Abstract
Diabetes is an independent risk factor for the development of heart failure. Increased fatty acid (FA) uptake and deranged utilization leads to reduced cardiac efficiency and accumulation of cardiotoxic lipids, which is suggested to facilitate diabetic cardiomyopathy. We studied whether reduced FA uptake in the heart is protective against streptozotocin (STZ)-induced diabetic cardiomyopathy by using mice doubly deficient in fatty acid binding protein 4 (FABP4) and FABP5 (DKO mice). Cardiac contractile dysfunction was aggravated 8 weeks after STZ treatment in DKO mice. Although compensatory glucose uptake was not reduced in DKO-STZ hearts, total energy supply, estimated by the pool size in the TCA cycle, was significantly reduced. Tracer analysis with 13C6-glucose revealed that accelerated glycolysis in DKO hearts was strongly suppressed by STZ treatment. Levels of ceramides, cardiotoxic lipids, were similarly elevated by STZ treatment. These findings suggest that a reduction in total energy supply by reduced FA uptake and suppressed glycolysis could account for exacerbated contractile dysfunction in DKO-STZ hearts. Thus, enhanced FA uptake in diabetic hearts seems to be a compensatory response to reduced energy supply from glucose, and therefore, limited FA use could be detrimental to cardiac contractile dysfunction due to energy insufficiency.
Subject terms: Diabetes, Cardiovascular biology, Metabolomics, Metabolism
Introduction
Diabetic cardiomyopathy occurs among patients with diabetes independently of other cardiovascular risk factors including hypertension and ischemic heart disease1–3. Although severity of diabetic cardiomyopathy progresses from subtle structural and functional abnormalities to end-stage heart failure, typical diabetic cardiomyopathy displays left ventricular diastolic and systolic dysfunction, left ventricular hypertrophy and interstitial fibrosis4–8. It has been proposed that diabetic cardiomyopathy is induced by multifactorial mechanisms including increased myocardial fatty acid (FA) utilization and lipid storage, sustained hyperglycemia, reduced glucose consumption, compromised mitochondrial energetics, impaired Ca2+ handling, increased connective tissue content, increased reactive oxygen species (ROS) production, and enhanced inflammatory responses4–8. Among these possibilities of causative burdens, lipotoxicity has been suggested to have a significant role in the development of diabetic cardiomyopathy.
Abnormal FA utilization in the heart has been shown in diabetes in humans and animals4–11. FA uptake and utilization are presumably accelerated to compensate for decreased glucose use, which is often accompanied by increased expression of genes associated with FA utilization, such as peroxisome proliferator-activated receptor α (PPARα) and its target genes12–15. In cardiomyocytes, excessive FA is converted into other lipid intermediates such as neutral triacylglycerol (TG) and others with lipotoxic effects (e.g. diacylglycerol [DAG] and ceramides). Increased levels of ceramides can stimulate inflammatory signaling and facilitate ROS production, which in turn results in impairment of mitochondrial energetics. Reduced cardiac efficiency (cardiac work/oxygen consumption) by deranged FA use is also suggested to be a causative burden for diabetic cardiomyopathy. To date, the lipotoxicity hypothesis is prevailing as a key event driving cardiac dysfunction in diabetes.
Fatty acid binding protein 4 (FABP4) and FABP5, intracellular lipid chaperons, are abundantly expressed in capillary endothelial cells in the heart and skeletal muscle, in addition to adipocytes and macrophages16,17. We recently reported that FABP4/5 in capillary endothelium facilitates FA transport from circulation into these tissues (known as trans-endothelial FA transport), particularly in the heart16. Importantly, in mice with the genetic deletion of both FABP4 and FABP5 (double knockout, DKO), cardiac FA uptake is reduced by 30% compared to wild-type (WT) while compensatory glucose uptake is 20-fold higher. We further found that a reduction in FA use with elevated glucose uptake renders DKO hearts more susceptible to cardiac dysfunction under increased workload by transverse aortic constriction18. Cardiac dysfunction in DKO-TAC mice is associated with a reduction in total energy supply relative to energy demand, which is partially rescued by supplementation of medium chain FA. These findings provided an important notion that long chain FA are pivotal fuels to generate sufficient energy for preserved cardiac contraction under increased workload and not toxic for pressure-overloaded hearts. We also demonstrated that FABP4/5 DKO hearts are a useful model to evaluate contribution of reduced FA utilization to pathophysiology of heart failure models18,19.
In this study, we tested the hypothesis whether reduced FA utilization in DKO mice is protective against diabetic cardiomyopathy generated by streptozotocin (STZ) treatment. Contrary to our expectation, we found that cardiac contractile dysfunction was exacerbated in DKO-STZ mice. Total energy supply, estimated by the pool size in the tricarboxylic acid (TCA) cycle, was reduced in DKO-STZ hearts, which could account for reduced contractile function in DKO-STZ hearts. Our data suggest that enhanced FA utilization in diabetic hearts occurs to compensate for reduced glucose use, and therefore, that limitation of FA utilization is not always beneficial for diabetic cardiomyopathy.
