Abstract
Chronic inflammation is a causative factor of many cancers, although it originally acts as a protective host response to the loss of tissue homeostasis. Many inflammatory conditions predispose susceptible cells, most of which are of epithelial origin, to neoplastic transformation. There is a close correlation between digestive tract (DT) cancer and chronic inflammation, such as esophageal adenocarcinoma associated with Barrett’s esophagus, helicobacter pylori infection as the cause of stomach cancer, hepatitis leading to liver cirrhosis and subsequent cancer, and colon cancer linking to inflammatory bowel diseases and schistosomiasis. A prominent feature of malignant transformation of DT tract epithelial cells is their adoption of somatic gene mutations resulting in abnormal expression of proteins that endow the cells with unlimited proliferation as well as increased motility and invasive capabilities. Many of these events are mediated by Gi-protein coupled chemoattractant receptors (GPCRs) including formyl peptide receptors (FPRs in human, Fprs in mice). In this article, we review the current understanding of FPRs (Fprs) and their function in DT cancer types as well as their potential as therapeutic targets.
Keywords: digestive tract cancer, chronic inflammation, formyl peptide receptors
An Overview of Formyl Peptide Receptors
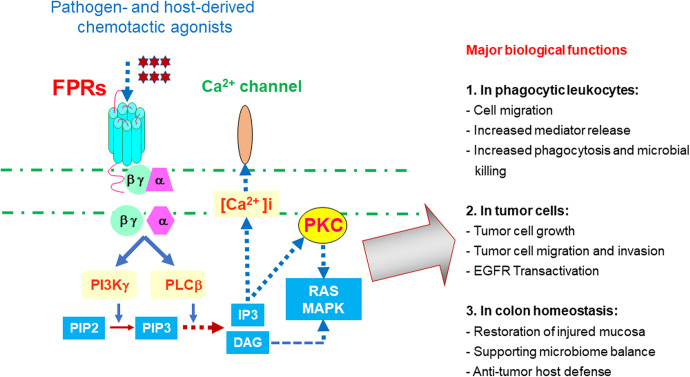
Formyl peptide receptors (FPRs in human, Fprs in mice) are members of the seven transmembrane, G-protein coupled chemoattractant receptor (GPCRs) family, which includes “classical chemoattractant GPCRs” and “chemokine GPCRs,” critical to immune cell trafficking during infection, inflammation, immune responses, and cancer progression,1,2 FPRs (Fprs) belong to the “classical chemoattractant GPCRs,”3 initially identified on neutrophils but have thus far been observed in many cell types including immune cells and cells of the non-hematopoietic origin.4-8 There are three FPRs in human: FPR1, FPR2, and FPR3, which share about 70% identity at the amino acid level. FPR1 responds with high affinity to bacterial and mitochondrial formylated peptides, such as formyl-methionyl-leucyl-phenylalanine (fMLF) produced by Gram- bacteria including E.Coli.4 FPR2 is a more promiscuous GPCR responding to fMLF with lower affinity, but also to a variety of viral, bacterial, host-derived and synthetic peptides, many of which are not formylated and without sequence homology.9 Activation of FPR1 and FPR2 by chemotactic agonists dissociates trimetric G-proteins coupled to the intracellular domains of the receptors, followed by a signaling cascade leading to cell activation (Figure 1).10 Unlike FPR1 and FPR2, the expression of human FPR3 is more restricted to monocytes and probably also dendritic cells (DCs), with only one better defined host-derived, endogenous peptide agonist, F2 L,11 presumably participating in DC recruitment in vivo.
Figure 1.
Signaling and function of FPRs. The binding of FPRs on leukocytes and epithelial cells by pathogen or host-derived agonists triggers Gi-protein-coupled signaling cascade resulting in increased calcium (Ca2+) mobilization, cell migration, mediator release, proliferation and activation of transcription factors involved in new gene transcription. In normal myeloid cells, FPRs (Fprs) mainly mediate cell recruitment, increased phagocytosis, and microbial killing. In colon epithelial cells, FPRs (Fprs) participate in mucosal repair, balance of microbiome, and anti-cancer host defense. Activation of FPR1 in glioblastoma cells transactive the receptor for the epidermal growth factor and two receptors cooperated to exacerbate the malignant phenotype of tumor cells.
The mouse FPR (mFPR or Fpr) gene family consists of at least 8 members including Fpr1 and Fpr2, as well as several genes without definitive cellular products.9 Fpr1 is considered as an orthologue of human FPR1, whereas Fpr2 is structurally and functionally closer to human FPR2.12,13 Although the mouse counterpart of human FPR3 is not well defined, murine Fpr2 shares the human FPR3 ligand F2 L.14,15 and is thus suggested to act as a counterpart of both FPR2 and FPR3.2,16 Table 1 lists major agonists of FPRs (Fprs) better known so far and their capacity to interact with one or more FPRs (Fprs).
Table 1.
Promiscuous Ligand Recognition by FPRs.
| Source Diseases | Agonists | FPR1 | FPR2 | FPR3 |
|---|---|---|---|---|
| (Fpr1) | (Fpr2) | (Fpr3) | ||
| Bacteria Infection | fMLF (E. Coli) | ++++ | ++ | |
| Listeria peptides | ++++ | +++ | ||
| Stomach cancer | Hp(2-20) | - | +++ | ++ |
| Host Inflammation | Serum amyloid A | - | ++++ | |
| Alzheimer’s | Aβ42, Humanin | + | ++++ | |
| Prion disease | Prp106-126 | - | +++ | |
| Angiogenesis | Annexin 1(ANXA1) | ++ | +++ | ++ |
| Tissue damage | Mitochondria pep | +++ | +++ | |
| F2 L (Heme) | - | ++ | ++++ | |
| Coagulation | uPAR frag | - | +++ | |
| Host defense | LL37 (CRAMP) | - | +++ | |
| Cathepsin G | +++ | |||
| Cancer | ANXA1 | ++ | +++ |
+ Denotes the relative potency of the ligands to activate a given FPR References: 1, 2, 16).
