Abstract
Temperate fruit trees belonging to Prunus species have the ability to suspend (induce dormancy) and resume growth periodically in response to environmental and seasonal conditions. Endodormancy release requires the long-term accumulation of chill. Upon accumulation of cultivar-specific chill requirements, plants enter the state of ecodormancy, which means the ability to grow has been restored, depending on the fulfilment of heat requirements. As many different metabolic pathways are implicated in endodormancy release, we have performed a metabolomic analysis, using the ultra-high-performance liquid chromatography–quadrupole time-of-flying (UPLC–QToF) technique. We assayed flower buds in different stages of endodormancy in four almond cultivars with different flowering times: the extra-early Desmayo Largueta, the late Antoñeta, the extra-late Penta, and the ultra-late Tardona. An orthogonal projection to latent-structure discriminant-analysis model was created to observe differences between endodormant and ecodormant flower buds. The metabolites showing the most significant variation were searched against the Metlin, HMDB, and KEGG libraries, which allowed us to identify 87 metabolites. These metabolites were subsequently assigned to specific pathways, such as abscisic acid biosynthesis, phenylpropanoid biosynthesis, and D-sorbitol metabolism, among others. The two metabolites that exhibited the most significant variations in all the cultivars studied with fold changes of up to 6.49 were ascorbic acid and prunasin. For the first time, these two metabolites have been proposed as potential biomarkers for endodormancy release in almond. Given the high synteny present between the Rosaceae species, these results could be extrapolated to other important crops like peach, plum, cherry, or apricot, among others.
Subject terms: Plant breeding, Secondary metabolism
Introduction
Dormancy in temperate fruit species, like almond (Prunus dulcis (Mill.) D. A. Webb), is a defense state that allows trees to survive adverse conditions during winter. Endodormant buds are resistant to low temperatures1, whereas ecodormant buds, flowers, and young fruits are susceptible to frosts, which can result in important economic loss for farmers2. Dormancy is divided into three different stages: endodormancy, ecodormancy, and paradormancy3. Endodormancy, or winter dormancy, is controlled by the flower bud itself and is released by the accumulation of a certain quantity of chill. These requirements are specific for each cultivar and unless they are fulfilled, even under suitable climate conditions, the buds remain endodormant and unable to flower4,5. When endodormancy is released, growth is resumed upon response to warm temperatures during spring, a state defined as ecodormancy, which ends with flowering. In summer, buds enter paradormancy and stop their growth; this process is mainly governed by apical dominance. Finally, the dormancy cycle is closed when buds shift gradually to the endodormant stage in autumn6.
Given the importance of temperatures in the dormancy cycle, climate change can negatively affect the vegetative development, reproduction, and productivity of almond and other temperate tree species due to (1) global warming, which prevents chilling accumulation during winter7,8 and (2) late or spring frosts, which severely damage reproductive organs like ecodormant buds, flowers, and young fruits. During ecodormancy, the cold hardiness of Prunus species like sweet cherry (Prunus avium L.) and several other species is lower than that exhibited by other tree species9. Moreover, the frost resistance of peach (Prunus persica (L.) Batsch) and other Prunus species flower buds was found to decrease rapidly in response to heat accumulation during ecodormancy and even faster after a mild winter10.
Therefore, in order to counteract the effects of climate change in temperate fruit trees, breeding programs are focused on obtaining extra-late flowering cultivars with higher chilling requirements (CR) to prevent production loss due to late or spring frosts11. These chilling requirements are measured in mild-winter areas using the dynamic model (Chill Portions (CP))4. However, the breeding process is time- and cost-consuming, as it takes ~10–12 years to obtain a new almond cultivar. Moreover, climate change also slows down the fulfillment of CR of these cultivars, requiring the application of endodormancy-release agrochemicals to reach endodormancy release12. In order to assist breeding programs, and to better understand the molecular mechanisms underlying endodormancy release, several molecular studies have been performed. For many years, highly dense linkage maps have made it possible to identify quantitative trait loci (QTL) for flowering date and CR in almond and sweet cherry13,14. Later, genetic and transcriptomic studies have unveiled differentially expressed genes during endodormancy release, such as DAM (DORMANCY-ASSOCIATED MADS-BOX) family genes, which were described for the first time in peach15.
Many different metabolites that play a role in endodormancy maintenance and release have been described. Genes responsible for the synthesis of abscisic acid (ABA), for instance, are known to suffer a drop in expression, suggesting that ABA plays a crucial role as an endodormancy maintainer16,17. Other metabolites like sugars, implicated in the energy supply and developmental signals18,19, have also been suggested to play a role in endodormancy release. Moreover, further secondary or specialized metabolites, such as flavonoids, have been described as a vital factor related to endodormancy release in tomato, controlled by the gene SlAN1120. Redox metabolism also has a great impact on endodormancy release, since H2O2 decreases naturally in almond, Japanese pear (Pyrus pyrifolia (Burm. Fil.) Nakai), and other species after endodormancy release21,22. Finally, some Phe-derived metabolites like the cyanogenic glucoside prunasin and its derivatives might play an important function in endodormancy release in almond and sweet cherry, in which they show a significant increase during endodormancy release23.
Owing to the huge number of metabolites that could be involved in endodormancy release24,25, we have performed, for the first time, a nontargeted analysis during endodormancy in four almond cultivars whose flowering times range from extra-early to ultra-late and whose CR ranges from low (21 CP) to high (56 CP)26. As a result, we have been able to identify specific metabolite variations in one or more cultivars. We envisage that the variation in metabolites from different pathways found in this kind of study will provide a first step in finding a future biomarker for endodormancy release in almond and also in developing a modulator of endodormancy release, which will likely be applicable to other temperate fruit tree species, as well.
Results
Determination of endodormancy release
Bud development in the growth chamber showed that the endodormancy-release date was December 19 for Desmayo Largueta, December 26 for Antoñeta, January 23 for Penta, and February 6 season 2017–2018 for the ultra-late Tardona cultivar (Fig. 1).
Fig. 1. Sample-assay collection.
A Phenological stages of Tardona in the different periods of dormancy, according to Felipe 197749. B Diagram of the four studied cultivars from November 7 to March 21: orange for the extra-early Desmayo Largueta (D) cultivar, purple for the late Antoñeta (A) cultivar, blue for the extra-late Penta (P) cultivar, and green for the ultra-late Tardona (T) cultivar. C Chill accumulation during the endodormancy and ecodormancy period with the endodormancy-release date. For each cultivar, the dormancy-release time is marked with a triangle
Metabolomic profiling
In total, a set of 51 samples was analyzed for exhaustive cultivar fingerprinting. The model built is shown in Supplementary Fig. S1, in which we can observe the separation between endodormant and ecodormant buds on the x axis. Good-quality parameters for the model (Q2 > 0.84) were observed. The features that most contributed to the creation of the OPLS-DA were represented with a feature-importance plot (Supplementary Fig. S2). The model was validated using the OPLS-DA Overview (Supplementary Fig. S3). The separation between endodormant and ecodormant buds was mainly attributed to the fluctuation of 87 metabolites, which were remarkably significant according to a Volcano Plot analysis; of these 87 metabolites, 34 were detected in the negative-ionization mode and 53 in the positive-ionization mode (Supplementary Tables S3 and S4).