Results
Deterioration of left ventricular contractile dysfunction in STZ-treated DKO mice
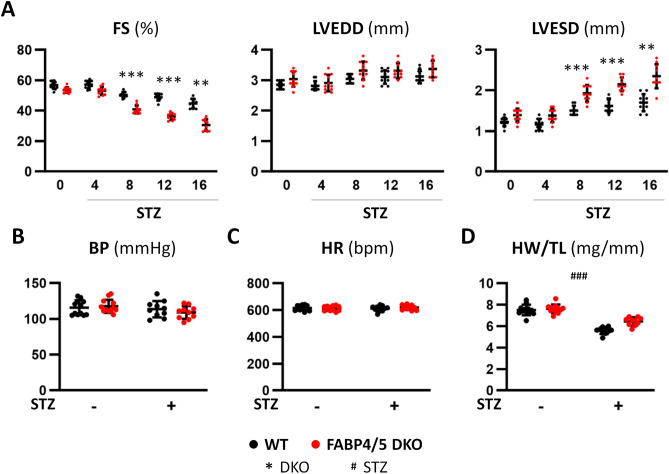
To study whether reduced FA use influences the cardiac function in the context of diabetes by insulin deficiency, cardiac function was assessed by echocardiography for 16 weeks after STZ treatment. Body weight was significantly reduced by STZ treatment, which was accompanied by disappearance of abdominal and subcutaneous adipose tissue (Supplementary Fig. S1). While functional difference was not observed between DKO and WT mice at baseline, a decrease in fractional shortening (FS) and an increase in left ventricular systolic diameter (LVESD) was more remarkable in DKO-STZ mice at all measured time points after 8 week (Fig. 1A). Wall thickness of interventricular septum (IVS) and posterior wall (PW) was not different among all groups (Supplementary Fig. S2). Systolic blood pressure and heart rate were not significantly different between the groups with and without STZ treatment (Fig. 1B,C). Cardiac atrophy was observed by heart weight/tibial length (HW/TL) ratio and cross sectional area (CSA) in STZ-treated mice (Fig. 1D and Supplementary Fig. S3). These findings suggest that contractile dysfunction in DKO-STZ mice is more prominent compared to that in WT-STZ although cardiac atrophy is induced by STZ in both mice to a similar extent.
Figure 1.
Cardiac contractile dysfunction was more deteriorated in DKO-STZ mice. (A) Cardiac function was estimated by echocardiography before and 4, 8, 12 and 16 weeks after STZ treatment (n = 10–12). FS fractional shortening, LVEDD left ventricular end-diastolic diameter, LVESD left ventricular end-systolic diameter. (B,C) Systolic blood pressure (BP) and heart rate (HR) were not significantly different. (D) Heart weight/tibial length (HW/TL) ratio was significantly reduced 16 weeks after STZ treatment in both mice. **p < 0.01, ***,###p < 0.001.
Glucose uptake is not reduced in DKO-STZ hearts
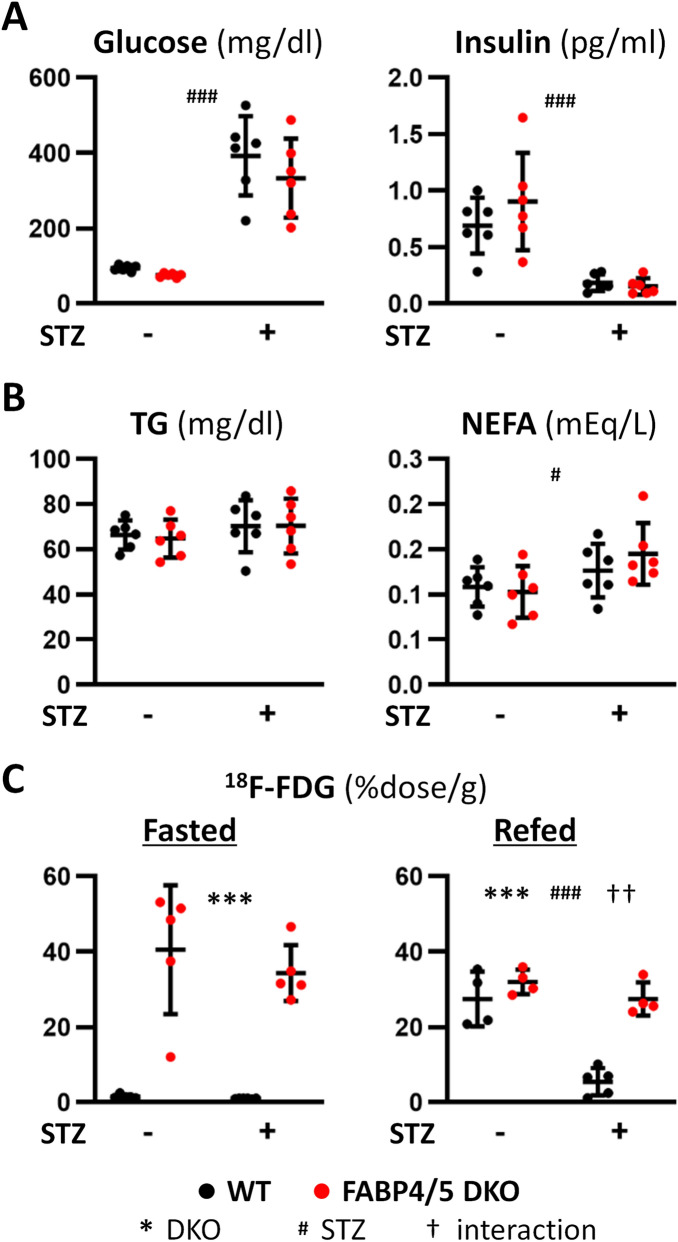
We examined metabolic alteration by STZ treatment. Blood glucose levels were markedly increased by STZ treatment while insulin levels were reduced (Fig. 2A). Serum levels of non-esterified FA (NEFA) were increased by STZ treatment (Fig. 2B). We next examined effects of diabetes on uptake of 18F-FDG, glucose tracer. 18F-FDG uptake was 20-fold higher in DKO hearts compared to WT at baseline to compensate for reduced FA uptake (Fig. 2C). 18F-FDG uptake was not changed by STZ treatment in both groups in the fasted state (Fig. 2C). However, 18F-FDG uptake was markedly reduced after refeeding in WT-STZ mice, but not in DKO-STZ mice. These finding suggest that insulin-dependent glucose uptake by the heart is significantly reduced during postprandial period in WT-STZ mice and that glucose uptake is constantly enhanced without an effect of insulin in DKO-STZ.
Figure 2.
