Abstract
Background:
Neovascularization plays a crucial pathogenic role in tumor development and vascular endothelial growth factor (VEGF-A) is a key signaling element that drives angiogenesis, thereby facilitating hepatocellular cancer (HCC) growth and metastasis. We aimed to define the relationship between serum VEGF-A levels and clinical outcomes in a cohort of Turkish patients with HCC.
Methods:
We enrolled and prospectively followed 84 patients with HCC in our study. Serum VEGF-A levels were measured and we assessed the association between VEGF-A levels and clinical features.
Results:
Forty-eight patients had cirrhosis while 35 patients were noncirrhotic. Serum VEGF-A levels were significantly lower in HCC patients with cirrhosis compared to non-cirrhotic HCC patients (p = 0.03).In terms of viral hepatitis subtype, 36 (%42.8) of patients were hepatitis B virus (HBV) positive and 8 (%9.5) of patients were hepatitis C virus (HCV) positive. Patients with serum VEGF-A levels ≥100 pg/mL had significantly lower OS rates than patients with serum VEGF-A level <100 pg/mL (p = 0.01). The OS rates were 5.8 and 14.2 months, respectively (p = 0.02). The median OS was 7.38 months (95% CI: 5.89-13.79 months). We observed a significant relationship between serum VEGF-A level and tumor size. Patients with tumor size ≤ 5cm had lower VEGF-A levels than patients with VEGF-A levels <5 cm. The VEGF-A levels were 132.7 and 342.1 pg/mL, respectively (p < 0.001). The median follow-up was 32 months.
Conclusions:
Serum VEGF-A level, a biological marker of angiogenesis, is an independent predictor of survival in patients with HCC. Serum VEGF-A levels may be utilized to predict response to treatment targeting serum VEGF-A in patients with HCC.
Keywords: hepatitis B, hepatocellular cancer, overall survival, prognosis, vascular endothelial growth factor-A
Introduction
The circulating levels of vascular endothelial growth factor (VEGF) have been reported by several studies as a prognostic factor in patients with various types of cancers.1-4 Cancer’s growth capability depends on angiogenesis which has a significant role in cancer progression.5 Angiogenesis is a complex process encompassing multiple biological events and is controlled by growth factors.6 The VEGF-A is a growth factor that belongs to the VEGF family and is the most important surrogate biomarker of cancer angiogenesis.7,8 The healthy or abnormal cells, like cancer cells, tissue stroma, pituitary follicular cells, macrophages, hepatocytes, and endothelial cells can produce VEGF-A for regeneration or growth.9,10 Hypoxia is the main factor leading to the production of VEGF A. Reduced tissue oxygenation stimulates new blood vessel formation to overcome hypoxia through VEGF-A production. Hypoxia-inducible transcription factors (HIFs) increase transcription of the VEGF gene that regulates VEGF-A expression.11,12
Hepatocellular cancer is a hypervascular tumor, and VEGF plays an important role in HCC vascularization.13,14 The association between increased serum VEGF-A and advanced-stage HCC, characterized by vascular invasion and metastasis, has been reported previously.15,16 Additionally, the correlation between microscopic venous invasion and intrahepatic metastasis and high serum VEGF-A levels has been demonstrated in a trial that may transform the treatment landscape of HCC.17 Despite the well-described association between serum VEGF-A levels and clinicopathologic features in patients with HCC, the impact of circulating VEGF-A on overall survival (OS) and prognosis in patients with HCC is still uncertain. We aimed, with this study, to investigate the prognostic value of circulating VEGF-A level in a cohort of Turkish HCC patients with different stages of the disease.
