Abstract
Objective:
The present study aims to analyze the clinical characteristics and etiology of transarterial chemoembolization (TACE) in the treatment of hepatocellular carcinoma (HCC) complicated with acute respiratory distress syndrome (ARDS), in order to improve the early diagnosis rate and cure rate.
Methods:
A total of 816 patients with primary HCC received 2,200 TACE treatments from January 2014 to May 2018. Among these patients, 6 patients developed ARDS after TACE. The clinical data, lesion characteristics, laboratory tests, treatment process and prognosis of 6 patients were retrospectively analyzed.
Results:
The longest lesion diameter ranged within 5.0-10.2 cm (mean: 6.6 cm) in the 6 patients with primary HCC. Among these patients, 4 patients had lesions mainly located in the left lateral lobe of the liver, while 5 patients had no hepatic arteriovenous fistula detected before TACE. Nedaplatin, epirubicin and iodinated oil suspension chemoembolization were used in all 6 patients during TACE, and all of them experienced ARDS symptoms within 24-48 hours after TACE. However, no clear pathogenic bacteria were incubated in the sputum culture after the onset of the disease. Diffused exudative changes of both lungs were found in the chest X-ray, and the oxygenation index (PaO2/FiO2) was within 100-300 mmHg. The symptoms of 6 patients improved after 3-6 days of hormone therapy.
Conclusion:
In this study, we found that although the incidence of ARDS after TACE was low in the treatment for HCC, the symptoms after onset were serious, and the early hormone therapy may be beneficial to improve the prognosis and reduce mortality. Further research with larger samples is still needed to confirm the pathogenesis of ARDS after TACE in the treatment for HCC.
Keywords: acute respiratory distress syndrome, hepatocellular carcinoma, transcatheter arterial chemoembolization, lipiodol embolism, pneumonia
Introduction
Respiratory complications after transarterial chemoembolization (TACE) of primary hepatocellular carcinoma (HCC) are commonly observed in pulmonary ectopic embolisms caused by hepatic arteriovenous fistula, abnormal communication of branch openings and anatomical variations, and present with acute cough, chest tightness, dyspnea and chest pain. Acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) is relatively rare, and has an incidence of less than 0.2%. However, the consequences are serious, and can even lead to death.1-3 TACE is an effective method for the palliative treatment of HCC. Postoperative complications of ARDS are rare, but the consequences are relatively serious. Improving the understanding of the postoperative complications of ARDS after TACE would be helpful for its early diagnosis and treatment, and reduction in fatality rate.
Previous studies had reported that the cause of primary HCC after TACE complicated with ALI or ARDS was suspected to be ectopic iodinated oil embolism in lung tissues.4-6 High density iodinated oil was found on the CT scan of some cases.7 The postmortem examination also revealed a fat droplet embolism in the arterioles of lung tissues.6 However, the mechanism of diffused exudation of both lungs was unclear. Some researchers have considered that iodinated oil particles are decomposed into free fatty acids by intrapulmonary lymphocyte esterase when these enter the pulmonary lymphoid-microvenous anastomotic network through the communicating branch. Then, these free fatty acids constantly accumulate in the capillary pool, and the concentration would continue to increase. Finally, these are metabolized to toxic fatty acids, resulting in pulmonary edema, and leading to decreased ventilation-ventilation function and pulmonary embolism. At the same time, these fatty acid particles can lead to a decrease in pulmonary surfactants, thereby expediting the production of atelectasis and pneumonia, and aggravating the symptoms of the pulmonary embolism.8
Recently, the clinical characteristics of TACE in treatment of primary HCC complicated with respiratory distress syndrome remains unclear. Therefore, we conducted this study to analyze the clinical characteristics and etiology of TACE in the treatment of HCC complicated with ARDS in order to improve the early diagnosis rate and cure rate. In the present study, relevant cases in our department were analyzed, as follows.