The Major Functions of FPRS (FPRS)
One of the most important and well characterized functions of FPRs (Fprs) is their capacity to mediate myeloid cell chemotaxis in response to agonists derived from bacteria, damaged cells and other sources, including also many synthetic peptides. When activated, the receptors also enhance neutrophil phagocytosis of dead tissues and bacteria.17 mediator (such as ROS) generation, NET (neutrophil extracellular trap) formation, and cytokine release.18 These receptors are additionally reported to promote wound healing by recruiting inflammatory cells and maintain gut mucosal integrity (Figure 1).19,20 Therefore, FPRs (Fprs) are critical sentinels of host innate defense. However, like many GPCRs, FPRs (Fprs) also possess the dark side of their function, i.e. promoting cancer progression by their aberrant expression in cancer cells for abnormal growth, survival and invasion.21,22
More recently, a fascinating aspect of the functions of FPRs, thanks to the availability of genetically engineered mice, has emerged, which represents an important conceptual advance in understanding these receptors in disease models from a temporal and spatial point of view. The first piece of evidence was obtained from a mouse model of allergic airway inflammation, in which a chemokine GPCR CCR2 mediates the mobilization of DC precursors from the circulation to the perivascular region in an antigen stimulated, inflamed lung, where the cells lost the functional CCR2 but instead they gained the expression of high levels of Fpr2 to direct the cells across the tissue to peribronchiolar regions, where DC became mature and express a homing chemokine GPCR, CCR6, for eventual cell homing to draining lymph nodes to trigger an adaptive immune response.23 This new paradigm thus positions Fpr2 as an essential player in a sequential DC “chemotaxis signal relay” that also involves chemokine GPCRs (CCR2-Frp2-CCR6) to complete orchestrated host immune responses. This relay of GPCR functions in a stepwise trafficking of DCs was later found to be imitated by neutrophils in at least two models of bacterial infection, namely Listeria and E. coli, both requiring rapid accumulation of neutrophils at the sites of infection for clearance of the invading pathogens.18,24 In both models, neutrophils rapidly accumulate in the liver in response to intravenously injected pathogens in response to bacterial chemotactic peptides that activate both Fpr1 and Fpr2. This Fpr-mediated “first wave” of neutrophil recruitment proves to be vital for the host to establish in a timely manner a defense line that would be later further consolidated by subsequent waves of neutrophils accumulation in response to CXCR2 chemokines elicited by bacterial lipoproteins or LPS through TLRs expressed by liver cells. In addition, in a sterile ear skin wound, a rapid neutrophil “swarming” was initiated by leukotriene B4 that activates its classical chemoattractant GPCR, followed by Fpr2 and CxcR2, a receptor for the mouse IL-8 (CXCL8) analogue CxcL2.25 These novel findings indicate the necessity to take consideration of the co-operation of multiple chemoattractant GPCRs for leukocyte trafficking as host responses to pathogen invasion and tissue injury, in which Fprs are indispensable participants.
The Role of FPRs (FPRS) in the Progression of DT Cancers
Chronic inflammation is a prolonged form of protective host response to tissue damage, which however has also been recognized as a causative factor of many cancers. In fact, inflammation and neoplasia co-develop into “wounds that do not heal.”21 Leukocytes, mainly myeloid cells such as neutrophils, monocytes, macrophages, and eosinophils, produce soluble factors including metabolites of arachidonic acid, cytokines, chemokines, and free radicals, may benefit the development of inflammation-associated cancer.26 A variety of inflammatory conditions promote susceptible cells, such as epithelial cells in the DT, to undergo neoplastic transformation. This is particularly true in segments of the DT in which chronic inflammation is associated with cancers in multiple locations, including helicobacter pylori infection in the development of gastric cancer, esophageal adenocarcinoma associated with reflux esophagitis (Barrett’s esophagus), hepatitis promoting liver cirrhosis and subsequent cancer, and colon cancer linked to inflammatory bowel diseases (IBD, chronic ulcerative colitis and Crohn’s disease) and schistosomiasis.1,21 A prominent feature of malignant transformation of DT epithelial cells is their acquirement of somatic gene mutations culminating in abnormal expression of proteins that enable unlimited cell proliferation, increased motility and invasiveness, many of which are directly mediated by GPCRs, including FPRs (Fprs) (Table 2). In the following chapters, we briefly review the role of FPRs (Fprs) in the carcinogenic process, or in contrast suppression, of DT cancers, in hope to benefit the development of novel anti-cancer strategies.
Table 2.
The Role of FPRs (Fprs) in the Progression of DT Cancer.