Patients and Methods
Patients
We included patients who admitted to our hospital with prior HCC diagnosis or diagnosed at the time of admission, between November 2014 and May 2017. All data were obtained prospectively from patients at the Cancer Institute of the Hacettepe and the serum samples were collected during the first admission of patients. The pathological or radiological findings that are in line with AASLD and EASL guidelines for HCC diagnosis were utilized. The patients who had a diagnosis of hepatocellular carcinoma based on either histopathological or radiological findings were included. The typical radiological findings of HCC in imaging techniques obtained by multidetector CT scan or dynamic contrast-enhanced MRI were used to confirm the diagnosis in cirrhotic patients in the absence of tissue sample. Additionally, the patients with HCC who were 18 years or older with any level of serum α-fetoprotein, all stages of the disease which assessed by the Barcelona Clinic Liver Cancer (BCLC) system, and any score of Child-Turcotte-Pugh scoring system were included to the study. In addition to the typical radiological findings for the diagnosis of liver cirrhosis, laboratory results such as liver function and thrombocytopenia, and clinical findings such as non-malignant acids, hepatic encephalopathy, splenomegaly, and esophageal varices were used to confirm the diagnosis of cirrhosis. The biopsy was performed in patients whose cirrhotic status had not been confirmed with laboratory, clinical, or radiologically (MRI or ultrasonography) findings, in this way both HCC diagnosis was made and cirrhotic status was clarified. The serum bilirubin, albumin, and INR as laboratory parameters, and ascites and hepatic encephalopathy as clinical findings were used for calculating the Child-Turcotte-Pugh (CTP) score. The BCLC staging system was used to determine the disease stage. All treatment decisions which may include surgical or non-surgical treatment modalities were discussed in our multidisciplinary tumor boards, ultimate treatment decisions were recommended based on the consensus opinion. Diverse first-line treatment modalities that include both systemic and local approaches were included in data variables. The first-line treatments that performed to patients according to their disease stage were determined. These treatments were local ablative therapies such as radiofrequency (RFA) and microwave ablation (MWA), local palliative treatments such as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE), surgical resection, systemic treatments such as tyrosine kinase inhibitors treatment and cytotoxic systemic therapy, and the best supportive care therapy (BSC). Overall survival was calculated for all patients according to clinical or laboratory parameters. The Body Mass Index (BMI) of patients is calculated by dividing the bodyweight of the individual (in kilograms) by the square of the individual’s height (in meters). This estimates the BMI in kilograms per square meter (kg/m2) and classified into 4 subgroups of patients according to WHO International Classification like underweight (BMI <18.5), normal weight(BMI ≥18.5 to 24.9), overweight (≥25.0 to 29.9) and obesity (BMI ≥30).
Circulating VEGF-A Measurement
The patient’s plasma samples were collected at the time of initial HCC diagnosis or at the time of patient inclusion to study. After the collection of blood samples for the measurement of circulating VEGF-A, they were stored at − 80 degrees until measurement. To measure VEGF-A levels in the circulation, plasma samples were analyzed in duplicate using the Human VEGF-A (Vascular Endothelial Cell Growth Factor A) ELISA Kit (Elabscience, catalog no: E-EL-H0111) according to the manufacturer’s instructions.
Ethical Aspects
This study was planned and conducted in accordance with the Helsinki Declaration. Accordingly, the feasibility and suitability of the study were obtained from the Ethics Committee of Hacettepe University Faculty of Medicine (Approval date and number: 04.03.2015 and 15/153).
Assessment of the Study Outcomes and Statistical Analysis
The primary outcome of interest was to assess if there is an association between circulating VEGF-A levels and clinicopathologic features in patients with hepatocellular cancer. The median overall survival (OS) was defined as the time from the blood draw date to death or censorship, and for patients who lost to follow-up were censored at the date they were last known to be alive. Hereby, the median OS was calculated for all patients and the Log rank test was used to compare the median OS values. Differences in patient characteristics and their circulating VEGF-A levels were compared, all categorical variables, number of cases and percentage of patients in each category were provided, and Chi-Square (X2) or the Fisher’s exact test was used to test for statistical differences between the groups. Survival rates were estimated by the Kaplan-Meier method and the log-rank test was used to compare OS rates between groups. Multivariable and univariable relation between survival and the covariates were investigated using the Cox proportional hazards model. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. The time-dependent receiver operating characteristic (ROC) curve and area under curve (AUC) analyses were used to evaluate the sensitivity and specificity of the investigated biomarker. When a significant cut-off value observed, sensitivity, specificity values were presented. While evaluating the area under the curve, a 5% type-I error level was used to accept statistically significant predictive value of the test variable. Statistical significance was taken as P < 0.05, and all tests were 2-sided. Analyses were performed using SPSS version 22 statistical software (IBM Corporation, Somers, New York, USA).