Glutathione metabolism
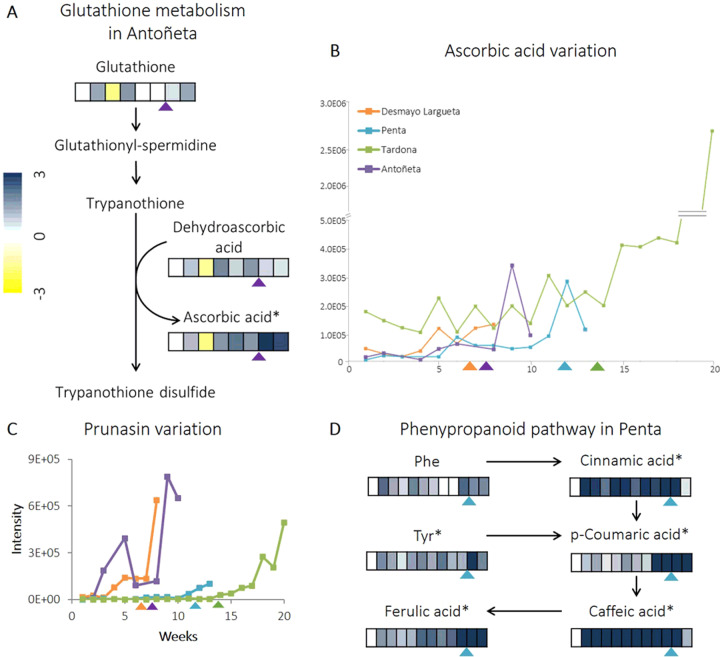
Ascorbic acid, a by-product in glutathione metabolism, showed a sharp increase (FC > 2) during endodormancy release in the extra-early Desmayo Largueta, the extra-late Penta, and the ultra-late Tardona, while it varied right after endodormancy release in the late Antoñeta (Fig. 2A, B). Additionally, we managed to detect a small increase in dehydroascorbic acid and glutathione (GSH), but it was not as significant as that seen in ascorbic acid.
Fig. 2. Glutathione metabolism and amino-acid-based pathways.
A A heatmap showing the evolution of glutathione, dehydroascorbic acid, and ascorbic acid across the 8 weeks from endo- to ecodormancy evaluated in flower buds of the late cultivar Antoñeta. Values represent the means of FC from three biological replicates. B Ascorbic acid content variation in the four cultivar studied. A dashed line marks the dormancy-release date. Asterisks show the metabolites whose variation meets the requirements for being considered significant. C Variation of prunasin content in the four cultivar studied. D Phenylpropanoid-pathway heatmap showing the evolution of cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, and their precursors Phe or Tyr, across the 12 weeks from endo- to ecodormancy in the flower buds of the extra-late Penta cultivar. Values represent the means of FC from three biological replicates. Triangles mark the dormancy-release date. Asterisks show the metabolites whose variation meets the requirements for being considered significant. The extra-early Desmayo Largueta is in orange, the late Antoñeta in purple, the extra-late Penta in blue and the ultra-late Tardona in green
Amino-acid-based pathways
The first specialized metabolite was prunasin, a Phe-derived metabolite (Fig. 2C), which showed a strong increase (FC > 3) during and after the endodormancy release in the four cultivars analyzed.
Other Phe-derived metabolites identified to belong to the phenylpropanoid biosynthetic pathway, where most of the metabolites and intermediates presented a significant increase. This could be seen in the extra-late Penta cultivar, whose endodormancy-release date was estimated in week 12 of sampling, whereas the other cultivars did not present such variations (Fig. 2D). p-Coumaric acid showed an FC of 5.48 between weeks 9 and 10, two weeks before endodormancy was released.
Similarly, ferulic acid showed a steady increase and reached a maximum during weeks 11 and 12 with an FC of 4.51. In contrast, from the beginning of endodormancy, caffeic aid and cinnamic acid exhibited a similar increase, with an FC of 3.46 and 3.03, respectively. Therefore, cinnamic acid, p-coumaric acid, caffeic acid, and ferulic acid all peaked at the moment of endodormancy release, followed by a sharp drop, with the exception of p-coumaric acid, which kept increasing after endodormancy release (Fig. 2D). It is remarkable that almost all of the compounds experienced a great increase over time, even presenting FCs over four. In contrast, no significant variation was observed in their precursors, Tyr and Phe.
The biosynthesis of Pro showed a significant fluctuation in the ultra-late Tardona cultivar, while it remained invariable in the other cultivars (Supplementary Fig. S4A). Glu also fluctuated, but in the opposite way, suffering drops, while Pro experienced an increase. Neither fluctuation was relevant, however. On the other hand, the intermediate metabolite 1-pyrroline increased during endodormancy release with an FC of 2.08.
The last amino acid detected was Trp, which presented some variation in the extra-early Desmayo Largueta cultivar (Supplementary Fig. S4B). The amount of Trp fluctuated during endodormancy, increasing with the release of endodormancy to a maximum FC of 1.94. On the contrary, the Trp-derived metabolites like tryptamine showed a gradual decrease over time, down to an FC of −4.14.
A similar pattern to that observed for prunasin could be observed in 2-methylbutylamine (Supplementary Fig. 4C), which is a by-product of Ile degradation. This compound showed a huge increase just before endodormancy release in the extra-early Desmayo Largueta and the extra-late Penta cultivars only.
The ABA biosynthetic pathway
In the biosynthesis of ABA, metabolites like violaxanthin, abscisic alcohol, and ABA presented variations in the extra-late Penta cultivar (Fig. 3A), but no variations in the rest of the cultivars. ABA showed a gradual and significant drop at the end of endodormancy, falling to an FC of −2.11, whereas variations in violaxanthin and abscisic alcohol were not significant.
Fig. 3. Abscisic acid (ABA) biosynthetic pathway and D-sorbitol metabolism.
A A heatmap showing the variation of violaxanthin, abscisic alcohol, and ABA across the 12 time points from endo- to ecodormancy in the flower buds of the extra-late Penta cultivar. Values represent the means of FC from three biological replicates. A dashed line marks the dormancy-release date. Asterisks show the metabolites whose variation meets the requirements for being considered significant. B A heatmap showing the variation of D-glucose, D-sorbitol, D-fructose-2-P, D-fructose-2,6-P2, and D-sorbitol-6-P across the eight time points from endo- to ecodormancy in the flower buds of the extra-early Desmayo Largueta cultivar. Values represent the means of FC from three biological replicates. Triangles mark the dormancy-release date. Asterisks show the metabolites whose variation meets the requirements for being considered significant
D-sorbitol metabolism
In the sorbitol metabolism, variations were detected in five different metabolites in the extra-early Desmayo Largueta cultivar (Fig. 3B) that remained constant in the other cultivars. Sorbitol and sorbitol-6-phosphate, with FCs of −4.18 and 2.84, respectively, experienced a huge drop and increase. D-glucose also exhibited a dramatic drop upon endodormancy release. Consequently, D-sorbitol-6-phosphate experienced a huge rise, as did the immediate precursors of the glycolysis, D-fructose-2,6-biphosphate and D-fructose-2-phosphate, which showed an FC of 2.06 and 1.23, respectively.