| DT cancer | FPRs | Biological function | Refs |
|---|---|---|---|
| OSCC | FPRs | The agonist LL-37 might act as a tumor suppressor, and with its
derivatives inhibit OSCC cell growth by inducing cell
death ANXA1 was implicated as a tumor suppressor in OSCC |
31,32 33-36 |
| EC | FPRs | ANXA 1 expression has a significant correlation to the status of ESCC differentiation | 38 |
| FPRs | No clear information about FPRs in the pathogenesis of EAC | 39,40 | |
| GC | FPR1 | In vitro, FPR1 suppresses GC cell growth | 45-47 |
| Higher FPR1 expression is associated with poor clinical results in patients | 48 | ||
| Up-regulated ANXA1 expression is involved in cancer invasion and lymph node metastasis and were implicated in poor prognosis of patients | 49,50 | ||
| FPR2 | Promotes the invasion and metastasis of GC cells and predicts
the poor prognosis of patients Hp(2-20) promotes migration and proliferation of gastric epithelial cells by interacting with FPR2 in vitro |
51 44 |
|
| LL-37 contributes to the balance between host mucosal defense and H. pylori viability that governs chronic infection | 56-58 | ||
| HCC | FPR1 | Participates in the inflammatory courses in the liver and the progression of HCC | 63-66 |
| PC | FPRs | ANXA1 participates in many pathophysiological processes in PC cells, over-expressed in PC tissues from patients and contributes to the malignant phenotype of PC cells and their metastatic potential through FPR-independent pathways. | 68-71 |
| CRC | FPR1 | A risk factor in the prognosis of CRC | 74 |
| FPR2 | Supports the malignant transformation of colon epithelial cells | 74-76 | |
| Fpr2 | Protects normal colon mucosa from inflammation and carcinogenesis in mice | 77 | |
| FPR2 (Fpr2) | The agonists LL-37/CRAMP directly inhibit tumorigenesis in the colon and maintains colon microbiota balance | 78-84 |
Abbreviations: DT: digestive tract; FPRs (Fprs in mice), formyl peptide receptors; OSCC, oral squamous cell carcinoma; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma; GI, gastrointestinal; ANXA1, annexin 1; GC, gastric cancer; HCC, hepatocellular carcinoma; PC, pancreatic carcinoma; CRC, colorectal cancer.
Oral Squamous Cell Carcinoma (OSCC)
OSCC is an aggressive tumor type that occurs at several sites of the oral mucosa of DT. It is also the most common type of head and neck cancer.27 Despite advances in its treatment, the 5-year survival rate of OSCC wanders at ∼50%, which is mostly due to late diagnosis.28 LL-37 is a neutrophil-derived antimicrobial peptide that mediates chemotaxis of inflammatory cells via activation of FPRs, mainly FPR2 (Table 1).29,30 Interestingly, LL-37 might act as a tumor suppressor in OSCC. Immunohistochemical analyses showed that lower expression of LL-37 in OSCC tissues compared to the normal mucosa tissues is correlated with poor differentiation and increased lymph node metastasis,31 implicating a protection exerted by this anti-microbial peptide. In addition, the regulation of the gene for LL-37 in several oral cancer cell lines involves higher DNA methylation status in the promoter region, potentially the cause of the lower expression of the peptide. It had also been shown that incubation of a C-terminal domain peptide of LL-37 (aa 109-135) induces caspase-independent apoptosis in OSCC SAS-H1 cells but not in human gingival fibroblasts or HaCaT cells.32 Thus, LL-37 and its derivatives inhibit OSCC cell growth by inducing cell death.
The anti-inflammatory protein annexin 1 (ANXA1), a 37-kDa member of the annexin superfamily, is a steroid-regulated protein implicated in certain beneficial activities of glucocorticoids.33 ANXA1 is important in cancer progression because it was released by necrotic glioblastoma cells to stimulate live tumor cells as an autocrine/paracrine factor to stimulate FPR1 for tumor progression.34 However, ANXA1 has been reported to activate both FPR1 and 2 (Fpr1, 2) to exert an anti-inflammatory function.8 In Tca-8113 and SCC-9 cell study, ANXA1 was also found to be a potential tumor suppressor in OSCC.35 These un-orthodox roles of LL-37 and ANXA1 are not entirely in accordance with the conventional conception of FPRs as their receptors. Nevertheless, FPRs do mediate the chemotactic activity of these ligands for tumor cells. Therefore, it is postulated that LL-37 and ANXA1 may elicit skewed signaling cascade via FPRs in OSCC cells, or they may also activate other membrane sensors that transmit growth inhibition signals. This calls for more extensive studies of FPRs in OSCC progression.
Esophageal Cancer (EC)
Esophageal cancer remains an important cause of cancer-related death in under-developed countries, and squamous cell carcinoma (ESCC) is the predominant cancer type.36 The lower expression of the ANXA 1 is correlated with ESCC tumorigenesis.37 Analysis of the protein profiles of 24 pairs of ESCC and adjacent normal epithelia showed a positive correlation between ANXA 1 expression and the status of tumor differentiation. Although most of the upper esophageal cancers are of the squamous cell origin, the incidence of esophageal adenocarcinoma (EAC) of the lower esophageal section has risen rapidly over the past three decades.38 Esophageal adenocarcinoma is a typical model of an inflammation-associated cancer.39 Various proinflammatory states are known to be involved, including obesity and gastro-esophageal reflux disease. Release of proinflammatory mediators such as IL-6, IL-8 (CXCL8) and TGF-β by activated epithelial cells and infiltrating leukocytes promotes esophageal cell transformation, growth and invasion via activation of intracellular signaling pathways involving NF-kB and STAT3. However, there is no clear information about FPRs in the pathogenesis of EAC, which awaits clarification.
Gastric Cancer (GC)
GC or cancer of the stomach is divided into two main classes: gastric cardia cancer (cancer of the top inch of the stomach, where it meets the esophagus) and non-cardia gastric cancer (cancer in all other areas of the stomach).40 GC is the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide.41 Although remarkable achievements in surgical and other therapeutic options have been obtained, the overall 5-year survival rate of GC patients remains low,42 due to the advanced stage at diagnosis and the more vicious malignant nature of invasion and metastasis of the disease.
All members of FPR family, including FPR1, FPR2, and FPR3, are expressed in human GC cells,43 but only the functions of FPR1 and FPR2 have been better understood in GC.