Results
Baseline Patient Characteristic
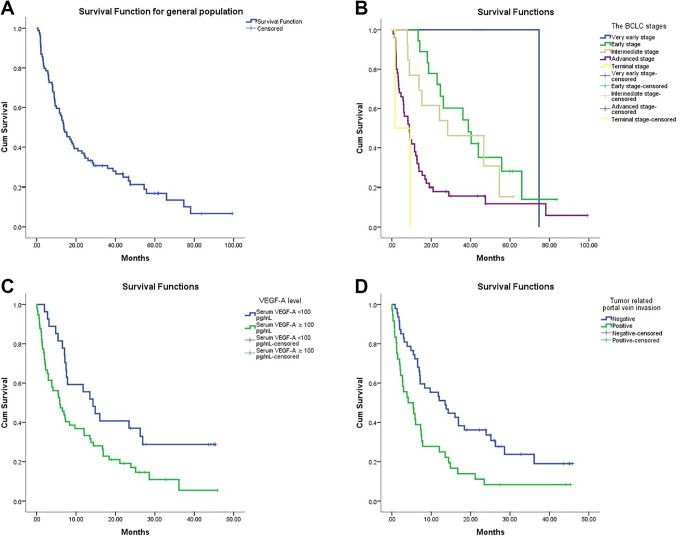
Between November 2014 and May 2017, a total of 84 patients with hepatocellular cancer were enrolled to the study. The demographic and clinical features of all patients with HCC are listed in Table 1. In the patients’ characteristics table; in the Child-Turcotte-Pugh classification, tumor nodularity, and portal vein invasion groups 1 patient could not be reported due to insufficient related information; a similar situation was valid in the largest tumor size and the body mass index groups, for 2 and 9 patients, respectively. All included patients agreed to provide blood samples and consented at time of the participating the study. In this study, the number of male gender patients were dominant, 71 (84.5%) of the patients were male and 13 (15.5%) were female. The median age of 84 patients was 64 (range 19-90). The patients were classified according to the CTP scoring system, 58 (69%) of patients were CTP class A, 22 (26.2%) patients CTP class B and 3 (3.6%) patients were CTP class C. In terms of viral hepatitis, hepatitis B (HBV) was much more common than hepatitis C (HCV) in our patient cohort: there were 36 patients had HBV and 8 patients had HCV infection. As expected, the majority of patients were cirrhotic: 48 of patients had cirrhosis and 35 didn’t have cirrhosis. A total of 69 patients of the HCC population had died at the time of the final analysis. The estimated median survival, defined as the time from blood draw date to death or censorship was 7.29 months (95% CI: 4.43-10.1months). In general population, the estimated median overall survival as the time defined from date of diagnosis to the date of death or the last follow-up date rate was 13.7 months (95% CI: 9.54-17.92 months) (Figure 1A). Additionally, the median overall survivals of the HCC patients according to the BCLC staging system were calculated, and there was statistically significant difference in OS of patients according to BCLC stages. The median OS from the time of diagnosis for very early stage, early stage, intermediate stage, advanced stage, and terminal stage were 74.6, 38.7, 28.3, 8.8 and 1.6 months, respectively (p < 0.001) (Figure 2B). The mean serum level of VEGF-A according to the BCLC tumor stages was 132.9 (132.9 -132.9) in patients with very early-stage, 148.8 (55.5-286.3) in early-stage, 149.3 (26.3-498.9) in intermediate-stage, 318.2 (28.5-1414.1) in advanced-stage, and was 650.2 (457.4-843.1) in terminal-stage (Table 2). The difference between the mean serum level of VEGF-A of the tumor stages was significant (p = 0.02). In addition, a statistically significant positive correlation was found between serum VEGF-A and tumor stage, and serum VEGF-A level was increasing as the patient’s stage advanced (p = 0.003).
Table 1.
Baseline Demographic and Clinical Features of the Patients with HCC.
| Number | Percentage | |||
|---|---|---|---|---|
| Total patients(n) | 84 | 100% | ||
| Median age of all patients | 64 (19-90) | 100% | ||
| Median age | Female | 65 (29-85) | 19% | |
| Male | 64 (19-90) | 81% | ||
| Gender | Female | 13 | 15.5% | |
| Male | 71 | 84.5% | ||
| Cirrhosis status |
No | 35 | 42.2% | |
| Yes | 48 | 57.8% | ||
| Child-Turcotte-Pugh | A | 58 | 69.9% | |
| B | 22 | 26.5% | ||
| C | 3 | 3.6% | ||
| Tumor nodularity |
Uninodular | 34 | 41% | |
| Multinodular | 49 | 59% | ||
| The largest tumor size | ≤ 5 cm | 30 | 36.6% | |
| >5 cm | 52 | 63.4% | ||
| Portal Vein invasion | No | 47 | 56.6% | |
| Yes | 36 | 43.4% | ||
| Hepatitis Infection | HBV | Positive | 36 | 42.9% |
| Negative | 48 | 57.1% | ||
| HCV | Positive | 8 | 9.6% | |
| Negative | 76 | 90.4% | ||
| The BCLC stage | Very early | 1 | 1.2% | |
| Early | 18 | 21.4% | ||
| Intermediate | 13 | 15.5% | ||
| Advanced | 50 | 59.5% | ||
| Terminal | 2 | 2.4% | ||
| The first-line treatments | Surgery | 9 | 10.7% | |
| RFA or MVA | 7 | 8.3% | ||
| TACE or TARE | 22 | 26.2% | ||
| Systemic cytotoxic treatment | 24 | 28.6% | ||
| Tyrosine kinase | 9 | 10.7% | ||
| BSC | 4 | 4.8% | ||
| Not reported | 9 | 10.7% | ||
| Body Mass Index (BMI) | Normal weight | 30 | 40% | |
| Overweight | 30 | 40% | ||
| Obese | 15 | 20% | ||
Abbreviations: The BCLC: Barcelona Clinic Liver Cancer, TACE: transarterial chemoembolization, TARE: transarterial radioembolization, RFA: radiofrequency ablation, MWA: micro wave ablation, TKIs: tyrosine kinase inhibitors, BSC: best supportive care.
