Abstract
Objectives:
The clinical benefit of conventional disease-modifying antirheumatic drugs (cDMARDs) for treating ankylosing spondylitis (AS) is generally limited to improvements in peripheral arthritis. However, cDMARDs could be conditionally considered as alternatives to established drugs for improving axial manifestations in exceptional circumstances. However, there are few studies of the impact of cDMARDs on radiographic progression outcomes. Therefore, we investigated the effectiveness of cDMARDs on radiographic progression in AS.
Methods:
Among 1280 AS patients at a single hospital from 2000 to 2018, 301 who had been treated with sulfasalazine (SSZ) or methotrexate (MTX) were enrolled. For each patient, the entire follow-up period was split into 1-year intervals. Each interval was classified as either an “on-cDMARD” interval, which was a period of treatment with SSZ alone, MTX alone, or a combination of SSZ and MTX, or an “off-cDMARD” interval, which was a period without cDMARD treatment. Radiographic progression was scored using the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS). The relationship between cDMARD use and radiographic progression within the intervals, defined as the rate of mSASSS progression, was investigated using linear models with adjustment for potential confounding covariates and for clustering among observations from the same patient.
Results:
The 732 on-cDMARD intervals and 1027 off-cDMARD intervals were obtained from enrolled patients. In multivariable regression analysis, there was no significant association between cDMARDs and the rate of mSASSS progression (β = −0.081, p = 0.418). The mean adjusted mSASSS change per year was 0.610 from on-cDMARD intervals and 0.691 from off-cDMARD intervals.
Conclusion:
Treatment with cDMARDs may not reduce radiographic progression in AS patients.
Keywords: ankylosing spondylitis, disease-modifying antirheumatic drugs, radiographic progression, sulfasalazine, methotrexate
Introduction
Conventional disease-modifying antirheumatic drugs (cDMARDs) are widely used to treat rheumatic diseases. For patients with spondyloarthritis (SpA), cDMARDs have been recommended to treat peripheral arthritis.1–3 However, in situations where non-steroidal anti-inflammatory drugs (NSAIDs) are contraindicated or not tolerated, or in resource-poor settings where tumor necrosis factor (TNF) inhibitors are not readily accessible, cDMARDs could be cautiously considered as an alternative.1–3
Although guidelines state that the conditional use of cDMARDs should be considered primarily in exceptional circumstances and with low expectations for efficacy in patients with predominantly axial manifestations, some previous studies have shown that cDMARDs offer some benefit for improving axial symptoms.4,5 Indeed, cDMARDs still constitute a portion of the treatment armamentarium for patients with ankylosing spondylitis (AS) in some regions, and the prescription frequency varies between countries, ranging from 10% to 87%.3,6–9 National reimbursement policies or financial constraints might partly contribute to inter-country variation.6,7,10 However, given the nature of slowly progressing disease, there have been few examinations of the anti-inflammatory effect of cDMARDs on the axial skeleton over a long observation period among large patient samples. Additionally, no studies have evaluated the impact of cDMARDs on radiographic progression using validated outcome measures in patients with AS.
Previously, we demonstrated TNF inhibitor effectiveness for slowing radiographic progression using time intervals during treatment.11 However, it was difficult to evaluate the effect of cDMARDs on radiographic progression because the study design was focused on TNF inhibitors. Therefore, we investigated the effectiveness of cDMARDs on spinal radiographic progression from real-world longitudinal data based on a different design than previously used.
Materials and methods
Patients and clinical assessment
Data from a single-center cohort of patients with AS between January 2001 and December 2018 were retrospectively reviewed. Among a total of 1280 patients who satisfied the modified New York criteria,12 301 patients treated with sulfasalazine (SSZ) or methotrexate (MTX) who also had at least two sets of spinal radiographs were included. This study was conducted in accordance with the Helsinki Declaration and was approved by the Hanyang University Seoul Hospital Institutional Review Board (HYUH 2014-04-010). The need for patient consent was waived by the institutional review board because of the retrospective nature of our study.
Demographic and clinical features including age, sex, disease duration, human leukocyte antigen (HLA)-B27 positivity, history of uveitis, peripheral arthritis, serum erythrocyte sediment rate (ESR) and C-reactive protein (CRP) levels, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and concomitant drugs were obtained from patient medical records.
Radiographic assessment
Radiographic images of the cervical and lumbar spine were obtained from 1280 patients and were independently scored according to the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) by two radiologists.13 The average number (standard deviation) (SD) of mSASSS measurements per patient during the entire period was 4.6 (1.2) and the average interval (SD) between mSASSS measurements was 2.4 (0.7) years. Two readers who were blinded to patients’ clinical data scored radiographs in chronological order. The intra-class correlation coefficient (ICC) values for intra-observer reliability [ICC = 0.978 (95% confidence interval (CI)): 0.976, 0.979] and inter-observer reliability [ICC = 0.946 (95% CI: 0.941, 0.950)] were both excellent.
