Abstract
Purpose of review
To advance a re-conceptualized prevention, treatment, and care continuum (PTCC) for HIV-associated TB in prisons, and to make recommendations for strengthening prison health systems and reducing HIV-associated TB morbidity and mortality throughout the cycle of pre-trial detention, incarceration, and release.
Recent findings
Despite evidence of increased HIV-associated TB burden in prisons compared to the general population, prisoners face entrenched barriers to accessing anti-TB therapy, anti-retroviral therapy, and evidence-based HIV and TB prevention. New approaches, suitable for the complexities of healthcare delivery in prisons, have emerged that may address these barriers, and include: novel TB diagnostics, universal test and treat for HIV, medications for addiction treatment, comprehensive transitional case management, and peer navigation, among others.
Summary
Realizing ambitious international HIV and TB targets in prisons will only be possible by first addressing the root causes of the TB/HIV syndemic, which are deeply intertwined with human rights violations and weaknesses in prison health systems, and, second, fundamentally re-organizing HIV and TB services around a coordinated PTCC. Taking these steps can help ensure universal access to comprehensive, good-quality, free and voluntary TB/HIV prevention, treatment, and care, and advance efforts to strengthen health resourcing, staffing, information management, and primary care access within prisons.
Keywords: prisons, inmates, HIV, tuberculosis, health systems
INTRODUCTION
Though global tuberculosis (TB) incidence and mortality for people living with HIV (PLHIV) has declined over the last decade, HIV-associated TB still afflicts over 1 million people annually and accounts for one-third of all TB deaths[1]. Disproportionately affected are key and vulnerable populations, including prison inmates and other detained people. Inmates endure pervasive structural violence both within and outside prison as a result of draconian anti-drug policies, systemic racial and ethnic discrimination, inequitable access to healthcare, and criminalization of sex work, drug use, and homosexuality[2–4]. Due to a complex interplay among these and other factors, the prevalence of HIV and TB in prisons is several-fold higher than in neighboring communities[5].
Compounding these challenges, health systems and resourcing in prisons are at best constrained and at worst non-existent, and prisons health programming rarely involves systematic efforts to ensure an integrated prevention, treatment, and care continuum (PTCC) for numerous diseases, including HIV-associated TB[6]. As a result, access to anti-TB therapy (ATT) and anti-retroviral therapy (ART) for inmates with HIV-associated TB is woefully inadequate: less than one-third of countries[7] even offer ART in prisons; where in-prison ART is available, structural barriers such as a lack of certain ART regimens and inmate hesitancy to disclose their status to corrections officers and other treatment gatekeepers hinders access[7]—for example, 74% of Malaysian inmates did not receive ART despite qualifying per national guidelines[8]; and, although 63 countries globally offer ATT in prisons[2], inmate ATT initiation and completion rates remain suboptimal[9].
It is not possible to combat HIV-associated TB in prisons without first addressing the root causes of the epidemic, which are deeply intertwined with human rights violations and weaknesses in prison and community health systems. Human rights law, including the recently adopted UN Standard Minimum Rules for the Treatment of Prisoners, or “Mandela Rules”[10], unequivocally calls for every prisoner to “enjoy the same standards of health care that are available in the community”[10], and for safeguarding prisoner rights to timely preventive and curative medical care and freedom from torture and ill treatment, among others[2,6,10]. Collectively, these rights advance a notion of health care in prisons equipped to deliver a cohesive PTCC for HIV-associated TB during pre-trial detention, throughout incarceration, and after release.
In this article, we present an integrated PTCC for HIV-associated TB that acknowledges the logistical and political complexities of healthcare delivery in prisons, and that demonstrates the need for greater investment in prison health system strengthening. We also synthesize recent epidemiological and other evidence on HIV-associated TB in prisons, paying particular attention to reports from low- and middle-income countries (LMICs) and highlighting promising policies and practices for realizing ambitious End TB Strategy and UNAIDS 90-90-90 targets[11,12]. Throughout this article, we use the term “prison,” “correctional facility,” and “carceral setting” to represent any facility detaining an individual accused of, arrested for, or convicted of a crime—whom we refer to interchangeably as “prisoner” or “inmate”—including police holding cells, jails, pre-trial detention centers, and correctional facilities. We define “prison health system strengthening” as strategic resourcing for, and reforms of, the following health system building blocks ideally intended to provide inmates with highly accessible, equitable, effective, and cost-efficient health services within the complex prison environment: 1) leadership, management, and governance structures; 2) human resources for health; 3) health information systems; 4) clinical and laboratory infrastructure; and 5) health commodity logistics and supply chain management.
HIV-ASSOCIATED TB EPIDEMIOLOGY IN PRISONS
Limited data characterize the epidemiology of HIV-associated TB in prisons[13], with current reports tending to estimate HIV prevalence among inmates with active TB, or active TB prevalence among prisoners living with HIV, as opposed to describing the incidence and prevalence of HIV-associated TB or TB/HIV co-infection (which encompasses both latent and active TB) among all inmates within a carceral setting[5,13]. Rigorous estimates that include all inmates reflect a range of TB/HIV co-infection prevalence in prisons varying by region, from <1% in Latin America and Western Europe to >5% in parts of sub-Saharan Africa (SSA)[5,14,15]. Relative risk of inmates developing active TB given HIV, or having HIV given active TB, varies, with reported risk ratios ranging between 2.0—10.75[13]. While limited, existing evidence indicates that HIV-associated TB prevalence is likely to be higher in prison populations than the general community, particularly in regions with a generalized TB/HIV syndemic like SSA or where injection drug use is more prevalent, such as Eastern Europe and Central Asia[5,16]. Insights into the differential burden of HIV-associated TB experienced by incarcerated adolescents and young adults and female and transgender inmates are severely limited by few reports presenting data disaggregated by age, sex, gender identity, or key population status[5,17,18]. Similarly, aggregated prevalence data may mask meaningful differences in HIV-associated TB epidemiology across criminal justice jurisdictions and facility types due to the distinct TB screening practices and inmate demographics encountered in these different components of the carceral system. For example, in California, USA, HIV-associated TB was more frequently seen in state prisons where inmates with injection drug use history are more likely to be incarcerated[19].
Emerging data suggests a high burden of latent tuberculosis infection (LTBI) among HIV-positive inmates, particularly in LMICs[20–24]. In a large Iranian prison, among 173 inmates with known HIV status seen 6 months after negative TST testing, the odds of developing LTBI was ten times as high among HIV-positive as HIV-negative inmates, reinforcing HIV infection as a known risk factor for LTBI acquisition in prisons[24].