Figure 1.
A. In general population, the estimated median overall survival rate was estimated by the Kaplan-Meier (the time defined from date of diagnosis to the date of death or the last follow-up date), Figure 1 B. The survival rates of HCC patients according to the BCLC staging system were estimated by the Kaplan-Meier, Figure 1 C. The survival rates of HCC patients according to serum VEGF-A level were estimated by the Kaplan-Meier, Figure 1 D. The survival rates of HCC patients according to tumor related portal vein invasion status were estimated by the Kaplan-Meier.
Figure 2.
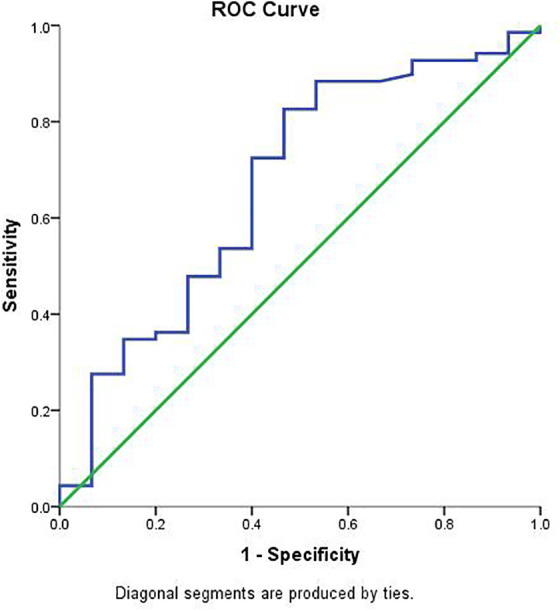
ROC curve was obtained by plotting the relationship between the specificity and the sensitivity at different cut-off levels of the serum VEGF-A.
Table 2.
Circulating the VEGF-A Level According to Different Features of Patients With HCC.
| Patients characteristic | Groups of variable | The mean circulating VEGF-A level (range) | P value |
|---|---|---|---|
| Age |
≤ 60 | 241.6 (26.3-1414.1) | 0.6 |
| >60 | 272.5 (45.7-1367.1) | ||
| Gender | Female | 277.5 (39.1-277.1) | 0.8 |
| Male | 258.5 (26.3-1414.1) | ||
| Cirrhosis status | No | 322.5 (28.5-1414.1) | 0.03* |
| Yes | 193.9 (26.3-772.1) | ||
| The largest tumor size | ≤ 5 cm | 132.7 (26.3-345.9) | <0.001* |
| >5 cm | 342.1 (28.5-1414.1) | ||
| Number of Tumor lesions | Uninodularity | 260.7 (28.5-1414.1) | 0.9 |
| Multinodularity | 265.1 (26.3-1367.1) | ||
| Lymph node involvement | No | 258.1 (28.5-1414.1) | 0.8 |
| Yes | 270.2 (30.4-849.2) | ||
| Portal vein involvement | No | 212.3 (26.3-1367.1) | 0.06 |
| Yes | 332.1 (28.5-1414.1) | ||
| Serum AFP level | ≤ 400 | 174.8 (26.3-1367.1) | 0.001* |
| >400 | 407.2 (76.3-1414.1) | ||
| Serum ALT value | ≤ 40 | 225.8 (26.3-1072.9) | 0.1 |
| >40 | 318.4 (28.5-1414.1) | ||
| Serum AST level |
≤45 | 179.6 (28.9-1072.9) | 0.012* |
| >45 | 324.4 (26.3-1414.1) | ||
| Serum Bilirubin | ≤ 2 | 243.5 (28.5-1367.1) | 0.2 |
| >2 | 362.4 (26.3-1414.1) | ||
| Hepatitis infection (HBV or HCV) | Negative | 342.9 (26.3-1414.1) | 0.006* |
| Positive | 174.5 (28.5-843.1) | ||
| Albumin | ≤3.5 | 307.6 (28.9-1367.1) | 0.3 |
| >3.5 | 241.1 (26.3-1414.1) | ||
| INR | ≤1.2 | 262.9 (28.5-1367.1) | 0.9 |
| >1.2 | 255.9 (26.3-1414.1) | ||
| The BCLC stage | Very early | 132.9 (132.9 -132.9) | 0.02* |
| Early | 148.8 (55.5-286.3) | ||
| Intermediate | 149.3 (26.3-498.9) | ||
| Advanced | 318.2 (28.5-1414.1) | ||
| Terminal | 650.2 (457.4-843.1) | ||
| The first-line treatment | Surgery | 181.6 (60.6-286.3) | 0.3 |
| Systemic cytotoxic treatment | 331.3 (28.5-1414.1) | ||
| Tyrosine kinase | 379.7 (30.4-1367.1) | ||
| RFA or MVA | 123.8 (79.1-170.3) | ||
| TACE or TARE | 211.5 (26.3-772.1) | ||
| BSC | 382.5 (86.9-843.1) | ||
| Not reported | 212.1 (55.5-480.2) | ||
| Child-Turcotte-Pugh class | A | 242.5 (28.5-1367.1) | 0.07 |
| B | 270.6 (26.3-1414.1) | ||
| C | 618.8 (457.4-843.1) |
