Abstract
Research on avian sex determination has focused on the chicken. In this study, we established the utility of another widely used animal model, the Japanese quail (Coturnix japonica), for clarifying the molecular mechanisms underlying gonadal sex differentiation. In particular, we performed comprehensive gene expression profiling of embryonic gonads at three stages (HH27, HH31 and HH38) by mRNA-seq. We classified the expression patterns of 4,815 genes into nine clusters according to the extent of change between stages. Cluster 2 (characterized by an initial increase and steady levels thereafter), including 495 and 310 genes expressed in males and females, respectively, contained five key genes involved in gonadal sex differentiation. A GO analysis showed that genes in this cluster are related to developmental processes including reproductive structure development and developmental processes involved in reproduction were significant, suggesting that expression profiling is an effective approach to identify novel candidate genes. Based on RNA-seq data and in situ hybridization, the expression patterns and localization of most key genes for gonadal sex differentiation corresponded well to those of the chicken. Our results support the effectiveness of the Japanese quail as a model for studies gonadal sex differentiation in birds.
Subject terms: Development, Gene expression
Introduction
In birds, males are homogametic (ZZ) and females are heterogametic (ZW). The sex determination mechanism involving gene(s) on the sex chromosome is highly conserved among birds. In chickens, sex determination is thought to occur after embryonic day (E) 4.5, corresponding to Hamburger and Hamilton stage1 24 (HH24). After sex determination, the gonads differentiate to testes or ovaries according to the sex chromosomal constitution of cells. However, until around E6–6.5 (HH29), the gonads are considered “bipotential,” indicating that they are able to differentiate into either testes or ovaries. At E6–6.5 (HH29), gonads begin morphological differentiation into testes in ZZ embryos or ovaries in ZW embryos. Sex-specific gonadal morphology emerges at this time, and genes important for ovary or testis development show sex-biased expression.
Genes involved in gonadal sex differentiation have been analysed extensively in chickens2,3. Several key genes involved in mammalian gonadal sex differentiation are conserved in chickens, including genes up-regulated in males (e.g., doublesex and mab-3-related transcription factor 1 (DMRT1), sex-determining region Y-box 9 (SOX9), anti-Mullerian hormone (AMH) and hemogen (HEMGN)), genes upregulated in females (e.g., forkhead box L2 (FOXL2) and cytochrome P450 family 19 subfamily A member 1 (CYP19A1, also known as aromatase)), and genes upregulated in both sexes (e.g., nuclear receptor subfamily 5 group A member 1 (NR5A1, also known as SF-1))4–15. Gene expression has been evaluated in embryonic gonads from E4.5 (HH24) to E6 (HH29) by RNA-sequencing in the chicken16,17. These data indicated that over 1,000 genes are transcribed in a sex-biased manner at E6.5 (HH29), and the majority of these become biased between E4.5 (HH24) and E6 (HH29). Novel genes and pathways that are activated in a sex-specific manner at the time of gonadal sex differentiation have been identified, emphasizing the utility of the RNA-seq approach.
The chicken is a common animal model. A number of transgenic chickens have been generated by retroviral infection18–21 and transposon systems22,23. For several genes involved in gonadal sex differentiation, over-expression or knockdown experiments have been performed4,5,8–10,24,25. Genetic editing tools, such as transcription activator-like effector nuclease (TALEN) and clustered regulatory interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CRISPR/Cas9), have been applied to modify the chicken genome. Null mutants have been generated for two egg white genes, ovalbumin and ovomucoid26,27, as well as DEAD-box helicase 4 (DDX4; also known as vasa), which is essential for the proper formation and maintenance of germ cells28. However, these methods are dependent on germline-mediated chimerism, which is time-consuming because individuals in the first generation are always mosaic. In addition, the introduction of mutations in female-specific genes on the W chromosome is nearly impossible because female-derived primordial germ cells are difficult to culture29,30.
We propose that the Japanese quail (Coturnix japonica) is a useful model for studies of the molecular mechanisms underlying gonadal sex differentiation. The Japanese quail is a small species in the family Phasianidae and is a well-established animal model for biological research. The Japanese quail benefits from its easier handling and shorter generation time than those of other Phasianidae species, such as the chicken. It requires only 16 days to hatch and 6 to 8 weeks of age to reach sexual maturity. In addition, the Japanese quail shows excellent reproductive performance (egg production, fertility and hatchability), comparable or superior to those of the chicken31. The Organization for Economic Co-operation and Development (OECD) recommends the use of the Japanese quail as a model for avian safety assessments32. Developing embryos of the Japanese quail can be easily cultured ex vivo and manipulated. In particular, it is worth noting that in vitro fertilization33,34 and complete culture from the single-cell stage to hatching35 have only been demonstrated in the Japanese quail to date. Current in vitro technologies in the species could be powerful tools for the production of genome-edited animals. Thus, the Japanese quail may be an effective alternative to the chicken as a laboratory research animal.
In this study, we performed comprehensive gene expression profiling of embryonic gonads at HH27 (just after sex determination and before morphological differentiation between ZZ and ZW embryonic gonads), HH31 (after morphological differentiation) and HH38 (proceeding differentiation) in the Japanese quail by mRNA-seq. We further classified genes according to the extent of change between stages and performed GO analyses to establish the potential functions of these genes in reproduction. Our results demonstrate that expression profiling using RNA-seq data for embryonic gonads is effective for the identification of novel candidate genes involved in gonadal sex differentiation. Further analyses indicated that the expression levels and localization of key genes for gonadal sex differentiation were consistent with those in chicken embryos, except for HEMGN. Based on our findings, the Japanese quail is an effective model for the identification of novel genes involved in gonadal sex differentiation.