Materials and Methods
Study Subject
The present study is a clinical retrospective study. The main subjects comprised of patients with HCC complicated with ARDS from January 2014 to May 2018. The diagnostic basis of HCC is upon clinical diagnosis or pathological diagnosis. The diagnostic criteria for ARDS was based on the Berlin Definition of ARDS,3 which included the contents as follows: (1) within 1 week of known clinical insult or new or worsening respiratory symptoms; (2) chest imaging showed bilateral opacities that could not be fully explained by effusion, lobe/lung collapse, or nodules; (3) respiratory failure that could not be fully explained by cardiac failure or fluid overload; and objective assessment was needed (eg, echocardiography) to exclude hydrostatic edema if no risk factor was presented; (4) hypoxemia: mild (200 mmHg < PaCO2/FiO2 ≤ 300 mmHg with PEEP or CAPA ≥ 5 cmH2O), moderate (100 mmHg < PaCO2/FiO2 ≤ 200 mmHg with PEEP or CAPA ≥ 5 cmH2O), severe (PaCO2/FiO2 ≤ 100 mmHg with PEEP or CAPA ≥ 5 cmH2O).
Ethical Statement: Our study was approved by Ethics Committee of Peiking University Shenzhen Hospital. All patients provided written informed consent prior to enrollment in the study.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) patients definitely diagnosed with HCC, (2) patients complicated with ARDS during clinical treatment, and (3) patients >18 years old. Exclusion criteria: (1) patients with incomplete clinical data, (2) patients with lacking pathological diagnostic data, and (3) patient who refused to undergo TACE.
TACE Procedure
All patients with TACE indications and contraindications were assessed based on the literature standard.2 The tumor feeding arteries were confirmed by routine angiography before chemoembolization, in order to determine the abnormal traffic, hepatic arteriovenous (portal vein) fistula, portal vein patency, and collateral artery blood supply. All 6 patients were treated with epirubicin (10-30 mg), nedaplatin (50 mg) and iodinated oil suspension. The maximum dose of the suspension was not more than 20 ml. According to the condition, it was determined whether gelatin sponge particles would be added until the forward blood flow of the tumor blood supply artery stagnated.
Methods of Diagnosis and Treatment After Symptoms of ARDS
These patients were instructed to stay in bed, and receive electrocardiogram (ECG) monitoring, blood oxygen saturation detection, and oxygen inhalation. In addition, corticosteroids, anti-inflammatory, expectorant and symptomatic support treatment were administered. The completion of the laboratory examination included blood routine, arterial blood gas analysis, coagulation function, D-dimer, sputum culture and virus antibody. The auxiliary examination included chest radiography or chest computed tomography (CT) and ECG. The chest radiography was reviewed every 1-2 days during the treatment.
Statistical Analysis
For the present study, SPSS 20.0 statistical software was used to process the data. The measurement data were expressed as mean ± standard deviation (x ± SD). The enumeration data were expressed in percentage (%). P < 0.05 was considered statistically significant.
Results
General Data
The present study included 816 patients with HCC, who were diagnosed clinically or pathologically in our hospital, and received 2,200 TACE treatments. Among these patients, 6 patients experienced ARDS after the procedure. The clinical data of these included patients are presented in Table 1.
Table 1.
Clinical Data of ARDS After TACE for Hepatocellular Carcinoma.
| Serial number | Gender | Age | BCLC staging | Liver function Child-Pugh classification | Lesion distribution | The longest path of the lesion | Visible tumor thrombus |
|---|---|---|---|---|---|---|---|
| 1 | Male | 74 | C | A | S3, S6 | 6.0cm | Yes |
| 2 | Male | 35 | B | A | S2, S3 | 5.5cm | No |
| 3 | Male | 53 | B | A | S2, S3 | 5.0cm | No |
| 4 | Male | 38 | C | A | S6, S8 | 5.4cm | No |
| 5 | Male | 63 | B | A | S4 | 10.2cm | No |
| 6 | Male | 55 | B | B | S4 | 7.2cm | No |
TACE Procedure
Six patients underwent the intraoperative radiography. Among these patients, 1 patient had a small hepatic arteriovenous (portal vein) fistula, while 1 patient had a tumor thrombus formation in the left branch of the portal vein. However, no definite hepatic arteriovenous fistula was found. The main blood supply arteries included the left hepatic artery (4 patients), right hepatic artery (2 patients), and right phrenic artery (1 patient). The TACE procedure was smoothly performed for all patients, and no abnormality was found in blood pressure and blood oxygen saturation during the intraoperative monitoring. However, 4 patients complained of abdominal pain and discomforts during the procedure, while 1 patient received symptomatic treatment with tramadol, without the symptoms of cough, chest distress and dyspnea.