The ROC curve and AUC analyses were performed to determine the optimal cutoff values for serum VEGF-A level and 100pg/mL value was chosen as the optimal cut-off value with 73.9% sensitivity and 53.3% specificity. The AUC value was 0.669 (95% = 0.509–0.829) (p = 0.041) in predicting OS (Figure 2). We therefore finally stratified patients into 2 groups using 100pg/mL as the serum VEGF-A cutoff value. Based on serum VEGF-A levels, there was significant difference between overall survival of patients with serum VEGF-A level <100 pg/mL and patient with serum VEGF-A levels ≥100 pg/mL (p = 0.01) (Figure 3C). The median OS rates of patients with <100 pg/mL versus ≥100 pg/mL were 14.3 and 5.9 months, respectively. In terms of serum AFP level, patients were divided into 2 groups: patients with AFP level ≤ 400ng/ml versus AFP level >400 ng/ml. Notably, we found statistically significant positive correlation between serum VEGF-A and AFP levels. Patients with serum VEGF-A level lower than 100pg/mL tended to have lower levels of serum AFP level lower (p = 0.001). The univariate cox regression analysis was conducted to determine the effect of serum VEGF-A levels, serum AFP levels, portal vein involvement status, AST, ALT, bilirubin, the CTP classes, LDH, lymph node status, age, and gender on survival (Table 3). These all parameters had significant effect on survival except gender of patients. The significant prognostic factors identified by the univariate analysis were entered into a Cox proportional hazard model for multivariate analysis. Serum VEGF-A ≥100 pg/ml (p = 0.002), and portal vein invasion positive (p < 0.001) were identified as independent prognostic factors (Table 4). Among these clinical features of HCC patients, tumor related vascular invasion status were positive in 36 patients (42.9%), and in 47 (56%) of patients were negative. The median OS of patients differed with regard to tumor vascular invasion status, mOS was 4.1 months in patients with tumor vascular invasion while it was 13.5 months in patients with tumors having no vascular invasion (p = 0.002) (Figure 4D). We also evaluated the laboratory parameters of patients with HCC. The median serum LDH level was 250.5 (76-1073), ALT 35(8-268), AST 50(16-620), ALP 153.5(56-1222), GGT 144.5(25-1719), and AFP 128.5(1.2-286.748). Our patients were assessed and classified according to their weight, 30 of patients were overweight, 15 of patients were obese, and 30 of patients were normal weight; there was no relation between VEGF-A and BMI subgroups. There was no significant difference between the first-line treatments in terms of their mean serum VEGF-A levels (p = 0.3). Among the first-line treatments, the mean serum VEGF-A level, in surgery resection group was 181.6 (60.6-286.3), in systemic cytotoxic treatment group was 331.3 (28.5-1414.1), in tyrosine kinase inhibitors group was 379.7 (30.4-1367.1), in the local ablative group (RFA or MVA) was 123.8 (79.1-170.3), in the local palliative group (TACE or TARE) was 211.5 (26.3-772.1), in BSC group was 382.5(86.9-843.1), and in patients group whose the first-line treatment couldn’t be determined was 212.1 (55.5-480.2). The mean values of circulating VEGF-A according to different treatment modalities and other clinical or laboratory features were calculated and listed in Table 2. The median follow-up time was 59.7 months (range 37.9–81.4 months).