Results
Gene expression profiling
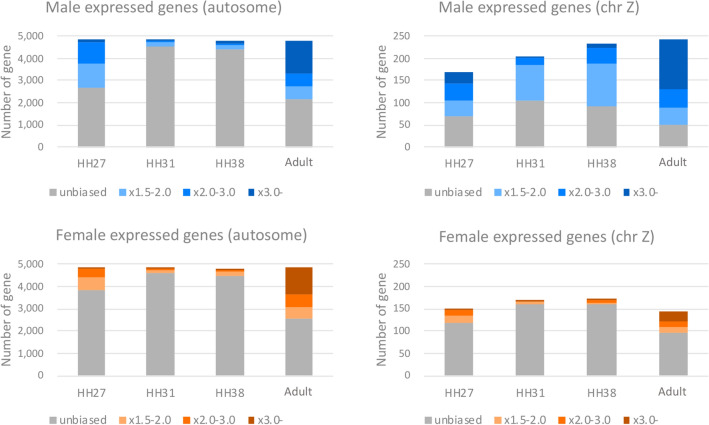
We conducted RNA-seq analysis focusing on changes in the expression level of genes. Total RNAs extracted form gonads of over than five individuals of each stage and sex were used. The RNA-seq data were used to obtain gene expression profiles for Japanese quail gonads at four developmental time points in males and females: HH27 (just after sex determination and before morphological differentiation between ZZ and ZW embryonic gonads), HH31 (after morphological differentiation), HH38 (proceeding differentiation) and adult. An MA-plot of TMM normalized FPKM is shown in Fig. S1. To confirm trends of gene expression patterns at each of the stages and chromosomes, the top 5,000 genes with high FPKM value at each samples are shown in Fig. 1, Fig. S2 and Table S1. We classified the top 5,000 genes into the following four groups according to fold change between sexes at the same stage: unbiased (- × 1.5 fold change), × 1.5–2.0, × 2.0–3.0 and × 3.0- fold change. A proportion of genes with fold-change × 2.0- on the autosomes was confirmed to be higher in the adult testis (43.1%) and ovary (36.6%) than at the other three early developmental time points. We also confirmed that the tendency did not change even if the threshold value of fold-change (× 1.5 or × 3.0) was changed. In males, genes located on the Z chromosome showed a similar pattern to that of autosomal genes, and the proportion of Z chromosomal genes with fold-change × 2.0- was higher than that on the autosomes in all developmental stages. It was also confirmed that the proportion of genes with fold change × 2.0- (× 1.5- and × 3.0, too) on chromosome Z is higher in males than in females at all stages. In particular, genes with fold-change × 2.0- accounted for 63.8% and 23.6% of Z chromosomal genes in adult males and females, respectively, and likewise genes with fold-change × 1.5- accounted for 79.4% and 34.0%, respectively. The top 3,000 and 10,000 genes showed similar features as the top 5,000 genes (Table S2 and S3). We also confirmed that similar results were obtained using GFOLD and isoDE2, which are designed for gene expression analysis using RNA-seq data without biological replicates (Figs. S3 and S4).
Figure 1.
Top 5,000 genes with high FPKM value in male (ZZ) and female (ZW) at the following four stages: embryonic gonads at HH27, HH31, and HH38, and the adult testis or ovary. Bar graphs were showed separately for autosomes (left side) or the Z chromosome (right side). Genes were classified into the following four groups according to fold change between sexes at the same stage: unbiased (grey), × 1.5–2.0 fold change (light blue for male and light orange for female, respectively), × 2.0–3.0 (blue and orange), and × 3.0- (dark blue and brown).
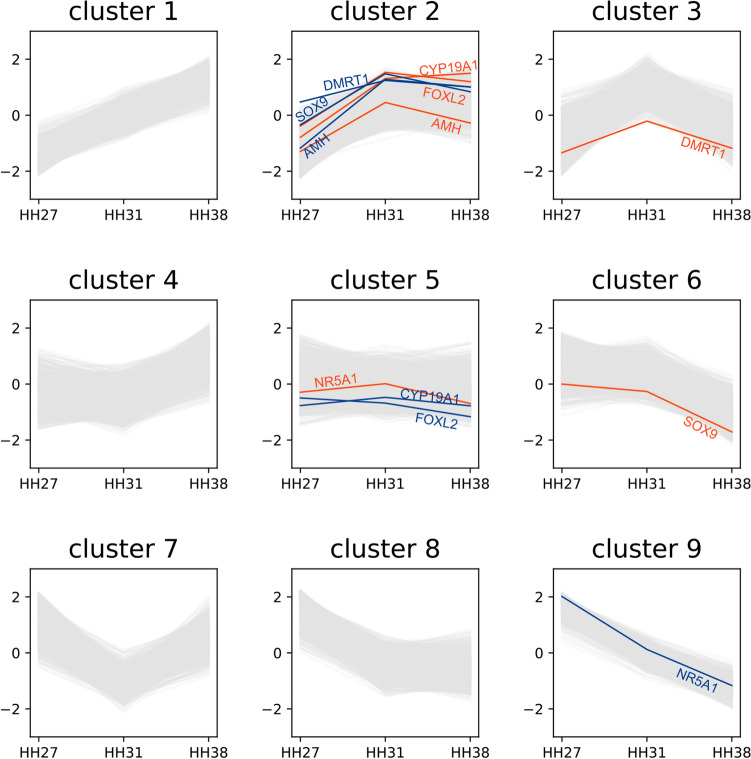
Focusing on the 4,815 genes with TMM normalized FPKM values of ≥ 30, we classified expression patterns into nine clusters according to the extent of change in two comparisons, HH27–HH31 and HH31–HH38 (Table 1 and Fig. 2; 3 × 3 clusters). Cluster 2 (up–flat), including 495 and 310 genes expressed in males and females, respectively, contained several genes involved in gonadal sex differentiation, including DMRT1, SOX9 and AMH in males (blue lines in cluster 2, Fig. 2), and FOXL2 and CYP19A1 in females (orange lines in cluster 2, Fig. 2). These genes displayed a common trend in which the expression level increased during HH27–HH31 and remained constant thereafter. Among the genes classified into cluster 2, those whose fold-change between males and females was ≥ 2 at HH31 comprised 52 genes in males (Table S4) and 40 genes in females (Table S5). NR5A1, which activates CYP19A1 in the chicken ovary, was assigned to cluster 5 (flat–flat) for females. HEMGN, involved in testis differentiation in the chicken, was excluded from the clustering analysis owing to its low expression level in the Japanese quail.