Flavonoids
Two different groups of flavonoids were identified among almond samples. The first was anthocyanins, in which case two different metabolites were found. The anthocyanin petunidin-3-glucoside presented an FC > 3 in the extra-early and in the extra-late cultivars. We could also detect malvidin-3-glucoside, although its variation was not significant (Supplementary Fig. S5A and B). The second group was flavonols, in which case flavonol quercetin-3-(6”-malonylglucoside) fluctuated in the extra-early Desmayo Largueta cultivar only with an FC of 2.10 (Supplementary Fig. S5C).
Metabolite–metabolite correlation analysis during endodormancy release
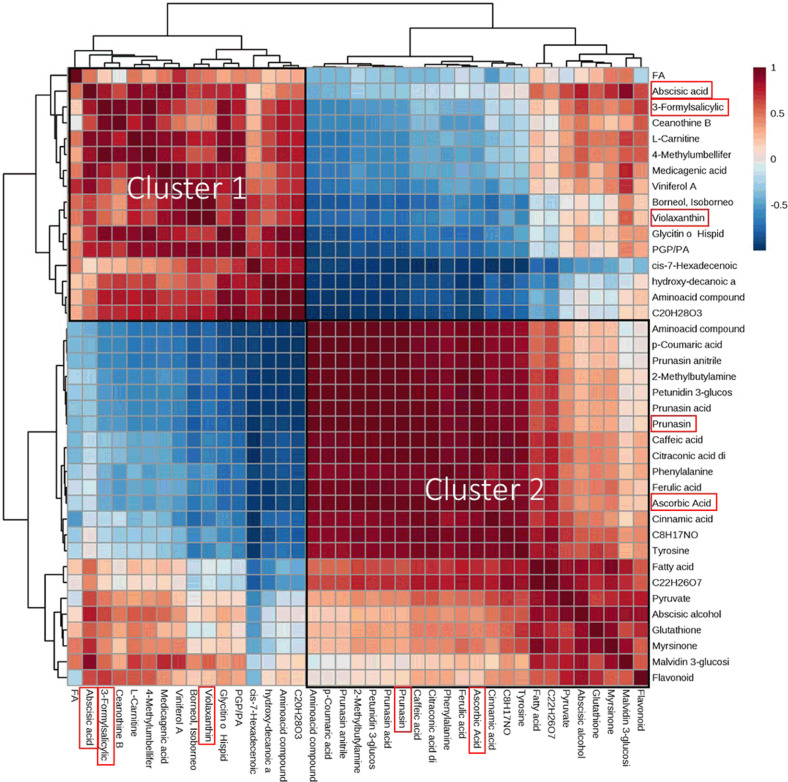
The correlation heatmap of the extra-late cultivar Penta showed two different clusters (Fig. 4). Cluster 1 grouped together metabolites belonging to the same pathway, such as violaxanthin and ABA, with a deep correlation with 3-formylsalicylic acid; cluster 2 grouped together metabolites showing the same trend, like those involved in the phenylpropanoid biosynthesis pathway, ABA biosynthesis, glutathione metabolism, or anthocyanin biosynthesis (cluster 2).
Fig. 4. Correlation heatmap cluster of the extra-late Penta cultivar.
Two different clusters of metabolites are shown, grouping together either the metabolites that are in the same pathway or the metabolites that present the same kind of variation. The complete name of the metabolites with ~ can be found in Supplementary Table S3
We also observed two different clusters in the correlation heatmaps of the extra-early cultivar Desmayo Largueta (Supplementary Fig. S6) and the late cultivar Antoñeta (Supplementary Fig. S7); the ultra-late Tardona cultivar, on the other hand, showed just one cluster (Supplementary Fig. S8). As in the heatmap of the extra-late Penta cultivar, we could observe that metabolites from the same pathways and with similar variation patterns were located in the same cluster.
Discussion
Glutathione metabolism
Previous studies have explained the importance of glutathione metabolism and especially ascorbic acid as an antioxidant metabolite against ROS during endodormancy. For example, in Japanese pear and sweet cherry, ROS increases during endodormancy and decreases afterward upon endodormancy release25,27. In our study, a significant increase in ascorbic acid was observed in the four cultivars studied (Fig. 2B). Future studies should focus on ROS measurement to confirm whether this also happens in almond. Moreover, our results are in concordance with the previous findings in Japanese pear and other species, where the activity of ascorbate peroxidase increased during endodormancy release. This fact indicates that the transition of the endodormancy stage may involve the removal of free radicals through a system using ascorbic acid as an antioxidant by ascorbate peroxidase27. It has been demonstrated in grapevine leaves (Vitis vinifera L.) that a rise of the ascorbic acid-level group with an increase in flavonols is implicated in the degradation of H2O228. This agrees with the trend shown by these metabolites in our samples, where their levels increased during endodormancy release in order to decrease ROS.
Furthermore, ascorbic acid has also been described as being related to the ABA-gibberellin (GA) balance, implicated in seed germination in rice (Oryza sativa L.). In fact, an ABA increase with a concomitant decrease in GA produced a drop in ascorbic acid, keeping the seed dormant29. On the contrary, we could observe an increase in ascorbic acid in all the cultivars assayed; moreover, this increase was accompanied by a simultaneous drop in ABA in the extra-late Penta cultivar. The relationship described between ascorbic acid and ABA in rice seeds agrees with our observations, since, in seeds, an ABA increase is responsible for keeping the seed dormant, but in flower buds, ABA levels must decrease in order to reach endodormancy release. This hypothesis will be validated in further studies with more cultivars. Overall, ascorbic acid represents a potential biomarker for endodormancy release in almond.
Amino-acid-based pathways
As previous studies in almond and sweet cherry23 have shown, prunasin increases during and after endodormancy release (Fig. 2C). Furthermore, those studies found a concomitant increase in prunasin anitrile and prunasin amide23, which we did not find in our study. All things considered, prunasin could be used as a potential biomarker for endodormancy release.
In peach, it has been described that the accumulation of phenylpropanoids coupled with a drop in ABA is necessary for reaching endodormancy release30, as shown in the extra-late Penta cultivar (Fig. 2D). Moreover, in sweet cherry flower buds, caffeic acid has been found to increase in the early stages of endodormancy and drop right after endodormancy release31, in agreement with our observations in the extra-late Penta cultivar (Fig. 2D). However, these authors did not find a cinnamic acid increase as we did. In contrast, other metabolites like p-coumaric acid and ferulic acid increased before, during, and 2 weeks after endodormancy release, indicating a potential role as biomarkers for these specialized metabolites in the transition from endodormancy to ecodormancy. In fact, the 4-COUMARATE:CoA LIGASE-LIKE 1 (4-CLL1) gene has recently been described in almond endodormancy release (Prudencio et al. 202032). This tendency indicates that a large concentration of this metabolite is still necessary after endodormancy release, suggesting its metabolization into other products such as its CoA derivatives. Very recently, a homolog to this gene has been described in apricot (Prunus armeniaca L.), the 4-CLL7 gene, which shows a steep increase during and after endodormancy release33.
