer hair cells. Journal of Cell Science, 121, 3347–3356. [DOI] [PubMed] [Google Scholar]
- Lemaillet G, Walker B, & Lambert S (2003). Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. Journal of Biological Chemistry, 278, 27333–27339. 10.1074/jbc.M303327200 [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, Abrams CS (2002). Pleckstrin homology domains and the cytoskeleton. FEBS Letters. 513, 71–76. 10.1016/s0014-5793(01)03243-4 [DOI] [PubMed] [Google Scholar]
- Leshchyns’ka I, Sytnyk V, Morrow JS, & Schachner M (2003). Neural cell adhesion molecule (NCAM) association with PKCβ2 via βI-spectrin is implicated in NCAM-mediated neurite outgrowth. Journal of Cell Biology, 161, 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C (2018). The axon initial segment: An updated viewpoint. The Journal of Neuroscience, 38, 2135–2145. 10.1523/JNEUROSCI.1922-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Clerc N, Rueda-Boroni F, Montersino A, Dargent B, & Castets F (2017). Ankyrin G membrane partners drive the establishment and maintenance of the axon initial segment. Frontiers in Cellular Neuroscience, 11, 6 10.3389/fncel.2017.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Potier J, Caillol G, Debarnot C, RuedaBoroni F, & Dargent B (2015). Nanoscale architecture of the axon initial segment reveals an organized and robust scaffold. Cell Reports, 13, 2781–2793. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Vacher H, Fache M-P, d’Ortoli SA, Castets F, Autillo-Touati A, & Dargent B (2011). End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proceedings of the National Academy of Sciences of the United States of America, 108, 8826–8831. 10.1073/pnas.1018671108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, & Willard M (1981). Fodrin: Axonally transported polypeptides associated with the internal periphery of many cells. The Journal of Cell Biology, 90, 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, & Bennett V (1996). Identification of the spectrin subunit and domains required for formation of spectrin/adducin/actin complexes. Journal of Biological Chemistry, 271, 15695–15702. [DOI] [PubMed] [Google Scholar]
- Lorenzo DN, Badea A, Davis J, Hostettler J, He J, Zhong G, et al. (2014). A PIK3C3-ankyrin-B-dynactin pathway promotes axonal growth and multiorganelle transport. Journal of Cell Biology, 207, 735e752 10.1083/jcb.201407063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo DN, Badea A, Zhou R, Mohler PJ, Zhuang X, & Bennett V (2019). βII-spectrin promotes mouse brain connectivity through stabilizing axonal plasma membranes and enabling axonal organelle transport. Proceedings of the National Academy of Sciences of the United States of America, 116(31), 15686–15695. 10.1073/pnas.1820649116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo DN, & Bennett V (2017). Cell-autonomous adiposity through increased cell surface GLUT4 due to ankyrin-B deficiency. Proceedings of the National Academy of Sciences of the United States of America, 114(48), 12743–12748. 10.1073/pnas.1708865114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo DN, Li MG, Mische SE, Armbrust KR, Ranum LP, & Hays TS (2010). Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. The Journal of Cell Biology, 189, 143–158. 10.1083/jcb.200905158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchiodi-Albedi F, Ceccarini M, Winkelmann JC, Morrow JS, & Petrucci TC (1993). The 270 kDa splice variant of erythrocyte beta-spectrin (beta I sigma 2) segregates in vivo and in vitro to specific domains of cerebellar neurons. Journal of Cell Science, 106, 67–78. [DOI] [PubMed] [Google Scholar]
- Michaely P, & Bennett V (1995). Mechanism for binding site diversity on ankyrin. Comparison of binding sites on ankyrin for neurofascin and the cl-/HCO3-anion exchanger. Journal of Biological Chemistry, 270, 31298–31302. 10.1074/jbc.270.52.31298 [DOI] [PubMed] [Google Scholar]
- Michaely P, Tomchick DR, Machius M, & Anderson RG (2002). Crystal structure of a 12 ANK repeat stack from human ankyrinR. The EMBO Journal, 21, 6387–6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler PJ, Davis JQ, & Bennett V (2005). Ankyrin-B coordinates the Na/K ATPase, Na/ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biology, 3, e423 10.1371/journal.pbio.0030423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler PJ, Gramolini AO, & Bennett V (2002). The ankyrin-b c-terminal domain determines activity of ankyrin-b/g chimeras in rescue of abnormal inositol 1,4,5-trisphosphate and ryanodine receptor distribution in ankyrin-b (−/−) neonatal cardiomyocytes. Journal of Biological Chemistry, 277, 10599–10607. 10.1074/jbc.M110958200 [DOI] [PubMed] [Google Scholar]