Circulating levels of metabolites and uptake of glucose tracer by hearts in the absence or the presence of STZ treatment. Samples were collected after a 6 h fast (n = 6). (A) Serum levels of glucose and insulin. (B) Serum levels of TG and NEFA. (C) Uptake of 18F-FDG, glucose tracer, by hearts. TG, triacylglycerol; NEFA, non-esterified fatty acids. #p < 0.05, ††p < 0.01, ***,###p < 0.001.
Ketolysis is accelerated in DKO hearts irrespective of STZ treatment
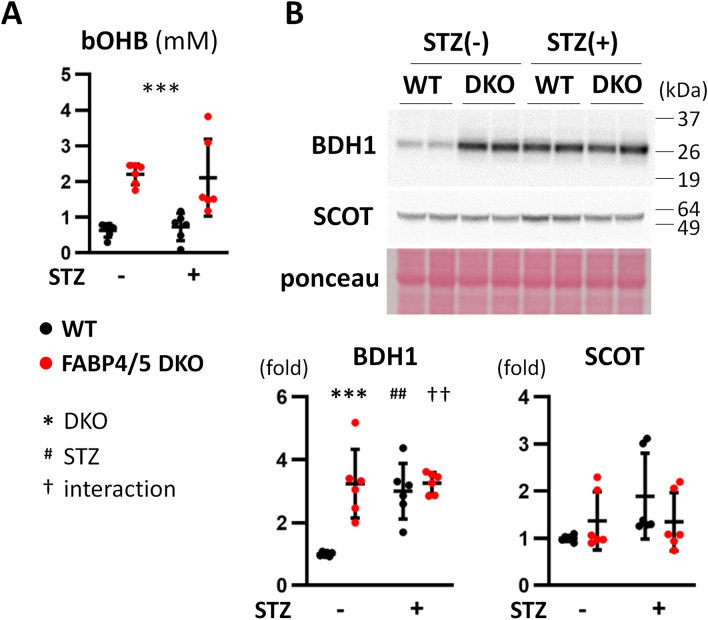
Ketone bodies are crucial fuels for hearts in diseased conditions, such as diabetes and heart failure20. We found that serum levels of β-hydroxybutyrate (bOHB) were increased in DKO mice after a 6 h fast irrespective of STZ treatment (Fig. 3A). Expression of BDH1, a ketolytic enzyme, was enhanced in DKO-control, WT-STZ and DKO-STZ hearts compared to WT-control whereas that of SCOT, another ketolytic enzyme, was not changed (Fig. 3B and Supplementary Fig. S8). These findings suggest that ketone body utilization seems to be facilitated in DKO hearts to compensate for reduced FA supply irrespective of STZ treatment.
Figure 3.
Circulating ketone body and ketolytic enzyme are increased in DKO hearts. (A) Serum levels of bOHB, β-hydroxybutyrate (n = 6). (B) Protein expression of ketolytic enzymes, BDH1 and SCOT, was assessed by western blot analysis (n = 6). ##,††p < 0.01, ***p < 0.001.
Facilitated glycolysis in DKO hearts is diminished by STZ treatment
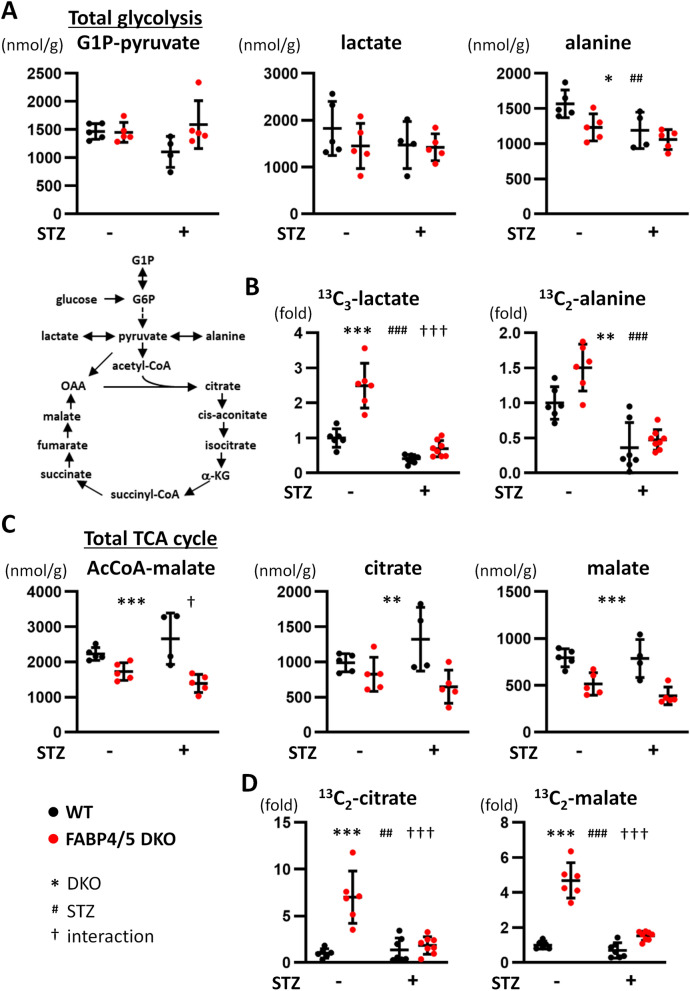
To examine cellular metabolic changes in DKO hearts, metabolome analysis was carried out. Despite a marked increase in glucose uptake in DKO mice (Fig. 2C), total metabolites in glycolysis (G1P to pyruvate) and levels of lactate were comparable between WT and DKO hearts irrespective of STZ treatment (Fig. 4A). Levels of alanine were lower in DKO hearts and reduced by STZ treatment (Fig. 4A). Tracer study with 13C6-glucose revealed that levels of 13C3-lactate and 13C2-alanine were significantly elevated in DKO hearts compared to WT, and the glycolytic flux was suppressed by STZ treatment (Fig. 4B). These data suggest that accelerated glycolytic rate in DKO hearts is significantly reduced by insulin deficiency despite a fact that enhanced glucose uptake is maintained.
Figure 4.