Time intervals according to cDMARD treatment
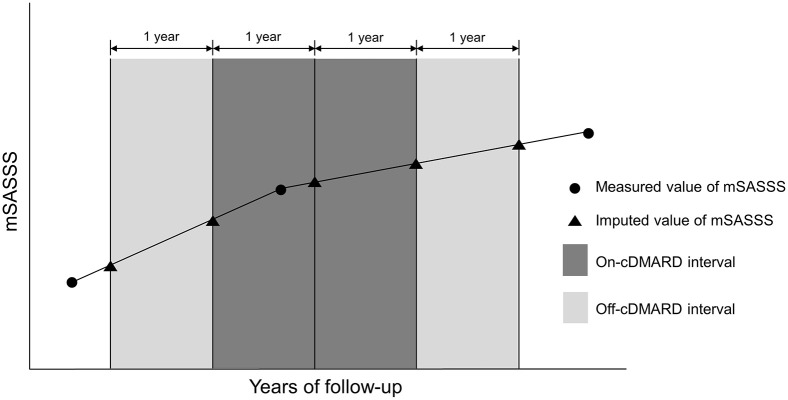
Time intervals were created for each of the 301 patients who were included in the analysis. The intervals were based on cDMARD treatment and the period of exposure to TNF inhibitors was not included. To capture as much data as possible, the interval included the period from the baseline to immediately before TNF inhibitor initiation if the treatment was switched to TNF inhibitors during the entire follow-up period. Then, the follow-up period was split into 1-year intervals and radiographic progression within each interval was estimated according to the rate of mSASSS increase (i.e. progression) over a year. Each interval was classified as either an “on-cDMARD” interval, which was the treatment period with SSZ alone, MTX alone, or a combination of SSZ and MTX, or an “off-cDMARD” interval, which was the period without cDMARD treatment. SSZ, MTX, NSAIDs, and glucocorticoids were considered to be sustained during the interval if they were prescribed during more than half of the interval. The mSASSS values at the beginning and end points in each interval were imputed with a linear interpolation method assuming a linear course during the time interval (Figure 1). ESR, CRP, and BASDAI were also imputed at each time point of the intervals by linear interpolation.
Figure 1.
Definition of on-cDMARD intervals and off-cDMARD intervals. The mSASSS values at the beginning and end points in each interval were imputed with a linear interpolation method using the values measured before and after each time point.
cDMARD, conventional disease-modifying antirheumatic drug; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score.
Statistical analyses
The relationship between radiographic progression, which was measured as the rate of mSASSS progression, and clinical factors was investigated based on interval-level data. Highly skewed clinical factors were transformed using a log or a square-root function. To account for correlations between repeated measurements, we used linear models with an exchangeable correlation structure estimated by generalized estimating equations. Any variables with a potentially significant impact on the outcome (p-value ⩽ 0.1) in the univariate models were entered into the multivariable regression models. HLA-B27 was excluded due to its high frequency of positives and non-significant p-value. Additionally, CRP and BASDAI were excluded due to a high correlation with ESR and many missing values, respectively. The impact of cDMARDs on radiographic progression was analyzed in the multivariable model with adjustment for potential confounding covariates and imputation of missing values (model 1). In model 2, the impact of SSZ and MTX were estimated individually. The mean mSASSS change per year according to whether cDMARDs were administered was also calculated from the multivariable models with confounding factors fixed. All statistical analyses were performed using the R statistical language version 3.5.1. p-values less than 0.05 were considered statistically significant.
Results
Patient characteristics
Of the 1280 patients enrolled, 301 patients who had been treated with cDMARDs and who had sufficient clinical data and sets of radiographs to create at least one on-cDMARD interval and one off-cDMARD interval were selected. Patient clinical characteristics are described in Table 1. Most patients were male (86.0%), positive for HLA-B27 (97.0%), and the mean (SD) age at diagnosis was 31.4 (9.2) years. Eye involvement and peripheral arthritis was observed in 41.4% and 60.8% of cases, respectively.
Table 1.
Baseline demographic characteristics of the patients with ankylosing spondylitis.
| Variable | No. with data | Value, mean (SD) or n (%) |
|---|---|---|
| Age at diagnosis, mean (SD), years | 301 | 31.41 (9.17) |
| Male, n (%) | 301 | 259 (86.0%) |
| Follow-up duration, mean (SD), years | 301 | 6.36 (3.42) |
| HLA-B27 positive, n (%) | 299 | 290 (97.0%) |
| Eye involvement, n (%) | 268 | 111 (41.4%) |
| Peripheral joint involvement, n (%) | 268 | 163 (60.8%) |
HLA, human leukocyte antigen; SD, standard deviation.