- Muresan V, Stankewich MC, Steffen W, Morrow JS, Holzbaur EL, & Schnapp BJ (2001). Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: A role for spectrin and acidic phospholipids. Molecular Cell, 7, 173–183. [DOI] [PubMed] [Google Scholar]
- Nelson AD, Caballero-Florán RN, Rodríguez Díaz JC, Hull JM, Yuan Y, Li J, et al. (2018). Ankyrin-G regulates forebrain connectivity and network synchronization via interaction with GABARAP. Molecular Psychiatry. 10.1038/s41380-018-0308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor MW, Cai X, Stone MR, Bloch RJ, & Thompson SM (2011). The actin binding domain of betaI-spectrin regulates the morphological and functional dynamics of dendritic spines. PLoS One, 6, e16197 10.1371/journal.pone.0016197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, et al. (2006). Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. The Journal of Neuroscience, 26, 5230–5239. 10.1523/jneurosci.0425-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara O, Ohara R, Yamakawa H, Nakajima D, & Nakayama M (1998). Characterization of a new beta-spectrin gene which is predominantly expressed in brain. Brain Research Molecular Brain Research, 57, 181–192. [DOI] [PubMed] [Google Scholar]
- Pan L, Yan R, Li W, & Xu K (2018). Super-resolution microscopy reveals the native ultrastructure of the erythrocyte cytoskeleton. Cell Reports, 22, 1151–1158. 10.1016/j.celrep.2017.12.107 [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, et al. (2006). A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. The Journal of Neuroscience, 26, 2599–2613. 10.1523/jneurosci.4314-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papal S, Cortese M, Legendre K, Sorusch N, Dragavon J, Sahly I, et al. (2013). The giant spectrin bV couples the molecular motors to phototransduction and usher syndrome type I proteins along their trafficking route. Human Molecular Genetics, 22, 3773–3788. [DOI] [PubMed] [Google Scholar]
- Patzke C, Acuna C, Giam LR, Wernig M, & Sudhof TC (2016). Conditional deletion of L1CAM in human neurons impairs both axonal and dendritic arborization and action potential generation. Journal of Experimental Medicine, 213, 499–515. 10.1084/jem.20150951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins EM, Clarkson YL, Sabatier N, Longhurst DM, Millward CP, Jack J, et al. (2010). Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. Journal of Neuroscience, 30(14), 4857–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, & Peles E (2003). The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews Neuroscience, 4, 968–980. 10.1038/nrn1253 [DOI] [PubMed] [Google Scholar]
- Pollerberg GE, Burridge K, Krebs KE, Goodman SR, & Schachner M (1987). The 180-kD component of the neural cell adhesion molecule N-CAM is involved in cell-cell contacts and cytoskeleton-membrane interactions. Cell Tissue Research, 250(1), 227–236. 10.1007/bf00214676 [DOI] [PubMed] [Google Scholar]
- Puchkov D, Leshchyns’ka I, Nikonenko AG, Schachner M, & Sytnyk V (2011). NCAM/spectrin complex disassembly results in PSD perforation and postsynaptic endocytic zone formation. Cerebral Cortex, 21, 2217–2232. [DOI] [PubMed] [Google Scholar]
- Qu F, Lorenzo DN, King SJ, Brooks R, Bear JE, & Bennett V (2016). Ankyrin-B is a PI3P effector that promotes polarized α5β1-integrin recycling via recruiting RabGAP1L to early endosomes. eLife, 5, e20417 10.7554/eLife.20417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN (2004). It’s “juxta” potassium channel! Journal of Neuroscience Research, 76, 749–757. 10.1002/jnr.20073 [DOI] [PubMed] [Google Scholar]
- Rasband MN (2010). The axon initial segment and the maintenance of neuronal polarity. Nature Reviews Neuroscience, 11, 552 10.1038/nrn2852 [DOI] [PubMed] [Google Scholar]
- Rasband MN, & Peles E (2015). The nodes of Ranvier: Molecular assembly and maintenance. Cold Spring Harbor Perspectives in Biology, 8, a020495–a020495. 10.1101/cshperspect.a020495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen FG, & Schachner M (1984). Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. The EMBO Journal, 3, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Redwine WB, Vale RD, & Carter AP (2018). The cytoplasmic dynein transport machinery and its many cargoes. Nature Reviews Molecular Cell Biology, 19, 382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer BM, Zagon IS, & Goodman SR (1986). Brain spectrin (240/235) and brain spectrin (240/235E): Two distinct spectrin subtypes with different locations within mammalian neural cells. The Journal of Cell Biology, 102, 2088–2097. 10.1083/jcb.102.6.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief M, Pascual J, Saraste M, & Gaub HE (1999). Single molecule force spectroscopy of spectrin repeats: Low unfolding forces in helix bundles. Journal of Molecular Biology, 286, 553–561. 10.1006/jmbi.1998.2466 [DOI] [PubMed] [Google Scholar]