Table 3.
Cox Regression Univariate Analysis and Prognostic Factors for Survival.
| Hazard Ratio (95% CI)¥ | P value | |
|---|---|---|
| VEGF-A ≥100 vs. VEGF-A <100 | 1.99 (1.16-3.39) | 0.012* |
| Bilirubin >2 vs. Bilirubin ≤ 2 | 2.17 (1.18-4.02) | 0.013* |
| Portal vein invasion positive vs. negative | 2.36 (1.45-3.85) | 0.001* |
| AFP >400 vs. AFP ≤ 400 | 1.95 (1.19-3.18) | 0.008* |
| Male vs. Female | 0.78 (0.40 -1.48) | 0.44 |
| LDH >215 vs. LDH ≤ 215 | 3.24 (1.74-6.05) | <0.001* |
| AST >45 vs. AST ≤ 45 | 2.64 (1.57-4.44) | <0.001* |
| ALT >40 vs. ALT ≤ 40 | 1.70 (1.05-2.76) | 0.031* |
| CTP class B vs. CTP class A | 2.03 (1.19-3.45) | 0.009* |
| CTP class C vs. CTP class A | 39.1 (8.9-170.7) | <0.001* |
| Lymph node positive vs. negative | 1.77 (1.04-3.02) | 0.037* |
| Age >60 vs. Age ≤ 60 | 1.93 (1.12-3.31) | 0.017* |
Abbreviations: * statistically significant, VEGF-A vascular endothelial growth factor A, AFP α-fetoprotein, ALT alanine transaminase, AST aspartate transaminase, LDH lactic acid dehydrogenase, CTP The Child-Turcotte-Pugh system, TACE: transarterial chemoembolization, TARE: transarterial radioembolization, RFA: radiofrequency ablation, MWA: micro wave ablation, TKIs: tyrosine kinase inhibitors, BSC: best supportive care, p < 0.05 was considered as significant.
Table 4.
Independent Prognostic Factors by Multivariate Analysis.
| Variable | Relative risk of death (95% confidence interval) |
P-value |
|---|---|---|
| VEGF-A ≥100 vs. VEGF-A < 100 | 2.47 (1.38-4.45) | 0.002* |
| Portal vein invasion positive vs. negative | 2.56 (1.51-4.33) | <0.001* |
Discussion
Our prospective study demonstrated the significant prognostic effect of circulating VEGF-A on survival in patients with HCC. Additionally, in this study, we were able to show the relationship between circulating VEGF-A level and several clinical and pathological features of HCC. These results indicate that VEGF-A may be a potential prognostic biomarker of patients with HCC who especially have higher serum levels than determined cut-off value.
In this study, about 57.8% of Turkish HCC patients have cirrhosis; therefore, we were able to compare VEGF levels between cirrhotic and non-cirrhotic patients groups. Our cirrhotic HCC patients had significantly lower circulating VEGF-A levels than HCC patients with non-cirrhotic liver. Although some studies have already investigated the relationship between serum VEGF-A and cirrhotic status, the knowledge about the association between circulating levels of VEGF and liver cirrhosis is limited. In one of these studies, there was no statistically significant difference between VEGF levels of patients with cirrhotic and non-cirrhotic liver. Another study that has investigated this relationship has demonstrated the down-regulated serum levels of VEGF in the presence of portal hypertension which may be associated with the grade of hepatocyte regeneration.18,19 In this context, our study unravels and highlights relationship between cirrhotic status and VEGF in HCC patients.