Time interval characteristics
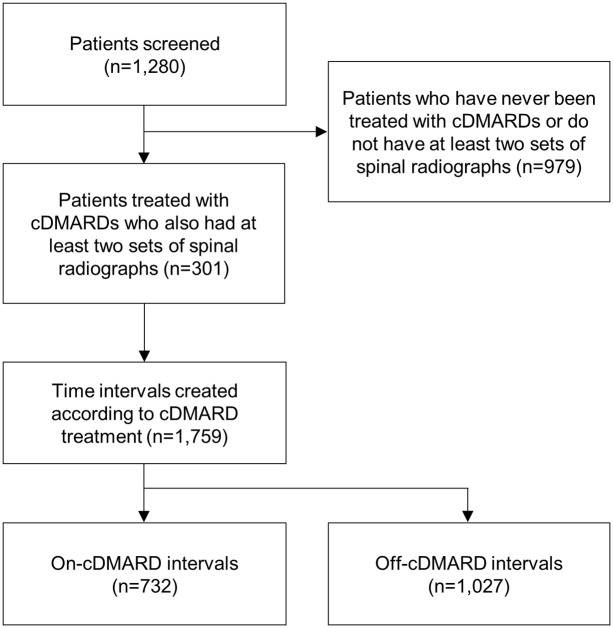
Among the 301 patients, 1759 intervals comprising 732 on-cDMARD intervals and 1027 off-cDMARD intervals were obtained (Figure 2). Among the on-cDMARD intervals, the number of intervals for SSZ treatment alone, MTX treatment alone, and combined SSZ and MTX was 704, 146, and 118, respectively. Clinical characteristics based on the intervals are summarized in Table 2. Gender, HLA-B27 positivity, eye involvement, and peripheral arthritis were investigated according to cDMARD interval. Additionally, mean (SD) values for age, ESR, CRP, BASDAI, and mSASSS at the start of the intervals were calculated. The mean mSASSS (SD) at the start of on-cDMARD intervals was 12.35 (13.20) and the mean at the start of off-cDMARD intervals was 14.18 (15.42).
Figure 2.
Flowchart of creating time intervals for each of the patients.
cDMARDs, conventional disease-modifying antirheumatic drugs.
Table 2.
Clinical characteristics of time intervals classified according to cDMARD treatment.
| Variable | Total intervals (n = 1759) |
On-cDMARD interval (n = 732) | Off-cDMARD interval (n = 1027) | |
|---|---|---|---|---|
| No. with data | Value, mean (SD) or n (%) | |||
| Age at the interval start, mean (SD), years | 1759 | 30.84 (8.61) | 31.56 (9.17) | 30.33 (8.15) |
| Female, n (%) | 1759 | 221 (12.6%) | 113 (15.4) | 108 (10.5) |
| HLA-B27 positive, n (%) | 1749 | 1695 (96.9%) | 702 (96.7) | 993 (97.1) |
| Eye involvement, n (%) | 1596 | 604 (37.8%) | 258 (38.7) | 346 (37.2) |
| Peripheral joint involvement, n (%) | 1599 | 907 (56.7%) | 454 (68.4) | 453 (48.4) |
| ESR at the interval start, mean (SD), mm/hr | 1740 | 24.22 (23.50) | 29.16 (27.68) | 20.75 (19.32) |
| CRP at the interval start, mean (SD), mg/dL | 1740 | 1.52 (1.46) | 1.85 (1.88) | 1.28 (1.00) |
| BASDAI at the interval start, mean (SD) | 682 | 3.55 (1.82) | 3.93 (1.82) | 3.39 (1.80) |
| NSAIDs*, n (%) | 1759 | 1022 (58.1%) | 486 (66.4) | 536 (52.2) |
| Glucocorticoids*, n (%) | 1759 | 118 (6.7%) | 101 (13.8) | 17 (1.7) |
| cDMARDs*, n (%) | 1759 | 732 (41.6%) | SSZ, n = 704 (96.2) MTX, n = 146 (19.9) |
– |
| mSASSS change per year, mean (SD) | 1759 | 0.65 (1.42) | 0.55 (1.36) | 0.73 (1.46) |
Considered to be sustained during the interval if they were prescribed during more than half of the interval.