ARDS Occurrence
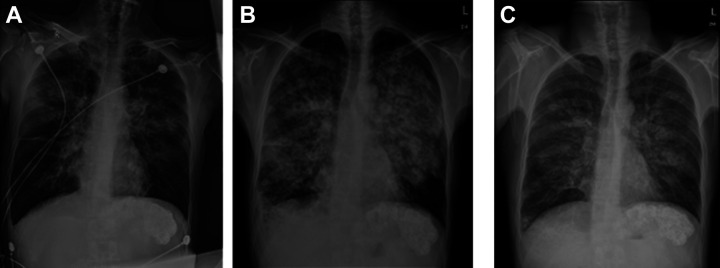
All 6 patients suffered from the dyspnea, cough, expectoration, and decrease in blood oxygen saturation within 24-48 hours after TACE. Among these patients, 2 patients had blood sputum, which was thick brick red sputum. The physical examination revealed that all patients had defused wet rales in both lungs. The chest radiography revealed diffused exudative changes in both lungs, which were particularly significant in the middle medial lung fields, with no clear high-density shadows of iodinated oil (Figure 1).
Figure 1.
Male, 35 years old, with sudden chest tightness, shortness of breath, dyspnea and large amount of sputum 32 hours after TACE. A: Reexamination of chest radiographs suggested a change in bilateral pulmonary exudation. B: Symptoms did not improve significantly on the third day after meropenem anti-infection treatment, and chest radiographs showed significant progress in bilateral pulmonary exudation. C: On the third day after the anti-inflammatory treatment of methylpredinone, the chest radiograph showed that the exudation in both lungs was significantly improved compared with the previous absorption.
Therapeutic Schemes
All 6 patients were advised to take bed rest, ECG monitoring, and high flow mask oxygen inhalation, but the improvement in blood oxygen saturation was not obvious. Furthermore, there were no clear pathogenic bacterial and fungal infections found in the bacterial and fungal culture of the sputum. In addition, no viral pneumonia was discovered in the 8 items of the viral test. After the onset of the disease, these patients received hormone (methylprednisolone injection of 20-40 mg, qd or bid), expectoration and anti-inflammatory treatment. The symptoms of these patients gradually improved. The chest radiography revealed that the exudative changes were absorbed and thinned in both lungs. The diagnoses and treatments are presented in Table 2.
Table 2.
Diagnosis and Treatment of Concurrent ARDS in Patients With Hepatocellular Carcinoma After TACE.
| Serial number | TACE times | Dosage of embolic drugs | Time of ARDS symptoms | Main clinical symptoms | Oxygenation index | Usage of hormone (methylprednisolone) | Symptom relief time after hormone use |
|---|---|---|---|---|---|---|---|
| 1 | The second time | 10ml | 28 hours after operation | Chest tightness, shortness of breath | 142 | 20mg 2 times / day 4 days | Day 3 |
| 2 | The third time | 10ml | 32 hours after operation | Chest tightness, shortness of breath, red sputum | 135 | 20mg 1 time / day 3 days | Day 2 |
| 3 | The first time | 8ml | 30 hours after operation | Chest tightness, cough, red sputum | 186 | 20mg 1 time / day 3 days | Day 3 |
| 4 | The fifth time | 6ml | 16 hours after operation | Chest tightness, shortness of breath | 205 | 20mg 1 time / day 6 days | Day 3 |
| 5 | The first time | 15ml | 32 hours after operation | Chest tightness, shortness of breath, expectoration | 110 | 40mg 1 time / day 6 days | Day 3 |
| 6 | The first time | 10ml | 26 hours after operation | Chest tightness, shortness of breath, dizziness | 188 | 20mg 1 time / day 3 days | Day 3 |
Discussion
The results revealed that the longest lesion diameter ranged within 5.0-10.2 cm (mean: 6.6 cm) in the 6 patients with primary HCC. Among these patients, 4 patients had lesions mainly located in the left lateral lobe of the liver, while 5 patients had no hepatic arteriovenous fistula detected before TACE. Nedaplatin, epirubicin and iodinated oil suspension chemoembolization were used for all 6 patients during TACE, and all of them experienced ARDS symptoms within 24-48 hours after TACE. However, no clear pathogenic bacteria were incubated in the sputum culture after the onset of the disease. The diffused exudative changes of both lungs were found in the chest X-ray, and the oxygenation index (PaO2/FiO2) was within 100-300 mmHg. The symptoms of 6 patients improved after 3-6 days of hormone therapy.