- Saifetiarova J, Taylor AM, & Bhat MA (2017). Early and late loss of the cytoskeletal scaffolding protein, ankyrin g reveals its role in maturation and maintenance of nodes of Ranvier in myelinated axons. The Journal of Neuroscience, 37, 2524–2538. 10.1523/JNEUROSCI.2661-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H, Tohyama J, Kumada T, Egawa K, Hamada K, Okada I, et al. (2010). Dominant-negative mutations in alpha-II spectrin cause west syndrome with severe cerebral hypomyelination, spastic quadriplegia, and developmental delay. American Journal of Human Genetics, 86, 881–891. 10.1016/j.ajhg.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi G, Orita S, Naito A, Maeda M, Igarashi H, Sasaki T, & Takai Y (1998). A novel brain-specific isoform of beta spectrin: Isolation and its interaction with Munc13. Biochemical and Biophysical Research Communications, 248, 846–851. [DOI] [PubMed] [Google Scholar]
- Salzer JL (2003). Polarized domains of myelinated axons. Neuron, 40, 297–318. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Nam YC, Moumen A, Keglowich L, Bouche E, Kuffner M, et al. (2010). L1 syndrome mutations impair neuronal L1 function at different levels by divergent mechanisms. Neurobiology of Disease, 40, 222–237. 10.1016/j.nbd.2010.05.029 [DOI] [PubMed] [Google Scholar]
- Scotland P, Zhou D, Benveniste H, & Bennett V (1998). Nervous system defects of AnkyrinB (−/−) mice suggest functional overlap between the cell adhesion molecule L1 and 440-kD AnkyrinB in premyelinated axons. The Journal of Cell Biology, 143, 1305–1315. 10.1083/jcb.143.5.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidenstein SC, D’Este E, Böhm MJ, Danzl JG, Belov VN, & Hell SW (2016). Multicolour multilevel STED nanoscopy of actin/spectrin organization at synapses. Scientific Reports, 6, 26725 10.1038/srep26725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, et al. (1998). A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nature Genetics, 18, 25–29. 10.1038/ng0198-25 [DOI] [PubMed] [Google Scholar]
- Šmít D, Fouquet C, Pincet F, Zapotocky M, & Trembleau A (2017). Axon tension regulates fasciculation/defasciculation through the control of axon shaft zippering. Elife. 6 Pii: e19907. doi: 10.7554/eLife.19907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schurmann B, et al. (2014). Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron, 84, 399–415. 10.1016/j.neuron.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotzik J-M, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, & Schultz C (2009). AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proceedings of the National Academy of Sciences of the United States of America, 106, 17564–17569. 10.1073/pnas.0909267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn PD, Huang CT, Yan R, Fan L, Tracy TE, Camargo CM, et al. (2019). Pathogenic tau impairs axon initial segment plasticity and excitability homeostasis. Neuron, 104(3), 458–470.e5. 10.1016/j.neuron.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher DW, Weglarz L, & DeSilva TM (1992). Properties of human red cell spectrin heterodimer (side-to-side) assembly and identification of an essential nucleation site. Journal of Biological Chemistry, 267, 14775–14782. [PubMed] [Google Scholar]
- Spratt PWE, Ben-Shalom R, Keeshen CM, Burke KJ Jr., Clarkson RL, Sanders SJ, & Bender KJ (2019). The autism-associated gene Scn2a contributes to dendritic excitability and synaptic function in the prefrontal cortex. Neuron, 103(4), 673–685. 10.1016/j.neuron.2019.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabach PR, & Morrow JS (2000). Identification and characterization of betaV spectrin, a mammalian ortholog of Drosophila betaH spectrin. Journal of Biological Chemistry, 275, 21385–21395. [DOI] [PubMed] [Google Scholar]
- Stankewich MC, Gwynn B, Ardito T, Ji L, Kim J, Robledo RF, et al. (2010). Targeted deletion of betaIII spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes. Proceedings of the National Academy of Sciences of the United States of America, 107, 6022–6027. 10.1073/pnas.1001522107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich MC, Tse WT, et al. (1998). A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proceedings of the National Academy of Sciences of the United States of America, 95, 14158–14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrbe S, Harms FL, Parrini E, Montomoli M, Mütze U, Helbig KL, et al. (2017). Delineating SPTAN1 associated phenotypes: From isolated epilepsy to encephalopathy with progressive brain atrophy. Brain, 140, 2322–2336. 