(A,B) Glycolytic flux was suppressed by STZ treatment. (A) Metabolic profiling in glycolysis pathway. G1P-pyruvate, total metabolites from G1P to pyruvate in glycolysis (n = 4–5). Hearts were isolated after a 6 h fast for metabolome analysis. (B) Tracer study with 13C6-glucose. After a 6 h fast, hearts were isolated 10 min after intraperitoneal injection of 13C6-glucose (n = 6). (C,D) The pool size in the TCA cycle was significantly reduced in DKO-STZ hearts. (C) Metabolic profiling in the TCA cycle. (n = 4–5). Hearts were isolated after a 6 h fast for metabolome analysis. (D) Tracer study with 13C6-glucose. After a 6 h fast, hearts were isolated 10 min after intraperitoneal injection of 13C6-glucose (n = 6). G1P glucose-1-phosphate, G6P glucose 6-phosphate, α-KG α-ketoglutarate, OAA oxaloacetate. *,†p < 0.05, **,##p < 0.01, ***,###,†††p < 0.001.
Accelerated glycolytic flux into the TCA cycle in DKO hearts is suppressed by STZ treatment
We next explored the amounts of metabolites in the TCA cycle. The pool size of total metabolites in the TCA cycle (acetyl-CoA to malate) was significantly lower in DKO hearts at baseline and the difference was enhanced by STZ treatment (Fig. 4C, interaction p < 0.05). Consistent with this, each metabolite in the TCA cycle such as citrate and malate exhibited tendency similar to the pool size (Fig. 4C). These findings suggest that increased FA use in diabetic WT hearts is compensation for reduced glucose utilization, which is lost in DKO-STZ hearts. Contrary to the reduction in the pool size in the TCA cycle, isotopomer analysis with 13C6-glucose exhibited that levels of 13C2-citrate and 13C2-malate were significantly increased in DKO hearts at baseline (Fig. 4D), which strongly suggests that accelerated glycolytic flux is compensation for reduced FA uptake. Although levels of 13C2-citrate and 13C2-malate were not markedly changed by-STZ treatment in WT, they were strongly reduced by STZ in DKO hearts (Fig. 4D, interaction p < 0.001). Thus, an accelerated glycolytic flux into the TCA cycle in DKO-STZ hearts is remarkably suppressed despite sustained enhancement of glucose uptake. We also measured levels of adenosine triphosphate (ATP) and phosphocreatine (PCr), reserve energy, to estimate creatine-phosphate energy shuttle. ATP levels were reduced by STZ treatment whereas PCr levels were not changed (Supplementary Fig. S4). There was a decline in ATP levels even in WT-STZ hearts despite maintained cardiac contraction. Because it is known that a decline in PCr levels usually precedes a decrease in ATP21, interpretation of early reduction in ATP levels remains elusive. Together, metabolic flux from two major fuels, FA and glucose, into the TCA cycle seems to be more reduced in DKO-STZ hearts, which could account for enhanced reduction in the pool size in the TCA cycle (or reduced energy supply relative to energy expenditure, see the discussion in detail) and a resultant deterioration of contractile dysfunction. Our data also suggest that the pool size in the TCA cycle is likely to be a more promising marker to estimate energy status in the failing heart compared to PCr/ATP.
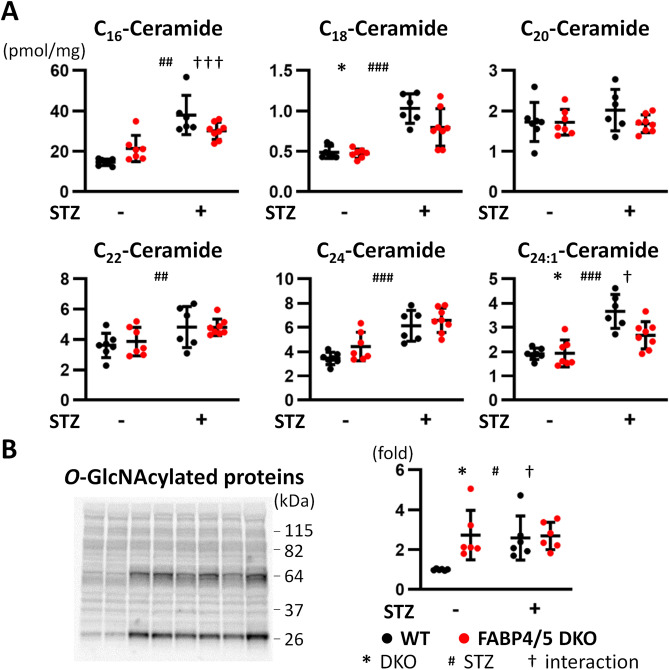
Levels of most ceramides are elevated by STZ treatment in WT and DKO hearts
Diabetes induces an imbalance between FA uptake and oxidation, which leads to accumulation of cardiotoxic lipids such as ceramides and DAG4–8. Amounts of most ceramides with different length of FAs were elevated by STZ treatment in both mice compared to control groups (Fig. 5A). However, there was interaction between genotype (DKO) and STZ treatment in C16- and C24:1-ceramides (Fig. 5A), indicating a significant reduction in DKO-STZ hearts compared to WT-STZ. Importantly, amounts of C16- and C24:1-ceramides occupy more than half of whole ceramides. Thus, accumulation of lipotoxic lipids does not account for exacerbation of cardiac dysfunction observed in DKO-STZ hearts.
Figure 5.
(A) Accumulation of cardiotoxic lipids was not enhanced in DKO-STZ hearts compared to WT-STZ. Levels of ceramides with different length of FAs; C16, C18, C20, C22, C24 and C24:1 (n = 6). (B) O-GlcNAcylation was similarly induced in WT-STZ and DKO-STZ hearts. O-GlcNAcylation of extracted proteins was estimated by western blot analysis with anti-GlcNAc antibody (n = 6). *,†p < 0.05, ##p < 0.01, ###,†††p < 0.001.