Although the association between HIV and multi-drug resistant tuberculosis (MDR-TB) among inmates has long been documented from prison reports of MDR-TB outbreaks[25], scarce studies shed new light on HIV-associated MDR-TB in prisons[26–28]. A few studies, mostly from SSA, suggest that HIV-associated MDR-TB is an emerging problem, but a lack of consistent HIV status reporting and application of molecular epidemiology hamper understanding of the scope of the problem and the transmission dynamics within prisons and between prisons and communities[9,14,29–31]. Countries of the former Soviet Union merit special attention as rates of MDR-TB and injection drug use among prison populations in these states rank among the world’s highest, yet few reports describe the extent of HIV co-infection in these populations or treatment outcomes for HIV-associated MDR-TB[16,28].
A COORDINATED PREVENTION, TREATMENT, AND CARE CONTINUUM FOR HIV-ASSOCIATED TB
TB and HIV services in prisons are often delivered ad hoc, and, where organized, tend to perpetuate deficiencies seen in the wider health system. In many LMIC health systems, HIV services are delivered in parallel to existing, and many times weakly constituted, TB programs as a consequence of having been established via disease-specific global health initiatives. In these countries, TB and HIV services have historically been provided through vertical programs despite evidence demonstrating superior outcomes with more integrated and comprehensive approaches[32–35]. With recent treatment advances, such as universal “test and treat” (UTT) for HIV, and improved screening and molecular diagnostic tools for TB, including Xpert MTB/RIF and LAM TB[36], there is a window of opportunity to bring such innovations into prison settings and reframe TB and HIV prevention, treatment, and care as a single, coordinated continuum (Figure 1). Such a harmonized PTCC offers the potential to generate new efficiencies for integrating TB/HIV service delivery in challenging prison settings, and to improve HIV-associated TB clinical outcomes throughout the cycle of pre-trial detention, incarceration, and return to the community (Figure 2).
Figure 1.
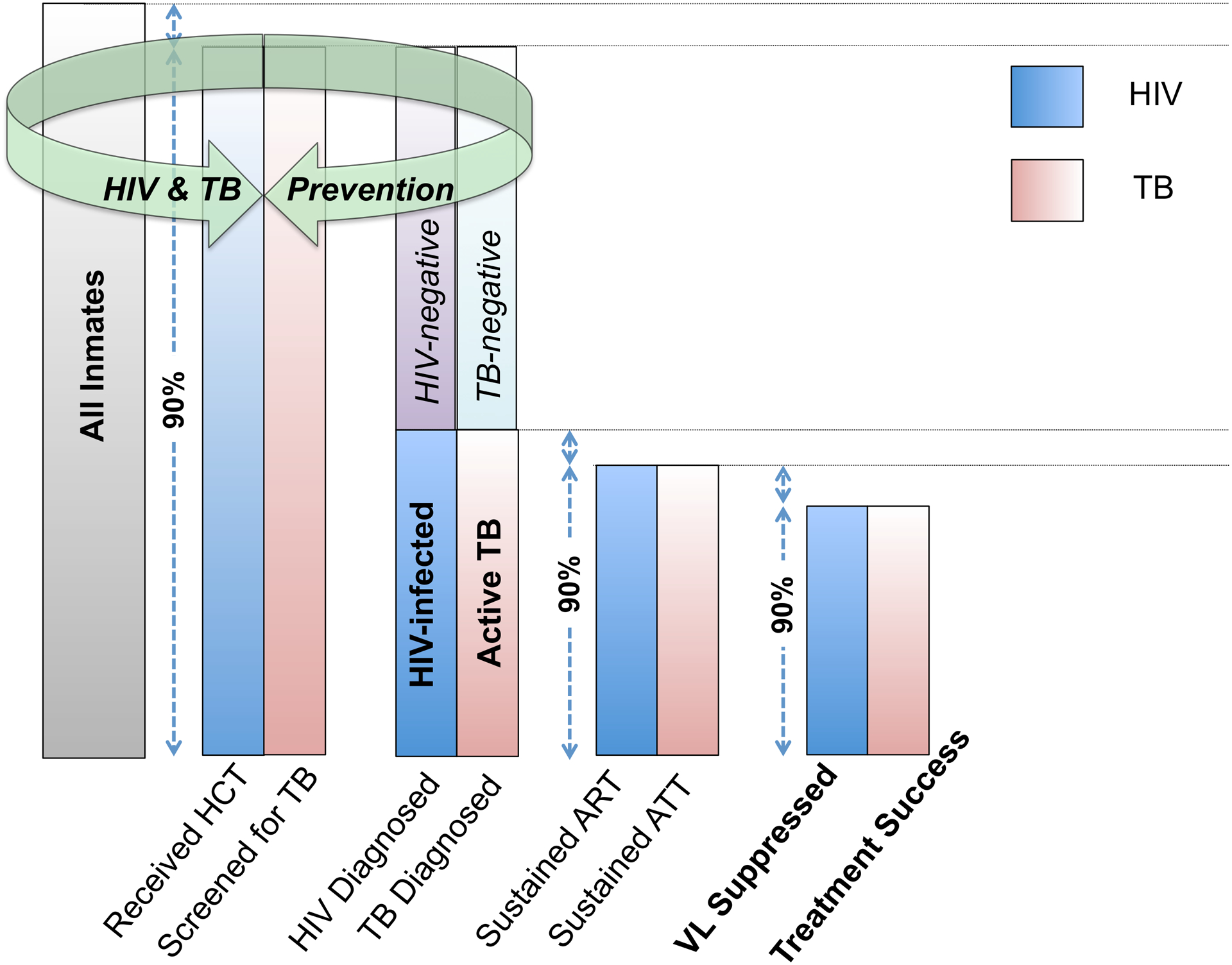
A coordinated prevention, treatment, and care continuum for HIV and TB in prisons. (HIV= Human immunodeficiency virus; TB= tuberculosis; HCT= HIV counseling and testing; ART= Anti-retroviral therapy; ATT= Anti-TB therapy; VL= Viral load).
Figure 2.