Another impressive finding of the study was that we observed higher circulating VEGF-A levels in HCC patients with portal vein involvement than in patients without. There was a positive correlation between high circulating VEGF levels and tumor involvement of portal vein. The patients with early-stage HCC, high circulating VEGF levels have been shown to be associated with venous invasion in previous studies. Therefore, VEGF-A may have a role in terms of HCC tumor invasiveness.17,20 It is believed that portal vein involvement can trigger angiogenesis in various ways. One of these ways is the concerted effects of inflammation and hypoxia that have been associated with angiogenesis and were considered as 2 main drivers.21 After tissue damage, endothelial cells are activated and secreted a plethora of chemokines, ensued by increase in vascular permeability and migration of inflammatory cells.22 In the damaged tissue, inflammation occurs and leads to hypoxia due to altered blood flow. This induces production of hypoxia-inducing factors (HIFs) that upregulate the vascular endothelial growth factor (VEGF) gene and induces angiogenesis.23 These pathways may explain the relationship between circulating VEGF-A levels and portal vein involvement. Despite sufficient data that explain the potential mechanism of portal vein involvement in patients with HCC, there is limited published data on the relation of angiogenic VEGF-A level and portal vein involvement. Therefore, this study may shed some light on the association between angiogenic VEGF-A level and portal vein involvement.
The largest tumor size, portal vein involvement, and high serum AFP and AST level have been reported as prognostic factors in patients with HCC.24 The correlation between serum AFP levels and tumor differentiation, tumor burden, and early recurrence has been demonstrated especially in HCC patients who underwent tumor resection.25,26 One of the serum markers that reflect active chronic hepatitis is serum AST level, and it has been reported in the previous studies as an independent predictor of worse survival of HCC patients.27,28 In our patient population, we also found out serum AST ve AFP level as an independent predictive factor of survival, similar to previous studies, Table 3. Similarly, in a previous study, the largest tumor size and portal vein invasion have been associated with poor prognosis of HCC patients.28 Consequently, the prognostic value of serum AST, AFP level, the largest tumor size, portal vein involvement in HCC patients that have been reported were confirmed with our study.
Our prospective study demonstrated that circulating VEGF level was an independent prognostic factor for survival of patients with HCC. Similarly, in another study, patients who had circulating VEGF-A levels higher than 240 pg/mL had a worse survival than patients with lower levels of VEGF-A.16 Additionally, a statistically significant positive correlation was found between serum VEGF-A and tumor stage, and serum VEGF-A level was increasing as the patient’s stage advanced in our HCC patients’ population. The relation between circulating VEGF-A level and disease stage has been reported in Japanese patients with HCC. In this study, patients with metastatic disease had higher circulating VEGF-A level than localized disease.29 Our study has confirmed prospectively the results that have been reported in the literature regarding the association between circulating VEGF-A levels and the disease stage and survival of patients with HCC.
Our prospective study has some limitations. First, we haven’t evaluated other potential VEGF sources that may affect circulating VEGF-A levels such as platelet count. Although some reports point out that there might be a correlation between platelet count and circulating VEGF-A, the exact relationship between platelets and circulating VEGF-A remains unclear. Second, about 44% of patients had been included in our study after HCC diagnosed, and the types of treatment patients have received before the blood draw may have affected the serum VEGF-A level. Therefore, this situation should be taken into account when interpreting the results of the study.
In conclusion, this prospective study of Turkish patients with HCC has demonstrated the significant prognostic effect of circulating VEGF-A levels on survival of patients with HCC as reported in literature. Since the patients are at different stages and receiving different treatment methods, a significant relationship that was found between serum VEGF-A level and survival, as one of the results of our study need to be interpreted cautiously. Our results indicate that circulating VEGF-A level is a potential prognostic biomarker of patients with HCC and can be exploited in patient management. Similarly, we were able to investigate the relationship between circulating VEGF-A levels and clinicopathological features, such as the largest tumor size, serum AFP levels, tumor related portal vein involvement which stratified patients into prognostic subgroups. These results that obtained from circulating VEGF-A study may help to predict treatment response and guide treatment modalities, further studies will be worthwhile to elucidate its exact role.
Acknowledgments
Thanks to all our patients.
Authors’ Note: Approval date and number: 04.03.2015 and 15/153.