GlcNAcylation of proteins is similarly induced in WT-STZ and DKO-STZ hearts
Excessive glucose availability has also been explored as a contributing factor to cellular dysfunction in diabetes, which is known as glucotoxicity5,22,23. Hexosamine biosynthesis pathway (HBP) is an ancillary pathway of glucose metabolism to modify protein function via O-linked attachment of UDP-GlcNAc (referred to as O-GlcNAcylation)22–25. Given that accelerated glucose uptake was not suppressed by STZ treatment, we presumed that O-GlcNAcylation might be a key mechanism for reduced cardiac contraction. Interestingly, GlcNAcylated proteins were already enhanced in cellular extracts from DKO hearts without STZ treatment (Fig. 5B and Supplementary Fig. S2). In addition, similar distribution of GlcNAcylated proteins was observed in WT-STZ and DKO-STZ hearts (Fig. 5B and Supplementary Fig. S9). These findings suggest that O-GlcNAcylation is unlikely to be a cause of deteriorated contractile dysfunction observed in DKO-STZ hearts.
Other possible causes of contractile dysfunction in DKO-STZ hearts
It has been suggested that augmented inflammatory responses and mitochondrial damage are possible mechanisms to facilitate metabolic impairment in diabetic hearts4,5,7. Expression of pro-inflammatory cytokines, such as tumor necrosis factor (Tnf) and interlukin 1β (Il1b), was not induced in DKO-STZ hearts (Supplementary Fig. S5), which suggests that inflammation is not a major factor in etiology of reduced contraction in DKO-STZ hearts. Overt mitochondrial damage, estimated by protein expression of electron transport chain complex, was not observed in DKO-STZ hearts, either (Supplementary Fig. S6). Thus, inflammatory responses and mitochondrial damage are unlikely to be responsible for reduced contractility in DKO-STZ hearts.
Expression of a master regulator for FA catabolism, Ppara (peroxisome proliferator activated receptor α), was induced by STZ treatment in both mice (Supplementary Fig. S7). However, expression of other FA metabolism-related genes such as Cd36, Acadm (acly-CoA dehydrogenase medium chain), Acadl (acly-CoA dehydrogenase long chain) and Fabp3 (heart-type FABP) was lower in DKO-STZ hearts compared to WT-STZ. Reduction of their gene expression might be caused by a decrease in endogenous PPARA ligands due to reduced FA uptake. These findings suggest that transcriptional suppression for FA metabolism could further enhance a reduction in FA utilization in DKO-STZ hearts compared to WT-STZ.
Discussion
In this study, our results suggest that aggravation of contractile dysfunction in DKO-STZ hearts is due to energy insufficiency. Total energy supply relative to energy expenditure, estimated by the pool size in the TCA cycle, was significantly reduced in DKO-STZ hearts for the following reasons. First, FA uptake by DKO hearts is diminished due to reduced function of trans-endothelial FA transport as reported previously16. Second, the enhanced glycolytic rate was markedly suppressed in DKO-STZ hearts. Although compensatory glucose uptake was maintained, glycolytic flux into the TCA cycle was strongly suppressed. An increase in ketone body utilization was also insufficient for restoration of energy shortage. Thus, a reduction in total energy supply is caused by reduced FA uptake and suppressed glycolysis, which could account for exacerbated contractile dysfunction. We further suggest that in the aspect of energy homeostasis, enhanced FA uptake in diabetic hearts seems to be a compensatory response to reduced energy supply from glucose, and therefore, limited FA use could contribute to deterioration of cardiac contractile dysfunction in diabetes, rather than protection from lipotoxic effects.
Our study also addressed other possibilities of cardiac contractile dysfunction in diabetes in addition to energy shortage. We showed no increase in accumulation of cardiotoxic lipids such as ceramides in DKO-STZ hearts compared to WT-STZ. Further, O-GlcNAcylation was similarly induced in WT-STZ and DKO-STZ hearts. We also observed no obvious inflammatory responses and mitochondrial damage. These findings suggest that lipotoxicity, glucotoxicity, inflammatory responses and mitochondrial damage are not major reasons for enhanced contractile dysfunction in DKO-STZ hearts.
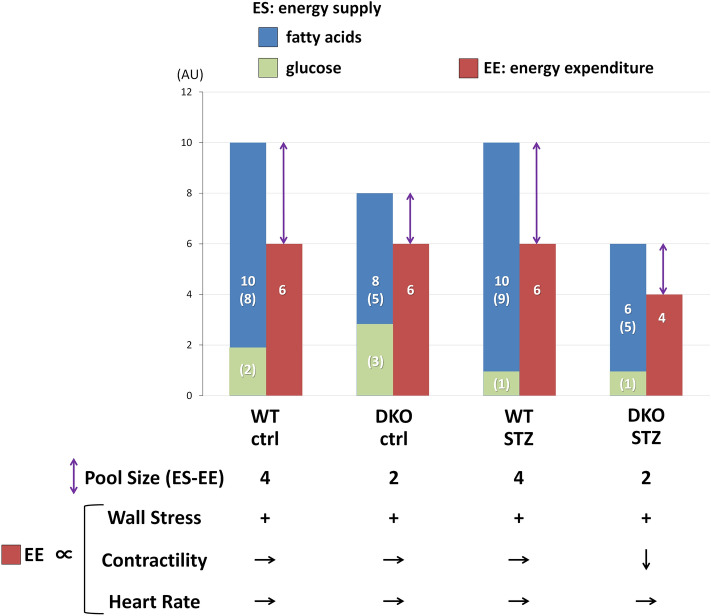
The pool size in the TCA cycle as a useful marker for energy status in vivo beating hearts
The pool size in the TCA cycle has been shown as a marker for energy status in isolated working hearts26–30. Keeping a constant amount of the pool size is necessary for normal function of energy production and cardiac contraction. Recently, we proposed that the pool size is also a useful marker for energy balance in vivo beating hearts under increased workload by transverse aortic constriction [38, 48]. As shown in Fig. 6, the concept of the pool size is also applied to account for our diabetic heart model. We suggest that the pool size is determined by balance between energy supply (ES) and energy expenditure (EE). ES depends on uptake and oxidation of energy substrates, mainly FA and glucose, and lesser amount of ketone bodies. EE is determined by three components, wall stress, contractility and heart rate. In DKO hearts at baseline, ES is smaller compared to WT-ctrl hearts, leading to the reduced pool size in the TCA cycle. The reduced ES in DKO hearts is sufficient for basal cardiac contraction since the reduced ES is still able to fulfill minimal EE required for normal function. In WT-STZ-hearts, FA utilization is enhanced to compensate for suppressed glycolysis, which can maintain total ES and cardiac contraction. In DKO-STZ hearts, however, FA uptake is limited in addition to suppressed glycolysis, which leads to a reduction in total ES. Importantly, cardiac contraction, a determinant for EE, is reduced in DKO-STZ hearts, which implies that the EE is smaller than minimal EE required for normal function. The pool size in DKO-STZ hearts is similar or reduced a little compared to DKO-control (Fig. 6), which further supports the notion that both ES and EE are reduced in DKO-STZ hearts. Thus, a reduction in the pool size in combination with a decrease in cardiac contraction reflects that the ES in DKO-STZ hearts is energetically insufficient for normal cardiac function.