Table 1.
Gene expression pattern in embryonic gonads of Japanese quail.
| Cluster | Expression pattern | Number of genes | Genes known to be involved in sex differentiation | ||||
|---|---|---|---|---|---|---|---|
| HH27-31 | HH31-38 | Male | Female | Total | Male | Female | |
| Cluster 1 | ↗ | ↗ | 320 | 192 | 512 | ||
| Cluster 2 | ↗ | → | 495 | 310 | 805 | DMRT1, SOX9, AMH | FOXL2, CYP19A1, AMH |
| Cluster 3 | ↗ | ↘ | 558 | 145 | 703 | DMRT1 | |
| Cluster 4 | → | ↗ | 363 | 974 | 1,337 | ||
| Cluster 5 | → | → | 139 | 1,353 | 1,492 | FOXL2, CYP19A1 | NR5A1 |
| Cluster 6 | → | ↘ | 562 | 1,282 | 1,844 | SOX9 | |
| Cluster 7 | ↘ | ↗ | 507 | 178 | 685 | ||
| Cluster 8 | ↘ | → | 1,090 | 256 | 1,346 | ||
| Cluster 9 | ↘ | ↘ | 781 | 125 | 906 | NR5A1 | |
| Total | 4,815 | 4,815 | 9,630 | ||||
Figure 2.
Expression patterns of nine clusters during gonadal sex differentiation. Between HH27 and HH31, gene expression levels increased in clusters 1, 2 and 3, remained constant in clusters 4, 5 and 6, and decreased in clusters 7, 8 and 9. Similarly, between HH31 and HH38, gene expression levels increased in clusters 1, 4 and 7, remained the same in clusters 2, 5 and 8, and decreased in clusters 3, 6 and 9. Blue and orange lines indicate genes related to gonadal differentiation in males (ZZ) and females (ZW), respectively.
GO analysis
To investigate the functional characteristics of the 92 genes with expression differences between males and females in cluster 2, we performed a GO analysis. In total, 13,243 of the 16,037 protein-coding genes of the Japanese quail were annotated to one or more GO terms. The criteria for significance were FDR < 0.05 and a hierarchical depth of up to three. Focusing on the biological process domain, 92 genes (52 in males and 40 in females) were assessed. GO terms related to developmental processes including reproductive structure development and developmental processes involved in reproduction were significant (Table 2).
Table 2.
GO terms in the "cluster 2" genes exhibiting different expression levels between males and females.
| # GO | Term | Ratio_in_study | Ratio_in_pop | Depth | p_fdr |
|---|---|---|---|---|---|
| GO:0,048,608 | reproductive structure development | 7/92 | 99/20,132 | 3 | 0.000 |
| GO:0,048,856 | anatomical structure development | 21/92 | 1279/20,132 | 2 | 0.002 |
| GO:0,032,502 | developmental process | 26/92 | 2064/20,132 | 1 | 0.002 |
| GO:0,003,006 | developmental process involved in reproduction | 8/92 | 207/20,132 | 2 | 0.008 |
| GO:0,048,869 | cellular developmental process | 18/92 | 1229/20,132 | 2 | 0.014 |
| GO:0,050,793 | regulation of developmental process | 17/92 | 1116/20,132 | 3 | 0.014 |
| GO:0,051,239 | regulation of multicellular organismal process | 18/92 | 1291/20,132 | 3 | 0.020 |
Genes with dimorphic expression involved in gonadal sex differentiation
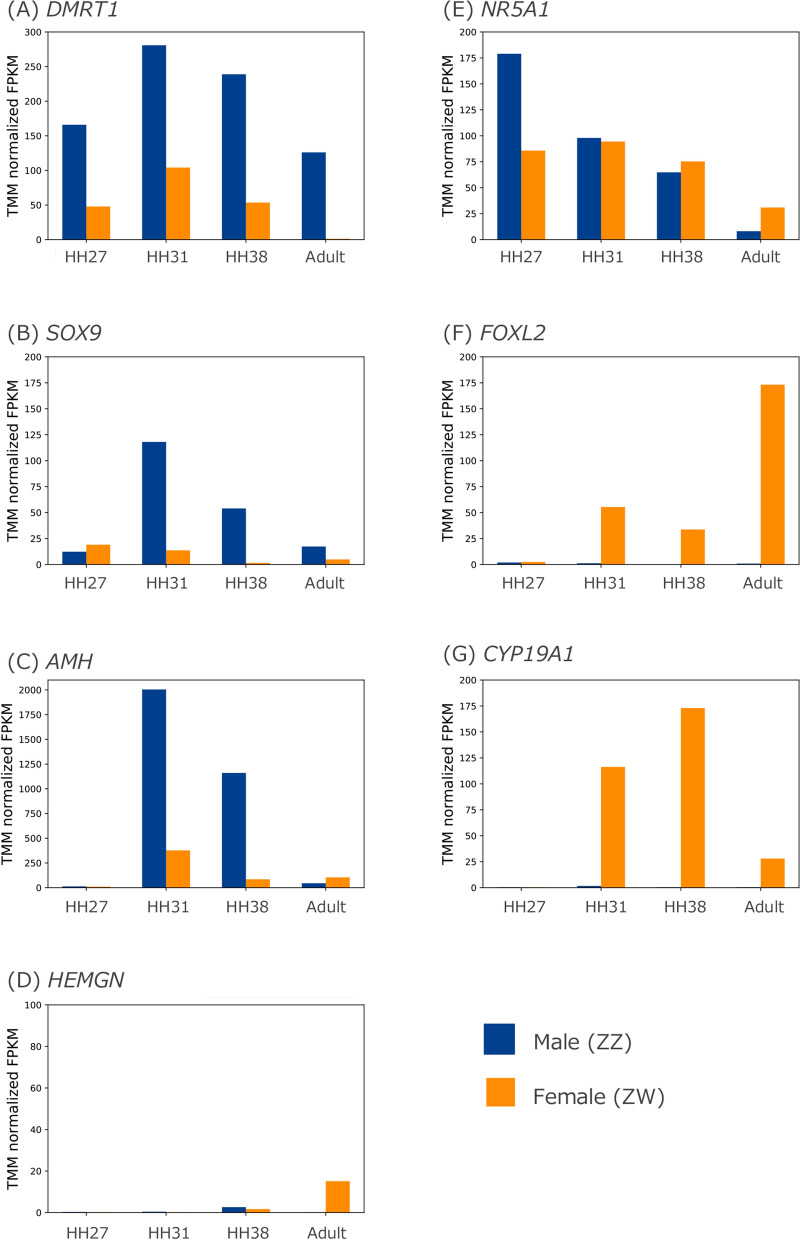
We analysed the expression patterns of seven genes involved in gonadal sex differentiation, DMRT1, SOX9, AMH, HEMGN, NR5A1, FOXL2 and CYP19A1, using RNA-seq data. We obtained RNA-seq data from the embryonic gonads of the Japanese quail at HH27, HH31 and HH38 and from the testis and ovary of an adult for comparisons between males (ZZ) and females (ZW). FPKM values normalized using the TMM method for seven genes were evaluated (Fig. 3).