BASDAI, Bath Ankylosing Spondylitis Activity Index; cDMARD, conventional disease-modifying antirheumatic drug; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HLA, human leukocyte antigen; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; MTX, methotrexate; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation; SSZ, sulfasalazine.
Association between covariates and radiographic progression
Table 3 shows the association between covariates and the rate of mSASSS progression. The following variables were identified as potentially related to the outcome in univariate regression analyses: age [β = 0.022 (95% CI: 0.010, 0.034) p < 0.001)], ESR (log) at the interval start [β = 0.195 (95% CI: 0.107, 0.283) p < 0.001], CRP (log) at the interval start [β = 0.285 (95% CI: 0.097, 0.472) p = 0.003], and eye involvement [β = 0.623 (95% CI: 0.298, 0.948) p < 0.001] were associated with an increase in the mSASSS progression rate. Meanwhile, female patients and patients with peripheral arthritis were associated with a reduction in the mSASSS progression rate [β = −0.290 (95% CI: −0.586, 0.006) p = 0.055 and β = −0.540 (95% CI: −0.857, −0.223) p = 0.001, respectively]. Taking NSAIDs or glucocorticoids was found to have no significant effect on mSASSS progression.
Table 3.
Association between covariates and the rate of mSASSS progression in the regression models.
| Variable | Univariate analysis |
Multivariable analysis* |
||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
|||||
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| Sex (female) | −0.290 (−0.586, 0.006) | 0.055 | −0.449 (−0.782, −0.117) | 0.008 | −0.440 (−0.775, −0.105) | 0.010 |
| Age | 0.022 (0.010, 0.034) | <0.001 | 0.012 (−0.001, 0.026) | 0.061 | 0.012 (−0.001, 0.025) | 0.076 |
| Eye involvement | 0.623 (0.298, 0.948) | <0.001 | 0.572 (0.264, 0.880) | <0.001 | 0.577 (0.268, 0.886) | <0.001 |
| Peripheral joint involvement | −0.540 (−0.857, −0.223) | 0.001 | −0.508 (−0.810, −0.206) | 0.001 | −0.513 (−0.817, −0.210) | 0.001 |
| HLA-B27 positivity | 0.065 (−0.415, 0.546) | 0.790 | − | − | − | − |
| ESR at the interval start (log) | 0.195 (0.107, 0.283) | <0.001 | 0.176 (0.087, 0.265) | <0.001 | 0.178 (0.088, 0.268) | <0.001 |
| CRP at the interval start (log)† | 0.285 (0.097, 0.472) | 0.003 | − | − | − | − |
| BASDAI at the interval start (square-root) | −0.039 (−0.455, 0.378) | 0.856 | − | − | − | − |
| NSAIDs | 0.004 (−0.195, 0.204) | 0.966 | − | − | − | − |
| Glucocorticoids | 0.097 (−0.231, 0.425) | 0.561 | − | − | − | − |
| cDMARDs | −0.078 (−0.290, 0.135) | 0.474 | −0.081 (−0.276, 0.115) | 0.418 | − | − |
| Sulfasalazine | − | − | − | − | −0.011 (−0.211, 0.189) | 0.913 |
| Methotrexate | − | − | − | − | −0.180 (−0.439, 0.078) | 0.172 |
Variables that were significant in univariate analysis at p-value ⩽ 0.1 were included.
Excluded from multivariable analysis due to its well-known high correlation with ESR.
BASDAI, Bath Ankylosing Spondylitis Activity Index; cDMARDs, conventional disease-modifying antirheumatic drugs; CI, confidence interval; HLA, human leukocyte antigen; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; NSAIDs, non-steroidal anti-inflammatory drugs.
Impact of cDMARDs on radiographic progression
Multivariable regression analysis was performed to confirm the association between cDMARDs and radiographic progression with adjustment for variables that were potentially significant in the univariate models (Table 3). In model 1, eye involvement and log-transformed ESR at the interval start were significantly associated with an increase in progression of radiographic damage [β = 0.572 (95% CI: 0.264, 0.880) p < 0.001 and β = 0.176 (95% CI: 0.087, 0.265) p < 0.001, respectively]. Being female and peripheral arthritis remained significantly associated with a reduced rate of mSASSS progression [β = −0.449 (95% CI: −0.782, −0.117) p = 0.008 and β = −0.508 (95% CI: −0.810, −0.206) p = 0.001, respectively]. In contrast, there was no significant association between cDMARD use and the rate of mSASSS progression with adjustment for independent significant confounders [β = −0.081 (95% CI: −0.276, 0.115) p = 0.418].