Figure 6.
Putative bar graph regarding the pool size in the TCA cycle. There are two points to understand the working hypothesis. First, the pool size in the TCA cycle is associated with balance between energy supply (ES) and energy expenditure (EE). Second, EE is determined by three components, wall stress, contractility and heart rate (see “Discussion” in detail). AU arbitrary unit.
Significance of lipotoxicity in diabetic cardiomyopathy
The highlight of this study is to show that facilitated FA utilization in diabetic hearts is necessary to keep total energy supply for normal cardiac function. This is the first report to demonstrate that accelerated FA use is beneficial rather than detrimental, which is a sharp contrast to a hitherto known concept of lipotoxicity in diabetic hearts. We presume that the discrepancy occurs because of distinct experimental conditions (ex vivo vs. in vivo) as described below.
In addition to accumulation of cardiotoxic lipids such as ceramides and DAG, it is also suggested that lipotoxic effects appear as a result of inefficient oxygen consumption (MVO2)4,5,7. While increased FA oxidation is associated with increased MVO2, increased MVO2 is not accompanied by an equivalent increase in cardiac contractility, which causes a reduction in cardiac efficiency (cardiac work/MVO2). Therefore, the reduced cardiac efficiency by excessive FA consumption has been considered to be one of most essential pathophysiological causes in diabetic hearts. However, the concept is established mostly by using isolated working hearts5,6. Caveats of such ex vivo studies are that experiments are performed under the same perfusate conditions (e.g. the same concentration of glucose, palmitate, insulin, Ca2+, etc.). In diabetic hearts in vivo, however, circulating components are considerably different from those in control hearts. If diabetic hearts are adapted to worse metabolic conditions to exert the best hemodynamic performance in vivo, the beneficial aspect of metabolic adaption is underestimated ex vivo under the same conditions. In this study, we exhibited for the first time that increased FA use in diabetic hearts is energetically compensatory in vivo, something which cannot be determined by ex vivo experiments. Future studies are needed to establish new methods to evaluate effects of metabolic adaptation in diabetic hearts in vivo.
Significance of glucotoxicity in diabetic cardiomyopathy
Because DKO-control hearts with normal cardiac contraction displays enhanced O-GlcNAcylation of proteins comparable with those of DKO-STZ, it is unlikely that O-GlcNAcylation alone causes reduced cardiac contractility in DKO-STZ hearts. We sought for the reason why our data are inconsistent with prevailing idea. O-GlcNAcylation is facilitated via the HBP depending on glucose availability in cells31,32 and ten to twenty % of all proteins are thought to be possible targets of O-GlcNAcylation. O-GlcNAcylation is controlled by a pair of enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) and the modification cycle is very rapid, which takes 1 min or even less31–33. Thus, the HBP has wide range of target proteins, which is rapidly, reversibly and non-selectively controlled by a pair of enzymes and transient nutritional state. Based on such characteristics of O-GlcNAc modification, we presume that comparable levels of O-GlcNAcylation should have similar effects on functions of target proteins. This notion is supported by our data that accelerated O-GlcNAcylation alone did not cause reduced cardiac contractile function in DKO-control hearts and that WT-STZ hearts did not exhibit contractile dysfunction at earlier phase of diabetes. Although it is proposed that O-GlcNAcylation of several candidate proteins can cause cardiac dysfunction22–25,34, our findings raise another possibility that some additional mechanisms exist to exacerbate protein function modified by O-GlcNAcylation in diabetic hearts during later period of diabetes.
Conclusions
Increased FA use in diabetic hearts is required for preservation of cardiac contraction to compensate for reduced energy supply from glucose. Therefore, limited FA utilization could be detrimental to cardiac contractile dysfunction in diabetic hearts, rather than protective against lipotoxicity.
Methods
Mice, type I diabetic model and sample preparation
All studies were approved by the Institutional Animal Care and Use Committee (Gunma University Graduate School of Medicine). Animal experiments were carried out according to the NIH guidelines (Guide for the Care and Use of Laboratory Animals). FABP4 and FABP5 double knockout (DKO) mice with the C57BL6j background were generated as described previously35. Control wild type mice (WT) and DKO−/− were obtained by breeding DKO+/− (Fabp4+/−Fabp5+/−) male and female mice. The mice were housed in a temperature-controlled room (20–26 °C) with a 12-h light/12-h dark cycle and given unrestricted access to water and standard chow (CE-2, Clea Japan, Inc.). For the type 1 diabetic model, 10–12-week-old male mice were treated with i.p. STZ in 0.1 M sodium citrate (pH 4.5) at a dose of 50 mg/kg for 5 days36. Blood was collected from the retro-orbital plexus, and then centrifuged at 1500 × g for 15 min at 4 °C to obtain the serum for measurement of biochemical parameters. After cervical dislocation, hearts were dissected, snap frozen in liquid nitrogen and stored at − 80 °C until further use.