Figure 3.
Comparison of expression patterns of seven genes between male (ZZ) and female (ZW) gonads using TMM normalized FPKM values. The expression patterns of DMRT1 (A), SOX9 (B), AMH (C), HEMGN (D), NR5A1 (E), FOXL2 (F) and CYP19A1 (G) are shown based on TMM normalized FPKM values in embryonic gonads at HH27, HH31, HH38 and the adult testis or ovary. Blue and orange bars indicate males (ZZ) and females (ZW), respectively.
DMRT1 expression was detected at HH27 and reached a peak at HH31 in males and females (Fig. 3A). Throughout all examined stages, expression levels were higher in males than in females. DMRT1 expression remained high in adult males, unlike the other six genes, which decreased over time. SOX9 expression was slightly higher in females than in males at HH27 (Fig. 3B). Beyond HH27, expression increased in males but remained at extremely low levels in females. Obvious expression of AMH was not detected at HH27; however, expression increased dramatically in males after HH31 (Fig. 3C). In adults, although the AMH expression level was low, it was higher in females than in males. Although HEMGN expression was slightly higher in males than in females at HH38, it was quite low at all embryonic stages in both sexes (Fig. 3D). In adults, HEMGN expression was only detected in females.
NR5A1 expression was approximately two-fold higher in males than in females at HH27 (Fig. 3E). NR5A1 expression was similar in males and females at HH31, since expression in males decreased. In adults, the expression pattern was reversed, with higher levels in females than in males. FOXL2 and CYP19A1 showed female-specific expression throughout all examined stages (Fig. 3F and G). A peak of FOXL2 expression was detected at embryonic stage HH31, and the highest expression among all stages was observed in adult females. CYP19A1 expression was higher than FOXL2 expression during embryonic stages, with a peak at HH38. Adult females showed low expression of CYP19A1.
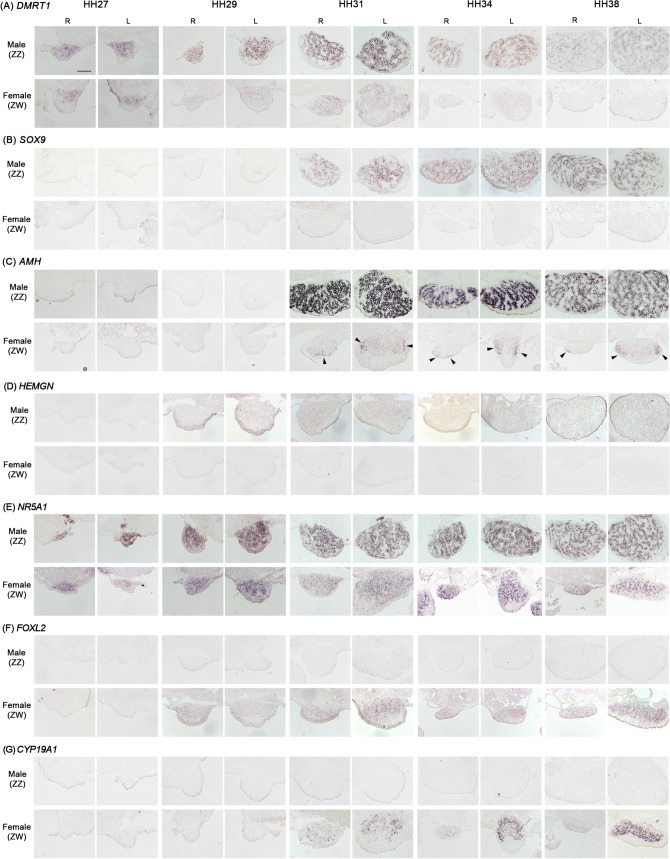
We used in situ hybridization to confirm the localization of these seven genes in Japanese quail embryonic gonads at five developmental stages, HH27, HH29, HH31, HH34 and HH38. DMRT1 signals were localized to the gonadal medulla of males (ZZ) at all examined stages (Fig. 4A) and in the gonadal medulla of females (ZW) from HH27. However, the signals were weaker in females than in males and almost undetectable after HH34. SOX9 signals were detected in the gonadal medulla of males from HH31 (Fig. 4B) and were not detected in female gonads at any stages. A similar pattern was observed for AMH hybridization in male gonads (Fig. 4C); however, the signals in males were the strongest among the three male-biased genes. Although the signals were weak in female gonads at all stages, expression was detected at HH31, 34 and 38 (arrowheads in Fig. 4C). In HEMGN, quite weak signals were observed in males from HH29 to HH38 (Fig. 4D). DMRT1 showed the earliest expression (from HH27) among all four male-biased genes.