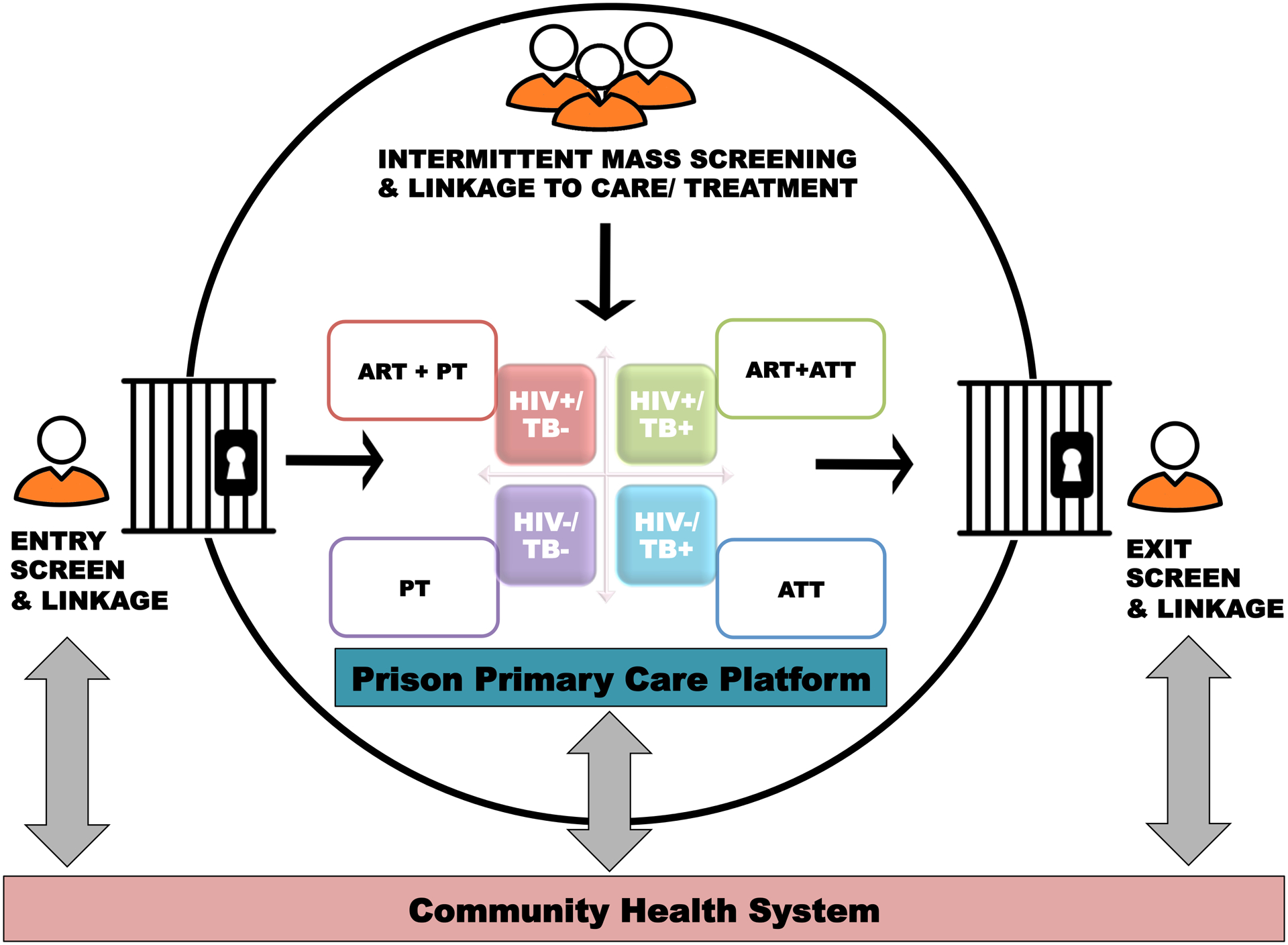
Operational framework for delivering integrated TB/HIV services linked to care and treatment in prison settings over the period spanning pre-trial detention, incarceration, and release to the community. (ART= antiretroviral therapy; TB= tuberculosis; ATT= anti-tuberculosis therapy; PT= preventive therapy).
Promising strategies for HIV-associated TB prevention, screening, and diagnosis
Over half of all countries globally expose inmates to overcrowding, and thus, increased risk of HIV and TB exposure[37,38]. Confronting this problem by reducing the number of people detained remains the most promising approach to decreasing HIV-associated TB burden in prisons long-term[5,39]. Yet the power to reduce incarceration rates lies outside the health sector, requiring reforms within the criminal justice system (Table 1)[2,39–46].
Table 1. Criminal justice reforms to reduce incarceration rates.
The following criminal justice reforms can help reduce prison overcrowding and, with it, the risk of HIV-associated TB in carceral settings.
| Reform/Action | Definition and Evidence | Target Stakeholders |
|---|---|---|
| Legislation and criminal law reform | Evidence supports decriminalization of behaviors associated with HIV transmission and unjust imprisonment, such as for drug-related offenses. In Portugal, de-criminalization of drug possession and use was associated with reduced HIV transmission[2,40,41] | Federal and state or provincial legislators and parliamentarians. |
| Eliminating arbitrary pre-trial detention | Pre-trial detention is common and may be extensive in under-resourced criminal justice systems. Eliminating this practice can reduce the number of people detained without a conviction, and can support efforts to mitigate prison overcrowding. Limited evidence suggests that this may be a cost-effective intervention to reduce inmate exposure to HIV and TB risk brought about by incarceration[42,43]. | Federal and state or provincial legislators and parliamentarians; Judges and courts at all levels of the criminal justice system; Public defendants |
| Release on personal recognizance, and non-custodial sentencing | These criminal justice interventions can also alleviate prison overcrowding and have been implemented in a number of countries globally[2,41,44,45]. | Federal and state or provincial legislators and parliamentarians; Judges and courts at all levels of the criminal justice system; Public defendants |
| Substance use disorder treatment for drug offenders outside carceral settings | Focusing on providing substance use treatment to non-violent drug offenders in medical settings would offload already burdened criminal justice systems, and more effectively, sustainably, and humanely address many of the root social and physiologic factors contributing to HIV transmission among people who use drugs[39,41,46]. | Judges and courts at all levels of the criminal justice system; Public defendants; Prison and Community Health System Administrators; Substance Use Treatment Program Managers |
For those who ultimately become incarcerated, upgrading prison infrastructure to meet international standards for daily cell occupancy, floor space, and air exchange can reduce TB transmission by ≥50%[47–49]. Robust TB infection control measures should also be in place to ensure prompt identification, isolation, and treatment of all active TB cases[50].
Integrated TB and HIV prevention should be a part of a comprehensive health assessment offered to every inmate at entry, exit, and regular intervals throughout incarceration. In high-burden settings, this should include, at a minimum: 1) non-coercive, opt-out HIV testing services[51,52] with linkage to HIV care and treatment for HIV-positive inmates, pre- and post-exposure prophylaxis (PEP, PrEP) for HIV-negative inmates, and access to condoms, lubricant, needle exchange, and medications for addiction treatment (MAT) for all inmates[47]; and 2) evidence-based TB screening, diagnosis, and treatment. To maximize service uptake, TB and HIV screening should be integrated and employ provider-initiated, active-case finding strategies that are voluntary but systematic[51]. Active case-finding is preferable to passive, triage-based approaches that may fail to curb HIV-associated TB prevalence even in prisons with longstanding, on-site TB treatment programs[29].