Multivariable model 2 analyzed the individual associations of SSZ and MTX with radiographic progression. Significant variables included eye involvement, log-transformed ESR at the start of the interval, being female, and peripheral joint involvement, as in model 1. Neither SSZ nor MTX were significantly associated with a reduced radiographic progression rate in their respective intervals [β = −0.011 (95% CI: −0.211, 0.189) p = 0.913 and β = −0.180 (95% CI: −0.439, 0.078) p = 0.172].
Adjusted mSASSS change per year according to cDMARD treatment
Mean adjusted mSASSS changes per year are shown in Table 4. mSASSS change per year with or without cDMARDs was estimated from multivariable regression model 1, while other covariates were fixed at their mean values (0.610 versus 0.691). Results according to monotherapy or combination therapy were also estimated from model 2 (0.485 for SSZ-MTX combination therapy, 0.665 for SSZ monotherapy, and 0.496 for MTX monotherapy).
Table 4.
Mean mSASSS change within cDMARD intervals estimated from the multivariable models.
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| cDMARD | Non-cDMARD | SSZ-MTX combination | SSZ | MTX | Non-SSZ and non-MTX | |
| Adjusted mSASSS change per year* | 0.610 | 0.691 | 0.485 | 0.665 | 0.496 | 0.676 |
Adjusted for sex, eye involvement, peripheral joint involvement, and ESR at the interval start (log).
cDMARD, conventional disease-modifying antirheumatic drug; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; MTX, methotrexate; SSZ, sulfasalazine.
Discussion
In this study, we showed that treatment with cDMARDs including SSZ and MTX does not decelerate radiographic damage progression in the spine. To our knowledge, this is the first study to show that cDMARDs have no inhibitory effect on radiographic progression using mSASSS values. Our results underpin the established recommendations for a limited role of cDMARDs for treating AS.
cDMARDs have been shown to prevent radiographic progression in rheumatoid arthritis (RA), as well as decrease pain and inflammation.14 However, cDMARDs in AS have been recognized as ineffective for suppressing inflammation in the axial skeleton,15–17 and they have only been investigated in a limited number of studies of patients with predominant axial disease.18,19 Although a placebo-controlled study showed that SSZ has some efficacy for improving axial manifestations, only a small proportion of the subjects reported an effect, and the observation period was relatively short given the slowly progressing nature of the disease.5 On the other hand, there is growing evidence that long-term treatment with biological DMARDs (bDMARDs), such as a TNF inhibitor or an interleukin (IL)-17 inhibitor, is associated with a preventive effect on spinal radiographic progression.20,21 In contrast to bDMARDs, our results showed that cDMARDs have no efficacy of retarding radiographic damage in the spine.
Unlike RA, the major pathological process associated with SpA is confined to the entheses, which are the interfaces between tendons or ligaments and bone, and the interface between cartilage and bone predominantly in the sacroiliac joints and spine.22 Mechanical stress or infection to these structures may trigger entheseal inflammation and adjacent osteitis, which is often followed by a subsequent tissue response process leading to syndesmophyte formation in the spine.23,24 However, cDMARDs, well known for their efficacy for synovitis, do not appear to effectively suppress entheseal inflammation.25,26 Furthermore, recent findings suggest that activation of the IL-23/IL-17 pathway is implicated in expanding entheseal inflammation and indirectly promoting new bone formation at entheseal sites.27 Additionally, TNF has an osteogenic differentiation effect that it might exhibit at enthesitis sites where osteoclasts and tendon-derived osteoblasts may be disconnected.28,29 In the context of immunopathology, this paradoxical action of cytokines in bone dynamics can partly account for pathogenic bone formation in AS.30 Thus, blockade of the mechanisms and involved cytokines related to enthesitis-induced bone formation would be a superior approach to cDMARDs whose anti-inflammatory effect on enthesitis is unclear. Indeed, IL-17 inhibition was observed to slow radiographic progression after 2 years of follow-up, and low radiographic changes were sustained at 4 years of follow-up.31,32
Additionally, according to studies based on magnetic resonance imaging (MRI), new syndesmophytes are more likely to develop at sites of prior inflammatory lesions in the spine and new bone formation may also proceed through a process of subsequent fat metaplasia following inflammation.33–35 Therefore, targeting early resolution of inflammatory lesions and prevention of reparative processes would support slowing radiographic progression.36 This inflammation-suppression-mediated effect has been confirmed in recent studies.11,37,38 However, the anti-inflammatory effect of cDMARDs has been shown to be weaker than that of TNF inhibitors in a randomized controlled study,39 and improvement of sacroiliac joint and spine inflammation according to MRI scores in patients with early axial SpA was significantly less in the SSZ-treated group than the etanercept-treated group.40,41 The inadequate efficacy of cDMARDs to suppress inflammation in the axial skeleton, despite previous reports of easing inflammatory back pain,5 would not in turn lead to an inhibitory effect on structural damage progression. Accordingly, based on our results and established findings from previous studies, bDMARDs outperform cDMARDs with respect to the ultimate therapeutic goal—inhibition of structural progression.