Some researchers have put forward the concept of fat embolism syndrome.9-11 It is speculated that the mechanism is the inflammatory reaction of lung tissues to iodinated oil, in which nitric oxide (NO), phospholipase A2, free radicals and pro-inflammatory cytokines (tumor necrosis factor-α, IL-1β and IL-10) play an important role in the pathogenesis. Macrophages in the alveoli also play a role in inducing nitric oxide synthase and promoting the production of NO in the lungs.12 The onset of all 6 patients in the present study ranged within 24-48 hours after TACE. It was speculated that the cause of ALI or ARDS after TACE is more likely to be the inflammatory reaction of lung tissues to iodinated oil, which is different from the immediate symptoms of mechanical embolism of iodinated oil.
In addition, it is noteworthy that regardless of whether the chemotherapeutic drugs used in TACE can induce or co-induce lung injury,13-15 the suspension formed by the iodinated oil and chemical drugs may cause disruptions on the direct or indirect immune response to lung tissues, including diffused alveolar damage, non-cardiogenic pulmonary edema, vasculitis alveolar hemorrhage, ARDS, and allergic reaction alveolitis. The combination of 2 chemotherapy drugs and iodinated oil in TACE in this group of patients may increase the chemotherapy drug-related pneumonia. After the onset of the disease, the chest radiography was reviewed, and bilateral pulmonary exudative lesions were found. These lesions were centered on the hilum of the lung. These were especially significant in the middle medial pulmonary field, but were comparatively not significant in the lateral field, which were not consistent with the mechanical pulmonary embolism.
In terms of treatment, the response of this group of patients to hormone therapy was good. On the second day of administration of hormone, the respiratory symptoms significantly improved in all patients. All patients were oxygenated with masks due to the significant decrease in oxygen saturation. Among these patients, 1 patient was ventilated with a ventilator. Although some researchers have considered that patients with respiratory symptoms within 6 hours and a clear diagnosis tend to be treated with early anticoagulant therapy and thrombolysis, when necessary,16,17 none of the patients in this group of patients were treated with anticoagulant drugs, such as heparin or low molecular weight heparin. The main reasons were as follows: (1) All patients had respiratory symptoms at 1 day after TACE, and the possibility of ALI or ARDS caused by mechanical embolism was low. (2) The dose of iodinated oil used for all patients during TACE did not exceed 20 ml. However, although a small amount of iodinated oil enters into the lung tissue through the communicating branch, the degree of mechanical embolism caused by the iodinated oil could not explain these serious clinical symptoms. (3) Anticoagulant therapy may increase the risk of pulmonary hemorrhage. However, the investigators consider that low-dose anticoagulant therapy may reduce the risk of pulmonary embolism after ARDS in the absence of significant risk of bleeding.
Therefore, the investigators consider that although the incidence of TACE complicated with ARDS is low, the symptoms after onset are more serious, and the fatality rate is higher. The ectopic embolism of iodinated oil and chemotherapeutic drug suspension, and the inflammatory reaction of lung tissues to iodinated oil and chemotherapeutic drugs may be the main causes of the disease. In order to reduce or avoid these complications, the hepatic pulmonary vascular communication, collateral circulation and arteriovenous fistula should be determined by angiography before embolization. Furthermore, the total amount of iodinated oil embolism should be controlled during the procedure to avoid excessive embolism. In addition, reducing the type of chemotherapeutic drugs may also be helpful to reduce the occurrence of complications. For the ARDS that occur after TACE, early diagnosis and the early use of hormone therapy may be an important treatment approach to improve the prognosis, and reduce the case fatality rate. Furthermore, the proper use of anticoagulant therapy may reduce the risk of secondary pulmonary embolism.