In the complex prison context, with often higher TB burden, screening PLHIV by the WHO-endorsed 4-symptom rule[53] alone may be insufficiently sensitive to discriminate between inmates who can safely receive preventive therapy versus those requiring further investigation for TB[53]. For example, in Malaysia, among 34 inmates with pulmonary TB diagnosed by liquid culture or Xpert, of whom 15 (44%) had HIV-associated TB, the WHO symptom screen would have missed 61.8% of cases if used alone[8]. In such high burden settings, universal TB screening should be performed by chest radiography (CXR), resources permitting. CXR has higher sensitivity than symptom screening, particularly among PLHIV on ART[53], and should be used as a pre-screen tool to identify presumptive TB cases requiring diagnostic testing[54]. CXR also has the potential to: increase TB screening throughput, especially when coupled with computer-aided detection (CAD) systems[55,56]; identify HIV-negative inmates requiring isolation and further work-up as part of routine infection control and clinical care[57]; and lower TB diagnostic testing costs by reducing the number needed to test[58]. Where CXR is not available, screening may be done by WHO 4-symptom screen or a clinical rule tailored to prison settings[53,59,60].
For all inmates with a positive TB screen, diagnostic work-up should proceed by sputum smear microscopy, nucleic acid amplification testing by Xpert MTB/RIF or line probe assay, and mycobacterial culture, where available[61]. In high-burden, low resource settings, Xpert MTB/RIF is the diagnostic tool of choice given its higher sensitivity, rifampin resistance-detection capabilities, and rapid turnaround time[47,53,62,63]. When used as part of an active TB case-finding strategy in prisons, Xpert MTB/RIF may support high-volume systematic screening[64] and result in high case detection[31]. Although testing by a single Xpert MTB/RIF assay alone may miss a substantial proportion of TB cases in prisons[65], testing with the newer Xpert MTB/RIF Ultra holds promise for improving HIV-associated TB case detection owing to improved diagnostic sensitivity, particularly for paucibacillary disease[36,66,67].
Once active TB has been ruled out, LTBI testing and treatment should be considered for all inmates[53]. Although not required to start preventive therapy in HIV-positive inmates, LTBI testing with either TST or interferon gamma release assay (IGRA)[53] may be used as no study has established superiority of one test over the other in PLHIV[53,61]. In high-income prison settings, however, IGRA may be preferred due to its lower cost per LTBI case detected[68].
Where TB transmission and incidence is high (≥100/100,000 population), WHO recommends ≥36 months of isoniazid preventive therapy (IPT) for PLHIV without active TB[53]. Thus, in countries with a TB/HIV syndemic, prisons may qualify as settings where an extended IPT course could be offered to HIV-positive inmates. WHO guidance notwithstanding, much remains unknown about delivering preventive therapy in prisons. Few reports document preventive therapy acceptability, adverse events, and completion rates, and no accounts describe secondary prevention efficacy among inmates with history of active TB or the utility of repeat or continuous IPT[53,69,70]. For example, for HIV-positive inmates with long sentences in high-turnover facilities, 36 months of IPT may be insufficient to confer sustained protection against LTBI and TB-related morbidity and mortality. Conversely, for prisoners detained a few months, a shorter course of a newer preventive regimen may be adequate to mitigate against these risks while also facilitating treatment completion.
New evidence for short course preventive therapy has emerged from the BRIEF-TB trial, which revealed that 1 month of daily isoniazid plus rifapentine (1HP) is non-inferior to 9 months of IPT on outcomes of incident TB disease or death from active TB or other cause, and results in fewer adverse events and higher rates of treatment completion for PLHIV[71]. These data suggest new possibilities for less logistically complex and resource intensive delivery of preventive treatment in prisons, extending findings from a US cohort study that demonstrated superior treatment completion for 91 HIV-negative inmates receiving 3 months of HP (85%) compared to 154 inmates who received 9 months of IPT (18%)[72]. While, collectively, these findings are cause for optimism, additional research is needed to define the drug-drug interactions between these LTBI regimens and newer anti-retrovirals like dolutegravir[73], and to elucidate their effects on active TB risk, ART timing, and TB-related morbidity and mortality in prisons.
HIV-associated TB Linkage, Treatment, and Care
While establishing an HIV-associated TB diagnosis is insufficient, in and of itself, to ensure timely HIV and TB treatment, few studies characterize the treatment steps for the HIV-associated TB PTCC in prisons[13]. Where characterized, evidence suggests it may be possible to coordinate TB/HIV care to achieve favorable clinical outcomes. For example, in a cohort of 496 inmates with active TB in Ethiopia, 91.5% (n/N=43/47) of those with HIV-associated TB and a documented outcome achieved WHO-defined treatment success[74]. Overall mortality in this cohort was low at 1.4%, explained, in part, by early and relatively high ART uptake among inmates with HIV-associated TB, 85% (n/N=46/54) of whom were either on ART at TB diagnosis or started ART within 3 months of ATT initiation[74]. Indeed, ensuring early ART for inmates during TB treatment—within 2 weeks for those with CD4 count <50 cells/μL and by 8–12 weeks for patients with CD4 count ≥50 cells/μL—confers an important survival benefit[75].
The advent of UTT may improve TB/HIV coordination for HIV-associated TB in prisons. Preliminary results from a UTT program in one large Zambian correctional facility revealed that 144 of 149 HIV-positive inmates (96.6%) underwent TB screening by Xpert MTB/RIF, and, of these, 10 were diagnosed with active TB by Xpert or clinically, with 100% receiving timely ATT and ART[76]. Though encouraging, these early results were achieved under study conditions, and the Zambian correctional system is currently under-capacitated to sustain such UTT services without additional resourcing[77]. Further programmatically relevant research is needed to identify strategies that can overcome fundamental and structural challenges with delivering dual treatment in prisons, providing ancillary services such as viral load monitoring, and ensuring linkage to, and continuation of, ATT and ART in the community following release[2,7].
Current evidence points to the myriad ways in which community release and inter-facility transfer interrupt the TB/HIV PTCC. In California, USA, inmates with active TB, including HIV-associated TB, were less likely to complete their ATT than comparable patients in the general population[19]. Studies from SSA echo these findings, with LTFU as high as 19% and 29% in Ethiopia and Zambia, respectively, for TB-treated inmates, including a substantial proportion with HIV-associated TB[9,74]. New approaches should be implemented and evaluated to reinforce a cohesive PTCC that spans pre-trial detention, incarceration, inter-prison transfer and release. Promising approaches include: comprehensive transitional case management[78], intensive peer navigation[79], multi-level interventions employing motivational interviewing[80], opioid substitution therapy for co-morbid drug dependence[81], and combination social welfare interventions[2,7].