In our multivariable regression analysis of clinical factors, ESR at the start of the interval was significantly associated with greater mSASSS change, as shown in previous reports,37,38 reflecting that baseline inflammation is a strong predictor of future radiographic progression. Eye involvement was also identified as a significant variable affecting radiographic progression. Given that uveitis presence in AS is associated with disease duration,42 the long observation period of our study may have increased uveitis incidence in this study, thus contributing to the strong correlations we found. Conversely, the mSASSS progression rate in patients with peripheral arthritis was significantly lower than in patients without peripheral arthritis, which is consistent with a previous finding.43 The inhibitory effect of NSAIDs on radiographic progression, which has been shown in some previous studies,44,45 was not confirmed in this study. There was no significant association between NSAID use, which was based on 1-year intervals with on/off periods of NSAID treatment, and a reduced mSASSS progression rate. However, because a considerable number of patients received on-demand NSAID treatment, it was difficult to collect complete information related to the dose and frequency of NSAID intake. Therefore, NSAID intake was based on the use of binary measurements and this might have influenced the outcome given that the effect of NSAIDs on structural progression may be dose-related.45
The mean adjusted mSASSS change per year calculated from the multivariable models was lower than that of our previous study, where the measured mSASSS value was 0.914 with TNF inhibitor therapy and 0.970 without TNF inhibitor therapy.11 The relatively lower mSASSS change value in this study might be attributable to the clinical characteristics of patients who have not been exposed to TNF inhibitors during the included observation period due to a lower risk of spinal damage progression. Meanwhile, according to two recent systematic reviews concerning the effect of therapy on radiographic progression, mean mSASSS change varied considerably between studies.20,21 Because different follow-up durations and different data-management approaches can heavily influence outcomes, especially given the slowly progressing nature and heterogeneity of AS and the added problem of confounding by indication, a direct comparison of our results with other studies would be not appropriate.
This study has several limitations. First, smoking, which has been shown to be predictive of spinal radiographic progression,46 was not included in the covariates due to too many missing values. There was a significant lack of available data on smoking status in medical records, forcing them to be excluded from the analysis. Thus, smoking was not adjusted for and this may have influenced the outcome. Additionally, other factors that may have an impact on structural damage progression, such as obesity47 or occupational status (white-collar versus blue-collar),48 were not included in the covariates. Second, we performed this study under the assumption that patients took their medicine regularly and as prescribed. Therefore, there may be unmeasured confounders, such as non-adherence or a discrepancy between the date of prescription and administration. Third, because this study was based on medical records during a long-term observation period with variability in follow-up periods, continuous variables were imputed by the interpolation method at a specific time point. Missing mSASSS data at beginning and end timepoints of the intervals were also handled by linear interpolation with consideration of the slow progression of spinal structural damage. Therefore, the imputed values may be different from the actual values, which could introduce unexpected bias.
However, given that a randomized placebo-controlled comparison of a cDMARDs treatment group with an untreated group in patients with axial SpA is not feasible, our results derived from real-world data have strength in that they reflect daily clinical practice. Furthermore, as the first study to show that cDMARDs are not effective in slowing spinal radiographic progression based on validated outcome measures, this could serve as a reference study for other countries where reimbursement regulations require routine use of cDMARDs before switching to TNF inhibitor therapies or where financial constraints limit the use of TNF inhibitors.6,7,49,50
Conclusion
Our study shows that cDMARDs have no significant effect in slowing radiographic progression in AS patients. Given the recent findings of the effectiveness of biologics in slowing spinal structural damage, use of TNF inhibitors or IL-17 inhibitors, rather than cDMARDs, should be considered to inhibit spinal damage especially for patients with a high risk of radiographic progression.
Acknowledgments
We are grateful to the nurses who helped collect patient data in the clinics for many years.
Footnotes
Contributors: BSK, JSO, SYP, and THK made contributions to the study conception and design. All authors contributed to data acquisition, analysis, or interpretation. SL and KBJ scored the spinal radiographs independently. JSO and SYP were responsible for the statistical analyses. THL and BSK drafted the manuscript and all coauthors were involved in critical revisions for maintenance of intellectual content. NB and THK provided administrative, technical, or material support. THK had full access to all study data and takes responsibility for data integrity and data-analysis accuracy. All authors approved the final version to be submitted for publication.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval: This study was approved by the Hanyang University Seoul Hospital Institutional Review Board (HYUH 2014-04-010).