Previous studies in grapevine have pointed to Pro as an osmotic factor that could protect flower buds against dehydration during winter34. This could explain the accumulation of 1-pyrroline, the immediate precursor of Pro, observed in the ultra-late Tardona cultivar (Supplementary Fig. S4A).
Finally, the Trp increase during endodormancy release (Supplementary Fig. S4B) is in concordance with previous findings in sweet cherry flower buds31. This increase could be caused by the high level of the activity-dormancy cycle, during which some compounds that varied during endodormancy are metabolized into others involved in growth and other physiological processes, like indole-3-acetic acid (IAA), a Trp-derived specialized metabolite35,36. However, this disagrees with some previous findings in sweet cherry, where genes responsible for the biosynthesis of IAA, such as YUC10, were downregulated during endodormancy release12. In Arabidopsis thaliana, strictosidine synthase has also been found to play a crucial role in the microsporogenesis process, during which strictosidine is needed for pollen-grain development10. In our study, this metabolite did not increase, probably due to the fact that this study was focused on the dormancy period and not on flowering. It has also been described in different apricot cultivars that microsporogenesis is related to endodormancy released, suggesting that an early microsporogenesis might be involved in a low rate of blooming37.
2-Methylbutylamine, a by-product of Ile metabolization38, showed a steep increase in the extra-early Desmayo Largueta cultivar and a gradual increase in the extra-late Penta cultivar (Supplementary Fig. S4C). To the best of our knowledge, this is the first time that this metabolite has been described in the endodormancy-release process.
The ABA biosynthetic pathway
ABA has been described as playing a crucial role in endodormancy release, and ABA levels have been found to decrease in order for plants to reach the ecodormancy phase39; these findings agree with our observations in the extra-late Penta cultivar (Fig. 3A). Additionally, ABA has also been described as being implicated in the modulation of other metabolic pathways that we have studied, such as glutathione metabolism, phenylpropanoid biosynthesis, and flavonoid biosynthesis29,30,40.
D-sorbitol metabolism
In peach flower buds, the accumulation of sorbitol is activated by chill, inhibiting the expression of the sorbitol catabolic genes41. This agrees with our results in the three cultivars with the highest CR (Antoñeta, Penta, and Tardona) (Fig. 1C and Supplementary Table S1), in which D-sorbitol may be acting as an osmolite and protecting flower buds against dehydration during winter. Nevertheless, a huge decrease in D-sorbitol with the concomitant accumulation of D-sorbitol-6-P was observed in the extra-early Desmayo Largueta cultivar (Fig. 3B), whose CR for endodormancy release is low. Moreover, the glycolysis metabolites D-fructose-2-P and D-fructose-2,6-P2 increased in the stages close to endodormancy release (Fig. 3B). It is known that during endodormancy release in red-rice seeds, the glycolysis and energy obtained increase42; these findings agree with the increase in the compounds from D-sorbitol metabolism that we could observe in the extra-early Desmayo Largueta cultivar, suggesting the role this metabolite plays in early endodormancy release.
Flavonoid metabolism
In sheepgrass (Leymus chinensis (Trin.) Tzvelev), during seed germination, high ABA levels downregulate anthocyanin biosynthesis through the inhibition of anthocyanidin synthase and anthocyanidin reductase by the bHLH92 transcription factor40. In our study, we observed a drop in ABA with a concomitant increase in the anthocyanin petunidin-3-glucoside during endodormancy release (Supplementary Fig. S5A, B). We, therefore, suggest that the anthocyanin increase seen in our samples is probably involved with the ABA drop previously explained in the extra-late Penta cultivar (Fig. 3A). Regarding flavonol biosynthesis, it has been described that under low temperatures, some flavonol-associated genes are repressed in apple (Malus domestica (Suckow) Borkh.)43. Accordingly, we were able to detect that flavonol biosynthesis increased during endodormancy release (Supplementary Fig. S5C), since once CR has been fulfilled, the flower bud is ready to awake and the genes are no longer downregulated by the chill.
Metabolite–metabolite correlation analysis during endodormancy release
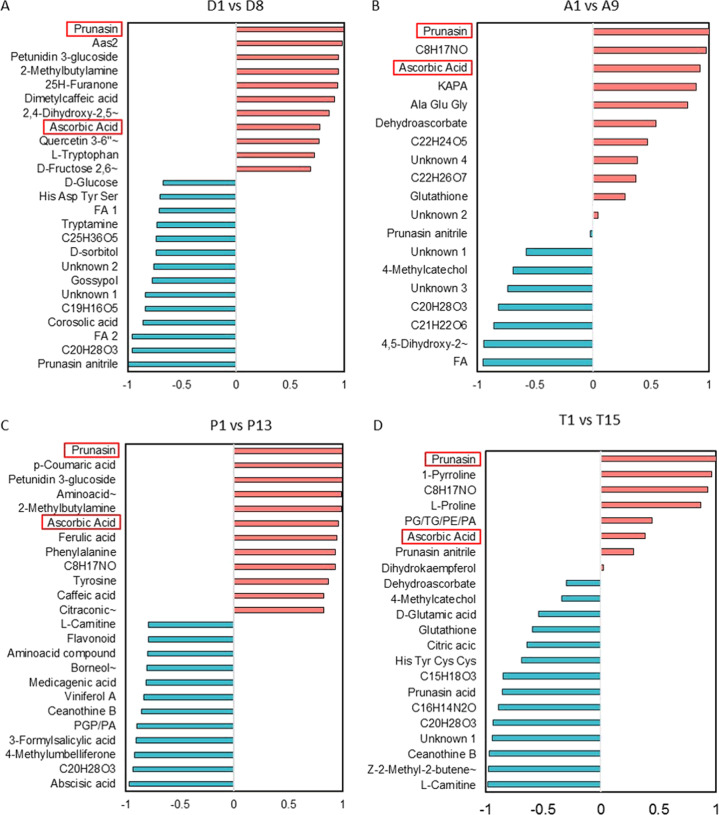
Correlation heatmaps showed how metabolites varied with respect to one another (Fig. 4 and Supplementary Figs. S6–S8), showing that metabolites with similar trends during endodormancy released clustered together. In order to corroborate the correlation between prunasin and the rest of the metabolites previously identified in the four cultivars, a pattern-searching plot was created facing endodormant (D1, A1, P1, and T1) against ecodormant (D8, A9, P13, and T15) flower buds (Fig. 5). A great number of metabolites showed a huge correlation with prunasin in all of our cultivars. The high level of relationship between ascorbic acid and prunasin—the only two metabolites that varied significantly in all our cultivars—was especially remarkable. The increases we observed in ascorbic acid and prunasin present a similar variation pattern (Fig. 2A–C). In fact, in peach, it has been described that both metabolites are connected under salt-stress scenarios, during which increases in salicylic acid (SA) and ABA cause a drop in cyanogenic glucoside and ascorbic acid levels44,45. This connection might also exist in the almond endodormancy-release process: in the extra-late Penta cultivar, ABA and 3-formylsalicylic acid (SA precursor) levels decreased (Fig. 3A), whereas ascorbic acid and prunasin levels increased (Fig. 2A–C, respectively), with a high negative correlation between prunasin, ABA, and the SA precursor.