10.1093/brain/awx195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns’ka I, Nikonenko AG, & Schachner M (2006). NCAM promotes assembly and activity dependent remodeling of the postsynaptic signaling complex. Journal of Cell Biology, 174(7), 1071–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Y, Gallo NB, Wang M, Yu J-R, & Van Aelst L (2019). Axo-axonic innervation of neocortical pyramidal neurons by GABAergic chandelier cells requires AnkyrinG-associated L1CAM. Neuron, 102, 358–372. 10.1016/j.neuron.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, & Hirikawa N (2000). Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. The Journal of Cell Biology, 148, 1255–1265. 10.1083/jcb.148.6.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Leshchyns’ka I, Welch JH, Diakowski W, Yang H, Schachner M, et al. (2012). Lipid raft-dependent endocytosis of close homolog of adhesion molecule L1 (CHL1) promotes neuritogenesis. Journal of Biological Chemistry, 287(53), 44447–44463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama J, Nakashima M, Nabatame S, Gaik-Siew C, Miyata R, Rener-Primec Z, et al. (2015). SPTAN1 encephalopathy: Distinct phenotypes and genotypes. Journal of Human Genetics, 60, 167–173. 10.1038/jhg.2015.5 [DOI] [PubMed] [Google Scholar]
- Tseng WC, Jenkins PM, Tanaka M, Mooney R, & Bennett V (2015). Giant ankyrin-G stabilizes somatodendritic GABAergic synapses through opposing endocytosis of GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America, 112, 1214–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvia S, Garver TD, & Bennett V (1997). The phosphorylation state of the FIGQY tyrosine of neurofascin determines ankyrin-binding activity and patterns of cell segregation. Proceedings of the National Academy of Sciences of the United States of America, 94, 12957–12962. 10.1073/pnas.94.24.12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsain N, Bordenave MD, Martinez GF, Jalil S, von Bilderling C, Barabas FM, et al. (2018). Remodeling of the actin/spectrin membrane-associated periodic skeleton, growth cone collapse and f-actin decrease during axonal degeneration. Scientific Reports, 8, 3007–3007. 10.1038/s41598-018-21232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnavicius L, Zhang K, Diamant AG, Motz C, Schlager MA, Yu M, et al. (2015). The structure of the dynactin complex and its interaction with dynein. Science, 347(6229), 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursitti JA, & Fowler VM (1994). Immunolocalization of tropomodulin, tropomyosin and actin in spread human erythrocyte skeletons. Journal of Cell Science, 107, 1633–1639. [DOI] [PubMed] [Google Scholar]
- Ursitti JA, Martin L, Resneck WG, Chaney T, Zielke C, Alger BE, et al. (2001). Spectrins in developing rat hippocampal cells. Developmental Brain Research, 129, 81–93. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos S, Gibaud S, Jimenez A, Caillol G, & Leterrier C (2019). Ultrastructure of the axonal periodic scaffold reveals a braid-like organization of actin rings. Nature Communications, 10(1), 5803 10.1038/s41467-019-13835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos YJ, de Walle HE, Bos KK, Stegeman JA, Ten Berge AM, Bruining M, … Hofstra RMW (2010). Genotype-phenotype correlations in L1 syndrome: A guide for genetic counselling and mutation analysis. Journal of Medical Genetics, 47, 169–175. 10.1136/jmg.2009.071688 [DOI] [PubMed] [Google Scholar]
- Wang C, Taki M, Sato Y, Tamura Y, Yaginuma H, Okada Y, & Yamaguchi S (2019). A photostable fluorescent marker for the superresolution live imaging of the dynamic structure of the mitochondrial cristae. Proceedings of the National Academy of Sciences U S A, 116(32), 15817–15822. 10.1073/pnas.1905924116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wei Z, Chen K, Ye F, Yu C, Bennett V, & Zhang M (2014). Structural basis of diverse membrane target recognitions by ankyrins. eLife, 3 10.7554/eLife.04353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yu C, Ye F, Wei Z, & Zhang M (2012). Structure of the ZU5-ZU5-UPA-DD tandem of ankyrin-B reveals interaction surfaces necessary for ankyrin function. Proceedings of the National Academy of Sciences of the United States of America, 109, 4822–4827. 10.1073/pnas.1200613109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Ortiz-González XR, Yum SW, Gill SM, White A, Kelter E, et al. (2018). βIV spectrinopathies cause profound intellectual disability, congenital hypotonia, and motor axonal neuropathy. American Journal of Human Genetics, 102, 1158–1168. 10.1016/j.ajhg.2018.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Miller R, Shaw R, & Shaw G (1996). The pleckstrin homology domain of human beta I sigma II spectrin is targeted to the plasma membrane in vivo. Biochemical and Biophysical Research Communications, 225, 420–426. [DOI] [PubMed] [Google Scholar]