ORCID iDs: Tae-Han Lee  https://orcid.org/0000-0001-7455-9534
https://orcid.org/0000-0001-7455-9534
Bon San Koo  https://orcid.org/0000-0002-4212-2634
https://orcid.org/0000-0002-4212-2634
Tae-Hwan Kim  https://orcid.org/0000-0002-3542-2276
https://orcid.org/0000-0002-3542-2276
Contributor Information
Tae-Han Lee, Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea.
Bon San Koo, Department of Internal Medicine, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
Bora Nam, Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea.
Ji Seon Oh, Department of Biomedical Informatics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Seo Young Park, Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Seunghun Lee, Department of Radiology, Hanyang University Hospital, Seoul, Korea.
Kyung Bin Joo, Department of Radiology, Hanyang University Hospital, Seoul, Korea.
Tae-Hwan Kim, Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, 222-1, Wangsimni-ro, Seongdong-gu, Seoul 04763, Korea.
References
- 1. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 2. Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019; 71: 1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tam LS, Wei JC, Aggarwal A, et al. 2018. APLAR axial spondyloarthritis treatment recommendations. Int J Rheum Dis 2019; 22: 340–356. [DOI] [PubMed] [Google Scholar]
- 4. Nissilä M, Lehtinen K, Leirisalo-Repo M, et al. Sulfasalazine in the treatment of ankylosing spondylitis: a twenty-six–week, placebo-controlled clinical trial. Arthritis Rheum 1988; 31: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 5. Braun J, Zochling J, Baraliakos X, et al. Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicentre randomised controlled trial. Ann Rheum Dis 2006; 65: 1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Relas H, Kautiainen H, Puolakka K, et al. Survival of disease-modifying antirheumatic drugs used as the first antirheumatic medication in the treatment of ankylosing spondylitis in Finland. A nationwide population-based register study. Clin Rheumatol 2014; 33: 1135–1138. [DOI] [PubMed] [Google Scholar]
- 7. Nair AM, Sandhya P, Yadav B, et al. TNFα blockers followed by continuation of sulfasalazine and methotrexate combination: a retrospective study on cost saving options of treatment in spondyloarthritis. Clin Rheumatol 2017; 36: 2243–2251. [DOI] [PubMed] [Google Scholar]
- 8. Bodur H, Ataman S, Buğdaycı DS, et al. Description of the registry of patients with ankylosing spondylitis in Turkey: TRASD-IP. Rheumatol Int 2012; 32: 169–176. [DOI] [PubMed] [Google Scholar]
- 9. Huscher D, Thiele K, Rudwaleit M, et al. Trends in treatment and outcomes of ankylosing spondylitis in outpatient rheumatological care in Germany between 2000 and 2012. RMD Open 2015; 1: e000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park E-J, Kim H, Jung SM, et al. The use of biological disease-modifying antirheumatic drugs for inflammatory arthritis in Korea: results of a Korean expert consensus. J Rheum Dis 2020; 27: 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koo BS, Oh JS, Park SY, et al. Tumor necrosis factor inhibitors slow radiographic progression in patients with ankylosing spondylitis: 18-year real-world evidence. Ann Rheum Dis 2020; 79: 1327–1332. [DOI] [PubMed] [Google Scholar]
- 12. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 13. Creemers MC, Franssen MJ, van’t Hof MA, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005; 64: 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finckh A, Liang MH, van Herckenrode CM, et al. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum 2006; 55: 864–872. [DOI] [PubMed] [Google Scholar]
- 15. Clegg DO, Reda DJ, Abdellatif M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral manifestations of the seronegative spondyloarthropathies: a department of Veterans affairs cooperative study. Arthritis Rheum 1999; 42: 2325–2329. [DOI] [PubMed] [Google Scholar]
- 16. Haibel H, Rudwaleit M, Braun J, et al. Six months open label trial of leflunomide in active ankylosing spondylitis. Ann Rheum Dis 2005; 64: 124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haibel H, Brandt HC, Song IH, et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis 2007; 66: 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J, Lin S, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2014; 11: CD004800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Veras MM, Liu C, et al. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2013; 2: CD004524. [DOI] [PubMed] [Google Scholar]
- 20. Karmacharya P, Duarte-Garcia A, Dubreuil M, et al. Effect of therapy on radiographic progression in axial spondyloarthritis: a systematic review and meta-analysis. Arthritis Rheumatol 2020; 72: 733–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baraliakos X, Gensler LS, D’Angelo S, et al. Biologic therapy and spinal radiographic progression in patients with axial spondyloarthritis: a structured literature review. Ther Adv Musculoskelet Dis 2020; 12: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet 1998; 352: 1137–1140. [DOI] [PubMed] [Google Scholar]