FPR1
The involvement of FPR1 in GC progression has been controversial. It is interesting that FPR1 is found to act as a tumor suppressor by inhibiting angiogenesis in GC xenograft experiments.44 FPR1 silencing (shFPR1) significantly enhanced the growth of GC cell-derived xenograft tumors. This is attributed to the augmented vessel density in tumor stroma and GC cell proliferation in the absence of FPR1.44 The genetic or pharmacologic regulation of FPR1 in GC cells is also achieved by ALOX5/15 expression and production of the SPMs Resolvin D1 (RvD1) and Lipoxin B4 (LXB4) that transduce “anti-inflammatory” signals through the receptor to reduce the rate of GC progression by inhibiting inflammatory and angiogenic processes in the tumor microenvironment.45 To support this notion, Otani et al. reported that a specific FPR1 polymorphism, which causes loss of FPR1 function, is positively correlated with the risk of GC.46 However, opposing results have also been reported in which FPR1 was found to be highly expressed in GC tissues, in particular in stage IV disease, in association with deeper invasion depth and poorer clinical outcome of the patients.47 GC patients with high FPR1 expression in tumors had lower overall survival rates than those with negative/low FPR1 expression. Cox multivariate analysis confirmed that FPR1 expression was an independent prognostic marker for poorer overall survival in GC patients. Thus, FPR1 expression is suggested as a predictor of outcome of GC patients treated with gastrectomy.47 These observations highlight the importance of taking into consideration of pro- versus anti-GC potential of FPR1 in designing treatment schemes.
FPR1 expression was significantly increased when ANXA1 was overexpressed in GC cells.47 ANXA1 expression positively correlated with the invasiveness of human GC cells both in vitro and in vivo. In vivo, ANXA1 regulates GC cell invasion through FPR1-associated extracellular signal regulated kinase/integrin β-1-binding protein pathway.48 Elevated ANXA1 expression was observed in 76 of 135 cases of GC and correlations were found between ANXA1 expression and the depth of organ wall invasion, lymphatic/venous involvement, and node metastasis. Thus, up-regulated ANXA1 expression is implicated in poor prognosis of GC patients,49 and ANXA1, similar to FPR1, is also suggested as as a prognostic biomarker in gastric (and colon) cancer and a potential target for treatment.
FPR2
FPR2 was expressed more frequently in GC cancerous tissues than in adjacent tissues and increased expression levels in cancerous tissues were correlated with the tumorigenesis, metastasis as well as the poor survival of the patients.50
The ability of FPR2 to promote tumorigenesis of GC cells is demonstrated in observation in which both FPR2-knockdown SGC7901 or XN0422 GC cells formed substantially smaller xenograft tumors in nude mice as compared with control mock cells.50
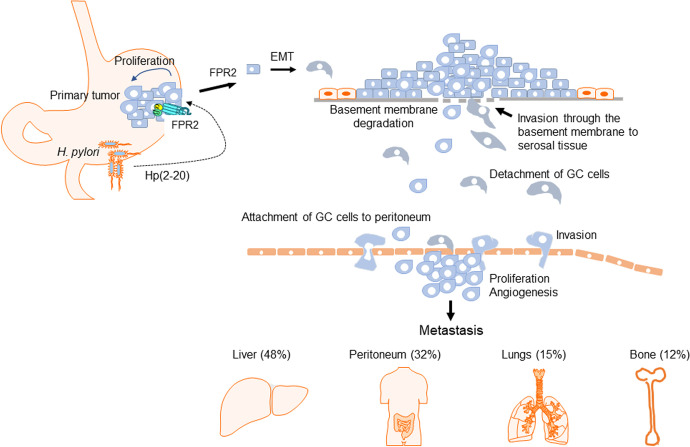
It has also been demonstrated that chronic inflammation resulted from Helicobacter (H) pylori infection is a major step in the initiation and development of GC. H. pylori is a Gram-negative spirochete infecting more than half of the world population, particularly in lesser developed countries, likely due to water contamination and poor living conditions. The bacterium colonizes the stomach of its host, where it attaches to the mucosal epithelia. Infection often persists for the lifetime of the host without treatment that eventually induces carcinogenesis in the stomach.51,52 Hp (2–20), an exogenous ligand of FPR2, is a cecropin-like peptide derived from H. pylori, as a recognized risk factor of GC.53 Hp (2–20) induces the migration and proliferation of GC cells by activating FPR243 that may exacerbate a pro-invasiveness of GC cells.
The ability of FPR2 to promote the invasion and metastasis of GC cells was supported by experiments in vitro and in vivo with GC cell line SGC7901 and primary GC cell XN0422, in which FPR2 deficiency significantly impaired the migratory and invasive potentials induced by FPR2 agonist peptides.50 FPR2 promotes the malignancy of GC cells by induction of epithelial-mesenchymal-transition (EMT) through ERK/MAPK pathways that converts tumor cells into an elongated, motile and invasive phenotype, pivotal for increased aggressiveness of tumor cells. The evidence for FPR2 to mediate EMT in GC cells was supported by the fact that treatment with FPR2 ligands decreased the expression of E-cadherin mRNA but enhanced vimentin, a process partially reversed by FPR2 knockdown, which did not entirely abolish the effect of FPR2 ligands on the expression of EMT-related molecules, possibly due to the presence of the prototype FPR1 in GC cells. Thus, FPR2 could be potentially used not only as a prognostic biomarker but also as a therapeutic target for GC patients. The potential involvement of FPR2 in GC progression is illustrated in Figure 2.
Figure 2.
The role of FPR2 in the progression of GC. H. pylori is a major carcinogenic of factor of human GC. Its infection causes chronic inflammation with abnormal growth of epithelial cells a mechanism of mucosal repairment, which forms the basis for malignant transformation. H. pylori produces an FPR2 agonist peptide HP (2-20) that stimulates GC cell migration, proliferation and EMT. This process results in increased malignancy of GC cells that more rigorously invades and penetrate the organ wall, accompanied by angiogenesis. The motile tumor cells thereafter metastasize through lymphatics and blood vessels to form lethal secondary lesions.