ACHIEVING UNIVERSAL HEALTH COVERAGE IN PRISONS REQUIRES A HEALTH SYSTEM STRENGTHENING APPROACH
Ending HIV-associated TB in prisons requires moving away from one-off interventions, and toward making several multidimensional investments in, and reforms of, criminal justice and prison health systems. First, reforms are needed to end discriminatory law enforcement and detention practices that oppress key, minority, and marginalized populations[4] already facing disproportionately high HIV and TB burden. Second, an appropriately resourced prison health system should be made freely available to all inmates, one able to serve as a primary care platform for delivering integrated prevention, treatment and care for HIV, TB, and other conditions, including substance use disorders. To enact this system, governments, with partner support where necessary, must: allow health institutions independent oversight[82] for delivering gender and disease-appropriate health services in prisons[83,84]; offer comprehensive, corrections-relevant health training to prison security and healthcare staff[83,84]; introduce electronic health information systems to monitor the PTCC for inmates undergoing transfer and release[77]; ensure uninterrupted access to essential health commodities, including condoms, lubricant, clean needles, ART, ATT, IPT, MAT, PEP, and PrEP[47]; provide adequate nutrition[85]; empower inmates to serve as their own healthcare decision-makers[84]; and deliver robust discharge planning, social welfare, and linkage to community services to reinforce care continuity for HIV-associated TB after release[2].
CONCLUSION
Prisoners face entrenched challenges of high TB/HIV burden and a fragmented TB/HIV PTCC. We argue that achieving ambitious international targets in prisons will only be possible by fundamentally re-organizing TB and HIV services around a coordinated PTCC, one that applies a “test and treat” strategy to ensure universal access to good-quality, free and voluntary TB and HIV screening and prevention for all inmates, and prompt linkage to ART and TB preventive therapy or ATT, as appropriate. UN adoption of the Mandela Rules has refocused the international community on the profound inequities experienced by prisoners, including those with HIV-associated TB. Realizing a world worthy of the Rules’ namesake will require new and sustained commitment to prisons health system strengthening and criminal justice reform on a scale commiserate with the magnitude of the HIV-associated TB problem currently faced by prisoners globally.
KEY POINTS.
Despite the disproportionately high HIV-associated TB burden in prisons, access to evidence-based TB/HIV treatment and prevention tools for prison inmates is limited in a majority of countries.
To improve HIV-associated TB clinical outcomes during pre-trial detention and incarceration, and after release to the community, we present an integrated TB/HIV prevention, treatment, and care continuum (PTCC) that coordinates disparate HIV and TB cascades and acknowledges the logistical and political complexities of healthcare delivery in prisons.
Recent evidence points to a number of promising interventions for strengthening the TB/HIV PTCC in prisons that warrant implementation and further study, including: inmate peer education and navigation; new TB diagnostics; new short course regimens for latent TB infection; comprehensive health training for prison security and healthcare staff; inmate empowerment for healthcare decision-making; medications for addiction treatment (MAT) for co-morbid drug dependence; and combination social welfare interventions.
Available reports of TB in prisons frequently do not present clinical and epidemiological findings disaggregated by HIV status, age band, sex, gender, or key population status, thereby hampering efforts to develop tailored health programs and research activities for HIV-associated TB in prison settings.
Ending HIV-associated TB in prisons requires moving away from one-off health interventions, and toward making multidimensional investments in, and reforms of, criminal justice and prison health systems.
Acknowledgements
This work was supported in part by the U.S. National Institute of Health (MEH: 1K01TW010272).
Footnotes
Conflicts of Interest:
The authors declare that they have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
∎ of special interest
∎∎ of outstanding interest
- 1.WHO. Global Tuberculosis Report. Geneva: World Health Organization, 2017. [Google Scholar]
- 2.Rubenstein LS, Amon JJ, McLemore M, Eba P, Dolan K, Lines R, et al. HIV, prisoners, and human rights. The Lancet. 2016;388(10050):1202–14. [DOI] [PubMed] [Google Scholar]
- 3.Beyrer C, Kamarulzaman A, McKee M. Prisoners, prisons, and HIV: time for reform. The Lancet. 2016;388(10049):1033–5. [DOI] [PubMed] [Google Scholar]
- 4.Shrage L. African Americans, HIV, and mass incarceration. The Lancet. 2016;388(10049):e2–e3. [DOI] [PubMed] [Google Scholar]
- 5.Dolan K, Wirtz AL, Moazen B, Ndeffo-mbah M, Galvani A, Kinner SA, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. The Lancet. 2016;388(10049):1089–102. [DOI] [PubMed] [Google Scholar]
- 6.Sander G, Lines R. HIV, Hepatitis C, TB, Harm Reduction, and Persons Deprived of Liberty: What Standards Does International Human Rights Law Establish? Health and human rights. 2016. December;18(2):171–82. [PMC free article] [PubMed] [Google Scholar]
- 7.Rich JD, Beckwith CG, Macmadu A, Marshall BDL, Brinkley-Rubinstein L, Amon JJ, et al. Clinical care of incarcerated people with HIV, viral hepatitis, or tuberculosis. The Lancet. 2016;388(10049):1103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Darraji HA, Altice FL, Kamarulzaman A. Undiagnosed pulmonary tuberculosis among prisoners in Malaysia: an overlooked risk for tuberculosis in the community. Tropical medicine & international health : TM & IH. 2016. August;21(8):1049–58. [DOI] [PubMed] [Google Scholar]
- 9.Hatwiinda S, Topp SM, Siyambango M, Harris JB, Maggard KR, Chileshe C, et al. Poor continuity of care for TB diagnosis and treatment in Zambian Prisons: a situation analysis. Tropical medicine & international health : TM & IH. 2018. February;23(2):243–50. [DOI] [PubMed] [Google Scholar]
- 10.UN. United Nations standard minimum rules for the treatment of prisoners (the Mandela rules) 2015. United Nations Economic and Social Council, 21 May 2015. [Google Scholar]
- 11.Partnership ST. The Paradigm Shift, 2016–2020: Global Plan to End TB. Geneva: Stop TB Partnership, 2015. [Google Scholar]
- 12.UNAIDS. Fast-Track: Ending the AIDS Epidemic by 2030. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS, 2014. [Google Scholar]