- 23. Schett G, Lories RJ, D’Agostino MA, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol 2017; 13: 731–741. [DOI] [PubMed] [Google Scholar]
- 24. Sari I, Haroon N. Disease modification in axial spondyloarthritis. Best Pract Res Clin Rheumatol 2018; 32: 427–439. [DOI] [PubMed] [Google Scholar]
- 25. Lehtinen A, Leirisalo-Repo M, Taavitsainen M. Persistence of enthesopathic changes in patients with spondylarthropathy during a 6-month follow-up. Clin Exp Rheumatol 1995; 13: 733–736. [PubMed] [Google Scholar]
- 26. Genc H, Duyur Cakit B, Nacir B, et al. The effects of sulfasalazine treatment on enthesal abnormalities of inflammatory rheumatic diseases. Clin Rheumatol 2007; 26: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 27. Gravallese EM, Schett G. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 2018; 14: 631–640. [DOI] [PubMed] [Google Scholar]
- 28. Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol 2014; 5: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magrey MN, Khan MA. The paradox of bone formation and bone loss in ankylosing spondylitis: evolving new concepts of bone formation and future trends in management. Curr Rheumatol Rep 2017; 19: 17. [DOI] [PubMed] [Google Scholar]
- 30. Sieper J, Poddubnyy D, Miossec P. The IL-23–IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol 2019; 15: 747–757. [DOI] [PubMed] [Google Scholar]
- 31. Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis 2017; 76: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 32. Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology (Oxford) 2019; 58: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009; 60: 93–102. [DOI] [PubMed] [Google Scholar]
- 34. Maksymowych WP, Morency N, Conner-Spady B, et al. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 2013; 72: 23–28. [DOI] [PubMed] [Google Scholar]
- 35. Machado PM, Baraliakos X, van der Heijde D, et al. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2016; 75: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 36. Aouad K, Ziade N, Baraliakos X. Structural progression in axial spondyloarthritis. Joint Bone Spine 2020; 87: 131–136. [DOI] [PubMed] [Google Scholar]
- 37. Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013; 65: 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molnar C, Scherer A, Baraliakos X, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2018; 77: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun J, van der Horst-Bruinsma IE, Huang F, et al. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum 2011; 63: 1543–1551. [DOI] [PubMed] [Google Scholar]
- 40. Song IH, Hermann KG, Haibel H, et al. Effects of etanercept versus sulfasalazine in early axial spondyloarthritis on active inflammatory lesions as detected by whole-body MRI (ESTHER): a 48-week randomised controlled trial. Ann Rheum Dis 2011; 70: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rios Rodriguez V, Hermann KG, Weiß A, et al. Progression of structural damage in the sacroiliac joints in patients with early axial spondyloarthritis during long-term anti-tumor necrosis factor treatment: six-year results of continuous treatment with etanercept. Arthritis Rheumatol 2019; 71: 722–728. [DOI] [PubMed] [Google Scholar]
- 42. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, et al. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 65–73. [DOI] [PubMed] [Google Scholar]
- 43. Kim TJ, Lee S, Joo KB, et al. The presence of peripheral arthritis delays spinal radiographic progression in ankylosing spondylitis: observation study of the Korean Spondyloarthropathy Registry. Rheumatology (Oxford) 2014; 53: 1404–1408. [DOI] [PubMed] [Google Scholar]
- 44. Wanders A, van der Heijde D, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005; 52: 1756–1765. [DOI] [PubMed] [Google Scholar]
- 45. Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German spondyloarthritis inception cohort. Ann Rheum Dis 2012; 71: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 46. Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 2012; 64: 1388–1398. [DOI] [PubMed] [Google Scholar]
- 47. Deminger A, Klingberg E, Geijer M, et al. A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther 2018; 20: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramiro S, Landewé R, van Tubergen A, et al. Lifestyle factors may modify the effect of disease activity on radiographic progression in patients with ankylosing spondylitis: a longitudinal analysis. RMD Open 2015; 1: e000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Can M, Aydin SZ, Niğdelioğlu A, et al. Conventional DMARD therapy (methotrexate-sulphasalazine) may decrease the requirement of biologics in routine practice of ankylosing spondylitis patients: a real-life experience. Int J Rheum Dis 2012; 15: 526–530. [DOI] [PubMed] [Google Scholar]
- 50. Khanna Sharma S, Kadiyala V, Naidu G, et al. A randomized controlled trial to study the efficacy of sulfasalazine for axial disease in ankylosing spondylitis. Int J Rheum Dis 2018; 21: 308–314. [DOI] [PubMed] [Google Scholar]