- Wang G, Simon DJ, Wu Z, Belsky DM, Heller E, O’Rourke MK, et al. (2019). Structural plasticity of actin-spectrin membrane skeleton and functional role of actin and spectrin in axon degeneration. eLife, 8, e38730 10.7554/eLife.38730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ou S-W, & Wang Y-J (2017). Distribution and function of voltage-gated sodium channels in the nervous system. Channels (Austin, Tex.), 11, 534–554. 10.1080/19336950.2017.1380758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ji T, Nelson AD, Glanowska K, Murphy GG, Jenkins PM, & Parent JM (2018). Critical roles of αII spectrin in brain development and epileptic encephalopathy. The Journal of Clinical Investigation, 128, 760–773. 10.1172/JCI95743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Pennise CR, Babcock GG, & Fowler VM (1994). Tropomodulin caps the pointed ends of actin filaments. The Journal of Cell Biology, 127, 1627–1635. 10.1083/jcb.127.6.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler A, & Teichberg VI (1998). Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO Journal, 17, 3931–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirgenes KV, Tesli M, Inderhaug E, Athanasiu L, Agartz I, Melle I, et al. (2014). ANK3 gene expression in bipolar disorder and schizophrenia. British Journal of Psychiatry, 205, 244–245. 10.1192/bjp.bp.114.145433 [DOI] [PubMed] [Google Scholar]
- Wu HC, Yamankurt G, Luo J, Subramaniam J, Hashmi SS, Hu H, & Cunha SR (2015). Identification and characterization of two ankyrin-B isoforms in mammalian heart. Cardiovascular Research, 107, 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhong G, & Zhuang X (2013). Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science, 339, 452–456. 10.1126/science.1232251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, & Cooper EC (2015). An ankyrin-g n-terminal gate and protein kinase ck2 dually regulate binding of voltage-gated sodium and KCNQ2/3 potassium channels. Journal of Biological Chemistry, 290, 16619–16632. 10.1074/jbc.M115.638932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Walder-Christensen KK, Kim N, Wu D, Lorenzo DN, Badea A, et al. (2019). ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proceedings of the National Academy of Sciences of the United States of America, 116, 15262–15271. 10.1073/pnas.1904348116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Walder-Christensen KK, Lalani S, Yan H, García-Prieto ID, Álvarez S, … Bennett V (2019). Neurodevelopmental mutation of giant ankyrin-G disrupts a core mechanism for axon initial segment assembly. Proceedings of the National Academy of Sciences of the United States of America, 116(39), 19717–19726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ogawa Y, Hedstrom KL, & Rasband MN (2007). betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. The Journal of Cell Biology, 176, 509–519. 10.1083/jcb.200610128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Li J, Ye F, Zhang Y, Zhang M, & Wang C (2020). Mechanistic insights into the interactions of dynein regulator Ndel1 with neuronal ankyrins and implications in polarity maintenance. Proceedings of the National Academy of Sciences of the United States of America, 117, 1207–1215. 10.1073/pnas.1916987117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Stevens SR, Leterrier C, Stankewich MC, & Rasband MN (2016). Developmental changes in expression of betaiv spectrin splice variants at axon initial segments and nodes of Ranvier. Frontiers in Cellular Neuroscience, 10, 304 10.3389/fncel.2016.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Susuki K, Zollinger DR, Dupree JL, & Rasband MN (2013). Membrane domain organization of myelinated axons requires βII spectrin. The Journal of Cell Biology, 203, 437–443. 10.1083/jcb.201308116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, He J, Zhou R, Lorenzo D, Babcock HP, Bennett V, & Zhuang X (2014). Developmental mechanism of the periodic membrane skeleton in axons. eLife, 3, e04581 10.7554/eLife.04581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, & Bloch RJ (1997). Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. The Journal of Cell Biology, 136, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, & Bennett V (1998). AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. The Journal of Cell Biology, 143, 1295–1304. 10.1083/jcb.143.5.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Han B, Xia C, & Zhuang X (2019). Membrane-associated periodic skeleton is a signaling platform for RTK transactivation in neurons. Science (New York, N.Y.), 365, 929–934. 10.1126/science.aaw5937 [DOI] [PMC free article] [PubMed] [Google Scholar]