FPR2 ligand LL-37 in GC
H. pylori infection plays a predominant role in the etiology of GC.54 The FPR2 ligand LL-37 (hCAP-18, FALL-39)55,56 was produced by gastric epithelium as well as chief and parietal cells in the fundic glands of normal gastric mucosa. LL-37, up-regulated in H. pylori-infected stomach of the patients, alone or in synergy with human defensin 1, was bactericidal for several H. pylori strains. Thus, LL-37 in the stomach may control the balance between host mucosal defense and H. pylori. Bleach of such a balance constitutes the cause of bacterial overexpansion and the consequent stomach inflammation-carcinogenesis.57
FPR1 in Hepatocellular Carcinoma (HCC)
As a malignancy that emerges from a background of chronic liver diseases,58 HCC is the fourth most common tumor worldwide with an extremely poor prognosis.59 Chronic inflammation and improper healing of hepatic injury are causative factors for cancer in the liver.60 The link between inflammation and liver cancer can be viewed from an extrinsic perspective for which viral infection and subsequent chronic inflammation drive oncogenesis.61 Functional FPR1 is detected in hepatocytes and promotes the production of acute phase proteins by the cells in response to the prototype FPR1 ligand, the bacterial fMLF.62 Thus, FPR1 expressed by hepatocytes may participate in the inflammatory courses in the liver, which acts as a cleaner of toxins in human body. It has been shown that the levels of chemokines in the liver are positively correlated with the severity of hepatic inflammation in chronic hepatitis C virus (HCV) infection63 by attracting the infiltration of leukocytes mainly myeloid cells into the liver.64 Given that HCC is typically an example of inflammation-associated cancer and FPR1 activation promotes the migration and ERK-dependent production of the neutrophil chemokine IL-8 (CXCL8) by HCC cells, FPR1 knockdown in HCC cells substantially reduced their tumorigenicity in nude mice, as an important piece of evidence that its presence in hepatic cells may act in a concerted inflammatory cycle in the liver to exacerbate the carcinoma development and progression.65
FPRs in Pancreatic Carcinoma (PC)
PC is one of the most aggressive GI malignancies, with an extremely low 5-year survival rate of 8%.66 ANXA1 participates in many pathophysiological processes in PC cells, including inhibition of cell proliferation, as well as regulation of cell migration, differentiation and death.67 ANXA1 is over-expressed in PC tissues from patients and despite its potential ability to transduce anti-inflammatory signals, and ANXA1 appears to be associated with malignant transformation and poor prognosis of PC.68 The regulatory activity of extracellular ANXA1 on tumor cells is mediated by FPRs.69 Therefore, ANXA1 may promote the metastasis of PC by favoring tumor cell invasion through its function as a cytoskeleton remodeling factor intracellularly and extracellularly through activation of FPRs on cell surface.68 However, it has also been reported that ANXA1 contributes to the malignant phenotype of PC cells and their metastatic potential through FPR-independent pathways.70 Therefore, ANXA1 as a multifaceted regulator of pathophysiological process in PC cells may exert its detrimental effect through various sensory molecules in the cells, FPRs among them.
FPRs in Colorectal Cancer
Colorectal cancer (CRC) is one of the most deadly cancers that affects a large world population.71 It has been well established that chronic inflammation is a major causative factor.72
FPR1 as a risk factor for poor prognosis of human CRC
Investigation of the expression of FPRs in primary human CRC revealed that the mRNA of FPRs, especially FPR1, was significantly higher in CRC tissues than in distant and adjacent non-tumor tissues, suggesting an association between FPR1 and CRC progression. FPR1 mRNA expression was also correlated with the size of tumors and serosal infiltration of CRC. In addition, FPR1 is found as a risk factor that predicts the prognosis of CRC. Absence of FPR1 increases the survival rate of colitis-associated CRC.73 These observations link FPR1 expressed in CRC cells to a more malignant nature.
FPR2 in exacerbated malignant behavior of human colon cancer cells
Compared to FPR1, FPR2 appears to be a more reliable maker in CRC that rigorously promotes the invasive phenotype of cancer cells.73 FPR2 was preferentially expressed in colon cancer (89%) than in rectal cancer (11%).19 The ability of FPR2 to promote the malignancy of CRC cells was demonstrated by experiments, in which human CRC cell line SW1116, its proliferation, migration, invasion, anti-apoptosis and pro-angiogenesis properties were attenuated by silencing FPR2 with shRNA, resulting in reduced tumorigenicity in xenograft models. Mechanistically, silencing FPR2 reduced the expression of proteins linked to EMT such as E-cadherin, N-cadherin, Snail, Slug and vimentin,74 indicating the capacity of FPR2 to support the malignant transformation of colon epithelial cells.
Fpr2 protection of mouse colon from inflammation and cancer
Colon cancer is believed to be consequences of chronic inflammatory responses of the epithelial layer. Clinically, patients with chronic ulcerative colitis or Crohn’s disease have a five-to seven-fold increase in the risk of developing CRC, as a result of persistent colon inflammation.26 beginning with focal proliferation of dysplastic cells, formation of benign adenomatous polyps, and transformation to malignant adenocarcinomas. It is noteworthy that in contrast to the reported capacity of FPR2 to promote colon cancer progression in human, the murine counterpart Fpr2 demonstrates a unique role in protecting colon mucosa from inflammation and carcinogenesis. This was demonstrated by observations in mice in which Fpr2 deficiency was associated with a prolonged chronic inflammation shown by greatly shortened colon, increased foci of ulcers in damaged mucosa and exacerbated inflammatory cell infiltration in ulcerative lesions, culminating in markedly increased number of adenomas.17 Thus, Fpr2 is essential for limiting the susceptibility of mouse colon to chronic inflammation-induced tumors. In this respect, Fpr2 (FPR2) appears to be a double-edged sword in that if the receptor was aberrantly expressed by transformed human CRC cells, its capacity to mediate normal epithelial migration, proliferation and mucosal restitution is exploited by tumor cells to exacerbate the malignant behavior.