Fig. 5. Pattern-searching plot between prunasin and the metabolites that showed variation from endo- to ecodormancy in the four cultivars studied.
Up to 25 of the top metabolites were grouped together with positive (salmon) or negative (cyan) correlation in the extra-early Desmayo Largueta (A) comparing stage 1 and stage 8 (D1 vs D8), in the late Antoñeta (A1 vs A9) (B), in the extra-late Penta (P1 vs P13) (C), and in the ultra-late Tardona (T1 vs T15) (D)
Besides, prunasin exhibited a high correlation with metabolites with similar behavior in the extra-early Desmayo Largueta cultivar (Fig. 5A), such as the flavonoids petunidin-3-glucoside, which also showed a high correlation in the extra-late Penta cultivar, and quercetin-3-(6 “-malonylglucoside); both flavonoids have been associated with seed germination and endodormancy release in sheepgrass and apple40. Trp and tryptamine also exhibited a high positive and negative correlation, respectively, with prunasin. The opposite correlation directions are probably due to the different trends of each metabolite (Supplementary Fig. S4), since tryptamine decreased during endodormancy release while Trp increased. This Trp behavior is probably due to the high level of the activity-dormancy cycle in this period35,36.
Prunasin acid, described for the first time in dormant and nondormant germinated seeds46, showed the highest negative correlation detected in the extra-early Desmayo Largueta cultivar (Fig. 5A). The same results were previously observed in the flower buds of the extra-early almond cultivars Achaak and Desmayo Largueta, while prunasin acid levels were minute in the extra-late cultivar as Penta23.
In the late Antoñeta cultivar (Fig. 5B), the unknown metabolite with the molecular formula C8H17NO, which is also found in the extra- and ultra-late cultivars Penta and Tardona, presented a high correlation with prunasin, suggesting that this metabolite might be associated with endodormancy release in late cultivars. Further studies should focus on the identification of this compound. KAPA, 7-keto-8-aminopelargonic acid, an Ala derivative in the biotin biosynthesis found in A. thaliana47, also exhibited a high correlation with prunasin. To the best of our knowledge, this is the first time that this metabolite has been described in the endodormancy-release process. With a high negative correlation, an unknown fatty acid (FA) was also found. FAs have been described as a source of ROS, either directly via β-oxidation or by an additional step of neoglucogenesis, producing monosaccharides48. In sweet cherry flower buds, hydrogen cyanamide treatment used to advance flower-bud dormancy decreased the expression of transcripts coding for the ROS-scavenging enzyme catalase 1 (CAT1) and manganese-containing superoxide dismutase (Mn-SOD)12. This agrees with the catalase-activity decrease observed in our study. Consequently, an increase in ROS, like H2O2, was found to be related to endodormancy release in sweet cherry12.
The highest correlations between prunasin and other metabolites were observed in the extra-late Penta cultivar (Fig. 5C); primary metabolites like Phe and Tyr and their derivatives, the phenylpropanoids p-coumaric acid, ferulic acid, and caffeic acid, were among the metabolites that presented the highest correlation with prunasin. The phenylpropanoid pathway has recently been described as varying during endodormancy release in five apricot cultivars, in which genes responsible for the biosynthesis of cinnamoyl CoA and p-coumaroyl-CoA exhibited a FC higher than 3.533. This trend is similar to that observed for cinnamic acid and p-coumaric acid in our samples, as well as to that found in prunasin; this fact would explain the high correlation between these metabolites and prunasin.
Finally, in the ultra-late Tardona cultivar (Fig. 5D), 1-pyrroline and Pro presented a high correlation with prunasin. Pro has been defined as being an osmotic protector during endodormancy release in grapevine flower buds34. With a negative correlation, compounds like prunasin acid and L-carnitine were detected. Prunasin acid results are in concordance with observations in previous studies in flower buds of the late S3067 and the extra-late Penta cultivars23. L-carnitine correlation was not only found in the ultra-late Tardona but also in the extra-late Penta, suggesting a possible link with the delayed flowering time. In peach, proteins responsible for carnitine transport were enhanced in endodormant flower buds10.
Conclusion
Production in temperate fruit species like almond relies on successful flowering, which only occurs upon adequate endodormancy release. Monitoring this multifaceted process is therefore crucial to assure high productivity. With this nontargeted metabolomic study, 87 compounds were identified; out of these compounds, prunasin and ascorbic acid were found to be significantly present in the four almond cultivars studied. Based on these results, the two best candidate biomarkers for endodormancy release in almond are prunasin and ascorbic acid. Further studies should focus on validation using more cultivars with different endodormancy-release requirements and in other almond-related species like apricot, peach, or sweet cherry, among others, but also considering different-year observation. The discovery of a biomarker for endodormancy release and the metabolic process behind it (Fig. 6) would suppose a great advance in monitoring the process, making it possible to predict the endodormancy-release date. Under unfavorable climate conditions, the biomarkers could boost the development and application of environmentally friendly modulators for endodormancy release, bringing forward or delaying the flowering time. This would counteract the effects of climate change, improving efficient and sustainable fruit production.
Fig. 6. Pathways and metabolites involved in endodormancy release.
Venn diagram shows the metabolites with a significant variation in one or more cultivars and the pathways (P) in which a metabolite varied significantly. The complete names of metabolites with ~ can be found in Supplementary Table S3
Materials and methods
Plant material
For this study, we used the following four almond cultivars from the experimental field of CEBAS-CSIC (Murcia, Spain; latitude: 38° 5’ 54.38” N, longitude: 1° 2’ 3.18” W): Desmayo Largueta (21- year-old tree grafted onto Garrigues seedling rootstock), Antoñeta (20-year-old tree grafted onto Garrigues seedling rootstock), and Penta and Tardona (17-year-old tree grafted onto Garrigues seedling rootstock). Desmayo Largueta is a traditional Spanish extra-early flowering cultivar, whereas Antoñeta (Ferragnès × Tuono), Penta (S5133 × Lauranne), and Tardona (S5133 × R1000) are late, extra-late, and ultra-late cultivars11, respectively, from the CEBAS-CSIC almond- breeding program11,26 (Supplementary Table S1). For each cultivar assayed, flower buds were collected weekly in three independent biological replicates from the same tree. Sampling started on Nov 7 (stage A = all cultivars fully endodormant) and lasted until March 21 (stage D = ecodormant stage in Tardona)49, covering the endodormant-to-ecodormant stage for each of the cultivars. In the case of Tardona, the sampling period was extended to stage D, previous to flowering time, to study the evolution of the metabolites that varied significantly during endodormancy and ecodormancy. In total, we collected 51 samples, which were labeled as D1–D8 for Desmayo Largueta, A1–A10 for Antoñeta, P1–P13 for Penta, and T1–T20 for Tardona (Fig. 1). Each cultivar has a different number of samples due to the different time periods required to reach endodormancy release.