Figure 4.
Expression patterns of seven genes in embryonic gonads of the Japanese quail. Expression patterns of DMRT1 (A), SOX9 (B), AMH (C), HEMGN (D), NR5A1 (E), FOXL2 (F) and CYP19A1 (G) were examined by in situ hybridization in frozen gonad sections of ZZ (male) and ZW (female) at HH27, HH29, HH31, HH34 and HH38. R and L indicate right and left gonads, respectively. Scale bar indicates 100 µm.
Relatively strong NR5A1 signals were observed in the gonadal medulla of males and females at all examined stages (Fig. 4E). Two female-biased genes, FOXL2 and CYP19A1, showed female-specific expression (Fig. 4F and G, respectively). FOXL2 was detected earlier than CYP19A1 (i.e., HH29 and HH31, respectively). The signals of both genes were localized to the gonadal medulla of females, but CYP19A1 expression was stronger than FOXL2 expression. Overall, the mRNA expression and localization patterns were consistent with the patterns reported in chicken embryos, with the exception of HEMGN.
Discussion
Genes involved in gonadal sex differentiation show sex-biased expression and regulate testicular or ovarian development. Several of these genes have been evaluated in knockout mouse models and in humans with disorders of sex development. However, gonadal sex differentiation is not well-understood and likely involves a number of unknown genes. In birds, the molecular mechanism underlying gonadal sex differentiation has implications for poultry breeding. We found that embryonic gonads at HH27 (just after sex determination) show the highest percentage of genes with sex-biased expression on the autosomes in the three embryonic stages. Many unknown genes are differentially expressed to promote gonadal differentiation after sex determination. The number of Z chromosomal genes showed male-biased expression was higher than that on the autosomes in all stages. We also confirmed that male-biased expression genes distributed throughout the Z chromosome, and was not biased to small regions (Fig. S5). This indicates that Z chromosomal genes are not completely dosage compensated in birds. Previous studies have reported that the mean expression levels of Z-linked genes are 1.6-fold higher in males than in females36–38. A similar result has been obtained in an RNA-seq analysis of blastoderms and embryonic gonads of chickens15. We could not identify W genes owing to insufficient sequence information for the Japanese quail. The enrichment of genome sequence information for the W chromosome of the species should be a focus of research.
We classified expression patterns into nine clusters according to the extent of change between HH27 and HH31 and between HH31 and HH38. Five genes involved in gonadal sex differentiation, DMRT1, SOX9 and AMH in males and FOXL2 and CYP19A1 in females, were assigned to cluster 2 (up–flat). These results indicate that genes involved in gonadal sex differentiation tend to increase after sex determination (HH27–HH31) and remain at a constant level during subsequent sex differentiation (HH31–HH38). Genes in cluster 2 were associated with GO terms related to developmental processes, including reproductive structure development, anatomical structure development, developmental process, developmental processes involved in reproduction, cellular developmental process and regulation of developmental process. Our expression profiling approach using RNA-seq data for embryonic gonads was effective for the identification of candidate genes not known to function on gonadal sex differentiation. In particular, the genes classified into cluster 2 (52 and 40 genes in males and females, respectively) (Table S4 and S5) are candidates for further functional assays.
The best candidate master testis-determining gene is Z-linked DMRT1 in birds lacking mammalian sex determinant Sry/SRY (sex-determining region Y). The chicken homolog of DMRT1 is expressed more highly in undifferentiated gonads of males than in females, and a high expression level is maintained in male gonads during testis development. In the Japanese quail, we also detected higher DMRT1 expression in male embryonic gonads at HH27, which was the earliest expressed male-biased genes, and the expression continued after HH27. These results indicate that DMRT1 is a conserved gene with key functions in testis determination in the Japanese quail. We also observed male-biased expression of SOX9 and AMH in embryonic gonads after HH31 in the Japanese quail. In placental mammals, SRY directly activates Sox9/SOX9 by binding to the Sox9/SOX9 enhancer together with NR5A1 in the undifferentiated gonads of XY embryos39. Subsequently, SOX9 directly regulates Amh/AMH expression by binding to the Amh/AMH proximal promoter together with other transcription factors40–47. However, in chickens, there is a time-lag between the initial expression of DMRT1 and SOX9, which occur at days 3.5 and 6.5, respectively48. Furthermore, AMH mRNA is expressed prior to SOX9 mRNA7. Therefore, other factors, probably chicken (birds)-specific factors, must be components of the molecular cascade between DMRT1 and SOX9. We further found that SOX9 is expressed later than DMRT1 and AMH in the Japanese quail, indicating that the molecular cascade between these genes is conserved in the two species.
By contrast, we did not detect significant HEMGN expression in Japanese quail gonads. HEMGN is located on the Z chromosome and was first reported as a chicken-specific factor for testis differentiation by mediating SOX9 regulation under DMRT110. In mice, Hemgn (also known as EDAG in humans) is a hematopoietic tissue-specific gene encoding a nuclear protein49. Although the gene is not expressed in the gonads during embryogenesis in mammals, HEMGN is expressed in hematopoietic tissues and in early embryonic gonads of male chickens10. In ZW embryonic gonads masculinized by aromatase inhibitor treatment, HEMGN expression is induced. ZW embryos overexpressing HEMGN have masculinized gonads with increases in the male marker genes DMRT1 and SOX9 and decreases in the female marker genes aromatase and FOXL2. Furthermore, the distribution of germ cells shows a testis-like pattern. These findings suggest that HEMGN is specifically involved in gonadal differentiation in the chicken. However, HEMGN mRNA expression has not been detected in embryonic gonads of the emu and zebra finch50. We confirmed a lack of detectable HEMGN expression in the Japanese quail, belonging to the order Galliformes, along with the chicken, although the identity of mRNA sequences of HEMGN is 86.24% between chicken (XM_430508) and Japanese quail (XM_032441476). Accordingly, the role of this gene in gonadal sex differentiation could be limited to the chicken and closely related species.