The protective role of FPR2 agonist LL-37 in the colon
LL-37 is expressed by normal colon epithelial cells. It is interesting that unlike normal human colon epithelial cells, CRC cells do not express or only express low levels of LL-37.75 Therefore, the absence of LL-37 is used as one of the biomarkers of human colon cancer.76 FK-16, a fragment of LL-37, corresponding to the aa residues 17 to 32, induces the death of colon cancer cells by activating caspase-independent apoptosis and autophagy,77 suggesting its direct anti-colon cancer activity. The colon carcinoma cell line HCT116 treated in vitro with FF/CAP18, a 27-residue analogue of LL-37, exhibits a marked loss in major metabolic profiles required for the proliferation of cancer cells.78 In addition, LL-37 inhibits EMT of colon cells and fibroblast-supported colon cancer cell proliferation.79 In mice, the LL-37analogue CRAMP reduces chemically induced colon cancer by inhibiting vimentin positive fibroblasts to produce collagen and disrupting tubulin distribution in fibroblasts.79 These studies indicate that LL-37/CRAMP inhibits tumorigenesis in the colon. In vivo, the capacity of CRAMP to protect colon homeostasis and anti- tumorigenic host defense was demonstrated by observations in CRAMP-/- mice,80 which exhibited exacerbated chemically induced chronic colitis, associated with delayed mucosal restoration and increased carcinomas.81 Importantly, CRAMP deficiency disrupted the balance of microbiota in mouse colon, indicating the importance of CRAMP in controlling the microbiota homeostasis, while the peptide also mediates epithelial cell migration and growth via FPRs. These observations reflect two-fold benefits of LL-37/CRAMP in the colon, i.e. by activating FPR2/Fpr2 in cancer cells to promote their apoptosis with reduced malignancy and its anti-microbial property to maintain a physiological homeostatic microbiota critical for host defense against inflammation and carcinogenesis.
Perspectives
FPRs as a subfamily of the classic chemoattractant GPCRs interact with the most diverse ligands among chemoattractant GPCRs and they are expressed by a great variety of cell types, notably cancer cells as well. The roles of FPRs in cancer progression are increasingly explored during the recent years. These receptors, in addition to mediating host defense against many pathological insults, have also been shown to be hijacked by a plethora of tumor cells including those of the human glioblastoma and breast cancer.82 In terms of interaction with chemotherapeutic drugs, overexpression of FPR1 may contribute to drug-resistance of bladder cancer and promotes tumor deterioration.83 On the other hand, FPR1 has been reported to increase chemotherapy-induced anticancer immune responses.84 As reviewed in this article, FPRs are also exploited by tumor cells of the DT system to their advantage. Although advances in new diagnosis and treatment have led to declining mortality rates, GI tract cancers remain a leading cause of death among all cancers. Therefore, a better understanding of the interactions between tumors and inflammation that involve FPRs and their ligands may lead to the discovery of novel molecular targets for therapeutic intervention, as shown by the role of FPR2/Fpr2 and the ligands LL-37/CRAMP as guardians of normal colon.
Acknowledgments
The authors thank Ms. Cheri A. Rhoderick for secretariat assistant. Cuimeng Tian was also supported in part by funding from Academic Research Project of Beijing Tuberculosis and Thoracic Tumor Research Institute/Beijing Chest Hospital, Capital Medical University, Beijing, China.
Abbreviations
- DT
digestive tract
- GPCRs
Gi-protein coupled chemoattractant receptors
- FPRs
formyl peptide receptors
- fMLF
formyl-methionyl-leucyl-phenylalanine
- DCs
dendritic cells
- NET
neutrophil extracellular trap
- IBD
inflammatory bowel diseases
- OSCC
oral squamous cell carcinoma
- ANXA1
annexin 1
- EC
esophageal cancer
- ESCC
squamous cell carcinoma
- EAC
esophageal adenocarcinoma
- GC
gastric cancer
- shFPR1
FPR1 silencing
- H. pylori
helicobacter pylori
- EMT
epithelial-mesenchymal-transition
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- PC
pancreatic carcinoma
- CRC
colorectal cancer
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Federal funds from the National Cancer Institute (NCI), National Institutes of Health (NIH), under Contract No. 535 HSN261200800001E, and by the Intramural Research Programs of the NCI, CCR, LCIM.