Determination of endodormancy release
The phenological stages of the flower buds were determined weekly using the forcing method as described by Prudencio et al.26 (Fig. 1). Three branches consisted of single mixed twigs; 1-year shoots with flower and vegetative buds (40-cm long and 5 mm in diameter) of each cultivar were picked and placed in a growth chamber (23 ± 1 °C, RH 40% during the 16 h of photoperiod and 20 ± 1 °C, RH 50% during the dark period). After making a fresh cut at the base of each branch, the branches were placed in a 5% sucrose solution with 0.1% aluminum sulfate in the growth chamber. After 10 days in the growth chamber, the phenological state of flower buds on the branches was evaluated. When 50% of the flower buds were in the B–C state49, the date of endodormancy release was established as it has been previously described13,50. Additionally, the accumulated chill during winter was measured using the dynamic model suitable for mild-winter areas4 (Fig. 1C and Supplementary Table S1).
UPLC–QToF–MS/MS analysis
All flower-bud samples collected (except P4 and A7) (three 50–100-mg replicates) were ground to a fine powder in liquid nitrogen, mixed with 200 µl of ACN:H2O (80:20) with 0.1% HCOOH (Sigma), mechanically shaken, sonicated for 3 × 30 s, and centrifuged for 10 min at 13,000×g, as previously described51. The supernatant was stored at −20 °C until further analysis. Samples were mixed with glipizide (Sigma) at 0.1 µg/ml as an internal standard and passed through a filter plate (0.22 µm, Millipore) by centrifugation (5 min, 1107×g).
UPLC–QToF–MS/MS was performed using a Waters ACQUITY UPLC I-Class System (Waters Corporation, Milford, MA, USA) coupled to a Bruker Daltonics QToF-MS mass spectrometer [maXis impact Series with a resolution ≥ 55000 FWHM Bruker Daltonics, Bremen, Germany], using ESI for both positive- [ESI ( + )] and negative- [ESI (−)] ionization modes.
UPLC separation was performed using an HSS T3 C18 100 × 2.1-mm column with 1.8 µm of size particle (Waters Corporation, Milford, MA, USA) at a flow rate of 0.3 mL/min. The separation was performed using H2O with formic acid at 0.01% (pH ~3.20) (PanReac AppliChem, Barcelona, Spain) as the weak mobile phase (A) and ACN with HCOOH at 0.01% (J. T. Baker, New Jersey, USA) as the strong mobile phase (B). The gradient started with 10% of B at 0 min, which was progressively increased up to 90% at minute 14; after that, it was held until minute 16 and then decreased to 10% at 10 s, where it remained until minute 18 (Supplementary Table S2). Nitrogen was used as the desolvation gas with a flux of 8 L/min and as the nebulizing gas with a pressure of 2.0 bar. The drying temperature was 200 °C and the column temperature was 40 °C. The voltage source was 4.0 kV for ESI (−) and 4.5 kV for ESI ( + ). The MS experiment was carried out using HR-QTOF-MS, applying 24 eV for ESI ( + ) and 20 eV for ESI (−) and using broadband collision-induced dissociation (bbCID). MS data were acquired over an m/z range of 45–1200 Da.
The external calibrant solution was delivered by a KNAUER Smartline Pump 100 with a pressure sensor (KNAUER, Berlin, Germany). The instrument was calibrated externally before each sequence with a 10 mM sodium formate solution. The mixture was prepared by adding 0.5 mL of formic acid and 1.0 mL of 1.0 M sodium hydroxide to an isopropanol/Milli Q water solution (1:1, v/v).
Data processing and statistical analysis
We were able to start obtaining the raw-intensity data of the analysis from the nontarget metabolomics data workflow using Profile Analysis 2.1 software (Bruker Daltonics); we obtained 654 features with a signal-to-noise ratio of 3 for ESI (−) and 6218 features with a signal-to-noise ratio of 5 for ESI ( + ). For statistical analysis, we used MetaboAnalyst 4.0 software (http://www.metaboanalyst.ca/MetaboAnalyst/faces/home.xhtml). We transformed our data in a logarithmic base and performed a Pareto scaling. Likewise, we made an Orthogonal Partial Least‐Square Discriminant Analysis model (OPLS-DA) (Supplementary Fig. S1), which is a supervised model, in order to test whether there were real differences between endodormant and ecodormant flower buds. Furthermore, to test the robustness of the model, we used the OPLS-DA overview to obtain the explained variance and the capability of prediction, represented by the R2 and Q2 scores, respectively (Supplementary Fig. S2). In addition, we created a feature-importance plot based on the covariance and the correlation of the features of the model. Finally, with the aim of observing which features presented significant variation between endodormant and ecodormant flower buds, we performed a Volcano plot analysis based on the P value and the fold change of the features of our samples. The statistical significance was set to a logarithm of fold change (FC) > 2 and a P value <0.05.
Metabolite identification
The most significant metabolite MS/MS spectra were acquired and searched in the following databases: METLIN (https://metlin.scripps.edu/landing_page.php?pgcontent=mainPage), the Human Metabolome Database (http://www.hmdb.ca/), and Lipid Maps (https://www.lipidmaps.org/). Additionally, we used prunasin, prunasin acid, prunasin anitrile, and amygdalin standards for the identification of those metabolites23. Subsequently, the KEGG PATHWAY database (https://www.genome.jp/kegg/pathway.html) was used to identify the different pathways the compounds belonged to. We also studied the behavior of the metabolites that were in the same pathway of metabolites showing significant variation, even if their variation was not significant.
Finally, to determine the statistical correlation between the significant compounds involved in the pathways, we measured the distance between our metabolites with a Pearson r correlation coefficient, creating a correlation-analysis heatmap and a pattern-searching plot using MetaboAnalyst 4.0 software.
Supplementary information
Acknowledgements
The authors are grateful to Dr. Mohammed Saddik Motawia (PLEN Department, Copenhagen University) for the prunasin and amygdalin derivatives used as standards, to D. Alberto Lucas García (Department of Plant Breeding, CEBAS-CSIC), for his technical help in the field, and to Dr. José Alberto Egea Larrosa for reviewing the statistical treatment. This work was funded by the projects “Mejora Genética del Almendro” (MINECO), “Breeding stone fruit species assisted by molecular tools” (Fundación Séneca), and “The molecular mechanisms to break flower bud dormancy in fruit trees (VKR023124)” by the Villum Foundation. R.S.-P. is grateful to MICINN for the “Ramón y Cajal” fellowship and “Grupo Operativo para el Diseño e Implantación de un Plan de Erradicación de la Almendra Amarga en España” (MAPAMA) to J.G.G., R.S.-P., and F.D. J.G.G. is also grateful to “Fundación Tatiana Pérez de Guzmán el Bueno” for this PhD fellowship.
Author contributions
R.S.-P. and F.D. conceived the original screening and research plan; A.S.P. and J.G.G. collected the samples. J.G.G., J.E.Y., and R.S.-P. performed the experiments based on a UPLC–QToF metabolomics analysis; J.G.G. analyzed the data. J.G.G., A.S.P., and R.S.-P. wrote the paper, with contributions from all the authors. R.S.-P. agrees to serve as the author responsible for contact and ensures communication.
Conflict of interest
The authors declare that they have no conflict of interest.