- 13.Edge CL, King EJ, Dolan K, McKee M. Prisoners co-infected with tuberculosis and HIV: a systematic review. Journal of the International AIDS Society. 2016;19(1):20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebrecherkos T, Gelaw B, Tessema B. Smear positive pulmonary tuberculosis and HIV co-infection in prison settings of North Gondar Zone, Northwest Ethiopia. BMC public health. 2016. October 18;16(1):1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gizachew Beza M, Hunegnaw E, Tiruneh M. Prevalence and Associated Factors of Tuberculosis in Prisons Settings of East Gojjam Zone, Northwest Ethiopia. International journal of bacteriology. 2017;2017:3826980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altice FL, Azbel L, Stone J, Brooks-Pollock E, Smyrnov P, Dvoriak S, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016. September 17;388(10050):1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinner SA, Snow K, Wirtz AL, Altice FL, Beyrer C, Dolan K. Age-Specific Global Prevalence of Hepatitis B, Hepatitis C, HIV, and Tuberculosis Among Incarcerated People: A Systematic Review. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2018. March;62(3s):S18–s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poteat TC, Malik M, Beyrer C. Epidemiology of HIV, Sexually Transmitted Infections, Viral Hepatitis, and Tuberculosis Among Incarcerated Transgender People: A Case of Limited Data. Epidemiol Rev. 2018. March 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDaniel CJ, Chitnis AS, Barry PM, Shah N. Tuberculosis trends in California correctional facilities, 1993–2013. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2017. August 1;21(8):922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro PD, Almeida IN, Kritski AL, Ceccato MD, Maciel MM, Carvalho WD, et al. Prevalence of latent Mycobacterium tuberculosis infection in prisoners. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2016. Sep-Oct;42(5):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez de Goicoechea-Saiz ME, Sternberg F, Portilla-Sogorb J. Prevalence and associated risk factors of latent tuberculosis infection in a Spanish prison. Revista espanola de sanidad penitenciaria. 2018;20(1):4–10. [PMC free article] [PubMed] [Google Scholar]
- 22.Sagnelli E, Starnini G, Sagnelli C, Monarca R, Zumbo G, Pontali E, et al. Blood born viral infections, sexually transmitted diseases and latent tuberculosis in italian prisons: a preliminary report of a large multicenter study. European review for medical and pharmacological sciences. 2012. December;16(15):2142–6. [PubMed] [Google Scholar]
- 23.Paiao DS, Lemos EF, Carbone AD, Sgarbi RV, Junior AL, da Silva FM, et al. Impact of mass-screening on tuberculosis incidence in a prospective cohort of Brazilian prisoners. BMC infectious diseases. 2016. October 3;16(1):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamani M, Mahmudian H, Majzoobi MM, Poorolajal J. Prevalence and incidence rates of latent tuberculous infection in a large prison in Iran. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2016. August;20(8):1072–7. [DOI] [PubMed] [Google Scholar]
- 25.Valway SE, Greifinger RB, Papania M, Kilburn JO, Woodley C, DiFerdinando GT, et al. Multidrug-resistant tuberculosis in the New York State prison system, 1990–1991. The Journal of infectious diseases. 1994. July;170(1):151–6. [DOI] [PubMed] [Google Scholar]
- 26.Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PloS one. 2009;4(5):e5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesfin YM, Hailemariam D, Biadgilign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PloS one. 2014;9(1):e82235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Droznin M, Johnson A, Johnson AM. Multidrug resistant tuberculosis in prisons located in former Soviet countries: A systematic review. PloS one. 2017;12(3):e0174373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seri B, Koffi A, Danel C, Ouassa T, Blehoue MA, Ouattara E, et al. Prevalence of pulmonary tuberculosis among prison inmates: A cross-sectional survey at the Correctional and Detention Facility of Abidjan, Cote d’Ivoire. PloS one. 2017;12(7):e0181995. [DOI] [PMC free article] [PubMed] [Google Scholar]; ∎ This cross-sectional study conducted in the largest prison in Côte d’Ivoire, 16 years after a prison TB program was first established there, demonstrates the significant limitations of passive TB case-finding approaches in reducing TB and MDR-TB prevalence in prisons in countries with substantial TB/HIV burden. Despite the long-standing availability of TB screening for prisoners presenting to the prison infirmary with TB symptoms, the study identified 88 cases of confirmed, probable, or possible TB among 943 inmates (9.3%) newly evaluated by the study—a TB prevalence 10 times that of the general population—with 9.6% of TB cases identified as being HIV-positive.
- 30.Ali S, Beckert P, Haileamlak A, Wieser A, Pritsch M, Heinrich N, et al. Drug resistance and population structure of M.tuberculosis isolates from prisons and communities in Ethiopia. BMC infectious diseases. 2016. November 21;16(1):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merid Y, Woldeamanuel Y, Abebe M, Datiko DG, Hailu T, Habtamu G, et al. High utility of active tuberculosis case finding in an Ethiopian prison. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2018. May 1;22(5):524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legido-Quigley H, Montgomery CM, Khan P, Atun R, Fakoya A, Getahun H, et al. Integrating tuberculosis and HIV services in low- and middle-income countries: a systematic review. Tropical medicine & international health : TM & IH. 2013. February;18(2):199–211. [DOI] [PubMed] [Google Scholar]
- 33.Rich ML, Miller AC, Niyigena P, Franke MF, Niyonzima JB, Socci A, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. Journal of acquired immune deficiency syndromes (1999). 2012. March 1;59(3):e35–42. [DOI] [PubMed] [Google Scholar]
- 34.Palazuelos D, Farmer PE, Mukherjee J. Community health and equity of outcomes: the Partners In Health experience. The Lancet Global Health. 2018;6(5):e491–e3. [DOI] [PubMed] [Google Scholar]
- 35.Uyei J, Coetzee D, Macinko J, Guttmacher S. Integrated delivery of HIV and tuberculosis services in sub-Saharan Africa: a systematic review. The Lancet Infectious Diseases. 2011;11(11):855–67. [DOI] [PubMed] [Google Scholar]
- 36.Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. The Lancet Infectious Diseases. 2018. [DOI] [PubMed] [Google Scholar]
- 37.Hella J, Morrow C, Mhimbira F, Ginsberg S, Chitnis N, Gagneux S, et al. Tuberculosis transmission in public locations in Tanzania: A novel approach to studying airborne disease transmission. J Infect. 2017. September;75(3):191–7. [DOI] [PubMed] [Google Scholar]
- 38.ICPR. World Prison Brief: Occupancy level (based on official capacity): Institute for Criminal Policy Research (ICPR); 2016. [cited April 30, 2018]. Available from: http://www.prisonstudies.org/highest-to-lowest/occupancy-level?field_region_taxonomy_tid=All.