The present study has the following limitations. First, the present study is an observational study, and not a prospective study. Therefore, there is still a certain risk of bias. Second, the present study is a single-center clinical study, and the sample size is small. Thus, there is a need to further increase the sample size and carry out multicenter clinical studies. Finally, since no control group was established in the present study, these results still needs to be confirmed through further studies.
Conclusion
In this study, we found that although the incidence of ARDS after TACE was low in the treatment for HCC, the symptoms after onset were serious, and the early hormone therapy may be beneficial to improve the prognosis and reduce mortality. Further research with larger samples is still needed to confirm the pathogenesis of ARDS after TACE in the treatment for HCC.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hong-Jian Yu  https://orcid.org/0000-0001-6912-6523
https://orcid.org/0000-0001-6912-6523
References
- 1. Meng YL, Hu HT, Li HL, et al. The clinical therapeutic effects of arsenic trioxide combined with transcatheter arterial chemoembolization in treating primary liver cancer with pulmonary metastases. Zhonghua Nei Ke Za Zhi. 2012;51(12):971–974. [PubMed] [Google Scholar]
- 2. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin. Definition. Jama. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 3. Bilbao JI, Martínez-Cuesta A, Urtasun F, Cosín O. Complications of embolization. Semin Intervent Radiol. 2006;23(2):126–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187(3):689–693. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe T, Yamashita T, Sugawara H, et al. Rapid progression of lung cancer following emergency caesarean section led to postpartum acute respiratory failure. Intern Med. 2019;58(7):991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatamaru K, Azuma S, Akamatsu T, et al. Pulmonary embolism after arterial chemoembolization for hepatocellular carcinoma: an autopsy case report. World J Gastroenterol. 2015;21(4):1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taupin D, Mukherjee V, Nathavitharana R, Green DA, Fridman D. Lipiodol embolism following transarterial chemoembolization: an atypical case. Crit Care Med. 2014;42(6):e481–e484. [DOI] [PubMed] [Google Scholar]
- 8. Silvestri RC, Huseby JS, Rughani I, Thorning D, Culver BH. Respiratory distress syndrome from lymphangiography contrast medium. Am Rev Respir Dis. 1980;122(4):543–549. [DOI] [PubMed] [Google Scholar]
- 9. Kosova E, Bergmark B, Piazza G. Fat embolism syndrome. Circulation. 2015;131(3):317–320. [DOI] [PubMed] [Google Scholar]
- 10. DeFroda SF, Klinge SA. Fat embolism syndrome with cerebral fat embolism associated with long-bone fracture. Am J Orthop (Belle Mead NJ). 2016;45(7):E515–E521. [PubMed] [Google Scholar]
- 11. Newbigin K, Souza CA, Torres C, et al. Fat embolism syndrome: state-of-the-art review focused on pulmonary imaging findings. Respir Med. 2016;113:93–100. [DOI] [PubMed] [Google Scholar]
- 12. Kao SJ, Chen HI. Nitric oxide mediates acute lung injury caused by fat embolism in isolated rat′s lungs. J Trauma. 2008;64(2):462–469. [DOI] [PubMed] [Google Scholar]
- 13. Liu B, Huang JW, Li Y, et al. Arsenic trioxide transarterial chemoembolization with and without additional intravenous administration of arsenic trioxide in unresectable hepatocellular carcinoma with lung metastasis: a single-blind, randomized trial. J Cancer Res Clin Oncol. 2015;141(6):1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinto AM, Nutu OA, Manso RSR, et al. Complications of transarterial chemoembolization (TACE) in the treatment of liver tumors. Cir Esp. 2018;96(9):560–567. [DOI] [PubMed] [Google Scholar]
- 15. Ishimaru H, Morikawa M, Sakugawa T, et al. Cerebral lipiodol embolism related to a vascular lake during chemoembolization in hepatocellular carcinoma: a case report and review of the literature. World J Gastroenterol. 2018;24(37):4291–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park CH, Han K, Hur J, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. 2017;151(2):316–328. [DOI] [PubMed] [Google Scholar]
- 17. Nhu QM, Knowles H, Pockros PJ, Frenette CT. Pulmonary complications of transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Respirol. 2016;6(3):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]