Contribution to the field statement
Endodormancy is a protector state that allows trees to survive under low winter temperatures. Its release relies on the accumulation of a specific quantity of chill units. This study aims to be the first approach in the discovery of a biomarker for endodormancy release in almond. Thanks to nontargeted metabolomics analysis, we detected a significant increase in prunasin and ascorbic acid during endodormancy release in the four cultivars studied. Further studies will focus on the validation of these proposed biomarkers with a wider scope, and the test and development of endodormancy modulators that would bring forward or delay endodormancy release. The results would enable us to adapt Prunus spp. culture to a climate-change scenario in which low chilling and late frosts would endanger their production.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-020-00427-5).
References
- 1.Singh RK, Svystun T, AlDahmash B, Jönsson AM, Bhalerao RP. Photoperiod- and temperature-mediated control of phenology in trees – a molecular perspective. N. Phytol. 2017;213:511–524. doi: 10.1111/nph.14346. [DOI] [PubMed] [Google Scholar]
- 2.Fennell A. Systems and approaches to studying dormancy: introduction to the workshop. Hortscience A Publ. Am. Soc. Hortcultural Sci. 1999;34:1172–1173. [Google Scholar]
- 3.Lang GA, Early JD, Matin GC, Darnell RLEndodormancy. Paradormancy, and ecodormancy—physiological terminology and classification for dormancy research. Hortscience. 1987;22:371–377. [Google Scholar]
- 4.Fishman S, Erez A, Couvillon GA. The temperature dependence of dormancy breaking in plants: computer simulation of processes studied under controlled temperatures. J. Theor. Biol. 1987;126:309–321. doi: 10.1016/S0022-5193(87)80237-0. [DOI] [Google Scholar]
- 5.Egea J, Ortega E, Martıń ez-Gómez P, Dicenta F. Chilling and heat requirements of almond cultivars for flowering. Environ. Exp. Bot. 2003;50:79–85. doi: 10.1016/S0098-8472(03)00002-9. [DOI] [Google Scholar]
- 6.Champagnat P. Bud dormancy, correlation between organs, and morphogenesis in woody plants. Sov. Plant Physiol. 1983;30:458–471. [Google Scholar]
- 7.Blanke M, Kunz A. Effect of climate change on pome fruit phenology at Klein-Altendorf—based on 50 years of meteorological and phenological records. Erwerbs-Obstbau. 2009;51:101–114. doi: 10.1007/s10341-009-0086-3. [DOI] [Google Scholar]
- 8.Martínez-Gómez P, Prudencio AS, Gradziel TM, Dicenta F. The delay of flowering time in almond: a review of the combined effect of adaptation, mutation and breeding. Euphytica. 2017;213:1–10.. doi: 10.1007/s10681-017-1974-5. [DOI] [Google Scholar]
- 9.Vitra A, Lenz A, Vitasse Y. Frost hardening and dehardening potential in temperate trees from winter to budburst. N. Phytol. 2017;216:113–123. doi: 10.1111/nph.14698. [DOI] [PubMed] [Google Scholar]
- 10.Leida C, Terol J, Martí G, et al. Identification of genes associated with bud dormancy release in Prunus persica by suppression subtractive hybridization. Tree Physiol. 2010;30:655–666. doi: 10.1093/treephys/tpq008. [DOI] [PubMed] [Google Scholar]
- 11.Dicenta F, Ortega E, Martinez-Gomez P, Sánchez-Pérez R, Gambin M, Egea J. Penta and tardona: two new extra-late flowering self-compatible almond cultivars. Acta Hortic. 2009;814:189–192. doi: 10.17660/ActaHortic.2009.814.24. [DOI] [Google Scholar]
- 12.Ionescu IA, López-Ortega G, Burow M, et al. Transcriptome and metabolite changes during hydrogen cyanamide-induced floral bud break in sweet cherry. Front. Plant Sci. 2017;8:1–17.. doi: 10.3389/fpls.2017.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez-Pérez R, Dicenta F, Martínez-Gómez P. Inheritance of chilling and heat requirements for flowering in almond and QTL analysis. Tree Genet Genomes. 2012;8:379–389. doi: 10.1007/s11295-011-0448-5. [DOI] [Google Scholar]
- 14.Castède S, Campoy JA, García JQ, et al. Genetic determinism of phenological traits highly affected by climate change in Prunus avium: flowering date dissected into chilling and heat requirements. N. Phytol. 2014;202:703–715. doi: 10.1111/nph.12658. [DOI] [PubMed] [Google Scholar]
- 15.Bielenberg DG, Wang Y, Fan S, Reighard GL, Scorza R, Abbott AG. A deletion affecting several gene candidates is present in the evergrowing peach mutant. J. Hered. 2004;95:436–444. doi: 10.1093/jhered/esh057. [DOI] [PubMed] [Google Scholar]
- 16.Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc. Natl Acad. Sci. USA. 2011;108:20236–41.. doi: 10.1073/pnas.1116325108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao X, Zhang J, Liu W, et al. The MKKK62-MKK3-MAPK7/14 module negatively regulates seed dormancy in rice. Rice. 2019;12:2–15.. doi: 10.1186/s12284-018-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson JV, Gesch RW, Jia Y, Chao WS, Horvath DP. Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant, Cell Environ. 2005;28:1567–1578. doi: 10.1111/j.1365-3040.2005.01393.x. [DOI] [Google Scholar]
- 19.Kaufmann H, Blanke M. Changes in carbohydrate levels and relative water content (RWC) to distinguish dormancy phases in sweet cherry. J. Plant Physiol. 2017;218:1–5. doi: 10.1016/j.jplph.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Liu J, Chen Y, et al. Tomato SlAN11 regulates flavonoid biosynthesis and seed dormancy by interaction with bHLH proteins but not with MYB proteins. Hortic. Res. 2018;5:27–44.. doi: 10.1038/s41438-018-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroda H, Sugiura T, Ito D. Changes in hydrogen peroxide content in flower buds of Japanese pear (Pyrus pyrifolia Nakai) in relation to breaking of endodormancy. J. Jpn. Soc. Hortic. Sci. 2002;71:610–616. doi: 10.2503/jjshs.71.610. [DOI] [Google Scholar]
- 22.Pérez FJ, Vergara R, Rubio S. H2O2 is involved in the dormancy-breaking effect of hydrogen cyanamide in grapevine buds. Plant Growth Regul. 2008;55:149–155. doi: 10.1007/s10725-008-9269-4. [DOI] [Google Scholar]
- 23.Del Cueto J, Ionescu IA, Pičmanová M, et al. Cyanogenic glucosides and derivatives in almond and sweet cherry flower buds from dormancy to flowering. Front. Plant Sci. 2017;8:1–16.. doi: 10.3389/fpls.2017.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagter M, Yde CC, Kjær KH. Metabolic fingerprinting of dormant and active flower primordia of ribes nigrum using high-resolution magic angle spinning NMR. J. Agric Food Chem. 2017;65:10123–10130. doi: 10.1021/acs.jafc.7b03788. [DOI] [PubMed] [Google Scholar]