- 39.Ndeffo-Mbah ML, Vigliotti VS, Skrip LA, Dolan K, Galvani AP. Dynamic Models of Infectious Disease Transmission in Prisons and the General Population. Epidemiol Rev. 2018. March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]; ∎ This sytematic review of dynamic models of HIV, TB, and other selected infectious diseases in prisons synthesizes data from 34 studies. Collectively, data from these included studies suggest that screening for and treating HIV, TB, and other infections in prisons may reduce the burden of these diseases among inmates and in the general community. The summarized evidence indicates that adopting measures to reduce rates of incarceration could lead to declines in HIV incidence among incarcerated people who inject drugs as well as new TB infections among all prisoners. Of note, of the 10 studies that met the authors’ inclusion criteria and focused on TB transmission dynamics, only 2 considered the contributions of HIV/TB co-infection in their models.
- 40.Csete J, Kamarulzaman A, Kazatchkine M, Altice F, Balicki M, Buxton J, et al. Public health and international drug policy. Lancet. 2016. April 2;387(10026):1427–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark N, Dolan K, Farabee D. Public health alternatives to incarceration for drug offenders. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2017. May 1;23(3):222–30. [DOI] [PubMed] [Google Scholar]
- 42.Todrys KW, Amon JJ. Criminal justice reform as HIV and TB prevention in African prisons. PLoS medicine. 2012;9(5):e1001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Open Society Justice Initiative. The socioeconomic impact of pretrial detention. . New York: Open Society Foundation, 2011. [Google Scholar]
- 44.Todrys KW, Amon JJ, Malembeka G, Clayton M. Imprisoned and imperiled: access to HIV and TB prevention and treatment, and denial of human rights, in Zambian prisons. Journal of the International AIDS Society. 2011;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.UNODC. Handbook on strategies to reduce overcrowding in prisons. New York: United Nations, 2013. [Google Scholar]
- 46.Macarayan E, Ndeffo-Mbah M, Beyrer C, Galvani AP. Philippine drug war and impending public health crisis. Lancet. 2016. December 10;388(10062):2870. [DOI] [PubMed] [Google Scholar]
- 47.Kamarulzaman A, Reid SE, Schwitters A, Wiessing L, El-Bassel N, Dolan K, et al. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet. 2016. September 10;388(10049):1115–26. [DOI] [PubMed] [Google Scholar]
- 48.Urrego J, Ko AI, da Silva Santos Carbone A, Paiao DS, Sgarbi RV, Yeckel CW, et al. The Impact of Ventilation and Early Diagnosis on Tuberculosis Transmission in Brazilian Prisons. The American journal of tropical medicine and hygiene. 2015. October;93(4):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnstone-Robertson S, Lawn SD, Welte A, Bekker LG, Wood R. Tuberculosis in a South African prison - a transmission modelling analysis. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2011. November;101(11):809–13. [PMC free article] [PubMed] [Google Scholar]
- 50.Segal-Maurer S. Tuberculosis in Enclosed Populations. Microbiol Spectr. 2017. March;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]; ∎ This traditional literature review comprehensively summarizes evidence on TB risk factors and TB control and prevention interventions from a variety of closed settings, including correctional facilities as well as homeless shelters, acute-care facilities, and long-term care facilities.
- 51.Tavoschi L, Vroling H, Madeddu G, Babudieri S, Monarca R, Vonk Noordegraaf-Schouten M, et al. Active Case Finding for Communicable Diseases in Prison Settings: Increasing Testing Coverage and Uptake Among the Prison Population in the European Union/European Economic Area. Epidemiol Rev. 2018. April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Public Health England. BBV bulletin: Quarterly update report of the introduction of opt-out BBV testing in prisons from PHE, NHS England and NOMS. London, United Kingdom: Public Health England (PHE), 2016. [Google Scholar]
- 53.WHO. Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management. Geneva, Switzerland: World Health Organization (WHO), 2018. [PubMed] [Google Scholar]
- 54.WHO. Chest radiography in tuberculosis detection: Summary of current WHO recommendations and guidance on programmatic approaches. Geneva, Switzerland: World Health Organization (WHO), 2016. [Google Scholar]
- 55.Muyoyeta M, Maduskar P, Moyo M, Kasese N, Milimo D, Spooner R, et al. The sensitivity and specificity of using a computer aided diagnosis program for automatically scoring chest X-rays of presumptive TB patients compared with Xpert MTB/RIF in Lusaka Zambia. PloS one. 2014;9(4):e93757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breuninger M, van Ginneken B, Philipsen RH, Mhimbira F, Hella JJ, Lwilla F, et al. Diagnostic accuracy of computer-aided detection of pulmonary tuberculosis in chest radiographs: a validation study from sub-Saharan Africa. PloS one. 2014;9(9):e106381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Degner NR, Joshua A, Padilla R, Vo HH, Vilke GM. Comparison of Digital Chest Radiography to Purified Protein Derivative for Screening of Tuberculosis in Newly Admitted Inmates. Journal of correctional health care : the official journal of the National Commission on Correctional Health Care. 2016. October;22(4):322–30. [DOI] [PubMed] [Google Scholar]
- 58.Philipsen RH, Sanchez CI, Maduskar P, Melendez J, Peters-Bax L, Peter JG, et al. Automated chest-radiography as a triage for Xpert testing in resource-constrained settings: a prospective study of diagnostic accuracy and costs. Scientific reports. 2015. July 27;5:12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 60.Harris JB, Siyambango M, Levitan EB, Maggard KR, Hatwiinda S, Foster EM, et al. Derivation of a tuberculosis screening rule for sub-Saharan African prisons. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2014. July;18(7):774–80. [DOI] [PubMed] [Google Scholar]
- 61.Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017. January 15;64(2):e1–e33. [DOI] [PubMed] [Google Scholar]
- 62.Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014. February 1;383(9915):424–35. [DOI] [PubMed] [Google Scholar]
- 63.WHO. Rapid Implementation of the Xpert MTB/RIF Diagnostic Test. Geneva, Switzerland: World Health Organization (WHO), 2011. [Google Scholar]
- 64.Zishiri V, Charalambous S, Shah MR, Chihota V, Page-Shipp L, Churchyard GJ, et al. Implementing a large-scale systematic tuberculosis screening program in correctional facilities in South Africa. Open forum infectious diseases. 2015. January;2(1):ofu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abed Al-Darraji HA, Abd Razak H, Ng KP, Altice FL, Kamarulzaman A. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PloS one. 2013;8(9):e73717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. The Lancet Infectious Diseases. 2018;18(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.WHO. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTF/RIF Ultra compared to Xpert MTB/RIF. . World Health Organization (WHO), 2017. [Google Scholar]