FOXL2 is a conserved gene involved in ovary development in vertebrates13–15. The expression patterns of FOXL2 and CYP19A1 are highly correlated in the developing ovary in chickens12. CYP19A1 is also important for ovary development because it encodes an enzyme (aromatase) responsible for converting androgens to estradiol. FOXL2 is expressed just prior to CYP19A1, suggesting that it directly or indirectly regulates aromatase transcription in chickens. We observed nearly identical expression patterns in the Japanese quail. FOXL2 was initially detected at HH29, before the expression of CYP19A1. These results indicate that the functions of two female-biased genes in ovary development are conserved in the Japanese quail. All examined genes other than HEMGN showed similar expression and localization patterns in embryonic gonads to those in the chicken, suggesting functional conservation. Our results confirm that these genes can be used as molecular markers for studies of gonadal sex differentiation in the Japanese quail. More broadly, our gene profiling and expression analyses are expected to contribute to the identification of novel candidate genes involved in gonadal sex differentiation in birds.
Materials and methods
Animals and ethics statement
Fertilized Japanese quail eggs were purchased from Motoki Corporation (Saitama, Japan). Fertilized eggs were incubated at 37.5 °C. The sex of each embryo was determined by PCR genotyping using genomic DNA as the template51. The developmental stage of each embryo was determined based on a previous report by Ainsworth et al.52.
All animal experiments described in this study were approved by the Institutional Animal Care and Use Committee of National University Corporation Hokkaido University and were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals issued by Hokkaido University. This study did not involve any human participants or specimens.
RNA extraction and cDNA synthesis
Total RNA was extracted from embryonic gonads of the Japanese quail at HH27, HH31, HH38, and adult testis and ovary using an RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. We used left gonads of embryo and left testes and ovaries of adult, because Japanese quail shows asymmetric development of gonads that right gonads are gradually depress and fail to develop in ZW female. RNA was treated with DNase I and reverse-transcribed using SuperScript III reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA) and an oligo(dT) primer.
RNA-seq
Total RNA was quantified using a Bioanalyzer and RNA 6000 Nano Kit (Agilent, Santa Clara, CA, USA). Total RNA with an RIN value exceeding 9.7 was used for subsequent experiments. Using 200 ng of total RNA as an input, the libraries for RNA-seq were prepared using the SureSelect Strand-Specific RNA Library Preparation Kit (Agilent) according to the manufacturer’s protocol. Then, 100-bp paired-end reads were obtained using the HiSeq3000 platform (Illumina, San Diego, CA, USA). Sequencing data have been deposited in the DDBJ Sequence Read Archive under accession numbers DRA007208 and DRA008738.
Gene expression profiling
Total RNAs were extracted from gonads of over than five individuals of each stage and sex. We conducted RNA-seq analysis using the extracted RNAs focusing on changes in the expression level of genes. Firstly, for the following analyses, low-quality and adaptor sequences in RNA-seq reads were removed using Platanus_trim v1.0.7 (https://platanus.bio.titech.ac.jp/pltanus_trim). Fragments per kilobase of transcript per million mapped reads (FPKM) were estimated using RSEM (1.3.0)53. We used RefSeq Japanese quail genome (Coturnix japonica 2.1, GCF_001577835.1) eliminating a mitochondrial sequence as a reference genome. In RSEM, RNA-seq reads were mapped to the reference genome using bowtie2-2.3.4 (–bowtie2-mismatch-rate 0.03)54. Next, to enable make comparisons of FPKM values between samples, we applied the trimmed mean of M-values (TMM) normalization method for FPKM values. The TMM normalized FPKM values were calculated using TCC (3.9)55. To confirm gene expression patterns at each of the stages or at autosome and chromosome Z, we classified top 5,000 genes with high FPKM value into the following four groups according to fold change between sexes at the same stage: unbiased (- × 1.5 fold change) and × 1.5–2.0, × 2.0–3.0 and × 3.0- fold change. To confirm statistically supported gene expression and fold changes between sexes, we also ran two existing tools, GFOLD v1.1.456 and isoDE2 v1.1.557 with default parameters. The two tools were designed for gene expression analysis using RNA-seq data without biological replicates.
Genes were classified according to their expression patterns at three stages (HH27, HH31 and HH38). The target genes for classification had ≥ 30 TMM normalized FPKM in at least one of six samples (males and females at three stages). The TMM normalized FPKM values were pre-processed according to the common practice of applying quantile normalization, taking the logarithm of data values and converting z-scores. To confirm differences of expression pattern between males and females, we normalized FPKM values of males and at once females for each gene. Based on the difference in the normalized values between HH27 and HH31, the genes in each sex were divided into three by k-means clustering using Euclidean distance (conducted by sklearn library v0.19.2, Python v3.7.0). By this clustering, the differences between the two stages were classified into the following three patterns: increase (up), no change (flat) and decrease (down). Similarly, classification was also performed between HH27 and HH38, and the genes were finally classified into nine groups according to the combination of the differences between HH27 and HH31 and between HH31 and HH38.
Gene ontology (GO) analysis
To obtain GO terms associated with Japanese quail genes, GO terms for homologous RefSeq chicken genes (GCF_000002315.4) were obtained. GO terms predicted using InterProScan (5.33–72.0)58 for Japanese quail protein sequences were merged with the previous GO terms obtained for chicken genes. Next, a GO enrichment analysis was conducted using goatools (0.9.5)59. Enriched GO terms with a false discovery rate (FDR) of < 0.05 and hierarchical depth of up to three in a sub-ontology, biological process (BP), were obtained.