- 25.Baldermann S, Homann T, Neugart S, et al. Selected plant metabolites involved in oxidation-reduction processes during bud dormancy and ontogenetic development in sweet cherry buds (Prunus avium L.) Molecules. 2018;23:1197–1220. doi: 10.3390/molecules23051197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prudencio AS, Martínez-Gómez P, Dicenta F. Evaluation of breaking dormancy, flowering and productivity of extra-late and ultra-late flowering almond cultivars during cold and warm seasons in South-East of Spain. Sci. Hortic. 2018;235:39–46. doi: 10.1016/j.scienta.2018.02.073. [DOI] [Google Scholar]
- 27.Takemura Y, Kuroki K, Jiang M, Matsumoto K, Tamura F. Identification of the expressed protein and the impact of change in ascorbate peroxidase activity related to endodormancy breaking in Pyrus pyrifolia. Plant Physiol. Biochem. 2015;86:121–129. doi: 10.1016/j.plaphy.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Pérez FJ, Villegas D, Mejia N. Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochemistry. 2002;60:573–580. doi: 10.1016/S0031-9422(02)00146-2. [DOI] [PubMed] [Google Scholar]
- 29.Ye N, Zhu G, Liu Y, et al. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J. Exp. Bot. 2012;63:1809–1822. doi: 10.1093/jxb/err336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leida C, Conejero A, Arbona V, et al. Chilling-dependent release of seed and bud dormancy in peach associates to common changes in gene expression. PLoS ONE. 2012;7:e35777–e35785. doi: 10.1371/journal.pone.0035777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michailidis M, Karagiannis E, Tanou G, et al. Metabolic mechanisms underpinning vegetative bud dormancy release and shoot development in sweet cherry. Environ. Exp. Bot. 2018;155:1–11. doi: 10.1016/j.envexpbot.2018.06.024. [DOI] [Google Scholar]
- 32.Prudencio, A. S., Hoeberichts, F. A., Dicenta, F., Martínez-Gómez, P. & Sánchez-Pérez, R. Identification of early and late flowering time candidate genes in endodormant and eco dormant almond flower buds. Tree Physiology, tpaa151, 10.1093/treephys/tpaa151 (2020). [DOI] [PMC free article] [PubMed]
- 33.Conrad, A. O., Yu, J. & Staton, M. E. et al. Association of the phenylpropanoid pathway with dormancy and adaptive trait variation in apricot (Prunus armeniaca). Tree Physiol.39, 1136–1148 (2019). [DOI] [PubMed]
- 34.Ben Mohamed H, Vadel AM, Geuns JMC, Khemira H. Biochemical changes in dormant grapevine shoot tissues in response to chilling: possible role in dormancy release. Sci. Hortic. 2010;124:440–447. doi: 10.1016/j.scienta.2010.01.029. [DOI] [Google Scholar]
- 35.Zhang J, Yan X, Su F, et al. Long-term N and P additions alter the scaling of plant nitrogen to phosphorus in a Tibetan alpine meadow. Sci. Total Environ. 2018;625:440–448. doi: 10.1016/j.scitotenv.2017.12.292. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Zhuo X, Zhao K, et al. Transcriptome profiles reveal the crucial roles of hormone and sugar in the bud dormancy of Prunus mume. Sci. Rep. 2018;8:1–15.. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartolini S, Viti R, Guerriero R. Xylem differentiation and microsporogenesis during dormancy of apricot flower buds (Prunus armeniaca L.) Eur. J. Hortic. Sci. 2006;71:84–90. [Google Scholar]
- 38.Maggini V, De Leo M, Mengoni A, et al. Plant-endophytes interaction influences the secondary metabolism in Echinacea purpurea (L.) Moench: an in vitro model. Sci. Rep. 2017;7:16924–31.. doi: 10.1038/s41598-017-17110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng C, Acheampong AK, Shi Z, et al. Abscisic acid catabolism enhances dormancy release of grapevine buds. Plant Cell Environ. 2018;41:2490–2503. doi: 10.1111/pce.13371. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Li X, Jia J, et al. bHLH92 from sheepgrass acts as a negative regulator of anthocyanin/proanthocyandin accumulation and influences seed dormancy. J. Exp. Bot. 2019;70:269–284. doi: 10.1093/jxb/ery335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloret A, Martínez-Fuentes A, Agustí M, Badenes ML, Ríos G. Chromatin-associated regulation of sorbitol synthesis in flower buds of peach. Plant Mol. Biol. 2017;95:507–517. doi: 10.1007/s11103-017-0669-6. [DOI] [PubMed] [Google Scholar]
- 42.Gianinetti A, Finocchiaro F, Bagnaresi P, et al. Seed dormancy involves a transcriptional program that supports early plastid functionality during imbibition. Plants. 2018;7:35–84. doi: 10.3390/plants7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porto DD, Bruneau M, Perini P, et al. Transcription profiling of the chilling requirement for bud break in apples: a putative role for FLC-like genes. J. Exp. Bot. 2015;66:2659–2672. doi: 10.1093/jxb/erv061. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Vivancos P, Bernal-Vicente A, Cantabella D, Petri C, Hernández JA. Metabolomics and biochemical approaches link salicylic acid biosynthesis to cyanogenesis in peach plants. Plant Cell Physiol. 2017;58:2057–2066. doi: 10.1093/pcp/pcx135. [DOI] [PubMed] [Google Scholar]
- 45.Bernal-Vicente A, Cantabella D, Petri C, Hernández JA, Diaz-Vivancos P. The salt-stress response of the transgenic plum line J8-1 and its interaction with the salicylic acid biosynthetic pathway from Mandelonitrile. Int. J. Mol. Sci. 2018;19:1–19.. doi: 10.3390/ijms19113519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pičmanová M, Neilson EH, Motawia MS, et al. A recycling pathway for cyanogenic glycosides evidenced by the comparative metabolic profiling in three cyanogenic plant species. Biochem. J. 2015;469:375–389. doi: 10.1042/BJ20150390. [DOI] [PubMed] [Google Scholar]
- 47.Pinon V, Ravanel S, Douce R, Alban C. Biotin synthesis in plants. The first committed step of the pathway is catalyzed by a cytosolic 7-keto-8-aminopelargonic acid synthase. Plant Physiol. 2005;139:1666–1676. doi: 10.1104/pp.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beauvieux R, Wenden B, Dirlewanger E. Bud dormancy in perennial fruit tree species: a pivotal role for oxidative cues. Front Plant Sci. 2018;9:657. doi: 10.3389/fpls.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felipe, A. J. Phenological states of almond. in Proc Third GREMPA Colloq (ed. International Center for Advance Mediterranean Agronomic Studies) 101–103 (Mediterranean Agronomic Institute of Zaragoza, 1977).
- 50.Ruiz D, Campoy JA, Egea J. Chilling and heat requirements of apricot cultivars for flowering. Environ. Exp. Bot. 2007;61:254–263. doi: 10.1016/j.envexpbot.2007.06.008. [DOI] [Google Scholar]
- 51.Gil Solsona R, Boix C, Ibáñez M, Sancho JV. The classification of almonds (Prunus dulcis) by country and variety using UHPLC-HRMS-based untargeted metabolomics. Food Addit. Contam Part A. 2018;35:395–403. doi: 10.1080/19440049.2017.1416679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.