Cardiac function and hemodynamic parameters
The in vivo cardiac function and the heart rate were assessed by transthoracic echocardiography (EUB-7500, Hitachi, Tokyo) in conscious mice as described previously19,37. Blood pressure data were obtained from the average of three-time measurements by the tail cuff method (MK-2000ST, Muromachi Kikai, Tokyo)18.
Measurement of blood metabolites
Serum levels of glucose (glutest sensor, Sanwa Kagaku, Aichi), triglyceride (Triglyceride E-test, Wako Chemical, Osaka), non-esterified fatty acid (NEFA C-test Wako Chemical, Osaka) and β-hydroxybutyrate (EnzyChrom Ketone Body Assay Kit, BioAssay Systems, California) and insulin (mouse Insulin ELISA, Mercodia, Sweden) were measured according to the manufacturer’s protocols18,19,37.
The biodistribution of 18F-FDG (2-Fluorodeoxyglucose)
Mice received intravenous injections of 18F-FDG (100 kBq) via the lateral tail vein in a volume of 100 μl. 18F-FDG was obtained from batches that were prepared for clinical PET imaging at Gunma University. The animals were sacrificed 2 h after injection. The isolated hearts were weighed and counted using a well-type gamma counter (ARC-7001, Hitachi-Aloka Medical)18,19,37.
Western blot analysis
Western blot analysis was done with antibodies against 3-hydroxybutyrate dehydrogenase 1 (BDH1, SCB, sc-134281, CA) and succinyl CoA:3-oxoacid CoA transferase (SCOT, SCB, sc-133988, CA) as described previously18,19,37. To avoid removal of GlcNAcylation of proteins, proteins were extracted with the RIPA buffer containing an O-GlcNAcase inhibitor, O-(2-acetamido-2-deoxy-d-glucopyranosylidenamino) N-phenylacarbamate (i.e. PUGNAc, 25 μM)38. GlcNAc modification was detected with O-GlcNAc antibody (CTD110.6, SCB, sc-59623, CA).
Metabolome analysis by capillary electrophoresis-mass spectrometry
The mice were anesthetized by isoflurane and the ventricles were immediately removed from the mice after a 6 h fast. The heart samples were freeze-clamped using aluminum blocks precooled in liquid nitrogen and maintained at – 80 ℃. Metabolome analyses were carried out as described elsewhere16,18,19,37.
Tracing study with 13C6-glucose
Six hours after fasting, 13C6-glucose (1 mg/g) was intraperitoneally injected into mice. Ten minutes later, the ventricles were isolated, and metabolome analyses were conducted as described previously18,19,37.
Measurement of ceramides
Ceramide measurement was performed by using a triple quadrupole mass spectrometer coupled with a liquid chromatograph (LCMS-8050 system, Shimadzu). Heart tissues were crashed to powder in liquid nitrogen, then rotated for 1 h at 4 °C in methanol containing 1 μM C18 ceramide (C17 base, d17:1/18:0) as an internal standard. The extract was separated on a reversed-phase C8 column (Kinetex C8, 2.1 mm × 150 mm, 2.6 μm, Phenomenex) by using a gradient of solvent A (water/methanol/formic acid 97/2/1) and B (methanol/acetone/water/formic acid 68/29/2/1) with a flow rate of 0.4 ml/min. The initial solvent composition was 60% B, and the following solvent gradient was applied: 60% for 1 min, increased linearly to 100% from 1 to 8 min, held at 100% B for 3 min, then returned to 60% B and maintained for 2 min. The column was maintained at 60 °C. The separated analytes were ionized by electrospray ionization, then measured by the mass spectrometer with selected reaction monitoring (SRM) mode. The SRM transition for analytes were m/z 538.5 > 264.2 [M + H]+ for C16 ceramide (d18:1/16:0), m/z 548.5 > 264.2 [M + H-H2O]+ for C18 ceramide (d18:1/18:0), m/z 576.5 > 264.2 [M + H-H2O]+ for C20 ceramide (d18:1/20:0), m/z 604.5 > 264.2 [M + H-H2O]+ for C22 ceramide (d18:1/22:0), m/z 630.5 > 264.2 [M + H-H2O]+ for C24:1 ceramide (d18:1/24:1), m/z 632.5.5 > 264.2 [M + H-H2O]+ for C24 ceramide (d18:1/24:0), and m/z 534.5 > 250.2 [M + H-H2O]+ for C18 ceramide (C17 base, d17:1/18:0). Peak area of each ceramide was divided by that of the internal standard, then applied to the standard curve made for each ceramide species for quantification. All of the ceramide standards were purchased from Avanti Polar Lipids.
Statistical analysis
Statistical analysis was performed with IBM SPSS (version 24, IBM, NY). The data are presented as mean ± standard deviation. Student’s t-test was performed for 2 groups’ comparison. Two-way analysis of variance (ANOVA) was used to analyze effects of genotype (WT vs. DKO), STZ treatment (control vs. STZ) and their interaction. A p value < 0.05 was considered to be statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001; main effect for genotype. #p < 0.05, ##p < 0.01, ###p < 0.001; main effect for STZ treatment. †p < 0.05, ††p < 0.01, †††p < 0.001; interaction between genotype and STZ treatment.
Ethics approval and consent to participate
All studies were approved by the Institutional Animal Care and Use Committee (Gunma University Graduate School of Medicine, 18-080).
Consent for publication
All authors have declared their consent for this publication.
Supplementary information
Acknowledgements
We thank Miki Matsui, Takako Kobayashi and Keiko Matsukura for excellent technical help.