In situ hybridization
A fragment of each gene was amplified by RT-PCR using cDNA obtained from gonads as the template. The sequences of primers are listed in Table S6. RNA extraction and cDNA synthesis were performed as described earlier. The CDS sequence of chicken NR5A1 was inserted in a pBluescript SK. The PCR products of other genes were subcloned using the pGEM T-Easy Vector System (Promega, Madison, WI, USA). cDNA clones were labelled using Digoxigenin RNA Labeling Mix (Roche, Basel, Switzerland) and T7, SP6 or T3 RNA polymerase (MAXIscript; Thermo Fisher Scientific). Hybridization to serial frozen sections was performed as described previously23. The incubation temperature was modified to 65–70 °C. Images were captured using a cooled CCD camera (DS-Ri1; Nikon, Tokyo, Japan) mounted on a Nikon ECLIPSE E800 microscope and were analysed using NIS ELEMENTS (Nikon).
Supplementary information
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 16H06279 (PAGS) and 18K19317.
Author contributions
M.O., T.I. and A.K. designed the study; M.O. and T.I. performed informatic analyses; S.M. and S.M. performed sample collection and expression analyses; M.S. and Y.S. performed RNA-seq analyses; M.O., S.M., M.S. and A.K. wrote the manuscript; M.O., S.M., T.I., S.M. and A.K. discussed the results. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Miki Okuno and Shuntaro Miyamoto.
Supplementary information
is available for this paper at 10.1038/s41598-020-77094-y.
References
- 1.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 2.Kuroiwa A. Sex-determining mechanism in avians. Adv. Exp. Med. Biol. 2017;1001:19–31. doi: 10.1007/978-981-10-3975-1_2. [DOI] [PubMed] [Google Scholar]
- 3.Hirst CE, Major AT, Smith CA. Sex determination and gonadal sex differentiation in the chicken model. Int. J. Dev. Biol. 2018;62:153–166. doi: 10.1387/ijdb.170319cs. [DOI] [PubMed] [Google Scholar]
- 4.Smith CA, et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- 5.Lambeth LS, et al. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 2014;389:160–172. doi: 10.1016/j.ydbio.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morais da Silva S, et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat. Genet. 1996;14:62–8. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 7.Oreal E, et al. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev. Dyn. 1998;212:522–532. doi: 10.1002/(SICI)1097-0177(199808)212:4<522::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 8.Lambeth LS, et al. Anti-müllerian hormone is required for chicken embryonic urogenital system growth but not sexual differentiation. Biol. Reprod. 2015;93:138. doi: 10.1095/biolreprod.115.131664. [DOI] [PubMed] [Google Scholar]
- 9.Lambeth LS, et al. Overexpression of anti-Müllerian hormone disrupts gonadal sex differentiation, blocks sex hormone synthesis, and supports cell autonomous sex development in the chicken. Endocrinology. 2016;157:1258–1275. doi: 10.1210/en.2015-1571. [DOI] [PubMed] [Google Scholar]
- 10.Elbrecht A, Smith RG. Aromatase enzyme activity and sex determination in chickens. Science. 1992;255:467–470. doi: 10.1126/science.1734525. [DOI] [PubMed] [Google Scholar]
- 11.Govoroun MS, et al. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev. Dyn. 2004;231:859–870. doi: 10.1002/dvdy.20189. [DOI] [PubMed] [Google Scholar]
- 12.Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–3243. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- 13.Wang DS, et al. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol. Endocrinol. 2007;21:712–725. doi: 10.1210/me.2006-0248. [DOI] [PubMed] [Google Scholar]
- 14.Pisarska MD, Barlow G, Kuo FT. Minireview: roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. Endocrinology. 2011;152:1199–1208. doi: 10.1210/en.2010-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayers, K. L. et al. RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome. Genome Biol. 14; R26 (2013). [DOI] [PMC free article] [PubMed]
- 16.Ayers KL, et al. Identification of candidate gonadal sex differentiation genes in the chicken embryo using RNA-seq. BMC Genom. 2015;16:704. doi: 10.1186/s12864-015-1886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salter D, et al. Gene insertion into the chicken germ line by retroviruses. Poult. Sci. 1986;65:1445–1458. doi: 10.3382/ps.0651445. [DOI] [PubMed] [Google Scholar]
- 18.Bosselman RA, et al. Germline transmission of exogenous genes in the chicken. Science. 1989;243:533–535. doi: 10.1126/science.2536194. [DOI] [PubMed] [Google Scholar]
- 19.Salter D, Crittenden L. Artificial insertion of a dominant gene for resistance to avian leukosis virus into the germ line of the chicken. Theor. Appl. Genet. 1989;77:457–461. doi: 10.1007/BF00274263. [DOI] [PubMed] [Google Scholar]
- 20.Harvey AJ, Ivarie R. Validating the hen as a bioreactor for the production of exogenous proteins in egg white. Poult. Sci. 2003;82:927–930. doi: 10.1093/ps/82.6.927. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Lin C, Wang X. PiggyBac transgenic strategies in the developing chicken spinal cord. Nucleic Acids Res. 2009;37:e141. doi: 10.1093/nar/gkp686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park TS, Han JY. PiggyBac transposition into primordial germ cells is an efficient tool for transgenesis in chickens. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9337–9341. doi: 10.1073/pnas.1203823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakata T, Ishiguro M, Aduma N, Izumi H, Kuroiwa A. Chicken hemogen homolog is involved in the chicken-specific sex-determining mechanism. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3417–3422. doi: 10.1073/pnas.1218714110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka R, Izumi H, Kuroiwa A. Androgens and androgen receptor signaling contribute to ovarian development in the chicken embryo. Mol. Cell Endocrinol. 2017;443:114–120. doi: 10.1016/j.mce.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Aduma N, Izumi H, Mizushima S, Kuroiwa A. Knockdown of DDX4 decreases the number of germ cells in male and female chicken embryonic gonads. Reprod. Fertil. Dev. 2018;31:847–854. doi: 10.1071/RD18266. [DOI] [PubMed] [Google Scholar]
- 26.Park TS, Lee HJ, Kim KH, Kim JS, Han JY. Targeted gene knockout in chickens mediated by TALENs. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12716–12721. doi: 10.1073/pnas.1410555111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 2016;6:23980. doi: 10.1038/srep23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor L, et al. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development. 2017;144:928–934. doi: 10.1242/dev.145367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Lavoir MC, et al. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- 30.Naito M, Harumi T, Kuwana T. Long-term culture of chicken primordial germ cells isolated from embryonic blood and production of germline chimaeric chickens. Anim. Reprod. Sci. 2015;153:50–61. doi: 10.1016/j.anireprosci.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 31.EI-Samee, L. D. A., EI-Wardany, I., Ali, N. G. & Abo-EI-Azab, O. M. A. Egg quality, fertility and hatchability of laying quails fed diets supplemented with organic zinc, chromium yeast or mannan oligosaccharides. Intl. J. Poult. Sci.11, 221–224 (2012).