Author Contributions: Concept—Suayib Yalcin, Sahin Lacin; Design—Suayib Yalcin, Sahin Lacin,; Supervision—Suayib Yalcin; Resources—Suayib Yalcin, Sahin Lacin; Data Collection and/or Processing—Suayib Yalcin, Sahin Lacin; Analysis and/or Interpretation—Suayib Yalcin, Sahin Lacin; Literature Search—Suayib Yalcin, Sahin Lacin; Writing Manuscript—Suayib Yalcin, Sahin Lacin; Critical Review—Suayib Yalcin, Sahin Lacin,
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Committee Approval: Ethics Committee of the Hacettepe University Faculty of Medicine
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sahin Lacin, MD  https://orcid.org/0000-0002-8770-9842
https://orcid.org/0000-0002-8770-9842
Informed Consent: Written Consent obtained
References
- 1. Salven P, Manpaa H, Orpana A, Alitalo K, Joensuu H. Serum vascular endothelial growth factor is often elevated in disseminated cancer. Clin Cancer Res. 1997;3(5):647–651. [PubMed] [Google Scholar]
- 2. Salven P, Teerenhovi L, Joensuu H. A high pretreatment serum vascular endothelial growth factor concentration is associated with poor outcome in non-Hodgkin’s lymphoma. Blood. 1997;90(8):3167–3172. [PubMed] [Google Scholar]
- 3. Kumar H, Heer K, Lee PW, et al. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res. 1998;4(5):1279–1285. [PubMed] [Google Scholar]
- 4. Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg. 2002;236(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333(26):1757–1763. [DOI] [PubMed] [Google Scholar]
- 6. Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and Ephrins in vascular development. Genes Dev. 1999;13(9):1055–1066. [DOI] [PubMed] [Google Scholar]
- 7. Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19(4):1207–1225. [DOI] [PubMed] [Google Scholar]
- 8. Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41:278–286. [DOI] [PubMed] [Google Scholar]
- 9. Hariawala MD, Horowitz JR, Esakof D, et al. VEGF improves myocardial blood flow but produces EDRF-mediated hypotension in porcine hearts. J Surg Res. 1996;63(1):77–82. [DOI] [PubMed] [Google Scholar]
- 10. Grone HJ, Simon M, Grone EF. Expression of vascular endothelial growth factor in renal vascular disease and renal allografts. J Pathol. 1995;177(3):259–267. [DOI] [PubMed] [Google Scholar]
- 11. Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet Dev. 2001;11(3):293–299. [DOI] [PubMed] [Google Scholar]
- 12. Kuwai T, Kitadai Y, Tanaka S, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105(2):176–181. [DOI] [PubMed] [Google Scholar]
- 13. Li XM, Tang ZY, Zhou G, Lui YK, Ye SL. Significance of vascular endothelial growth factor mRNA expression in invasion and metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res. 1998;17(1):13–17. [PubMed] [Google Scholar]
- 14. Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116(6):838–845. [DOI] [PubMed] [Google Scholar]
- 15. Yao DF, Wu XH, Zhu Y, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4(2):220–226. [PubMed] [Google Scholar]
- 16. Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol. 2007;14(6):1835–1845. [DOI] [PubMed] [Google Scholar]
- 17. Poon RT, Ng IO, Lau C, et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233(2):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poon RT, Lau CP, Cheung ST, Yu WC, Fan ST. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63(12):3121–3126. [PubMed] [Google Scholar]
- 19. Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5(4):296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li XM, Tang ZY, Qin LX, Zhou J, Sun HC. Serum vascular endothelial growth factor is a predictor of invasion and metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 1999;18(4):511–517. [PubMed] [Google Scholar]
- 21. Elpek GÖ. Angiogenesis and liver fibrosis. World J Hepatol. 2015;7(3):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruno A, Pagani A, Pulze L, et al. Orchestration of angiogenesis by immune cells. Front Oncol. 2014;4:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novo E, Povero D, Busletta C, et al. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. J Pathol. 2012;226(4):588–597. [DOI] [PubMed] [Google Scholar]
- 24. Mondazzi L, Bottelli R, Brambilla G, et al. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19(5):1115–1123. [PubMed] [Google Scholar]
- 25. Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64(8):1700–1707. [DOI] [PubMed] [Google Scholar]
- 26. Matsumoto K, Yoshimoto J, Sugo H, et al. Relationship between the histological degrees of hepatitis and the postoperative recurrence of hepatocellular carcinoma in patients with hepatitis C. Hepatol Res. 2002;23(3):196–201. [DOI] [PubMed] [Google Scholar]
- 27. Poon RT, Fan ST, Lo CM, et al. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18(5):1094–1101. [DOI] [PubMed] [Google Scholar]
- 28. Poon RT, Lau C, Yu WC, et al. High serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective study. Oncol Rep. 2004;11(5):1077–1084. [PubMed] [Google Scholar]
- 29. Jinno K, Tanimizu M, Hyodo I, et al. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998;33(3):376–382. [DOI] [PubMed] [Google Scholar]