Author contributions
T.I. designed the experiments. Y.U., R.K., M.R.A.A.S., N.K., H.S., and H.M. conducted investigations including production of STZ-induced cardiomyopathy models. H.O., T.H. and N.H. performed metabolome analyses. A.Y., H.H., Y.U. and M.R.A.A.S. carried out nuclear medicine experiments. Y.U., R.K. and T.I. analyzed the data. Y.U., R.K. and T.I. drafted the manuscript. M.K. and T.I. revised and edited it. All authors read and approved the final manuscript.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to MK (16H05294) and TI (17K09568), a grant from the Japan Cardiovascular Foundation and a grant from Gunma University Initiative for Advanced Research to MK.
Data availability
All data and materials are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yogi Umbarawan and Ryo Kawakami.
Supplementary information
is available for this paper at 10.1038/s41598-020-77895-1.
References
- 1.Regan TJ, et al. Evidence for cardiomyopathy in familial diabetes mellitus. J. Clin. Investig. 1977;60:884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 3.Rubler S, et al. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 4.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong CR, Clarke K, Levelt E. Metabolic remodeling in diabetic cardiomyopathy. Cardiovasc. Res. 2017 doi: 10.1093/cvr/cvx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016;12:144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis. Model Mech. 2009;2:454–466. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- 9.Peterson LR, et al. Fatty acids and insulin modulate myocardial substrate metabolism in humans with type 1 diabetes. Diabetes. 2008;57:32–40. doi: 10.2337/db07-1199. [DOI] [PubMed] [Google Scholar]
- 10.Herrero P, et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J. Am. Coll. Cardiol. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Peterson LR, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 12.Lee TI, et al. HDAC inhibition modulates cardiac PPARs and fatty acid metabolism in diabetic cardiomyopathy. PPAR Res. 2016;2016:5938740. doi: 10.1155/2016/5938740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TI, et al. Cardiac metabolism, inflammation, and peroxisome proliferator-activated receptors modulated by 1,25-dihydroxyvitamin D3 in diabetic rats. Int. J. Cardiol. 2014;176:151–157. doi: 10.1016/j.ijcard.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Finck BN, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Investig. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Depre C, et al. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J. Mol. Cell Cardiol. 2000;32:985–996. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- 16.Iso T, et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler. Thromb. Vasc. Biol. 2013;33:2549–2557. doi: 10.1161/ATVBAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto K, et al. Peroxisome proliferator-activated receptor-gamma in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. J. Am. Heart Assoc. 2013;2:e004861. doi: 10.1161/JAHA.112.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umbarawan Y, et al. Robust suppression of cardiac energy catabolism with marked accumulation of energy substrates during lipopolysaccharide-induced cardiac dysfunction in mice. Metabolism. 2017;77:47–57. doi: 10.1016/j.metabol.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Umbarawan Y, et al. Glucose is preferentially utilized for biomass synthesis in pressure-overloaded hearts: Evidence from fatty acid-binding protein-4 and -5 knockout mice. Cardiovasc. Res. 2018;114:1132–1144. doi: 10.1093/cvr/cvy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–284. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neubauer S. The failing heart—An engine out of fuel. N. Engl. J. Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 22.Ducheix S, Magre J, Cariou B, Prieur X. Chronic O-GlcNAcylation and diabetic cardiomyopathy: The bitterness of glucose. Front. Endocrinol. (Lausanne) 2018;9:642. doi: 10.3389/fendo.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahma MK, Pepin ME, Wende AR. My sweetheart is broken: Role of glucose in diabetic cardiomyopathy. Diabetes Metab. J. 2017;41:1–9. doi: 10.4093/dmj.2017.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferron M, Denis M, Persello A, Rathagirishnan R, Lauzier B. Protein O-GlcNAcylation in cardiac pathologies: Past, present, Future. Front. Endocrinol. (Lausanne) 2018;9:819. doi: 10.3389/fendo.2018.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailleux F, Gelinas R, Beauloye C, Horman S, Bertrand L. O-GlcNAcylation, enemy or ally during cardiac hypertrophy development? Biochim. Biophys. Acta. 1862;2232–2243:2016. doi: 10.1016/j.bbadis.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Gibala MJ, Young ME, Taegtmeyer H. Anaplerosis of the citric acid cycle: Role in energy metabolism of heart and skeletal muscle. Acta Physiol. Scand. 2000;168:657–665. doi: 10.1046/j.1365-201x.2000.00717.x. [DOI] [PubMed] [Google Scholar]
- 27.Russell RR, 3rd, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J. Clin. Investig. 1991;87:384–390. doi: 10.1172/JCI115008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell RR, 3rd, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am. J. Physiol. 1991;261:H1756–1762. doi: 10.1152/ajpheart.1991.261.6.H1756. [DOI] [PubMed] [Google Scholar]
- 29.Taegtmeyer H. On the inability of ketone bodies to serve as the only energy providing substrate for rat heart at physiological work load. Basic Res. Cardiol. 1983;78:435–450. doi: 10.1007/bf02070167. [DOI] [PubMed] [Google Scholar]
- 30.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem. J. 1980;186:701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17:52. doi: 10.1186/s12915-019-0671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellen KE, Thompson CB. Cellular metabolic stress: Considering how cells respond to nutrient excess. Mol. Cell. 2010;40:323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond MR, Hanover JA. A little sugar goes a long way: The cell biology of O-GlcNAc. J. Cell Biol. 2015;208:869–880. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joubert M, et al. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes. 2017;66:1030–1040. doi: 10.2337/db16-0733. [DOI] [PubMed] [Google Scholar]
- 35.Maeda K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu I, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J. Clin. Investig. 2010;120:1506–1514. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umbarawan Y, et al. Myocardial fatty acid uptake through CD36 is indispensable for sufficient bioenergetic metabolism to prevent progression of pressure overload-induced heart failure. Sci. Rep. 2018;8:12035. doi: 10.1038/s41598-018-30616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Facundo HT, et al. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2122–2130. doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials are available upon request.