ORCID iD: Ji Ming Wang  https://orcid.org/0000-0002-7381-5695
https://orcid.org/0000-0002-7381-5695
References
- 1. Chen K, Bao Z, Gong W, et al. Regulation of inflammation by members of the formyl-peptide receptor family. J Autoimmun. 2017;85:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23(11):541–548. [DOI] [PubMed] [Google Scholar]
- 3. Liang W, Chen K, Gong W, et al. The contribution of chemoattractant GPCRs, formylpeptide receptors, to inflammation and cancer. Front Endocrinol (Lausanne). 2020;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 5. Heo SC, Kwon YW, Jang IH, et al. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells. 2014;32(3):779–790. [DOI] [PubMed] [Google Scholar]
- 6. Kim MK, Min DS, Park YJ, et al. Expression and functional role of formyl peptide receptor in human bone marrow-derived mesenchymal stem cells. Febs Lett. 2007;581(9):1917–1922. [DOI] [PubMed] [Google Scholar]
- 7. VanCompernolle SE, Clark KL, Rummel KA, Todd SC. Expression and function of formyl peptide receptors on human fibroblast cells. J Immunol. 2003;171(4):2050–2056. [DOI] [PubMed] [Google Scholar]
- 8. Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5(5):831–840. [DOI] [PubMed] [Google Scholar]
- 9. Ye RD, Boulay F, Wang JM, et al. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61(2):119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cattaneo F, Parisi M, Ammendola R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci. 2013;14(4):7193–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SD, Kim JM, Jo SH, et al. Functional expression of formyl peptide receptor family in human NK cells. J Immunol. 2009;183(9):5511–5517. [DOI] [PubMed] [Google Scholar]
- 12. Hartt JK, Barish G, Murphy PM, Gao JL. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med. 1999;190(5):741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen K, Tang P, Bao Z, et al. Deficiency in Fpr2 results in reduced numbers of Lin(-)cKit(+)Sca1(+) myeloid progenitor cells. J Biol Chem. 2018;293(35):13452–13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devosse T, Guillabert A, D’Haene N, et al. Formyl peptide receptor-like 2 is expressed and functional in plasmacytoid dendritic cells, tissue-specific macrophage subpopulations, and eosinophils. J Immunol. 2009;182(8):4974–4984. [DOI] [PubMed] [Google Scholar]
- 15. Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189(4):657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krepel SA, Wang JM. Chemotactic ligands that activate g-protein-coupled formylpeptide receptors. Int J Mol Sci. 2019;20(14):3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen K, Liu M, Liu Y, et al. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J Clin Invest. 2013;123(4):1694–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang M, Gao JL, Chen K, et al. A critical role of formyl peptide receptors in host defense against Escherichia coli. J Immunol. 2020;204(9):2464–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiang Y, Yao X, Chen K, et al. The G-protein coupled chemoattractant receptor FPR2 promotes malignant phenotype of human colon cancer cells. Am J Cancer Res. 2016;6(11):2599–2610. [PMC free article] [PubMed] [Google Scholar]
- 20. Liu M, Chen K, Yoshimura T, et al. Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. Plos One. 2014;9(6):e90613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. [DOI] [PubMed] [Google Scholar]
- 22. Huang J, Chen K, Gong W, Dunlop NM, Wang JM. G-protein coupled chemoattractant receptors and cancer. Front Biosci. 2008;13:3352–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen K, Le Y, Liu Y, et al. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol. 2010;184(7):3331–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu M, Zhao J, Chen K, et al. G protein-coupled receptor FPR1 as a pharmacologic target in inflammation and human glioblastoma. Int Immunopharmacol. 2012;14(3):283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lammermann T, Afonso PV, Angermann BR, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(7454):371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16(2):217–226, 229; discussion 230-2 [PubMed] [Google Scholar]
- 27. Ettinger KS, Ganry L, Fernandes RP. Oral cavity cancer. Oral Maxillofac Surg Clin North Am. 2019;31(1):13–29. [DOI] [PubMed] [Google Scholar]
- 28. Gigliotti J, Madathil S, Makhoul N. Delays in oral cavity cancer. Int J Oral Maxillofac Surg. 2019;48(9):1131–1137. [DOI] [PubMed] [Google Scholar]
- 29. De Yang, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192(7):1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525–528. [DOI] [PubMed] [Google Scholar]
- 31. Chen X, Qi G, Qin M, et al. DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity. Oncotarget. 2017;8(17):27943–27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okumura K, Itoh A, Isogai E, et al. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett. 2004;212(2):185–194. [DOI] [PubMed] [Google Scholar]
- 33. Sena A, Grishina I, Thai A, et al. Dysregulation of anti-inflammatory annexin A1 expression in progressive Crohns disease. Plos One. 2013;8(10):e76969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang Y, Liu Y, Yao X, et al. Annexin 1 released by necrotic human glioblastoma cells stimulates tumor cell growth through the formyl peptide receptor 1. Am J Pathol. 2011;179(3):1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wan YM, Tian J, Qi L, Liu LM, Xu N. ANXA1 affects cell proliferation, invasion and epithelial-mesenchymal transition of oral squamous cell carcinoma. Exp Ther Med. 2017;14(5):5214–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41(3):210–215. [DOI] [PubMed] [Google Scholar]
- 37. Paweletz CP, Ornstein DK, Roth MJ, et al. Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res. 2000;60(22):6293–6297. [PubMed] [Google Scholar]
- 38. Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer. 2002;99(6):860–868. [DOI] [PubMed] [Google Scholar]
- 39. O’Sullivan KE, Phelan JJ, O’Hanlon C, et al. The role of inflammation in cancer of the esophagus. Expert Rev Gastroenterol Hepatol. 2014;8(7):749–760. [DOI] [PubMed] [Google Scholar]
- 40. El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124(5):1193–1201. [DOI] [PubMed] [Google Scholar]
- 41. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. [DOI] [PubMed] [Google Scholar]
- 42. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 43. de Paulis A, Prevete N, Rossi FW, et al. Helicobacter pylori Hp(2-20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J Immunol. 2009;183(6):3761–3769. [DOI] [PubMed] [Google Scholar]
- 44. Prevete N, Liotti F, Visciano C, et al. The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene. 2015;34(29):3826–3838. [DOI] [PubMed] [Google Scholar]