- 32.OECD. Test No. 223: Avian acute oral toxicity test, OECD guidelines for the testing of chemicals, Section 2, OECD Publishing. 2010.
- 33.Olszańska, B., Stepińska, U. & Perry, M. M. Development of embryos from in vitro ovulated and fertilized oocytes of the quail (Coturnix coturnix japonica). J. Exp. Zoo.l292, 580–586 (2002). [DOI] [PubMed]
- 34.Mizushima S, et al. The birth of quail chicks after intracytoplasmic sperm injection. Development. 2014;141:3799–3806. doi: 10.1242/dev.111765. [DOI] [PubMed] [Google Scholar]
- 35.Ono T, et al. A complete culture system for avian trasngenesis, supporting quail embryos from the single-cell stage to hatching. Dev. Biol. 1994;161:126–130. doi: 10.1006/dbio.1994.1014. [DOI] [PubMed] [Google Scholar]
- 36.Itoh Y, et al. Dosage compensation is less effective in birds than in mammals. J. Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold AP, Itoh Y, Melamed E. A bird’s-eye view of sex chromosome dosage compensation. Annu. Rev. Genomics Hum. Genet. 2008;9:109–127. doi: 10.1146/annurev.genom.9.081307.164220. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SO, Mathur S, Hattem G, Tassy O, Pourquie O. Sex-dimorphic gene expression and ineffective dosage compensation of Z-linked genes in gastrulating chicken embryos. BMC Genomics. 2010;11:13. doi: 10.1186/1471-2164-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 40.De Santa Barbara, P. et al. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol. Cell Biol. 18, 6653–6665 (1998). [DOI] [PMC free article] [PubMed]
- 41.Giuili G, Shen W, Ingraham H. The nuclear receptor SF-1 mediates sexually dimorphic expression of Mullerian Inhibiting Substance, in vivo. Development. 1997;124:1799–1807. doi: 10.1242/dev.124.9.1799. [DOI] [PubMed] [Google Scholar]
- 42.Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse mis gene promoter. Cell. 1999;99:409–419. doi: 10.1016/S0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 43.Nachtigal MW, et al. Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell. 1998;93:445–454. doi: 10.1016/S0092-8674(00)81172-1. [DOI] [PubMed] [Google Scholar]
- 44.Johnson PA, Kent TR, Urick ME, Giles JR. Expression and regulation of anti-mullerian hormone in an oviparous species, the hen. Biol. Reprod. 2008;78:13–19. doi: 10.1095/biolreprod.107.061879. [DOI] [PubMed] [Google Scholar]
- 45.Monniaux D, et al. Regulation of anti-Müllerian hormone production in domestic animals. Reprod. Fertil. Dev. 2012;25:1–16. doi: 10.1071/RD12270. [DOI] [PubMed] [Google Scholar]
- 46.Viger R, Mertineit C, Trasler J, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development. 1998;125:2665–2675. doi: 10.1242/dev.125.14.2665. [DOI] [PubMed] [Google Scholar]
- 47.Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 48.Smith CA, Smith MJ, Sinclair AH. Gene expression during gonadogenesis in the chicken embryo. Gene. 1999;234:395–402. doi: 10.1016/S0378-1119(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 49.Yang LV, Nicholson RH, Kaplan J, Galy A, Li L. Hemogen is a novel nuclear factor specifically expressed in mouse hematopoietic development and its human homologue EDAG maps to chromosome 9q22, a region containing breakpoints of hematological neoplasms. Mech. Dev. 2001;104:105–111. doi: 10.1016/S0925-4773(01)00376-8. [DOI] [PubMed] [Google Scholar]
- 50.Hirst CE, et al. Sex reversal and comparative data undermine the W chromosome and support Z-linked DMRT1 as the regulator of gonadal sex differentiation in birds. Endocrinology. 2017;158:2970–2987. doi: 10.1210/en.2017-00316. [DOI] [PubMed] [Google Scholar]
- 51.Fridolfsson AK, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. doi: 10.2307/3677252. [DOI] [Google Scholar]
- 52.Ainworth SJ, Stanley RL, Evans DJR. Developmental stages of the Japanese quail. J. Anat. 2010;216:3–15. doi: 10.1111/j.1469-7580.2009.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun J, Nishiyama T, Shimizu K, Kadota K. TCC: an R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics. 2013;14:219. doi: 10.1186/1471-2105-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng J, et al. GFOLD: a generalized fold change for ranking differentially expressed genes from RNA-seq data. Bioinformatics. 2012;28:2782–2788. doi: 10.1093/bioinformatics/bts515. [DOI] [PubMed] [Google Scholar]
- 57.Mandric I, et al. Fast bootstrapping-based estimation of confidence intervals of expression levels and differential expression from RNA-Seq data. Bioinformatics. 2017;33:3302–3304. doi: 10.1093/bioinformatics/btx365. [DOI] [PubMed] [Google Scholar]
- 58.Jones P, et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klopfenstein DV, et al. GOATOOLS: a python library for gene ontology analyses. Sci. Rep. 2018;8:10872. doi: 10.1038/s41598-018-28948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.