- 68.Overton K, Varma R, Post JJ. Comparison of Interferon-gamma Release Assays and the Tuberculin Skin Test for Diagnosis of Tuberculosis in Human Immunodeficiency Virus: A Systematic Review. Tuberculosis and respiratory diseases. 2018. January;81(1):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Darraji HA, Kamarulzaman A, Altice FL. Isoniazid preventive therapy in correctional facilities: a systematic review. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012. July;16(7):871–9. [DOI] [PubMed] [Google Scholar]
- 70.Den Boon S, Matteelli A, Ford N, Getahun H. Continuous isoniazid for the treatment of latent tuberculosis infection in people living with HIV. AIDS (London, England). 2016. March 13;30(5):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz JT, Omoz-Oarhe A, Jean Juste MA, Lama JR, Valencia JA, Badal-Faesen S, Moran LE, Fletcher CV, Nuermberger E, Chaisson RE ONE MONTH OF RIFAPENTINE/ISONIAZID TO PREVENT TB IN PEOPLE WITH HIV: BRIEF-TB/A5279. Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2018; Boston, Massachusetts2018. [Google Scholar]
- 72.Juarez-Reyes M, Gallivan M, Chyorny A, O’Keeffe L, Shah NS. Completion Rate and Side-Effect Profile of Three-Month Isoniazid and Rifapentine Treatment for Latent Tuberculosis Infection in an Urban County Jail. Open forum infectious diseases. 2016. January;3(1):ofv220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brooks KM, George JM, Pau AK, Rupert A, Mehaffy C, De P, et al. Cytokine-Mediated Systemic Adverse Drug Reactions in a Drug-Drug Interaction Study of Dolutegravir with Once-Weekly Isoniazid and Rifapentine. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018. February 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adane K, Spigt M, Dinant GJ. Tuberculosis treatment outcome and predictors in northern Ethiopian prisons: a five-year retrospective analysis. BMC Pulm Med. 2018. February 20;18(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]; ∎ This retrospective cohort study conducted among 496 inmates with active TB from four prisons in northern Tigray Regional State, Ethiopia describes low mortality and >90% treatment success in the cohort, as well as high early ART uptake among inmates with HIV-associated TB. This study also highlights persistent challenges with ascertaining outcomes, and ensuring appropriate linkage to care, for prison inmates experiencing inter-facility transfer or release to the community.
- 75.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016. October 01;63(7):e147–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herce MH; Hatwiinda S; Chilembo P; Siyambango M; Kashela L; Yenga C; Kayuni A; Moonga C; Topp S; Muyoyeta M; Chisela C; Magwende G; Moyo C; Reid SE. Early results of universal test and treat implementation in a large Zambian correctional facility IAS 2017; July 23–26, 2017; Paris, France: Abstract #WEPEB0591. [Google Scholar]
- 77.Topp SM, Moonga CN, Luo N, Kaingu M, Chileshe C, Magwende G, et al. Mapping the Zambian prison health system: An analysis of key structural determinants. Global public health. 2016. July 8:1–18. [DOI] [PubMed] [Google Scholar]
- 78.Loeliger KB, Altice FL, Desai MM, Ciarleglio MM, Gallagher C, Meyer JP. Predictors of linkage to HIV care and viral suppression after release from jails and prisons: a retrospective cohort study. The Lancet HIV. 2018;5(2):e96–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cunningham WE, Weiss RE, Nakazono T, Malek MA, Shoptaw SJ, Ettner SL, et al. Effectiveness of a Peer Navigation Intervention to Sustain Viral Suppression Among HIV-Positive Men and Transgender Women Released From Jail: The LINK LA Randomized Clinical Trial. JAMA internal medicine. 2018. April 1;178(4):542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]; ∎ This randomized clinical trial conducted among 356 HIV-positive inmates scheduled for release from a large county jail in Los Angeles, USA demonstrated that a comprehensive transitional care intervention led by peer navigators trained to counsel inmates on skills to overcome barriers to community HIV care can result in successful maintenance of viral suppression 12 months after release, compared with standard transitional case management.
- 80.Wohl DA, Golin CE, Knight K, Gould M, Carda-Auten J, Groves JS, et al. Randomized Controlled Trial of an Intervention to Maintain Suppression of HIV Viremia After Prison Release: The imPACT Trial. Journal of acquired immune deficiency syndromes (1999). 2017. May 1;75(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]; ∎ This randomized controlled trial evaluating a multi-dimensional transitional care intervention among 405 HIV-positive inmates being released from prisons in Texas and North Carolina, USA did not prevent declines in viral suppression at 24 weeks compared to standard care despite evidence of more frequent early care engagement by 6 weeks in the intervention arm. The study’s findings suggest that the factors affecting post-release linkage to community care are complex, and may not be fully addressed by a comprehensive intervention incorporating motivational interviewing, medical care coordination, and cell phone text message reminders.
- 81.Green TC, Clarke J, Brinkley-Rubinstein L, Marshall BDL, Alexander-Scott N, Boss R, et al. Postincarceration Fatal Overdoses After Implementing Medications for Addiction Treatment in a Statewide Correctional System. JAMA Psychiatry. 2018. February 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McLeod KE, Martin RE. Health in correctional facilities is health in our communities. CMAJ. 2018. March 12;190(10):E274–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Topp SM, Moonga CN, Luo N, Kaingu M, Chileshe C, Magwende G, et al. Exploring the drivers of health and healthcare access in Zambian prisons: a health systems approach. Health policy and planning. 2016. November;31(9):1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Topp SM, Sharma A, Moonga CN, Chileshe C, Magwende G, Henostroza G. Evaluation of a health system strengthening initiative in the Zambian prison system. BMJ Glob Health. 2018;3(1):e000614. [DOI] [PMC free article] [PubMed] [Google Scholar]; ∎ This paper describes evaluation outcomes for a unique project conducted in the Zambian prison health system between 2013–2018. The project aimed to strengthen structural, organisational and operational weaknesses within the prison health system that were impeding timely and effective health service delivery, including for TB and HIV. The project reported favorable hard and soft outcomes including improved awareness among high-level policy makers, which was formalised in a new inter-ministerial Memorandum of Understanding on prison health; strengthened recruitment of human resources for health and enhanced health-training for all prison personnel; and the establishment and formalisation of a series of facility-level prison health committees that innovatively included both inmates and prison officers.
- 85.Abera SF, Adane K. One-fourth of the prisoners are underweight in Northern Ethiopia: a cross-sectional study. BMC public health. 2017. May 15;17(1):449. [DOI] [PMC free article] [PubMed] [Google Scholar]