- 45. Prevete N, Liotti F, Illiano A, et al. Formyl peptide receptor 1 suppresses gastric cancer angiogenesis and growth by exploiting inflammation resolution pathways. Oncoimmunol. 2017;6(4):e1293213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Otani T, Ikeda S, Lwin H, et al. Polymorphisms of the formylpeptide receptor gene (FPR1) and susceptibility to stomach cancer in 1531 consecutive autopsy cases. Biochem Biophys Res Commun. 2011;405(3):356–361. [DOI] [PubMed] [Google Scholar]
- 47. Cheng TY, Wu MS, Lin JT, et al. Formyl peptide receptor 1 expression is associated with tumor progression and survival in gastric cancer. Anticancer Res. 2014;34(5):2223–2229. [PubMed] [Google Scholar]
- 48. Cheng TY, Wu MS, Lin JT, et al. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer-Am Cancer Soc. 2012;118(23):5757–5767. [DOI] [PubMed] [Google Scholar]
- 49. Sato Y, Kumamoto K, Saito K, et al. Up-regulated Annexin A1 expression in gastrointestinal cancer is associated with cancer invasion and lymph node metastasis. Exp Ther Med. 2011;2(2):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hou X, Ji C, Tang J, et al. FPR2 promotes invasion and metastasis of gastric cancer cells and predicts the prognosis of patients. Sci Rep-Uk. 2017;7(1):3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem. 2013;114(3):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011;17(35):3971–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wroblewski LE, Peek RJ, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23(4):713–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Asaka M, Dragosics BA. Helicobacter pylori and gastric malignancies. Helicobacter. 2004;9(suppl 1): 35–41 [DOI] [PubMed] [Google Scholar]
- 55. Tomasinsig L, Zanetti M. The cathelicidins—structure, function and evolution. Curr Protein Pept Sci. 2005;6(1):23–34. [DOI] [PubMed] [Google Scholar]
- 56. Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group a streptococcus. J Invest Dermatol. 2001;117(1):91–97. [DOI] [PubMed] [Google Scholar]
- 57. Hase K, Murakami M, Iimura M, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 2003;125(6):1613–1625. [DOI] [PubMed] [Google Scholar]
- 58. Bocca C, Novo E, Miglietta A, Parola M. Angiogenesis and fibrogenesis in chronic liver diseases. Cell Mol Gastroenterol Hepatol. 2015;1(5):477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. [DOI] [PubMed] [Google Scholar]
- 60. Yang YM, Kim SY, Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin Liver Dis. 2019;39(1):26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 62. McCoy R, Haviland DL, Molmenti EP, et al. N-formylpeptide and complement C5a receptors are expressed in liver cells and mediate hepatic acute phase gene regulation. J Exp Med. 1995;182(1):207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shields PL, Morland CM, Salmon M, et al. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163(11):6236–6243. [PubMed] [Google Scholar]
- 64. Apolinario A, Majano PL, Alvarez-Perez E, et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97(11):2861–2870. [DOI] [PubMed] [Google Scholar]
- 65. Zhang L, Wang H, Yang T, et al. Formylpeptide receptor 1 mediates the tumorigenicity of human hepatocellular carcinoma cells. Oncoimmunol. 2015;5(2):e1078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhu H, Li T, Du Y, Li M. Pancreatic cancer: challenges and opportunities. Bmc Med. 2018;16(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. Faseb J. 2007;21(4):968–975. [DOI] [PubMed] [Google Scholar]
- 68. Belvedere R, Bizzarro V, Popolo A, et al. Role of intracellular and extracellular annexin A1 in migration and invasion of human pancreatic carcinoma cells. Bmc Cancer. 2014;14(1):961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bizzarro V, Belvedere R, Dal Piaz F, Parente L, Petrella A. Annexin A1 induces skeletal muscle cell migration acting through formyl peptide receptors. Plos One. 2012;7(10):e48246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Belvedere R, Bizzarro V, Forte G, et al. Annexin A1 contributes to pancreatic cancer cell phenotype, behaviour and metastatic potential independently of Formyl Peptide Receptor pathway. Sci Rep-Uk. 2016;6(1):29660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dekker E, Tanis PJ, Vleugels J, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. [DOI] [PubMed] [Google Scholar]
- 72. Yang ZH, Dang YQ, Ji G. Role of epigenetics in transformation of inflammation into colorectal cancer. World J Gastroenterol. 2019;25(23):2863–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li S, Su N, Gong P, et al. The expression of formyl peptide receptor 1 is correlated with tumor invasion of human colorectal cancer. Sci Rep-Uk. 2017;7(1):5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lu J, Zhao J, Jia C, et al. FPR2 enhances colorectal cancer progression by promoting EMT process. Neoplasma. 2019;66(5):785–791. [DOI] [PubMed] [Google Scholar]
- 75. Ren SX, Cheng AS, To KF, et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72(24):6512–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lim R, Lappas M, Riley C, et al. Investigation of human cationic antimicrobial protein-18 (hCAP-18), lactoferrin and CD163 as potential biomarkers for ovarian cancer. J Ovarian Res. 2013;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ren SX, Shen J, Cheng AS, et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. Plos One. 2013;8(5):e63641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kuroda K, Fukuda T, Isogai H, et al. Antimicrobial peptide FF/CAP18 induces apoptotic cell death in HCT116 colon cancer cells via changes in the metabolic profile. Int J Oncol. 2015;46(4):1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cheng M, Ho S, Yoo JH, et al. Cathelicidin suppresses colon cancer development by inhibition of cancer associated fibroblasts. Clin Exp Gastroenterol. 2015;8:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang M, Liang W, Gong W, et al. The critical role of the antimicrobial peptide ll-37/ cramp in protection of colon microbiota balance, mucosal homeostasis, anti-inflammatory responses, and resistance to carcinogenesis. Crit Rev Immunol. 2019;39(2):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yoshimura T, McLean MH, Dzutsev AK, et al. The antimicrobial peptide CRAMP is essential for colon homeostasis by maintaining microbiota balance. J Immunology. 2018;200(6):2174–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou J, Xiang Y, Yoshimura T, et al. The role of chemoattractant receptors in shaping the tumor microenvironment. Biomed Res Int. 2014;2014:751392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jiang X, Lei T, Zhang M. Expression and functions of formyl peptide receptor 1 in drug-resistant bladder cancer. Technol Cancer Res Treat. 2018;17(5):1533034618769413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vacchelli E, Ma Y, Baracco EE, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–978. [DOI] [PubMed] [Google Scholar]